Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains—An In Vitro Study
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Organisms and Growth Conditions
4.2. Photosensitizer and Laser
4.3. Microscopic Evaluation of the Absorption of Photosensitizer Particles by Planktonic Cells of Candida Strains in Real Time
4.4. Experimental Groups and Photodynamic Inactivation of Candida spp. In Vitro
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Champeau, M.; Vignoud, S.; Mortier, L.; Mordon, S. Photodynamic therapy for skin cancer: How to enhance drug penetration? J. Photochem. Photobiol. B Biol. 2019, 197, 111544. [Google Scholar] [CrossRef]
- Wu, H.; Minamide, T.; Yano, T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig. Endosc. 2019, 31, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horne, T.K.; Cronje, M.J. Cancer Tissue Classification, Associated Therapeutic Implications and PDT as an Alternative. Anticancer. Res. 2017, 37, 2785–2807. [Google Scholar] [CrossRef] [Green Version]
- Salvi, G.E.; Stähli, A.; Schmidt, J.C.; Ramseier, C.A.; Sculean, A.; Walter, C. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 47, 176–198. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Gaspirc, B.; Sculean, A. Clinical and microbiological effects of multiple applications of antibacterial photodynamic therapy in periodontal maintenance patients. A randomized controlled clinical study. Photodiagnosis Photodyn. Ther. 2019, 27, 44–50. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Nowicka, J.; Pajączkowska, M.; Matys, J.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Dobrzyński, M.; Dominiak, M. Effects of Nd:YAG laser irradiation on the growth of Candida albicans and Streptococcus mutans: In Vitro Study. Lasers Med. Sci. 2018, 34, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Kim, M.; Choi, J.W.; Baek, J.-H.; Lee, S.H.; Baek, K. Antimicrobial photodynamic therapy efficacy against specific pathogenic periodontitis bacterial species. Photodiagnosis Photodyn. Ther. 2020, 30, 101688. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.B.; Gulati, M.; Johnson, A.D.; Nobile, C.J. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018, 16, 19–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrera, E.T.; Dias, H.B.; Corbi, S.C.T.; Marcantonio, R.A.; Bernardi, A.C.A.; Bagnato, V.S.; Hamblin, M.R.; Rastelli, A.N.S. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: A critical review. Laser Phys. 2016, 26, 12300. [Google Scholar] [CrossRef] [Green Version]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photo-chemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Calzavara-Pinton, P.; Rossi, M.T.; Sala, R.; Venturini, M. Photodynamic Antifungal Chemotherapy. Photochem. Photobiol. 2012, 88, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Mylona, V.; Anagnostaki, E.; Parker, S.; Cronshaw, M.; Lynch, E.; Grootveld, M. Laser-Assisted aPDT Protocols in Randomized Controlled Clinical Trials in Dentistry: A Systematic Review. Dent. J. 2020, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- D’Ilario, L.; Martinelli, A. Toluidine blue: Aggregation properties and structural aspects. Model. Simul. Mater. Sci. Eng. 2006, 14, 581–595. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Promising Alternative Therapeutics for Oral Candidiasis. Curr. Med. Chem. 2019, 26, 2515–2528. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; De Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Chaffin, W.L. Candida albicans Cell Wall Proteins. Microbiol. Mol. Biol. Rev. 2008, 72, 495–544. [Google Scholar] [CrossRef] [Green Version]
- Free, S.J. Fungal Cell Wall Organization and Biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar] [CrossRef]
- Linares, C.E.B.; Giacomelli, S.R.; Altenhofen, D.; Alves, S.H.; Morsch, V.M.; Schetinger, M.R.C. Fluconazole and amphotericin-B resistance are associated with increased catalase and superoxide dismutase activity in Candida albicans and Candida dubliniensis. Rev. Soc. Bras. Med. Trop. 2013, 46, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Johnson, F.; Giulivi, C. Superoxide dismutases and their impact upon human health. Mol. Asp. Med. 2005, 26, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Martchenko, M.; Alarco, A.-M.; Harcus, D.; Whiteway, M. Superoxide Dismutases inCandida albicans: Transcriptional Regulation and Functional Characterization of the Hyphal-inducedSOD5Gene. Mol. Biol. Cell 2004, 15, 456–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, H.-F.; Chen, C.-P.; Chen, Y.-C.; Chang, P.-H.; Tsai, T.; Chen, C.-T. The Use of Chitosan to Enhance Photodynamic Inactivation against Candida albicans and Its Drug-Resistant Clinical Isolates. Int. J. Mol. Sci. 2013, 14, 7445–7456. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.C.; Junqueira, J.C.; Rossoni, R.D.; Pereira, C.A.; Munin, E.; Jorge, A.O.C. Comparison of the photodynamic fungicidal efficacy of methylene blue, toluidine blue, malachite green and low-power laser irradiation alone against Candida albicans. Lasers Med. Sci. 2009, 25, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of Toluidine Blue—Mediated Antimicrobial Photodynamic Therapy on Candida spp. A Systematic Review. Antibiotics 2021, 10, 349. [Google Scholar] [CrossRef]
- Zeina, B.; Greenman, J.; Purcell, W.; Das, B. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Dermatol. 2001, 144, 274–278. [Google Scholar] [CrossRef]
- Zeina, B.; Greenman, J.; Corry, D.; Purcell, W. Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br. J. Dermatol. 2002, 146, 568–573. [Google Scholar] [CrossRef]
- Demidova, T.N.; Hamblin, M.R. Effect of Cell-Photosensitizer Binding and Cell Density on Microbial Photoinactivation. Antimicrob. Agents Chemother. 2005, 49, 2329–2335. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, R.F.; McCarron, P.; Tunney, M. Antifungal photodynamic therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef]
- Prates, R.A.; Kato, I.T.; Ribeiro, M.S.; Tegos, G.P.; Hamblin, M.R. Influence of multidrug efflux systems on methylene blue-mediated photodynamic inactivation of Candida albicans. J. Antimicrob. Chemother. 2011, 66, 1525–1532. [Google Scholar] [CrossRef]
- Jackson, Z.; Meghji, S.; MacRobert, A.; Henderson, B.; Wilson, M. Killing of the Yeast and Hyphal Forms of Candida albicans Using a Light-Activated Antimicrobial Agent. Lasers Med. Sci. 1999, 14, 150–157. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.; Tunney, M.; Woolfson, A.D. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J. Photochem. Photobiol. B Biol. 2007, 86, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Yue, H.; Wei, Y.; Huang, G. Genetic regulatory mechanisms of Candida albicans biofilm formation. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2017, 33, 1567–1581. [Google Scholar]
- Pinto, A.P.; Rosseti, I.B.; Carvalho, M.L.; da Silva, B.G.M.; Alberto-Silva, C.; Costa, M.S. Photodynamic Antimicrobial Chemotherapy (PACT), using Toluidine blue O inhibits the viability of biofilm produced by Candida albicans at different stages of development. Photodiagn. Photodyn. Ther. 2018, 21, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.G.M.; Carvalho, M.L.; Rosseti, I.B.; Zamuner, S.; Costa, M.S. Photodynamic antimicrobial chemotherapy (PACT) using toluidine blue inhibits both growth and biofilm formation by Candida krusei. Lasers Med. Sci. 2018, 33, 983–990. [Google Scholar] [CrossRef]
- Huang, M.-C.; Shen, M.; Huang, Y.-J.; Lin, H.-C.; Chen, C.-T. Photodynamic Inactivation Potentiates the Susceptibility of Antifungal Agents against the Planktonic and Biofilm Cells of Candida albicans. Int. J. Mol. Sci. 2018, 19, 434. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, G.G.; Felipe, M.P.; Costa, M.S. The photodynamic effect of methylene blue and toluidine blue on Candida albicans is dependent on medium conditions. J. Microbiol. 2009, 47, 619–623. [Google Scholar] [CrossRef]
- Garcia, B.A.; Panariello, B.H.D.; de Freitas Pontes, K.M.; Duarte, S. Regimen and different surfaces interfere with photodynamic therapy on Candida albicans biofilms. J. Microbiol. Methods 2020, 178, 106080. [Google Scholar] [CrossRef]
- Soares, B.M.; da Silva, D.L.; Sousa, G.R.; Amorim, J.C.F.; de Resende, M.A.; Pinotti, M.; Cisalpino, P.S. In vitro photodynamic inactivation of Candida spp. growth and adhesion to buccal epithelial cells. J. Photochem. Photobiol. B Biol. 2009, 94, 65–70. [Google Scholar] [CrossRef]
- Merigo, E.; Chevalier, M.; Conti, S.; Ciociola, T.; Fornaini, C.; Manfredi, M.; Vescovi, P.; Doglio, A. Antimicrobial effect on Candida albicans biofilm by application of different wavelengths and dyes and the synthetic killer decapeptide KP. Laser Ther. 2019, 28, 180–186. [Google Scholar] [CrossRef]
- Sherwani, M.A.; Tufail, S.; Khan, A.A.; Owais, M. Gold Nanoparticle-Photosensitizer Conjugate Based Photodynamic Inactivation of Biofilm Producing Cells: Potential for Treatment of C. albicans Infection in BALB/c Mice. PLoS ONE 2015, 10, e0131684. [Google Scholar] [CrossRef]
- Dai, T.; De Arce, V.J.B.; Tegos, G.P.; Hamblin, M.R. Blue Dye and Red Light, a Dynamic Combination for Prophylaxis and Treatment of Cutaneous Candida albicans Infections in Mice. Antimicrob. Agents Chemother. 2011, 55, 5710–5717. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Chien, H.-F.; Lin, M.-H.; Chen, C.-P.; Shen, M.; Chen, C.-T. Chitosan Inhibits the Rehabilitation of Damaged Microbes Induced by Photodynamic Inactivation. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Rodrigues, G.B.; Baruffi, M.D.; Holman, N.; Wainwright, M.; Braga, G. In vitro photodynamic inactivation of Candida species and mouse fibroblasts with phenothiazinium photosensitisers and red light. Photodiagnosis Photodyn. Ther. 2013, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Wiench, R.; Skaba, D.; Stefanik, N.; Kępa, M.; Gilowski, Ł.; Cieślar, G.; Kawczyk-Krupka, A. Assessment of sensitivity of selected Candida strains on antimicrobial photodynamic therapy using diode laser 635 nm and toluidine blue–In vitro research. Photodiagnosis Photodyn. Ther. 2019, 27, 241–247. [Google Scholar] [CrossRef]
- Nielsen, H.K.; Garcia, J.; Væth, M.; Schlafer, S. Comparison of Riboflavin and Toluidine Blue O as Photosensitizers for Photoactivated Disinfection on Endodontic and Periodontal Pathogens In Vitro. PLoS ONE 2015, 10, e0140720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosseti, I.B.; Chagas, L.R.; Costa, M.S. Photodynamic antimicrobial chemotherapy (PACT) inhibits biofilm formation by Candida albicans, increasing both ROS production and membrane permeability. Lasers Med. Sci. 2014, 29, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Barbério, G.S.; da Costa, S.V.; Silva, M.D.S.; de Oliveira, T.M.; Silva, T.C.; Machado, M.A.D.A.M. Photodynamic inactivation of Candida albicans mediated by a low density of light energy. Lasers Med. Sci. 2013, 29, 907–910. [Google Scholar] [CrossRef]
- Decraene, V.; Pratten, J.; Wilson, M. Cellulose Acetate Containing Toluidine Blue and Rose Bengal Is an Effective Antimicrobial Coating when Exposed to White Light. Appl. Environ. Microbiol. 2006, 72, 4436–4439. [Google Scholar] [CrossRef] [Green Version]
- Javed, F.; Samaranayake, L.P.; Romanos, G.E. Treatment of oral fungal infections using antimicrobial photodynamic therapy: A systematic review of currently available evidence. Photochem. Photobiol. Sci. 2014, 13, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; He, C.; Zhao, C.; Chen, X.; Hua, H.; Yan, Z. Characterization of oral candidiasis and the Candida species profile in patients with oral mucosal diseases. Microb. Pathog. 2019, 134, 103575. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ.US National Institutes of Health, Bethesda, Maryland, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 14 March 2021).
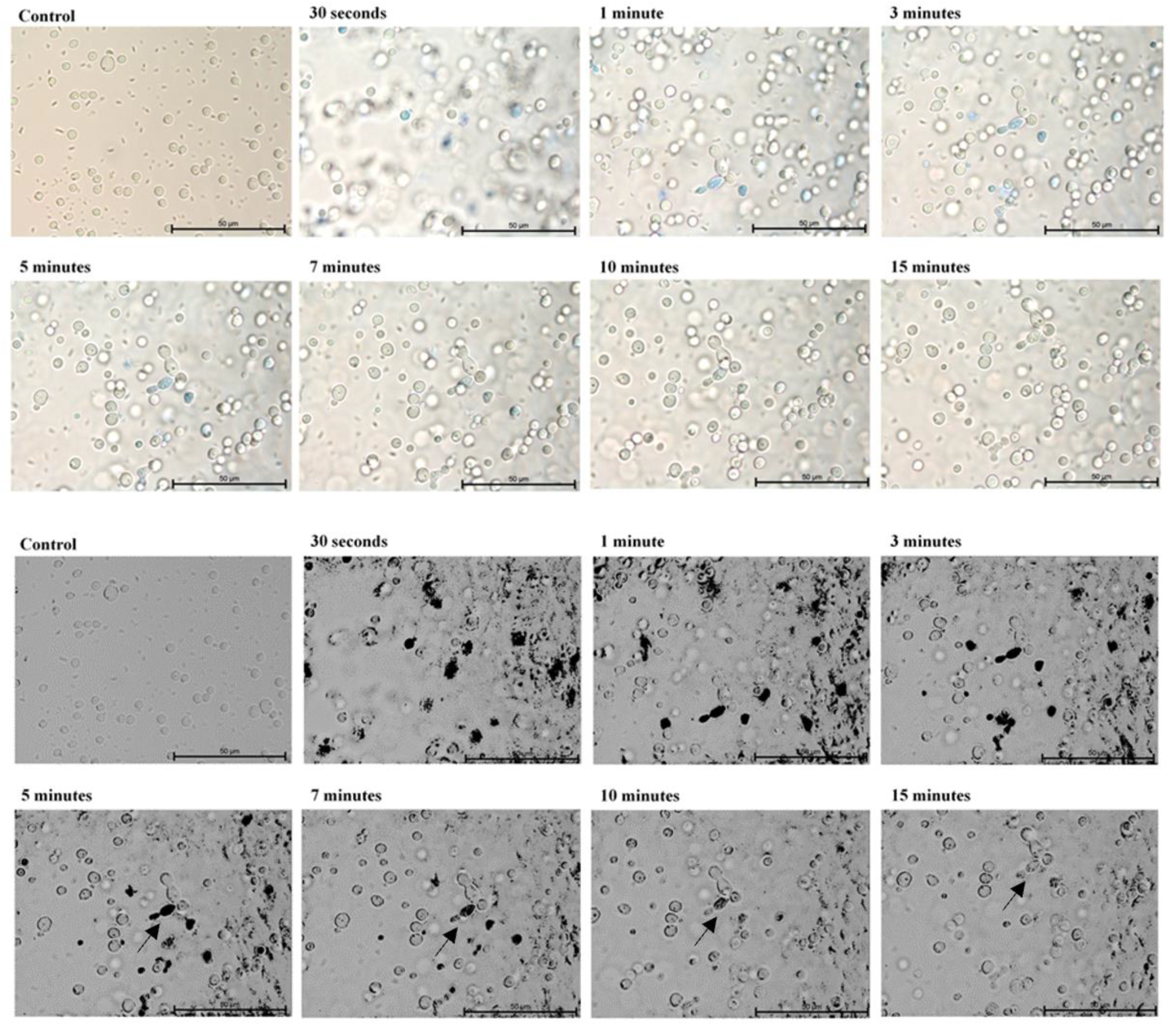
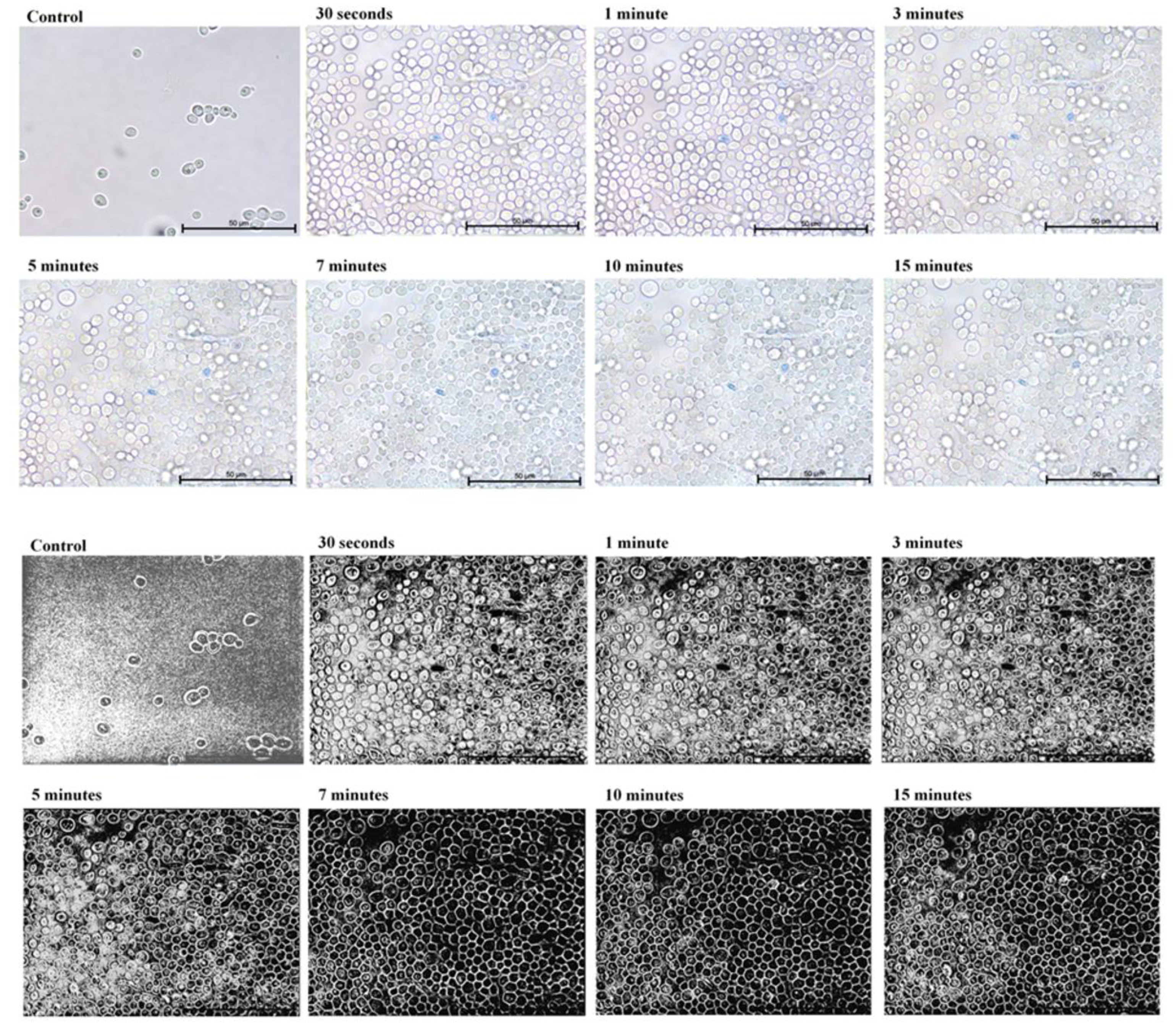
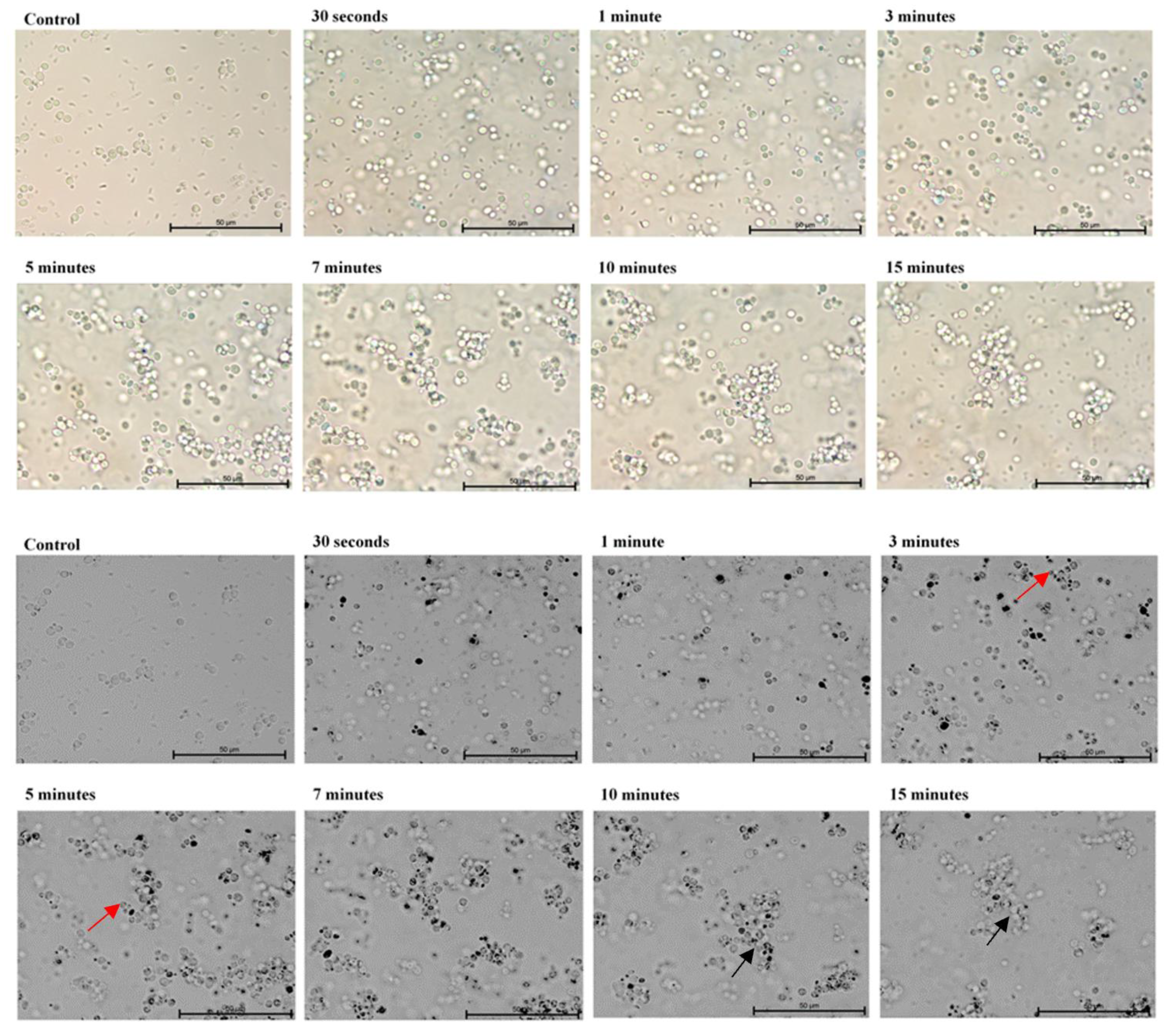
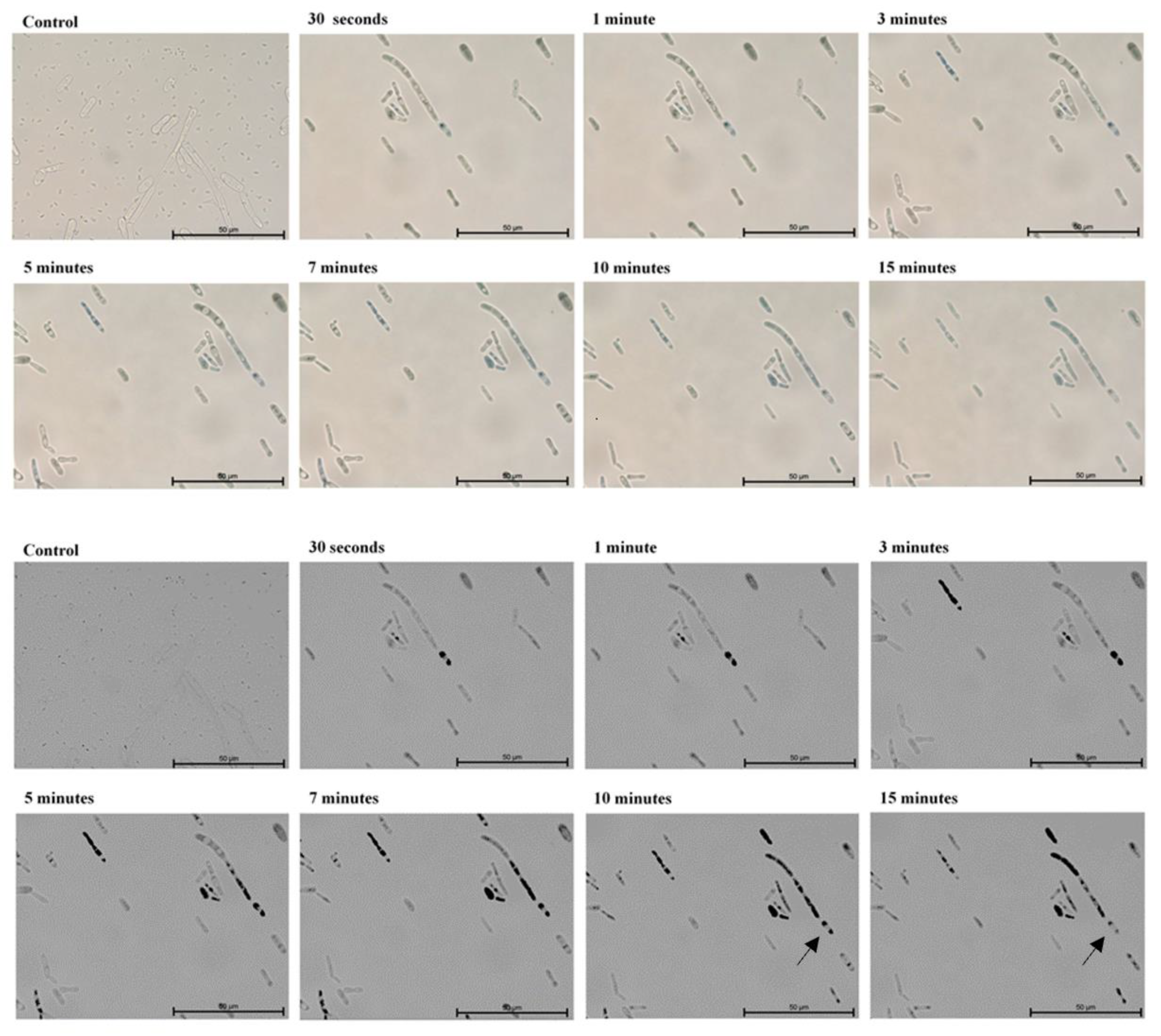
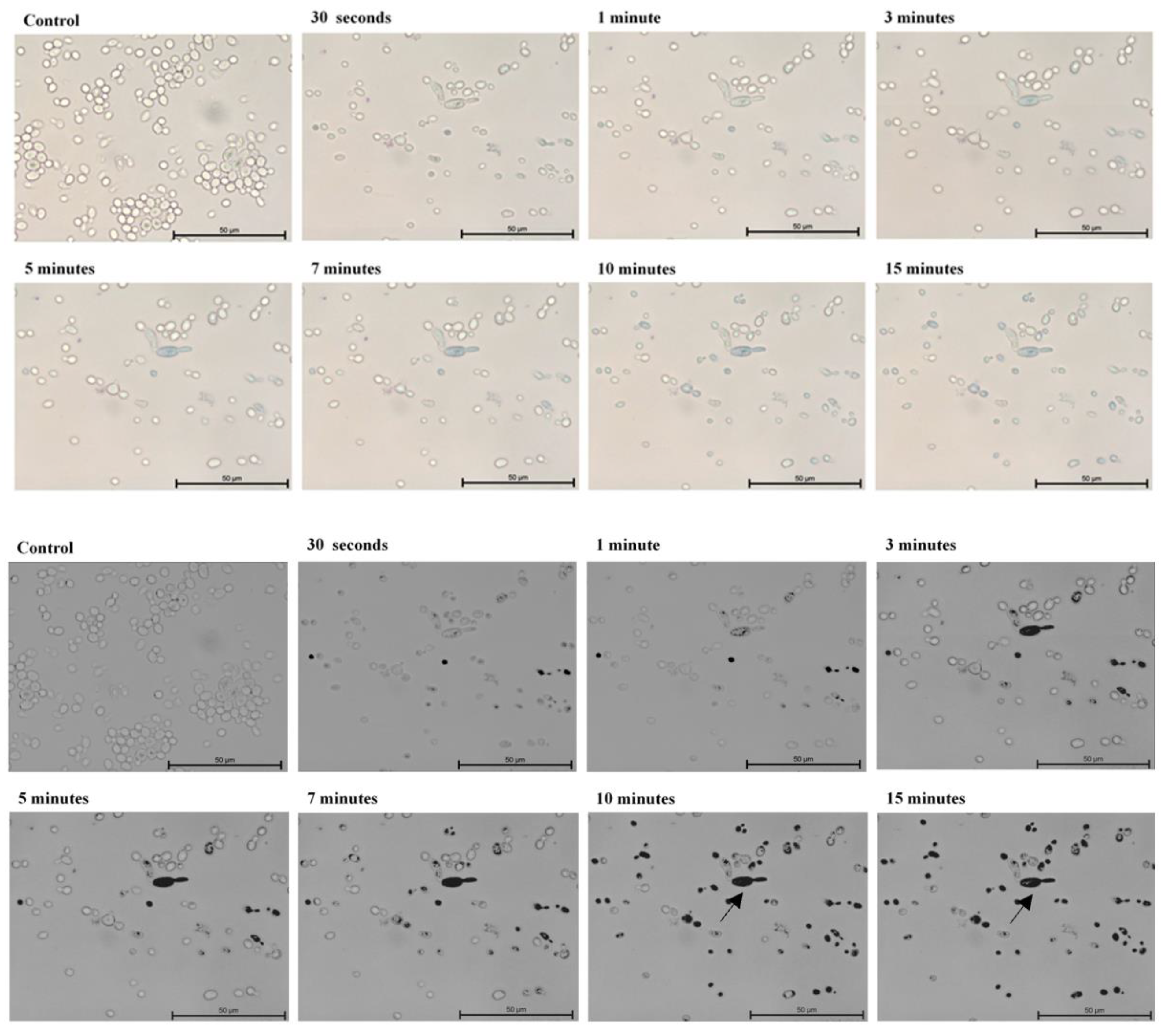
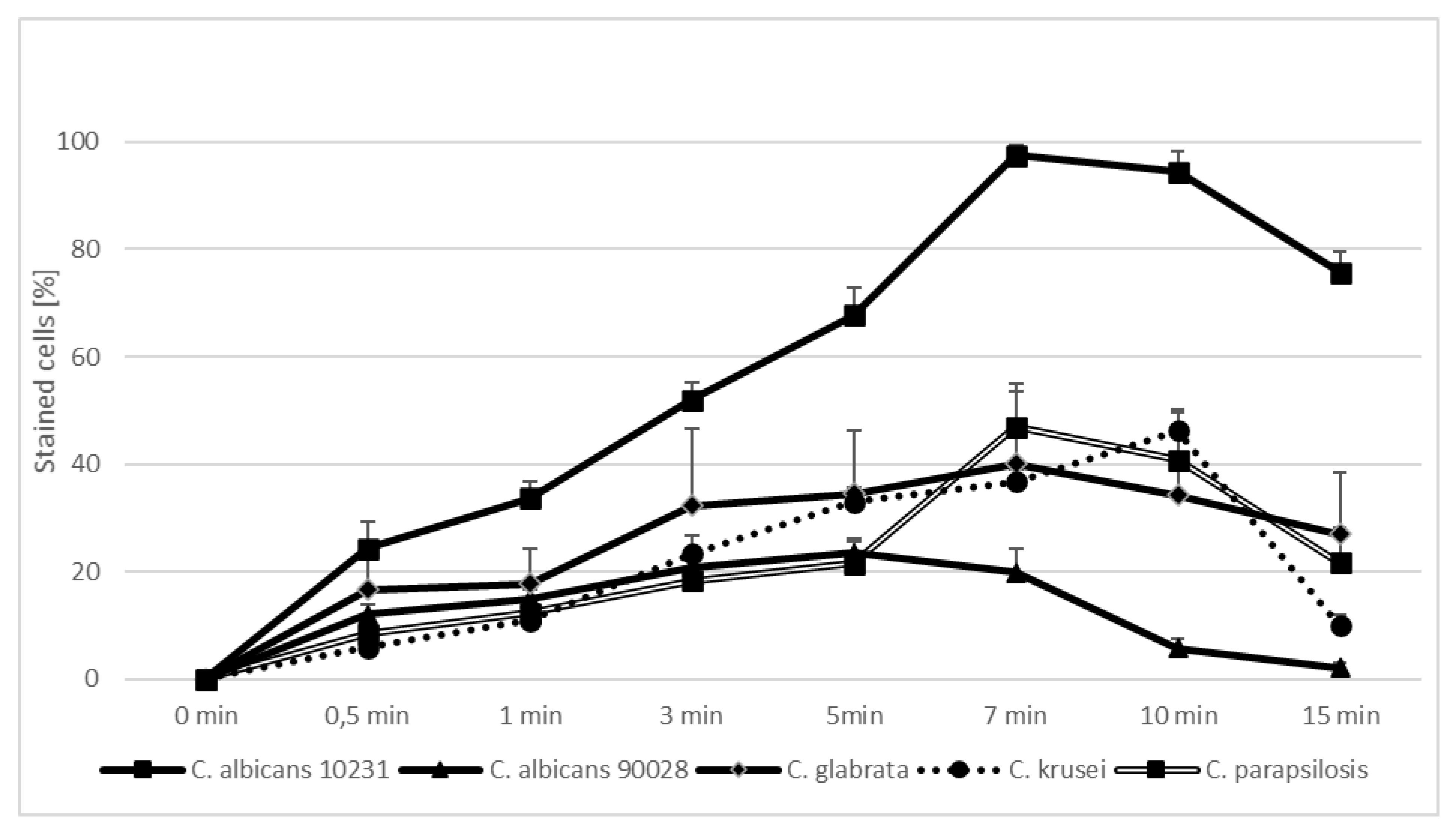

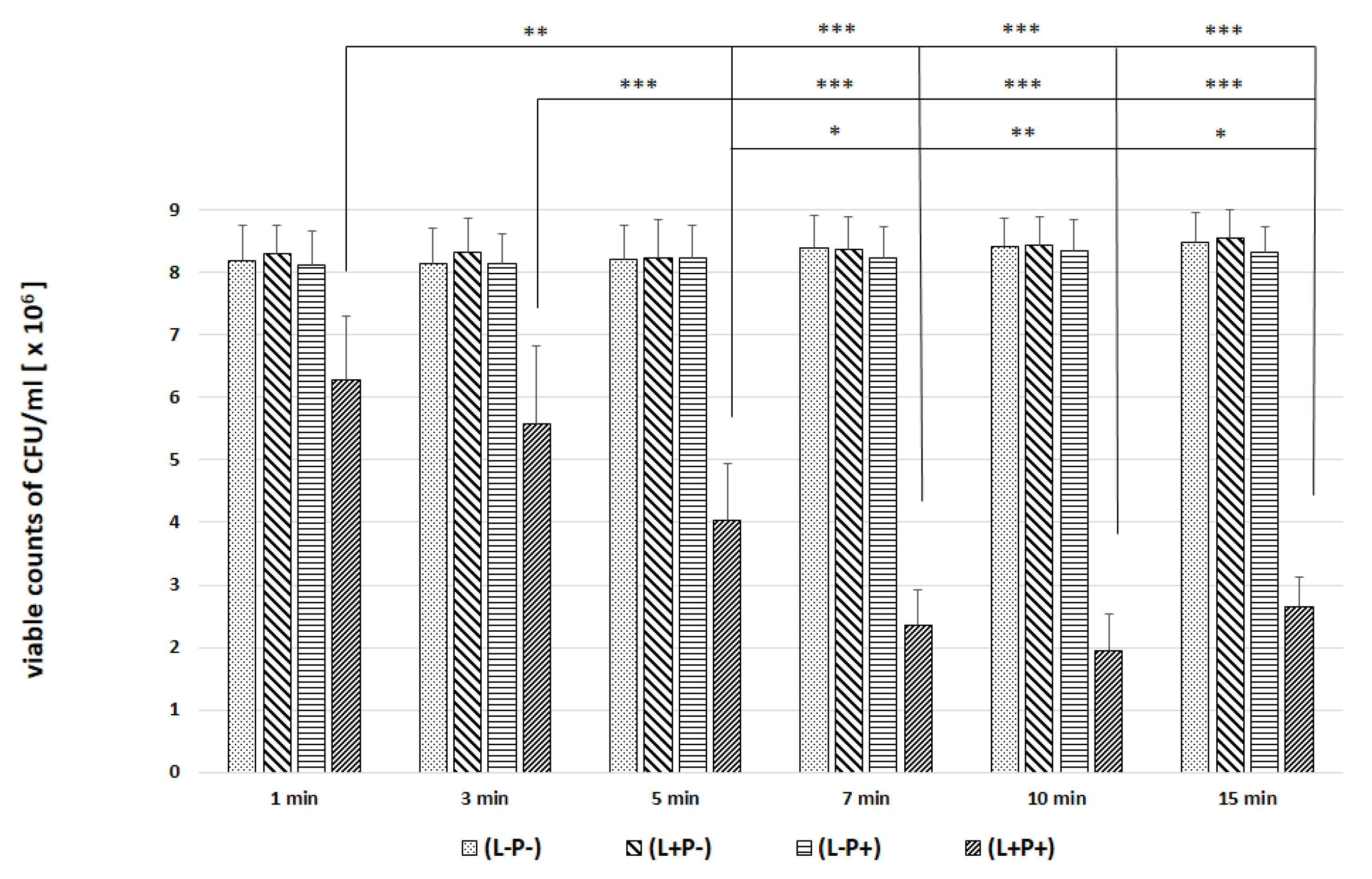
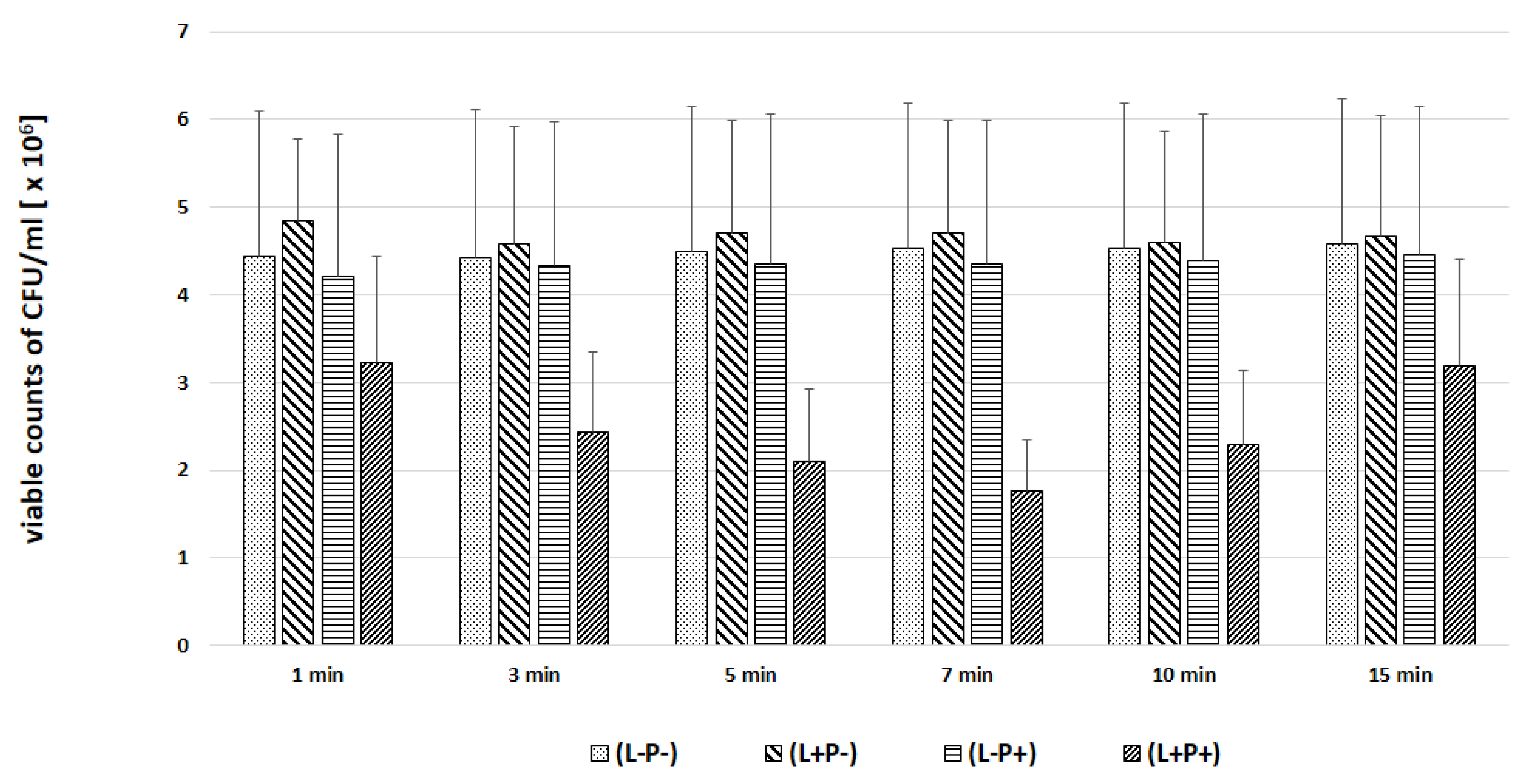



| Candida Strain (The Most Efficient Incubation Time) | The Highest Reduction of CFU/mL [%] | Assessment of TBO Uptake by Cells over Time [%] |
|---|---|---|
| C. albicans ATCC 90,028 (7 min) | 23.02 | 19.93 |
| C. albicans ATCC 10,231 (10 min) | 76.89 | 94.43 |
| C. glabrata (7 min) | 61.37 | 40.15 |
| C. krusei (3 min) | 59.40 | 23.39 |
| C. parapsilosis (7 min) | 46.81 | 47.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiench, R.; Nowicka, J.; Pajączkowska, M.; Kuropka, P.; Skaba, D.; Kruczek-Kazibudzka, A.; Kuśka-Kiełbratowska, A.; Grzech-Leśniak, K. Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains—An In Vitro Study. Int. J. Mol. Sci. 2021, 22, 10971. https://doi.org/10.3390/ijms222010971
Wiench R, Nowicka J, Pajączkowska M, Kuropka P, Skaba D, Kruczek-Kazibudzka A, Kuśka-Kiełbratowska A, Grzech-Leśniak K. Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains—An In Vitro Study. International Journal of Molecular Sciences. 2021; 22(20):10971. https://doi.org/10.3390/ijms222010971
Chicago/Turabian StyleWiench, Rafał, Joanna Nowicka, Magdalena Pajączkowska, Piotr Kuropka, Dariusz Skaba, Anna Kruczek-Kazibudzka, Anna Kuśka-Kiełbratowska, and Kinga Grzech-Leśniak. 2021. "Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains—An In Vitro Study" International Journal of Molecular Sciences 22, no. 20: 10971. https://doi.org/10.3390/ijms222010971
APA StyleWiench, R., Nowicka, J., Pajączkowska, M., Kuropka, P., Skaba, D., Kruczek-Kazibudzka, A., Kuśka-Kiełbratowska, A., & Grzech-Leśniak, K. (2021). Influence of Incubation Time on Ortho-Toluidine Blue Mediated Antimicrobial Photodynamic Therapy Directed against Selected Candida Strains—An In Vitro Study. International Journal of Molecular Sciences, 22(20), 10971. https://doi.org/10.3390/ijms222010971







