Impact of Maturation on Myocardial Response to Ischemia and the Effectiveness of Remote Preconditioning in Male Rats
Abstract
:1. Introduction
2. Results
2.1. Effect of Aging on the Basic Biometric Parameters of Rats
2.2. Characteristics of Isolated Hearts: Effect of Age/Maturation on Functional Parameters of Isolated Rat Hearts
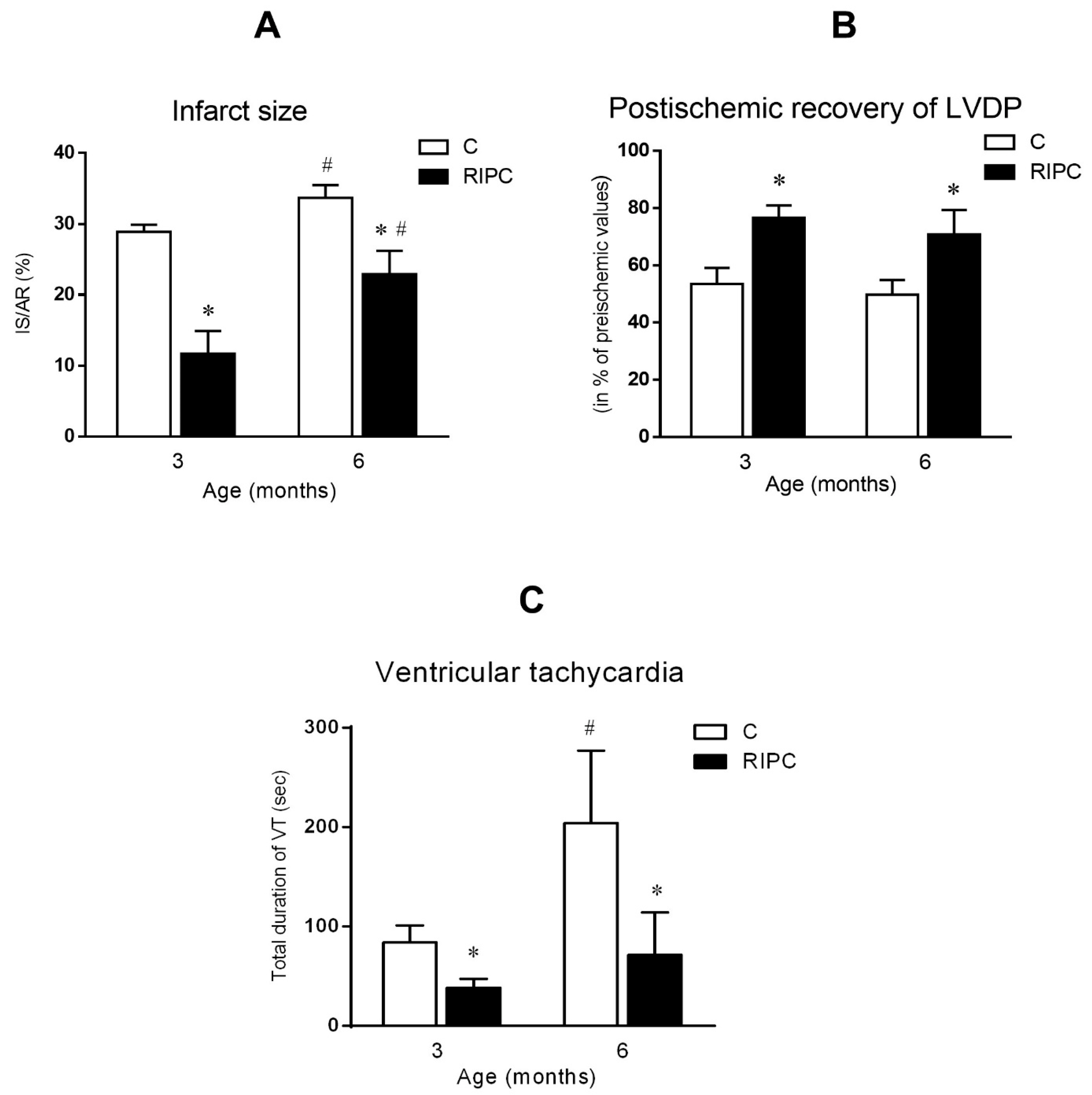
2.3. The Effect of Age and Remote Ischemic Preconditioning on Ischemia/Reperfusion Injury
2.4. The Effect of Age on the Changes in the Cell Signaling of Remote Ischemic Preconditioning
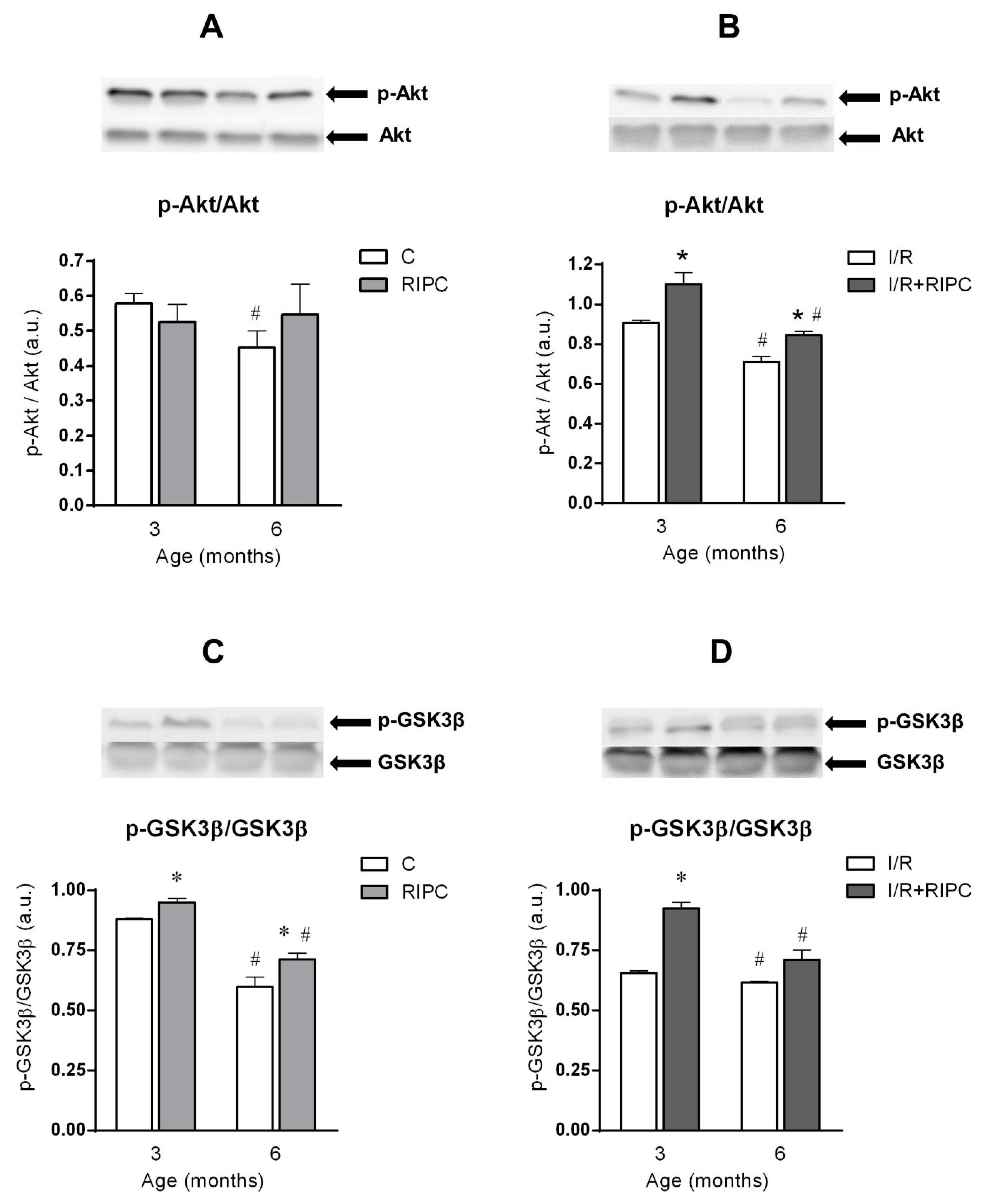
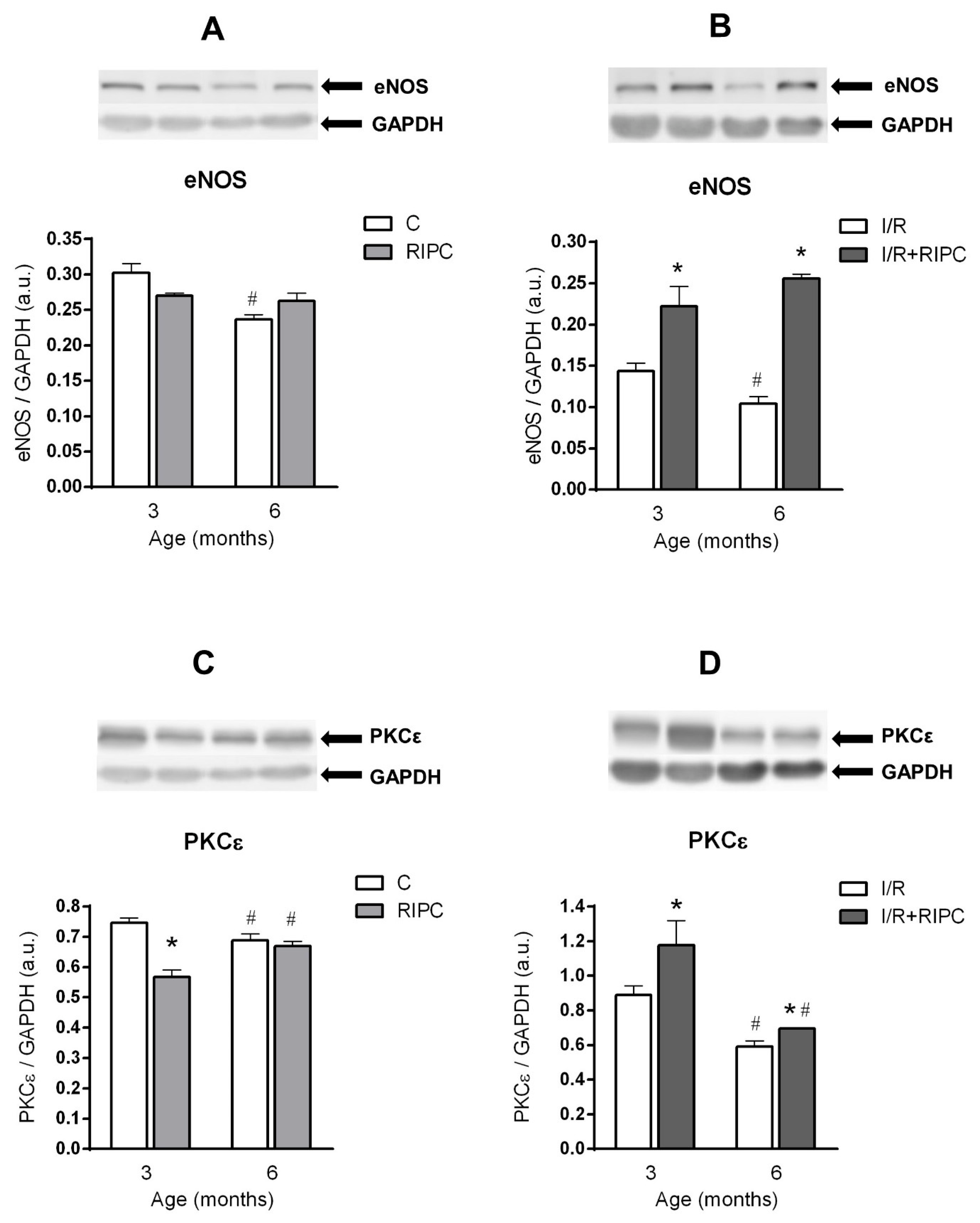
2.4.1. The Effect of Age and Remote Ischemic Preconditioning on the Expression of Selected RISK Pathway Proteins in Rat Myocardium at Baseline Conditions
2.4.2. The Effect of Age and Remote Ischemic Preconditioning on the Expression of Selected RISK Pathway Proteins in Rat Myocardium after Ischemia and Reperfusion
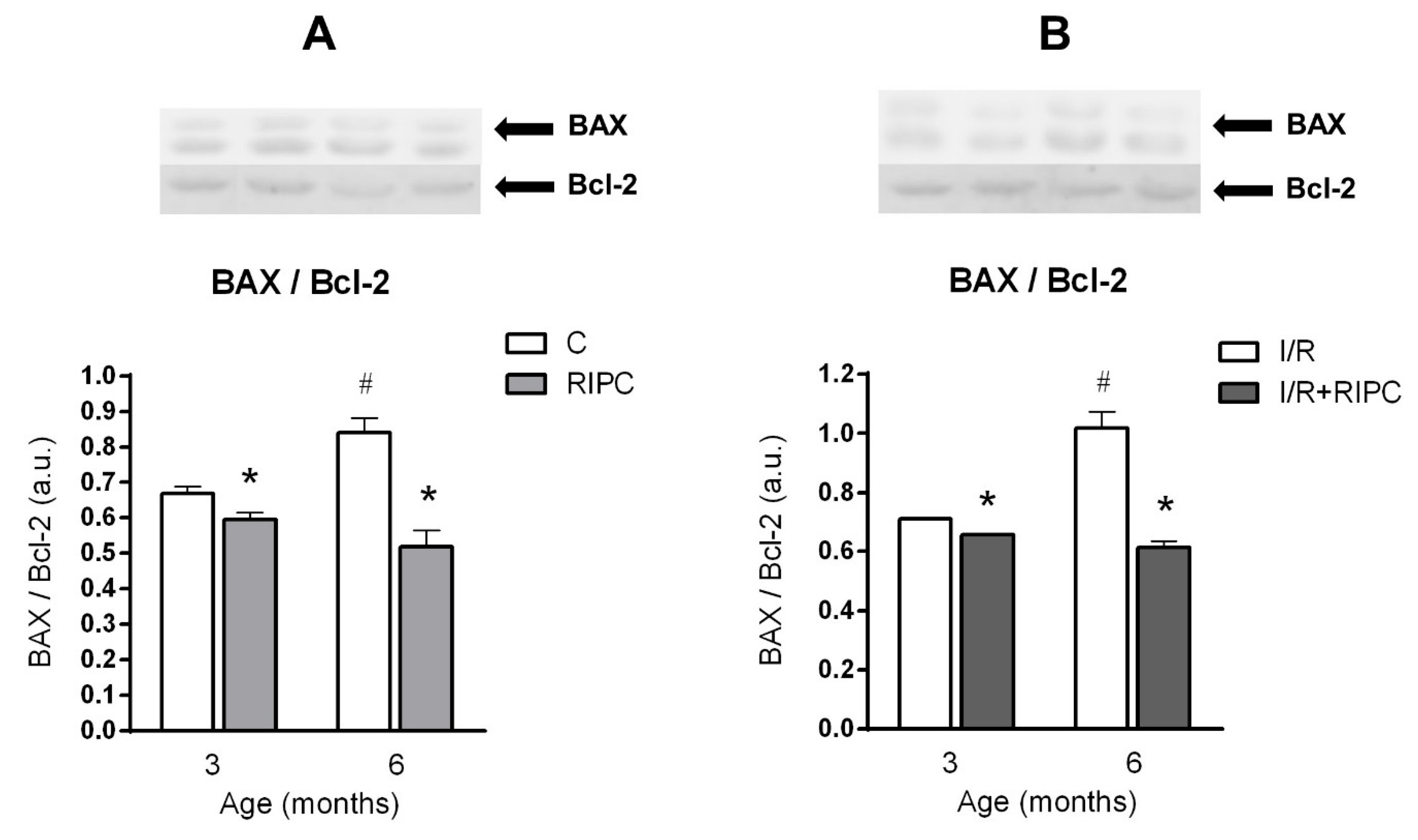
2.5. The Effect of Age and Remote Ischemic Preconditioning on the Expression of Selected Proteins of Pro- and Anti-Apoptotic Cascades in Rat Myocardium
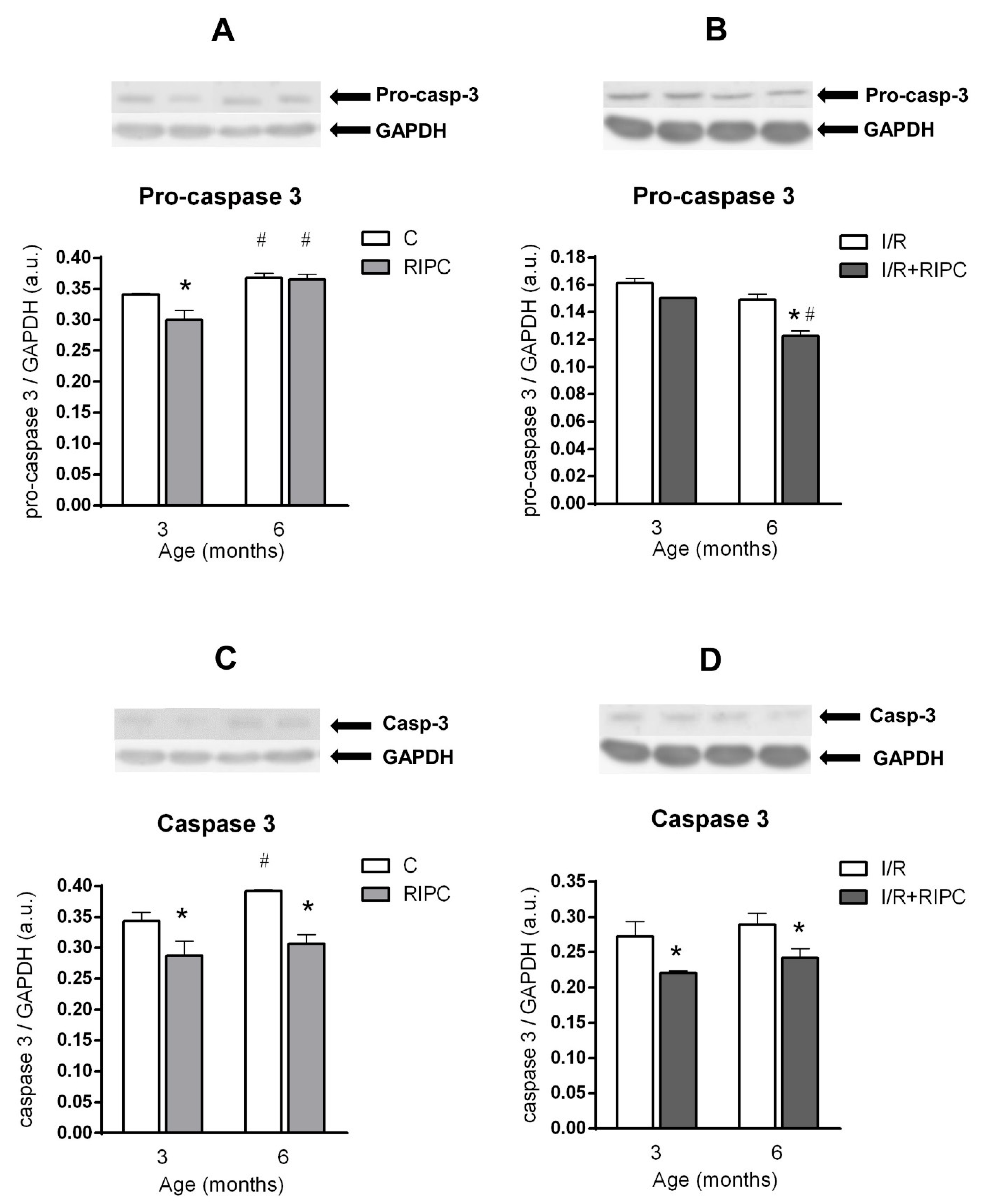
2.5.1. The Effect of Age and Remote Ischemic Preconditioning on the Expression of Selected Proteins of Pro- and Anti-Apoptotic Cascades in Rat Myocardium at the Baseline Condition
2.5.2. The Effect of Age and Remote Ischemic Preconditioning on the Expression of Selected Proteins of Pro- and Anti-Apoptotic Cascades in Rat Myocardium after Ischemia and Reperfusion
3. Discussion
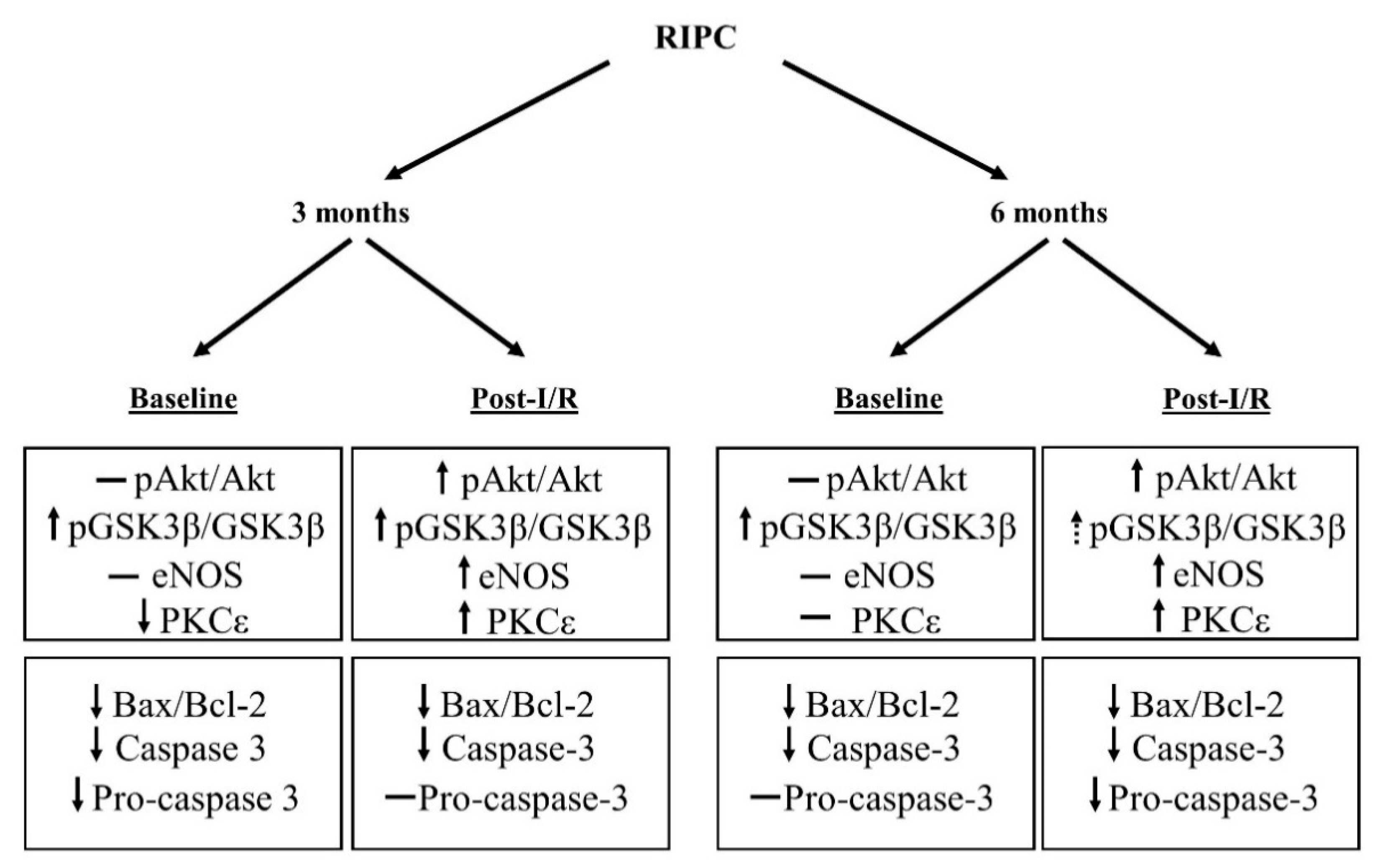
3.1. Cell Signaling of RIPC in Relation with Maturation
3.2. Effect of Maturation and Remote Preconditioning on Pro- and Anti-Apoptotic Mechanisms
4. Materials and Methods
4.1. Animals
4.2. Perfusion Technique
4.3. Experimental Protocols
4.4. Induction of Ischemia/Reperfusion
4.5. Quantification of Arrhythmias
4.6. Determination of Infarct Size
4.7. Preparation of Tissue Protein Fractions
4.8. Electrophoresis and Western Blot Analysis
4.9. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| BAX | pro-apoptotic protein from Bcl-2 protein family (B-cell lymphoma 2) |
| Bcl-2 | anti-apoptotic protein from Bcl-2 protein family |
| eNOS | endothelial nitric oxide synthase |
| ERK1/2 | extracellular signal-regulated kinases1/2 |
| IHD | ischemic heart disease |
| I/R | ischemia reperfusion |
| IS | infarct size |
| LV | left ventricle |
| LVDP | left ventricular developed pressure |
| MPTP | mitochondrial permeability transition pore |
| p-Akt | phosphorylated (activated) Akt (protein kinase B) |
| p-GSK3β p | phosphorylated (inactivated) glycogen synthase kinase 3 beta |
| PI3K | phosphoinositide 3-kinase |
| PKCε | protein kinase C epsilon |
| RIPC | remote ischemic preconditioning |
| RISK | Reperfusion Injury Salvage Kinase |
| VT | ventricular tachycardia |
References
- Khan, M.A.; Hashim, M.J.; Mustafa, H.; Baniyas, M.Y.; Al Suwaidi, S.K.B.M.; AlKatheeri, R.; Alblooshi, F.M.K.; Almatrooshi, M.E.A.H.; Alzaabi, M.E.H.; Al Darmaki, R.S.; et al. Global Epidemiology of Ischemic Heart Disease: Results from the Global Burden of Disease Study. Cureus 2020, 12, e934. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Cheung, M.M.H.; Kharbanda, R.K.; Konstantinov, I.E.; Shimizu, M.; Frndova, H.; Li, J.; Holtby, H.M.; Cox, P.N.; Smallhorn, J.F.; Van Arsdell, G.S.; et al. Randomized Controlled Trial of the Effects of Remote Ischemic Preconditioning on Children Undergoing Cardiac Surgery. J. Am. Coll. Cardiol. 2006, 47, 2277–2282. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Mwamure, P.K.; Venugopal, V.; Harris, J.; Barnard, M.; Grundy, E.; Ashley, E.; Vichare, S.; Di Salvo, C.; Kolvekar, S.; et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: A randomised controlled trial. Lancet 2007, 370, 575–579. [Google Scholar] [CrossRef]
- Albrecht, M.; Zitta, K.; Bein, B.; Wennemuth, G.; Broch, O.; Renner, J.; Schuett, T.; Lauer, F.; Maahs, D.; Hummitzsch, L.; et al. Remote ischemic preconditioning regulates HIF-1α levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: A pilot experimental study. Basic Res. Cardiol. 2013, 108, 314. [Google Scholar] [CrossRef]
- Venugopal, V.; Hausenloy, D.J.; Ludman, A.; Di Salvo, C.; Kolvekar, S.; Yap, J.; Lawrence, D.; Bognolo, J.; Yellon, D.M. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: A randomised controlled trial. Heart 2009, 95, 1567–1571. [Google Scholar] [CrossRef] [Green Version]
- White, S.K.; Frohlich, G.M.; Sado, D.M.; Maestrini, V.; Fontana, M.; Treibel, T.A.; Tehrani, S.; Flett, A.S.; Meier, P.; Ariti, C.; et al. Remote Ischemic Conditioning Reduces Myocardial Infarct Size and Edema in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2015, 8, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Ferdinandy, P.; Hausenloy, D.J.; Heusch, G.; Baxter, G.F.; Schulz, R. Interaction of Risk Factors, Comorbidities, and Comedications with Ischemia/Reperfusion Injury and Cardioprotection by Preconditioning, Postconditioning, and Remote Conditioning. Pharmacol. Rev. 2014, 66, 1142–1174. [Google Scholar] [CrossRef]
- Obas, V.; Vasan, R.S. The aging heart. Clin. Sci. 2018, 132, 1367–1382. [Google Scholar] [CrossRef]
- Ruiz-Meana, M.; Boengler, K.; Garcia-Dorado, D.; Hausenloy, D.J.; Kaambre, T.; Kararigas, G.; Perrino, C.; Schulz, R.; Ytrehus, K. Ageing, sex, and cardioprotection. Br. J. Pharmacol. 2020, 177, 5270–5286. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Garcia-Dorado, D.; Bøtker, H.E.; Davidson, S.M.; Downey, J.; Engel, F.B.; Jennings, R.; Lecour, S.; Leor, J.; Madonna, R.; et al. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2017, 113, 564–585. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Yellon, D.M. Reperfusion injury salvage kinase signalling: Taking a RISK for cardioprotection. Heart Fail. Rev. 2007, 12, 217–234. [Google Scholar] [CrossRef]
- Juhaszova, M.; Zorov, D.B.; Kim, S.-H.; Pepe, S.; Fu, Q.; Fishbein, K.W.; Ziman, B.D.; Wang, S.; Ytrehus, K.; Antos, C.L.; et al. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Investig. 2004, 113, 1535–1549. [Google Scholar] [CrossRef]
- Rossello, X.; Riquelme, J.A.; Davidson, S.M.; Yellon, D.M. Role of PI3K in myocardial ischaemic preconditioning: Mapping pro-survival cascades at the trigger phase and at reperfusion. J. Cell. Mol. Med. 2017, 22, 926–935. [Google Scholar] [CrossRef] [Green Version]
- Griecsová, L.; Farkašová, V.; Gáblovský, I.; Khandelwal, V.K.M.; Bernátová, I.; Tatarková, Z.; Kaplan, P.; Ravingerová, T. Effect of Maturation on the Resistance of Rat Hearts Against Ischemia. Study of Potential Molecular Mechanisms. Physiol. Res. 2015, 64, S685–S696. [Google Scholar] [CrossRef]
- Ravingerova, T.; Farkasova, V.; Griecsova, L.; Carnicka, S.; Murarikova, M.; Barlaka, E.; Kolar, F.; Bartekova, M.; Lonek, L.; Slezak, J.; et al. Remote Preconditioning as a Novel “Conditioning” Approach to Repair the Broken Heart: Potential Mechanisms and Clinical Applications. Physiol. Res. 2016, 65, S55–S64. [Google Scholar] [CrossRef]
- Schmidt, M.R.; Støttrup, N.B.; Michelsen, M.M.; Contractor, H.; Sørensen, K.E.; Kharbanda, R.K.; Redington, A.N.; Bøtker, H.E. Remote ischemic preconditioning impairs ventricular function and increases infarct size after prolonged ischemia in the isolated neonatal rabbit heart. J. Thorac. Cardiovasc. Surg. 2014, 147, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Behmenburg, F.; Heinen, A.; Vom Bruch, L.; Hollmann, M.W.; Huhn, R. Cardioprotection by Remote Ischemic Preconditioning is Blocked in the Aged Rat Heart in Vivo. J. Cardiothorac. Vasc. Anesth. 2017, 31, 1223–1226. [Google Scholar] [CrossRef]
- Turcato, S.; Turnbull, L.; Wang, G.-Y.; Honbo, N.; Simpson, P.C.; Karliner, J.S.; Baker, A.J. Ischemic preconditioning depends on age and gender. Basic Res. Cardiol. 2006, 101, 235–243. [Google Scholar] [CrossRef]
- Ledvenyiova, V.; Pancza, D.; Matejiková, J.; Ferko, M.; Bernatova, I.; Ravingerova, T. Impact of age and sex on response to ischemic preconditioning in the rat heart: Differential role of the PI3K–AKT pathway. Can. J. Physiol. Pharmacol. 2013, 91, 640–647. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Boengler, K.; Schulz, R.; Heusch, G. Loss of cardioprotection with ageing. Cardiovasc. Res. 2009, 83, 247–261. [Google Scholar] [CrossRef]
- Kostyak, J.; Hunter, J.; Korzick, D. Acute PKCδ inhibition limits ischaemia–reperfusion injury in the aged rat heart: Role of GSK-3β. Cardiovasc. Res. 2006, 70, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Korzick, D.H.; Kostyak, J.C.; Hunter, J.C.; Saupe, K.W. Local delivery of PKCε-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3β but not F1-ATPase inactivation. Am. J. Physiol. Circ. Physiol. 2007, 293, H2056–H2063. [Google Scholar] [CrossRef] [Green Version]
- Heinen, N.M.; Pütz, V.E.; Görgens, J.I.; Huhn, R.; Grüber, Y.; Barthuber, C.; Preckel, B.; Pannen, B.H.; Bauer, I. Cardioprotection by Remote Ischemic Preconditioning Exhibits a Signaling Pattern Different from Local Ischemic Preconditioning. Shock 2011, 36, 45–53. [Google Scholar] [CrossRef]
- Singh, B.; Randhawa, P.K.; Singh, N.; Jaggi, A.S. Investigations on the role of leukotrienes in remote hind limb preconditioning-induced cardioprotection in rats. Life Sci. 2016, 152, 238–243. [Google Scholar] [CrossRef]
- Sharma, R.; Randhawa, P.K.; Singh, N.; Jaggi, A.S. Possible role of thromboxane A2 in remote hind limb preconditioning-induced cardioprotection. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 1–9. [Google Scholar] [CrossRef]
- Galagudza, M.M.; Sonin, D.L.; Vlasov, T.D.; Kurapeev, D.I.; Shlyakhto, E.V. Remote vs. local ischaemic preconditioning in the rat heart: Infarct limitation, suppression of ischaemic arrhythmia and the role of reactive oxygen species. Int. J. Exp. Pathol. 2016, 97, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Donato, M.; Goyeneche, M.A.; Garces, M.; Marchini, T.; Pérez, V.; del Mauro, J.; Höcht, C.; Rodríguez, M.; Evelson, P.; Gelpi, R.J. Myocardial triggers involved in activation of remote ischaemic preconditioning. Exp. Physiol. 2016, 101, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.-H.; Kim, J.-H.; Lee, Y.-C. Mitochondrial ATP-Sensitive Potassium Channels Play a Role in Reducing Both Myocardial Infarction and Reperfusion Arrhythmia in Remote Ischemic Preconditioned Hearts. Anesthesiol. Pain Med. 2017, 7, e42505. [Google Scholar] [CrossRef] [Green Version]
- Randhawa, P.K.; Jaggi, A.S. Investigating the involvement of glycogen synthase kinase-3β and gap junction signaling in TRPV1 and remote hind preconditioning-induced cardioprotection. Eur. J. Pharmacol. 2017, 814, 9–17. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Yu, P.; Chen, M.; Peng, Q.; Wang, Z.; Dong, N. Remote Ischaemic Preconditioning and Sevoflurane Postconditioning Synergistically Protect Rats from Myocardial Injury Induced by Ischemia and Reperfusion Partly via Inhibition TLR4/MyD88/NF-κB Signaling Pathway. Cell. Physiol. Biochem. 2017, 41, 22–32. [Google Scholar] [CrossRef]
- You, L.; Pan, Y.-Y.; An, M.-Y.; Chen, W.-H.; Zhang, Y.; Wu, Y.-N.; Li, Y.; Sun, K.; Yin, Y.-Q.; Lou, J.-S. The Cardioprotective Effects of Remote Ischemic Conditioning in a Rat Model of Acute Myocardial Infarction. Med. Sci. Monit. 2019, 25, 1769–1779. [Google Scholar] [CrossRef]
- Heinen, A.; Behmenburg, F.; Aytulun, A.; Dierkes, M.; Zerbin, L.; Kaisers, W.; Schaefer, M.; Meyer-Treschan, T.; Feit, S.; Bauer, I.; et al. The release of cardioprotective humoral factors after remote ischemic preconditioning in humans is age- and sex-dependent. J. Transl. Med. 2018, 16, 112. [Google Scholar] [CrossRef]
- Whittington, H.J.; Harding, I.; Stephenson, C.I.M.; Bell, R.; Hausenloy, D.J.; Mocanu, M.M.; Yellon, D.M. Cardioprotection in the aging, diabetic heart: The loss of protective Akt signalling. Cardiovasc. Res. 2013, 99, 694–704. [Google Scholar] [CrossRef] [Green Version]
- Tani, M.; Suganuma, Y.; Hasegawa, H.; Shinmura, K.; Hayashi, Y.; Guo, X.; Nakamura, Y. Changes in Ischemic Tolerance and Effects of Ischemic Preconditioning in Middle-aged Rat Hearts. Circulation 1997, 95, 2559–2566. [Google Scholar] [CrossRef]
- Abete, P.; Testa, G.; Ferrara, N.; De Santis, D.; Capaccio, P.; Viati, L.; Calabrese, C.; Cacciatore, F.; Longobardi, G.; Condorelli, M.; et al. Cardioprotective effect of ischemic preconditioning is preserved in food-restricted senescent rats. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1978–H1987. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Hu, S.; Yang, S.; Chen, M.; Zhang, P.; Liu, J.; Abbott, G.W. Remote Liver Ischemic Preconditioning Protects against Sudden Cardiac Death via an ERK/GSK-3β-Dependent Mechanism. PLoS ONE 2016, 11, e0165123. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, M.; Zhang, P.; Liu, J.; Abbott, G.W. Remote ischemic preconditioning differentially attenuates post-ischemic cardiac arrhythmia in streptozotocin-induced diabetic versus nondiabetic rats. Cardiovasc. Diabetol. 2017, 16, 57. [Google Scholar] [CrossRef] [Green Version]
- Iemitsu, M.; Maeda, S.; Jesmin, S.; Otsuki, T.; Miyauchi, T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am. J. Physiol. Circ. Physiol. 2006, 291, H1290–H1298. [Google Scholar] [CrossRef]
- Ren, J.; Dong, F.; Cai, G.-J.; Zhao, P.; Nunn, J.M.; Wold, L.E.; Pei, J. Interaction between Age and Obesity on Cardiomyocyte Contractile Function: Role of Leptin and Stress Signaling. PLoS ONE 2010, 5, e10085. [Google Scholar] [CrossRef]
- Rybin, V.O.; Steinberg, S.F. Protein kinase C isoform expression and regulation in the developing rat heart. Circ. Res. 1994, 74, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.C.; Korzick, D.H. Age- and sex-dependent alterations in protein kinase C (PKC) and extracellular regulated kinase 1/2 (ERK1/2) in rat myocardium. Mech. Ageing Dev. 2005, 126, 535–550. [Google Scholar] [CrossRef]
- Zhu, J.; Rebecchi, M.J.; Glass, P.S.A.; Brink, P.R.; Liu, L. Interactions of GSK-3 with Mitochondrial Permeability Transition Pore Modulators during Preconditioning: Age-Associated Differences. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xuan, W.; Yan, R.; Tropak, M.B.; Jean-St-Michel, E.; Liang, W.; Gladstone, R.; Backx, P.H.; Kharbanda, R.K.; Redington, A.N. Remote preconditioning provides potent cardioprotection via PI3K/Akt activation and is associated with nuclear accumulation of β-catenin. Clin. Sci. 2011, 120, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Xu, B.; Cavalieri, T.A.; Hock, C.E. Age-related difference in myocardial function and inflammation in a rat model of myocardial ischemia-reperfusion. Cardiovasc. Res. 2002, 56, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Phaneuf, S.; Leeuwenburgh, C. Cytochrome c release from mitochondria in the aging heart: A possible mechanism for apoptosis with age. Am. J. Physiol. Integr. Comp. Physiol. 2002, 282, R423–R430. [Google Scholar] [CrossRef] [Green Version]
- Kwak, H.; Song, W.; Lawler, J.M. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006, 20, 791–793. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Wang, S.; Mao, X.; Yan, D.; Wong, S.S.; Xia, Z.; Irwin, M.G. Repeated Non-Invasive Limb Ischemic Preconditioning Confers Cardioprotection Through PKC-ε/STAT3 Signaling in Diabetic Rats. Cell. Physiol. Biochem. 2018, 45, 2107–2121. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Yan, M.; Wu, P.; Qing, Y.; Li, S.; Li, Y.; Dong, Z.; Xia, H.; Huang, D.; Xin, P.; et al. Combination of remote ischemic perconditioning and remote ischemic postconditioning fails to increase protection against myocardial ischemia/reperfusion injury, compared with either alone. Mol. Med. Rep. 2016, 13, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.-L.; Kong, F.-J.; Guo, J.-J.; Zhu, J.-B.; Shi, H.-T.; Li, Y.; Sun, R.-H.; Ge, J.-B. Hypercholesterolemia Abrogates Remote Ischemic Preconditioning-Induced Cardioprotection. Shock 2017, 47, 363–369. [Google Scholar] [CrossRef]
- Yang, S.; Abbott, G.W.; Gao, W.D.; Liu, J.; Luo, C.; Hu, Z. Involvement of glycogen synthase kinase-3β in liver ischemic conditioning induced cardioprotection against myocardial ischemia and reperfusion injury in rats. J. Appl. Physiol. 2017, 122, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Iliodromitis, E.K.; Andreadou, I.; Papalois, A.; Gritsopoulos, G.; Anastasiou-Nana, M.; Kremastinos, D.T.; Yellon, D.M. Investigating the Signal Transduction Pathways Underlying Remote Ischemic Conditioning in the Porcine Heart. Cardiovasc. Drugs Ther. 2012, 26, 87–93. [Google Scholar] [CrossRef]
- Andreadou, I.; Bibli, S.I.; Mastromanolis, E.; Zoga, A.; Efentakis, P.; Papaioannou, N.; Farmakis, D.; Kremastinos, D.T.; Iliodromitis, E.K. Transient carotid ischemia as a remote conditioning stimulus for myocardial protection in anesthetized rabbits: Insights into intracellular signaling. Int. J. Cardiol. 2015, 184, 140–151. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, X.-J.; Zong, Q.-F.; Zhang, G.-J.; Ye, H.-W.; Hu, J.; Gao, Q.; Guan, S.-D. Remote ischemic postconditioning protects the heart by upregulating ALDH2 expression levels through the PI3K/Akt signaling pathway. Mol. Med. Rep. 2014, 10, 536–542. [Google Scholar] [CrossRef] [Green Version]
- An, M.Y.; Li, Y.; Chen, W.H.; Zhang, Y.; Wu, Y.N.; Sun, K.; Pan, Y.Y.; Yin, Y.Q.; Lou, J.S. Effects of non-invasive remote ischemic conditioning on rehabilitation after myocardial infarction. Biochem. Biophys. Res. Commun. 2017, 488, 278–284. [Google Scholar] [CrossRef]
- Curtis, M.J.; Walker, M.J.A. Quantification of arrhythmias using scoring systems: An examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc. Res. 1988, 22, 656–665. [Google Scholar] [CrossRef]
- Ravingerová, T.; Matejíková, J.; Neckář, J.; Andelová, E.; Kolář, F. Differential role of PI3K/Akt pathway in the infarct size limitation and antiarrhythmic protection in the rat heart. Mol. Cell. Biochem. 2007, 297, 111–120. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

| Group | BW (g) | HW (mg) |
|---|---|---|
| 3 months | 250 ± 10 | 800 ± 14 |
| 6 months | 376 ± 35 * | 1100 ± 20 * |
| Group (Age) | HR (Beats/min) | LVSP (mmHg) | LVEDP (mmHg) | LVDP (mmHg) | +(dP/dt)max (mmHg/s) | −(dP/dt)max (mmHg/s) | CF (mL/min) |
|---|---|---|---|---|---|---|---|
| 3 months | 270 ± 7 | 87.6 ± 4.6 | 6.8 ± 1.7 | 87.0 ± 3.5 | 2339 ± 181 | 1581 ± 95 | 6.6 ± 0.7 |
| 6 months | 261 ± 7 | 87.7 ± 3.8 | 5.9 ± 0.8 | 93.0 ± 3.2 | 2846 ± 138 | 1748 ± 95 | 8.5 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kindernay, L.; Farkasova, V.; Neckar, J.; Hrdlicka, J.; Ytrehus, K.; Ravingerova, T. Impact of Maturation on Myocardial Response to Ischemia and the Effectiveness of Remote Preconditioning in Male Rats. Int. J. Mol. Sci. 2021, 22, 11009. https://doi.org/10.3390/ijms222011009
Kindernay L, Farkasova V, Neckar J, Hrdlicka J, Ytrehus K, Ravingerova T. Impact of Maturation on Myocardial Response to Ischemia and the Effectiveness of Remote Preconditioning in Male Rats. International Journal of Molecular Sciences. 2021; 22(20):11009. https://doi.org/10.3390/ijms222011009
Chicago/Turabian StyleKindernay, Lucia, Veronika Farkasova, Jan Neckar, Jaroslav Hrdlicka, Kirsti Ytrehus, and Tanya Ravingerova. 2021. "Impact of Maturation on Myocardial Response to Ischemia and the Effectiveness of Remote Preconditioning in Male Rats" International Journal of Molecular Sciences 22, no. 20: 11009. https://doi.org/10.3390/ijms222011009
APA StyleKindernay, L., Farkasova, V., Neckar, J., Hrdlicka, J., Ytrehus, K., & Ravingerova, T. (2021). Impact of Maturation on Myocardial Response to Ischemia and the Effectiveness of Remote Preconditioning in Male Rats. International Journal of Molecular Sciences, 22(20), 11009. https://doi.org/10.3390/ijms222011009







