The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery
Abstract
:1. Introduction
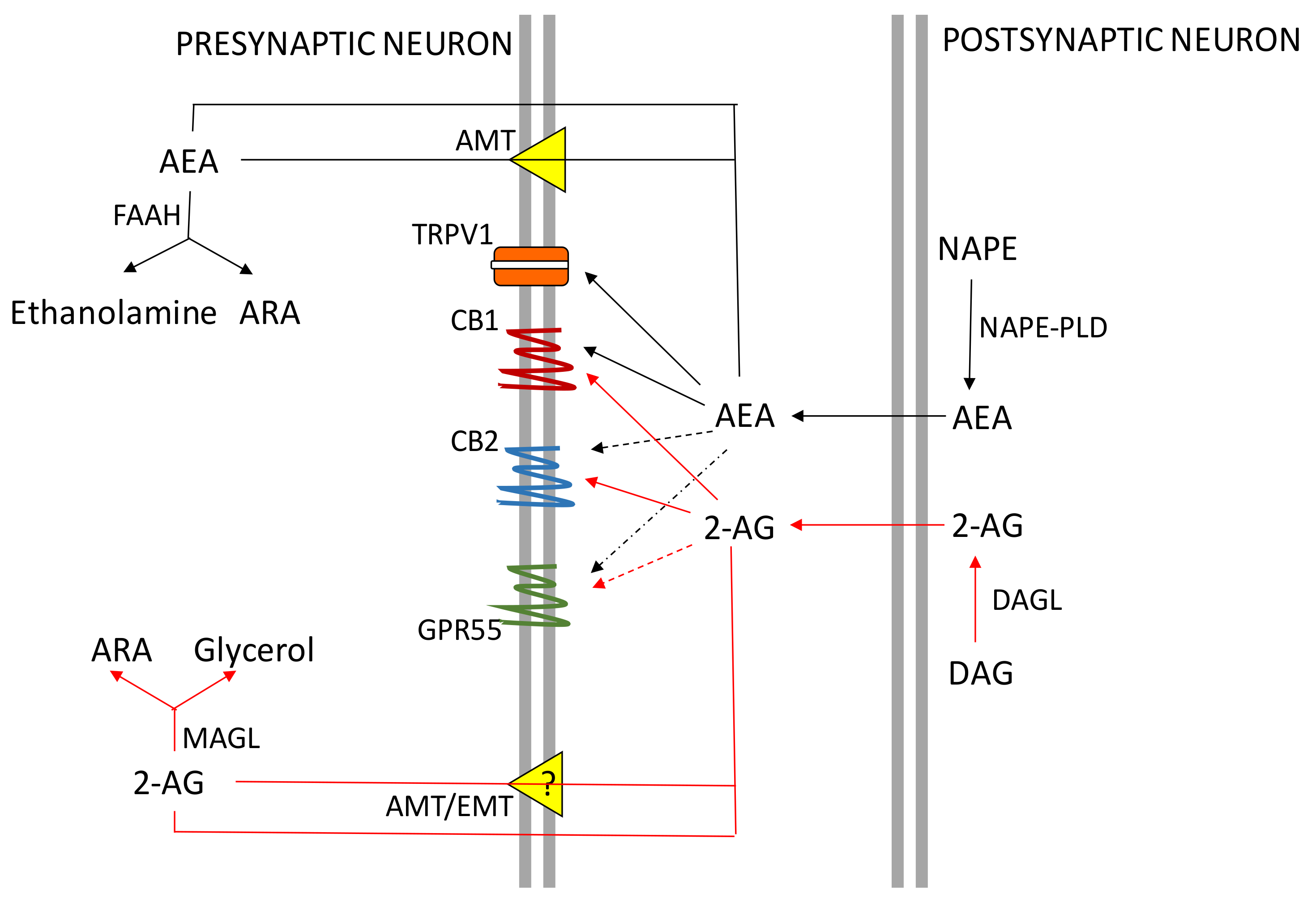
2. The ECS System
CB Receptors Expression in the Central Nervous System (CNS) and Peripheral Organs
3. The ES
3.1. Estrogens in the CNS and Reproductive Tract
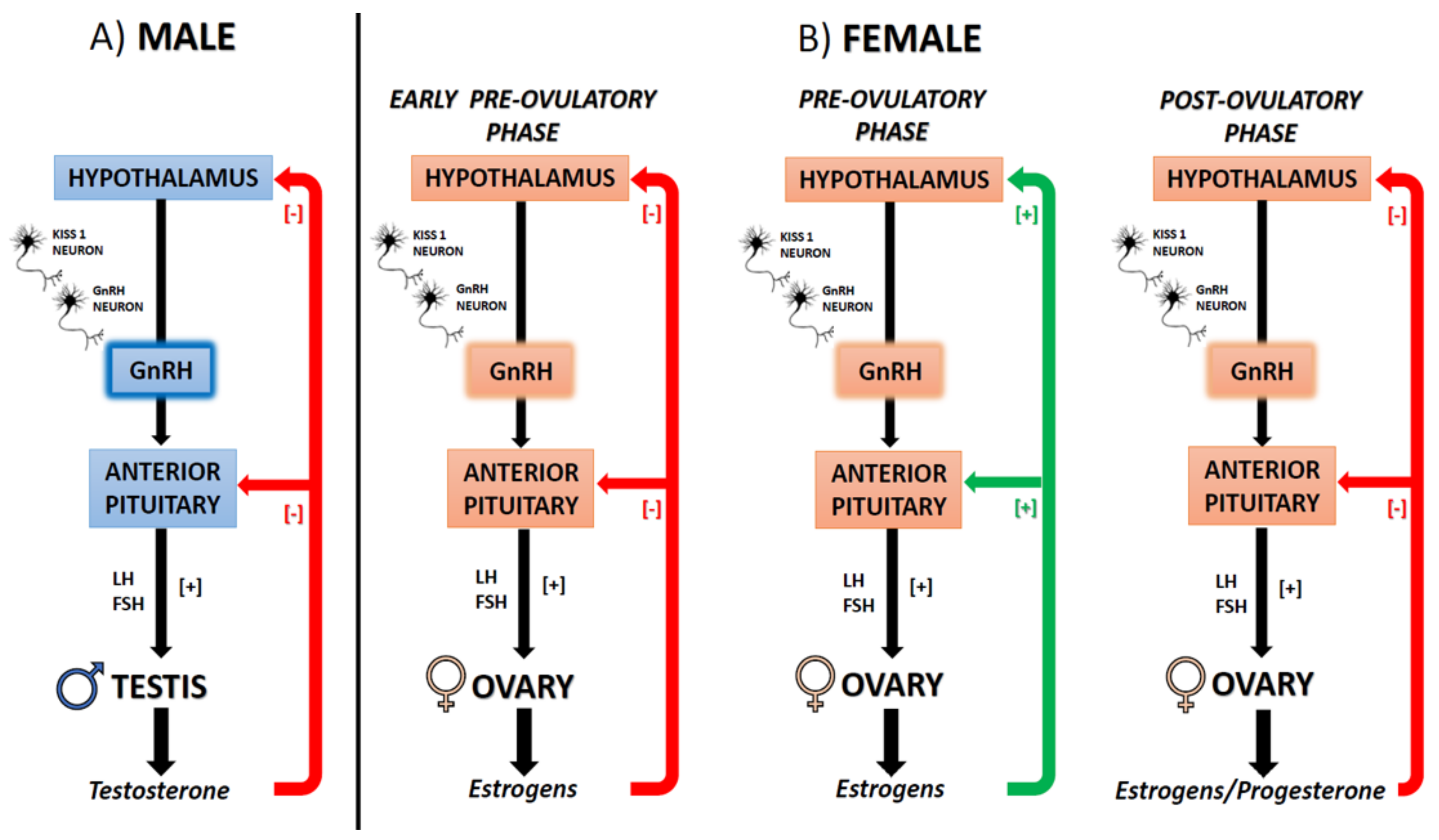
3.2. Different Functions of ES in Male and Female CNS
4. ECS and ES Interactions in the CNS
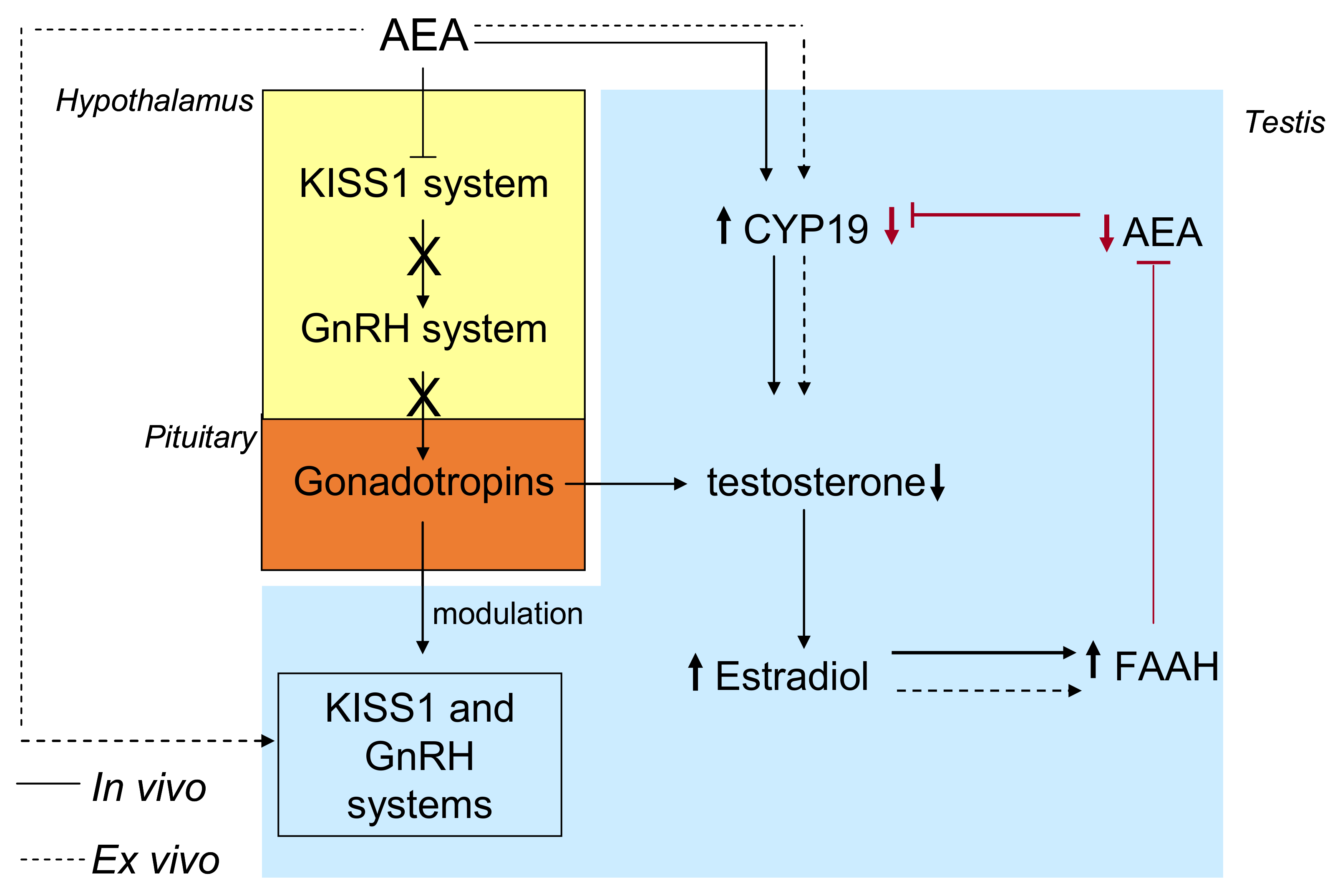
5. ECS, Estrogens, and Reproduction
5.1. The ECS-Dependent Modulation of GnRH and the Interplay with Estrogens
6. ES and ECS Modulation by Estrogens-Like Substances Affecting CNS and Gonads
7. ECS and ES Interactions in the Periphery
7.1. Interactions in Blood Cells
7.2. Interactions of ECS and ES in Cancer
8. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McPartland, J.M.; Matias, I.; Di Marzo, V.; Glass, M. Evolutionary origins of the endocannabinoid system. Gene 2006, 370, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Fasano, S.; Meccariello, R.; Cobellis, G.; Chianese, R.; Cacciola, G.; Chioccarelli, T.; Pierantoni, R. The endocannabinoid system: An ancient signaling involved in the control of male fertility. Ann. N. Y. Acad. Sci. 2009, 1163, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Elphick, M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3201–3215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatr. 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaoni, Y.; Mechoulam, R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Proto, M.C.; Gazzerro, P.; Di Croce, L.; Santoro, A.; Malfitano, A.M.; Pisanti, S.; Laezza, C.; Bifulco, M. Interaction of endocannabinoid system and steroid hormones in the control of colon cancer cell growth. J. Cell. Physiol. 2012, 227, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Willets, J.M.; Konje, J.C. The Endocannabinoid System and Sex Steroid Hormone-dependent cancers. Int. J. Endocrinol. 2013, 2013, 259676. [Google Scholar] [CrossRef] [Green Version]
- Cacciola, G.; Chioccarelli, T.; Fasano, S.; Pierantoni, R.; Cobellis, G. Estrogens and spermiogenesis: New insights from type 1 cannabinoid receptor knockout mice. Int. J. Endocrinol. 2013, 2013, 501350. [Google Scholar] [CrossRef]
- Dobovišek, L.; Hojnik, M.; Ferk, P. Overlapping molecular pathways between cannabinoid receptors type 1 and 2 and estrogens/androgens on the periphery and their involvement in the pathogenesis of common diseases (Review). Int. J. Mol. Med. 2016, 38, 1642–1651. [Google Scholar] [CrossRef] [Green Version]
- Lipina, C.; Hundal, H.S. The endocannabinoid system: ‘NO’ longer anonymous in the control of nitrergic signalling? J. Mol. Cell Biol. 2017, 9, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Layé, S.; Pierantoni, R.; et al. Impact of dietary fat on brain functions. Curr. Neuropharmacol. 2018, 16, 1059–1085. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R. Endocannabinoid System in Health and Disease: Current Situation and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 3549. [Google Scholar] [CrossRef] [PubMed]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, M.; Santoro, A.; Laezza, C.; Malfitano, A.M. Cannabinoid receptor CB1 antagonists state of the art and challenges. Vitam. Horm. 2009, 81, 159–189. [Google Scholar] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef]
- Klein, T.W.; Newton, C.; Larsen, K.; Lu, L.; Perkins, I.; Nong, L.; Friedman, H. The cannabinoid system and immune modulation. J. Leukoc. Biol. 2003, 74, 486–496. [Google Scholar] [CrossRef] [Green Version]
- Bialuk, I.; Winnicka, M.M. AM251, cannabinoids receptor ligand, improves recognition memory in rats. Pharmacol. Rep. 2011, 63, 670–679. [Google Scholar] [CrossRef]
- Litvin, Y.; Phan, A.; Hill, M.N.; Pfaff, D.W.; McEwen, B.S. CB1 receptor signaling regulates social anxiety and memory. Genes Brain Behav. 2013, 12, 479–489. [Google Scholar] [CrossRef]
- Morena, M.; Campolongo, P. The endocannabinoid system: An emotional buffer in the modulation of memory function. Neurobiol. Learn. Mem. 2014, 112, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, C.; Pisanti, S.; Laezza, C.; Malfitano, A.M.; Santoro, A.; Vitale, M.; Caruso, M.G.; Notarnicola, M.; Iacuzzo, I.; Portella, G.; et al. Anandamide inhibits adhesion and migration of breast cancer cells. Exp. Cell. Res. 2006, 312, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, G.; Chioccarelli, T.; Mackie, K.; Meccariello, R.; Ledent, C.; Fasano, S.; Pierantoni, R.; Cobellis, G. Expression of type-1 cannabinoid receptor during rat postnatal testicular development: Possible involvement in adult Leydig cell differentiation. Biol. Reprod. 2008, 79, 758–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchi, M.; Innocenzi, E.; Giannattasio, T.; Dolci, S.; Rossi, P.; Grimaldi, P. Cannabinoid Receptors Signaling in the Development, Epigenetics, and Tumours of Male Germ Cells. Int. J. Mol. Sci. 2019, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Cueto, C.; Benito, C.; Fernández-Ruiz, J.; Romero, J.; Hernández-Gálvez, M.; Gómez-Ruiz, M. Changes in CB(1) and CB(2) receptors in the post-mortem cerebellum of humans affected by spinocerebellar ataxias. Br. J. Pharmacol. 2014, 171, 1472–1489. [Google Scholar] [CrossRef] [Green Version]
- Lanciego, J.L.; Barroso-Chinea, P.; Rico, A.J.; Conte-Perales, L.; Callén, L.; Roda, E.; Gómez-Bautista, V.; López, I.P.; Lluis, C.; Labandeira-García, J.L.; et al. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J. Psychopharmacol. 2011, 25, 97–104. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Hernández, M.; Ramos, J.A. Cannabinoid–Dopamine Interaction in the Pathophysiology and Treatment of CNS Disorders. CNS Neurosci. Ther. 2010, 16, 72–91. [Google Scholar] [CrossRef]
- Lutz, B. Neurobiology of cannabinoid receptor signalling. Dialogues Clin. Neurosci. 2020, 22, 207–222. [Google Scholar]
- Stempel, A.V.; Stumpf, A.; Zhang, H.Y.; Özdoğan, T.; Pannasch, U.; Theis, A.K.; Otte, D.M.; Wojtalla, A.; Rácz, I.; Ponomarenko, A.; et al. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron 2016, 90, 795–809. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Y.; Gao, M.; Shen, H.; Bi, G.H.; Yang, H.J.; Liu, Q.R.; Wu, J.; Gardner, E.L.; Bonci, A.; Xi, Z.X. Expression of functional cannabinoid CB2 receptor in VTA dopamine neurons in rats. Addict. Biol. 2017, 22, 752–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, C.J.; Xi, Z.X. Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neurosci. Biobehav. Rev. 2019, 98, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Reiner, D.; Shen, H.; Wu, K.J.; Liu, Q.R.; Wang, Y. Time-dependent protection of CB2 receptor agonist in stroke. PLoS ONE 2015, 10, e0132487. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.H.; Stanford, I.M.; Woodhall, G.L. Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology 2009, 57, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Spinelli, C.C.; Martucciello, S.; Nori, S.L.; Capunzo, M.; Puca, A.A.; Ciaglia, E. Innate immunity and cellular senescence: The good and the bad in the developmental and aged brain. J. Leukoc. Biol. 2018, 103, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, S.J.; Marciano-Cabral, F.; Staab, A.; Ludwick, C.; Cabral, G.A. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int. Immunopharm. 2002, 2, 69–82. [Google Scholar] [CrossRef]
- Benito, C.; Kim, W.K.; Chavarria, I.; Hillard, C.J.; Mackie, K.; Tolon, R.M.; Williams, K.; Romero, J. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J. Neurosci. 2005, 25, 2530–2536. [Google Scholar] [CrossRef] [Green Version]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 2, 437–445. [Google Scholar] [CrossRef]
- Nephi, S. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 2010, 58, 1017–1030. [Google Scholar]
- Nilsson, S.; Makela, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- O’Lone, R.; Frith, M.C.; Karlsson, E.K.; Hansen, U. Genomic targets of nuclear estrogen receptors. Mol. Endocrinol. 2004, 18, 1859–1875. [Google Scholar] [CrossRef] [PubMed]
- Gottlicher, M.; Heck, S.; Herrlich, P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J. Mol. Med. 1998, 76, 480–489. [Google Scholar] [CrossRef]
- Liu, S.B.; Zhao, M.G. Neuroprotective effect of estrogen: Role of nonsynaptic NR2B-containing NMDA receptors. Brain Res. Bull. 2013, 93, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Haas, E.; Prossnitz, E.R.; Barton, M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol. Cell. Endocrinol. 2009, 308, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vail, G.; Roepke, T.A. Membrane-initiated estrogen signaling via Gq-coupled GPCR in the central nervous system. Steroids 2019, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Toran-Allerand, C.D. Estrogen and the brain: Beyond ER-α and ER-β. Exp. Gerontol. 2004, 39, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Losel, R.; Wehling, M. Nongenomic actions of steroid hormones. Nat. Rev. Mol. Cell. Biol. 2003, 4, 46–56. [Google Scholar] [CrossRef]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef]
- Leranth, C.; Shanabrough, M.; Redmond, D.E., Jr. Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J. Comp. Neurol. 2002, 447, 34–42. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Hojo, Y.; Kojima, H.; Ikeda, M.; Hotta, K.; Sato, R.; Ooishi, Y.; Yoshiya, M.; Chung, B.C.; Yamazaki, T.; et al. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain. Res. 2015, 1621, 147–161. [Google Scholar] [CrossRef]
- Marx, C.E.; Stevens, R.D.; Shampine, L.J.; Uzunova, V.; Trost, W.T.; Butterfield, M.I.; Massing, M.W.; Hamer, R.M.; Morrow, A.L.; Lieberman, J.A. Neuroactive steroids are altered in schizophrenia and bipolar disorder: Relevance to pathophysiology and therapeutics. Neuropsychopharmacology 2006, 3, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; De Vita, F.; Fisichella, A.; Colizzi, E.; Provenzano, S.; Lauretani, F.; Luci, M.; Ceresini, G.; Dall’Aglio, E.; Caffarra, P.; et al. DHEA and cognitive function in the elderly. J. Steroid Biochem. Mol. Biol. 2015, 145, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef]
- Hampson, E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology 1990, 15, 97–111. [Google Scholar] [CrossRef]
- Kimura, D. Sex differences in the brain. Sci. Am. 1992, 267, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, B.B.; Tulandi, T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J. Clin. Endocrinol. Metab. 1996, 81, 2545–2549. [Google Scholar]
- Smith, S. The effects of oestrogen and progesterone on GABA and glutamate responses at extrahypothalamic sites. In Neurosteroids and Brain Function; Costa, E., Paul, S.M., Eds.; Thieme Medical: New York, NY, USA, 1991; pp. 87–94. [Google Scholar]
- Kimoto, T.; Tsurugizawa, T.; Ohta, Y.; Makino, J.; Tamura, H.; Hojo, Y.; Takata, N.; Kawato, S. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology 2001, 142, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Kawato, S.; Hojo, Y.; Kimoto, T. Histological and metabolism analysis of P450 expression in the brain. Methods Enzymol. 2002, 357, 241–249. [Google Scholar]
- Kawato, S.; Yamada, M.; Kimoto, T. Brain neurosteroids are 4th generation neuromessengers in the brain: Cell biophysical analysis of steroid signal transduction. Adv. Biophys. 2003, 37, 1–48. [Google Scholar] [CrossRef]
- Hojo, Y.; Hattori, T.A.; Enami, T.; Furukawa, A.; Suzuki, K.; Ishii, H.T.; Mukai, H.; Morrison, J.H.; Janssen, W.G.; Kominami, S.; et al. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 865–870. [Google Scholar] [CrossRef] [Green Version]
- Hojo, Y.; Murakami, G.; Mukai, H.; Higo, S.; Hatanaka, Y.; Ogiue-Ikeda, M.; Ishii, H.; Kimoto, T.; Kawato, S. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol. Cell. Endocrinol. 2008, 290, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Murakami, G.; Tanabe, N.; Ishii, H.T.; Ogiue-Ikeda, M.; Tsurugizawa, T.; Mukai, H.; Hojo, Y.; Takata, N.; Furukawa, A.; Kimoto, T.; et al. Role of cytochrome p450 in synaptocrinology: Endogenous estrogen synthesis in the brain hippocampus. Drug Metab. Rev. 2006, 38, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Kretz, O.; Fester, L.; Wehrenberg, U.; Zhou, L.; Brauckmann, S.; Zhao, S.; Prange-Kiel, J.; Naumann, T.; Jarry, H.; Frotscher, M.; et al. Hippocampal synapses depend on hippocampal estrogen synthesis. J. Neurosci. 2004, 24, 5913–5921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fester, L.; Ribeiro-Gouveia, V.; Prange-Kiel, J.; von Schassen, C.; Bottner, M.; Jarry, H.; Rune, G.M. Proliferation and apoptosis of hippocampal granule cells require local oestrogen synthesis. J. Neurochem. 2006, 97, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Prange-Kiel, J.; Fester, L.; Zhou, L.; Lauke, H.; Carretero, J.; Rune, G.M. Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus 2006, 16, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Higo, S.; Hojo, Y.; Ishii, H.; Kominami, T.; Nakajima, K.; Poirier, D.; Kimoto, T.; Kawato, S. Comparison of sex-steroid synthesis between neonatal and adult rat hippocampus. Biochem. Biophys. Res. Commun. 2009, 385, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Tsurugizawa, T.; Ogiue-Ikeda, M.; Asashima, M.; Mukai, H.; Murakami, G.; Hojo, Y.; Kimoto, T.; Kawato, S. Local production of sex hormones and their modulation of hippocampal synaptic plasticity. Neuroscientist 2007, 13, 323–334. [Google Scholar] [CrossRef]
- Hojo, Y.; Higo, S.; Ishii, H.; Ooishi, Y.; Mukai, H.; Murakami, G.; Kominami, T.; Kimoto, T.; Honma, S.; Poirier, D.; et al. Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology 2009, 150, 5106–5112. [Google Scholar] [CrossRef]
- McEwen, B.S.; Alves, S.E. Estrogen actions in the central nervous system. Endocr. Rev. 1999, 20, 279–307. [Google Scholar] [CrossRef]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, S.C.; Korach, K.S. Estrogen Receptors: New Directions in the New Millennium. Endocr. Rev. 2018, 39, 664–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blakemore, J.; Naftolin, F. Aromatase: Contributions to Physiology and Disease in Women and Men. Physiology (Bethesda) 2016, 31, 258–269. [Google Scholar]
- Hess, R.A.; Cooke, P.S. Estrogen in the male: A historical perspective. Biol. Reprod. 2018, 99, 27–44. [Google Scholar] [CrossRef]
- Franks, S.; Hardy, K. Androgen Action in the Ovary. Front. Endocrinol. (Lausanne) 2018, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Cobellis, G.; Chioccarelli, T.; Ciaramella, V.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptins, estrogens and male fertility. Curr. Med. Chem. 2016, 23, 4070–4091. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M. Estradiol and the Developing Brain. Physiol. Rev. 2008, 88, 91–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gegenhuber, B.; Tollkuhn, J. Sex Differences in the Epigenome: A Cause or Consequence of Sexual Differentiation of the Brain? Genes 2019, 10, 432. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunk, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and Reproductive Effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef]
- Sharpe, R.M. The roles of oestrogen in the male. Trends Endocrinol. Metab. 1998, 9, 371–377. [Google Scholar] [CrossRef]
- Rochira, V.; Balestrieri, A.; Madeo, B.; Spaggiari, A.; Carani, C. Congenital estrogen deficiency in men: A new syndrome with different phenotypes; clinical and therapeutic implications in men. Mol. Cell. Endocrinol. 2002, 193, 19–28. [Google Scholar] [CrossRef]
- Jones, M.E.; Boon, W.C.; Proietto, J.; Simpson, E.R. Of mice and men: The evolving phenotype of aromatase deficiency. Trends Endocrinol. Metab. 2006, 17, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.E.; Liu, M.; Hurn, P.D. Brain aromatization: Classic roles and new perspectives. Semin. Reprod. Med. 2009, 27, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Buuse, M.; Simpson, E.R.; Jones, M.E. Prepulse inhibition of acoustic startle in aromatase knock-out mice: Effects of age and gender. Genes Brain Behav. 2003, 2, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; McInnes, K.J.; Gong, E.C.; Jones, M.E.; Simpson, E.R.; Boon, W.C. Estrogen deficient male mice develop compulsive behavior. Biol. Psychiatry 2007, 61, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Abraha´m, I.M.; Herbison, A.E. Major sex differences in non-genomic estrogen actions on intracellular signaling in mouse brain in vivo. Neuroscience 2005, 131, 945–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, D.D.; Segal, M. Morphological plasticity of dendritic spines in central neurons is mediated by activation of cAMP response element binding protein. Proc. Natl. Acad. Sci. USA 1997, 94, 1482–1487. [Google Scholar] [CrossRef] [Green Version]
- Frankfurt, M.; Gould, E.; Wolley, C.; McEwen, B.S. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: A Golgi study in the adult rat. Neuroendocrinology 1990, 51, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Carrer, H.F.; Aoki, A. Ultrastructural changes in the hypothalamic ventromedial nucleus of ovariectomized rats after estrogen treatment. Brain Res. 1982, 240, 221–233. [Google Scholar] [CrossRef]
- Frankfurt, M.; McEwen, B.S. Estrogen increases axodendritic synapses in the VMN of rats after ovariectomy. NeuroReport 1991, 2, 380–382. [Google Scholar] [CrossRef]
- Gould, E.; Woolley, C.; Frankfurt, M.; McEwen, B.S. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990, 10, 1286–1291. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.; McEwen, B.S.; Frankfurt, M. Estrogen-induction of dendritic spines in ventromedial hypothalamus and hippocampus: Effects of neonatal aromatase blockade and adult castration. Dev. Brain Res. 1995, 87, 91–95. [Google Scholar] [CrossRef]
- Amantea, D.; Spagnuolo, P.; Bari, M.; Fezza, F.; Mazzei, C.; Tassorelli, C.; Morrone, L.A.; Corasaniti, M.T.; Maccarone, M.; Bagetta, G. Modulation of the endocannabinoid system by focal brain ischemia in the rat is involved in neuroprotection afforded by 17 beta-estradiol. FEBS J. 2007, 274, 4464–4475. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Karacabely, E.S.; Gorzalka, B. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology 2007, 32, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Vallée, M.; Vitiello, S.; Bellocchio, L.; Hébert-Chatelain, E.; Reggio, P.H.; Ross, R.A.; Marsicano, G.; Piazza, P.V. Pregnenolone can protect the brain from cannabis intoxication. Science 2014, 343, 94–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmusson, A.M.; Marx, C.E.; Pineles, S.L.; Locci, A.; Scioli-Salter, E.R.; Nillni, Y.I.; Liang, J.J.; Pinna, G. Neuroactive steroids and PTSD treatment. Neurosci. Lett. 2017, 10, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Fasano, S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. Int. Rev. Cytol. 2002, 218, 69–141. [Google Scholar] [PubMed]
- Pinilla, L.; Aguilar, E.; Dieguez, C.; Millar, R.P.; Tena-Sempere, M. Kisspeptins and Reproduction: Physiological Roles and Regulatory Mechanisms. Physiol. Rev. 2012, 92, 1235–1316. [Google Scholar] [CrossRef]
- Moenter, S.M.; Silveira, M.A.; Wang, L.; Adams, C.J. Central aspects of systemic oestradiol negative- and positive-feedback on the reproductive neuroendocrine system. Neuroendocrinology 2020, 32, 12724. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Winuthayanon, W.; Korach, K.S. What’s new in estrogen receptor action in the female reproductive tract. J. Mol. Endocrinol. 2016, 56, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Meccariello, R.; Chianese, R.; Chioccarelli, T.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Cobellis, G. Intratesticular signals regulate germ cell progression and production of qualitatively mature spermatozoa in vertebrates. Front. Endocrinol. 2014, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in reproduction: Epigenetic effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Scafuro, M.; Meccariello, R. BPA and nutraceuticals, simultaneous effects on endocrine functions. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, R.; Santoro, A.; D’Angelo, S.; Morrone, R.; Fasano, S.; Viggiano, A.; Pierantoni, R. The epigenetics of the Endocannabinoid system. Int. J. Mol. Sci. 2020, 21, 1113. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dey, S.K.; Maccarrone, M. Jekyll and Hyde: Two Faces of Cannabinoid Signaling in Male and Female Fertility. Endocr. Rev. 2006, 27, 427–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovolin, P.; Cottone, E.; Pomatto, V.; Fasano, S.; Pierantoni, R.; Cobellis, G.; Meccariello, R. Endocannabinoids are involved in male vertebrate reproduction: Regulatory mechanisms at central and gonadal level. Front. Endocrinol. 2014, 5, 54. [Google Scholar] [CrossRef]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Giuffrida, A. N-Acylethanolamines in human reproductive fluids. Chem. Phys. Lipids 2002, 121, 211–227. [Google Scholar] [CrossRef] [Green Version]
- Meccariello, R.; Battista, N.; Bradshaw, H.B.; Wang, H. Updates in reproduction coming from the endocannabinoid system. Int. J. Endocrinol. 2014, 2014, 412354. [Google Scholar] [CrossRef]
- Brents, L.K. Marijuana, the Endocannabinoid System and the Female Reproductive System. Yale J. Biol. Med. 2016, 89, 175–191. [Google Scholar]
- Battista, N.; Meccariello, R.; Cobellis, G.; Fasano, S.; Di Tommaso, M.; Pirazzi, V.; Konje, J.C.; Pierantoni, R.; Maccarrone, M. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol. Cell. Endocrinol. 2012, 355, 1–14. [Google Scholar] [CrossRef]
- Cobellis, G.; Meccariello, R.; Chianese, R.; Chioccarelli, T.; Fasano, S.; Pierantoni, R. Effects of neuroendocrine CB1 activity on adult Leydig cells. Front. Endocrinol. 2016, 7, 47. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Syriac, A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J. Assist. Reprod. Genet. 2015, 32, 1575–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, O.S.; Holloway, A.C.; Raha, S. The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res. 2019, 12, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Yoshida, K.; Nishimura, H.; Harada, M.; Okajima, S.; Miyoshi, H.; Okamoto, Y.; Amamoto, T.; Watanabe, K.; Omiecinski, C.J.; et al. Δ(9)-Tetrahydrocannabinol disrupts estrogen-signaling through up-regulation of estrogen receptor β (ERβ). Chem. Res. Toxicol. 2013, 26, 1073–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiori, J.L.; Sanghvi, M.; O’Connell, M.P.; Krzysik-Walker, S.M.; Moaddel, R.; Bernier, M. EGF receptor and its ligands via destabilization of estrogen-related receptor α protein. Br. J. Pharmacol. 2011, 164, 1026–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Xie, H.; Guo, Y.; Zhang, H.; Takahashi, T.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; Cravatt, B.F.; Dey, S.K. Fatty acid amide hydrolase deficiency limits early pregnancy events. J. Clin. Investig. 2006, 116, 2122–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, N.E. The peripheral cannabinoid receptor knockout mice: An update. Br. J. Pharmacol. 2008, 153, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Wang, H.; Okabe, M.; Mackie, K.; Kingsley, P.J.; Marnett, L.J.; Cravatt, B.F.; Dey, S.K. Genetic loss of FAAH compromises male fertility in mice. Biol. Reprod. 2009, 80, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Dey, S.S. Endocannabinoid Signaling in Female Reproduction. ACS Chem. Neurosci. 2012, 3, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acone, G.; Trabucco, E.; Colacurci, N.; Cobellis, L.; Mackie, K.; Meccariello, R.; Cacciola, G.; Chioccarelli, T.; Fasano, S.; Pierantoni, R.; et al. Low type I cannabinoid receptor levels characterize placental villous in labouring delivery. Placenta 2009, 30, 203–205. [Google Scholar] [CrossRef]
- Trabucco, E.; Acone, G.; Marenna, A.; Pierantoni, R.; Cacciola, G.; Chioccarelli, T.; Mackie, K.; Fasano, S.; Colacurci, N.; Meccariello, R.; et al. Endocannabinoid System in First Trimester Placenta: Low FAAH and High CB1 Expression Characterize Spontaneous Miscarriage. Placenta 2009, 30, 516–522. [Google Scholar] [CrossRef]
- Li, Y.; Bian, F.; Sun, X.; Dey, S.K. Mice Missing Cnr1 and Cnr2 Show Implantation Defects. Endocrinology 2019, 160, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, G.; Ricci, G.; Cacciola, G.; Orlando, P.; Petrosino, S.; Cascio, M.G.; Bisogno, T.; De Petrocellis, L.; Chioccarelli, T.; Altucci, L.; et al. A Gradient of 2-Arachidonoylglycerol Regulates Mouse Epididymal Sperm Cell Start-Up. Biol. Reprod. 2010, 82, 451–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chioccarelli, T.; Cacciola, G.; Altucci, L.; Lewis, S.E.; Simon, L.; Ricci, G.; Ledent, C.; Meccariello, R.; Fasano, S.; Pierantoni, R.; et al. Cannabinoid Receptor 1 Influences Chromatin Remodeling in Mouse Spermatids by Affecting Content of Transition Protein 2 mRNA and Histone Displacement. Endocrinology 2010, 151, 5017–5029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chioccarelli, T.; Manfrevola, F.; Porreca, V.; Fasano, S.; Altucci, L.; Pierantoni, R.; Cobellis, G. The Cannabinoid Receptor CB1 Stabilizes Sperm Chromatin Condensation Status During Epididymal Transit by Promoting Disulphide Bond Formation. Int. J. Mol. Sci. 2020, 21, 3117. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, G.; Chioccarelli, T.; Altucci, L.; Viggiano, A.; Fasano, S.; Pierantoni, R.; Cobellis, G. Nuclear size as estrogen-responsive chromatin quality parameter of mouse spermatozoa. Gen. Comp. Endocrinol. 2013, 193, 2019. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, G.; Chioccarelli, T.; Altucci, L.; Ledent, C.; Mason, J.I.; Fasano, S.; Pierantoni, R.; Cobellis, G. Low 17beta-estradiol levels in CNR1 knock-out mice affect spermatid chromatin remodeling by interfering with chromatin reorganization. Biol. Reprod. 2013, 88, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, J.; Almada, M.; Silva, A.; Correia-da-Silva, G.; Teixeira, N.; Sá, S.I.; Fonseca, B.M. The endocannabinoid system expression in the female reproductive tract is modulated by estrogen. J. Steroid Biochem. Mol. Biol. 2017, 74, 40–47. [Google Scholar] [CrossRef]
- Ernst, J.; Grabiec, U.; Greither, T.; Fischer, B.; Dehghani, F. The endocannabinoid system in the human granulosa cell line KGN. Mol. Cell. Endocrinol. 2016, 423, 67–76. [Google Scholar] [CrossRef]
- Waleh, N.S.; Cravatt, B.F.; Apte-Deshpande, A.; Terao, A.; Kildu, T.S. Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene 2002, 291, 203–210. [Google Scholar] [CrossRef]
- Ciaramella, V.; Meccariello, R.; Chioccarelli, T.; Sirleto, M.; Fasano, S.; Pierantoni, R.; Chianese, R. Anandamide acts via kisspeptin in the regulation of testicular activity of the frog, Pelophylax esculentus. Mol. Cell. Endocrinol. 2016, 420, 75–84. [Google Scholar] [CrossRef]
- Grimaldi, P.; Pucci, M.; Di Siena, S.; Di Giacomo, D.; Pirazzi, V.; Geremia, R.; Maccarrone, M. The FAAH gene is the first direct target of estrogen in the testis: Role of histone demethylase LSD1. Cell. Mol. Life Sci. 2012, 69, 4177–4190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, G.; Gasperi, V.; Paro, R.; Barsacchi, D.; Cecconi, S.; Maccarrone, M. Follicle-stimulating hormone activates fatty acid amide hydrolase by protein kinase A and aromatase-dependent pathways in mouse primary Sertoli cells. Endocrinology 2007, 148, 1431–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, I.; Kalló, I.; Deli, L.; Vida, B.; Hrabovszky, E.; Fekete, C.; Moenter, S.M.; Watanabe, M.; Liposits, Z. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology 2010, 151, 5818–5829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meccariello, R.; Franzoni, M.F.; Chianese, R.; Cottone, E.; Scarpa, D.; Donna, D.; Cobellis, G.; Guastalla, A.; Pierantoni, R.; Fasano, S. Interplay between the endocannabinoid system and GnRH-I in the forebrain of the anuran amphibian Rana esculenta. Endocrinology 2008, 149, 2149–2158. [Google Scholar] [CrossRef] [Green Version]
- Bálint, F.; Liposits, Z.; Farkas, I. Estrogen Receptor Beta and 2-arachidonoylglycerol Mediate the Suppressive Effects of Estradiol on Frequency of Postsynaptic Currents in Gonadotropin-Releasing Hormone Neurons of Metestrous Mice: An Acute Slice Electrophysiological Study. Front. Cell. Neurosci. 2016, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, M.J.; Velasco, I.; Tena-Sempere, M. Novel mechanisms for the metabolic control of puberty: Implications for pubertal alterations in early-onset obesity and malnutrition. J. Endocrinol. 2019, 242, 51–65. [Google Scholar] [CrossRef]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota-Gut-Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef] [Green Version]
- Pucci, M.; Zaplatic, E.; Micioni Di Bonaventura, M.V.; Micioni Di Bonaventura, E.; Paolo De Cristofaro, P.; Maccarrone, M.; Cifani, C.; D’Addario, C. On the Role of Central Type-1 Cannabinoid Receptor Gene Regulation in Food Intake and Eating Behaviors. Int. J. Mol. Sci. 2021, 22, 398. [Google Scholar] [CrossRef]
- Washburn, N.; Borgquist, A.; Wang, K.; Jeffery, G.S.; Kelly, M.J.; Wagner, E.J. Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Neuroendocrinology 2013, 97, 160–175. [Google Scholar] [CrossRef] [Green Version]
- Meccariello, R.; Fasano, S.; Pierantoni, R. Kisspeptins, new local modulators of male reproduction: A comparative overview. Gen. Comp. Endocrinol. 2020, 299, 113618. [Google Scholar] [CrossRef] [PubMed]
- Motti, M.L.; Meccariello, R. Minireview: The epigenetic modulation of KISS1 in cancer and reproduction. Int. J. Environ. Res. Public Health 2019, 16, 2607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamikheirabad, M.; Behzadi, G.; Faghihi, M.; Raoofian, R.; Ejtemaei-Mehr, S.; Zuure, W.A.; Sadeghipour, H.R. A role for endocannabinoids in acute stress-induced suppression of the hypothalamic-pituitary-gonadal axis in male rats. Clin. Exp. Reprod. Med. 2013, 40, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobellis, G.; Cacciola, G.; Scarpa, D.; Meccariello, R.; Chianese, R.; Franzoni, M.F.; Mackie, K.; Pierantoni, R.; Fasano, S. Endocannabinoid system in frog and rodent testis: Type-1 cannabinoid receptor and fatty acid amide hydrolase activity in male germ cells. Biol. Reprod. 2006, 75, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meccariello, R.; Chianese, R.; Cacciola, G.; Cobellis, G.; Pierantoni, R.; Fasano, S. Type-1 cannabinoid receptor expression in the frog, Rana esculenta, tissues: A possible involvement in the regulation of testicular activity. Mol. Reprod. Dev. 2006, 73, 551–558. [Google Scholar] [CrossRef]
- Chianese, R.; Cobellis, G.; Pierantoni, R.; Fasano, S.; Meccariello, R. Non mammalian vertebrate models and the endocannabinoid system: Relationships with gonadotropin-releasing hormone. Mol. Cell. Endocrinol. 2008, 286, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Anandamidemodulates the expression of GnRH-II and GnRHRs in frog, Rana esculenta, diencephalon. Gen. Comp. Endocrinol. 2011, 173, 389–395. [Google Scholar] [CrossRef]
- Chianese, R.; Ciaramella, V.; Scarpa, D.; Fasano, S.; Pierantoni, R.; Meccariello, R. Anandamide regulates the expression of GnRH-I, GnRH-II and GnRHRs in frog testis. Am. J. Physiol. Endrocrinol. Metab. 2012, 303, 475–487. [Google Scholar] [CrossRef]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Hypothalamus-pituitary axis: An obligatory target for endocannabinoids to inhibit steroidogenesis in frog testis. Gen. Comp. Endocrinol. 2014, 205, 88–93. [Google Scholar] [CrossRef]
- Chianese, R.; Ciaramella, V.; Scarpa, D.; Fasano, S.; Pierantoni, R.; Meccariello, R. Endocannabinoids and endovanilloids: A possible balance in the regulation of the testicular GnRH signalling. Int. J. Endocrinol. 2013, 2013, 904748. [Google Scholar] [CrossRef]
- Ciaramella, V.; Chianese, R.; Pariante, P.; Fasano, S.; Pierantoni, R.; Meccariello, R. Expression analysis of Gnrh1 and Gnrhr1 in spermatogenic cells of rat. Int. J. Endocrinol. 2015, 2015, 982726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptin Receptor, GPR54, as a Candidate for the Regulation of Testicular Activity in the Frog, Rana esculenta. Biol. Reprod. 2013, 88, 73. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptin drives germ cell progression in the anuran amphibian Pelophylax esculentus: A study carried out in ex vivo testes. Gen. Comp. Endocrinol. 2015, 211, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptin regulates steroidogenesis and spermiation in the anuran amphibian Pelophylax esculentus testis. Reproduction 2017, 154, 403–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R.; Wada-Kiyama, Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ. Int. 2015, 83, 11–40. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Ratajczak-Wrona, W.; Górska, M.; Jabłońska, E. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef]
- Grimaldi, M.; Boulahtouf, A.; Delfosse, V.; Thouennon, E.; Bourguet, W.; Balaguer, P. Reporter cell lines for the characterization of the interactions between human nuclear receptors and endocrine disruptors. Front. Endocrinol. (Lausanne) 2015, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment: Review and Analysis of Its Occurrence and Bioaccumulation. Dose-Response 2015, 13, 1559325815598308. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Gao, P.; Xiang, P.; Zhang, X.; Cui, X.; Ma, L.Q. Molecular mechanisms of PFOA-induced toxicity in animals and humans: Implications for health risks. Environ. Int. 2017, 99, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Morck, T.J.; Sorda, G.; Bechi, N.; Rasmussen, B.S.; Nielsen, J.B.; Ietta, F.; Rytting, E.; Mathiesen, L.; Paulesu, L.; Knudsen, L.E. Placental transport and in vitroeffects of Bisphenol A. Reprod. Toxicol. 2010, 30, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Corbel, T.; Gayrard, V.; Puel, S.; Lacroix, M.Z.; Berrebi, A.; Gil, S.; Viguie, C.; Toutain, P.L.; Picard-Hagen, N. Bidirectional placental transfer of Bisphenol A and its main metabolite, Bisphenol A Glucuronide, in the isolated perfused human placenta. Reprod. Toxicol. 2014, 47, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, P.; D’Auria, R.; Viggiano, A.; Ciaglia, E.; Meccariello, R.; Russo, R.D.; Puca, A.A.; Vecchione, C.; Nori, S.L.; Santoro, A. Bisphenol A induces DNA damage in cells exerting immune surveillance functions at peripheral and central level. Chemosphere 2020, 254, 126819. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Viggiano, A.; Urbanek, K.; Cappetta, D.; Troisi, J.; Scafuro, M.; Guida, M.; Esposito, G.; Ciuffreda, L.P.; Rossi, F.; et al. Chronic exposure to low dose of bisphenol A impacts on the first round of spermatogenesis via SIRT1 modulation. Sci. Rep. 2018, 8, 2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boberg, J.; Axelstad, M.; Svingen, T.; Mandrup, K.; Christiansen, S.; Vinggaard, A.M.; Hass, U. Multiple endocrine disrupting effects in rats perinatally exposed tobutylparaben. Toxicol. Sci. 2016, 152, 244–256. [Google Scholar] [CrossRef]
- Smarr, M.M.; Sundaram, R.; Honda, M.; Kannan, K.; Louis, G.M.B. Urinary concentrations of parabens and other antimicrobial chemicals and their association with couples’ fecundity. Environ. Health Perspect. 2017, 125, 730–736. [Google Scholar] [CrossRef]
- Smith, K.W.; Souter, I.; Dimitriadis, I.; Ehrlich, S.; Williams, P.L.; Calafat, A.M.; Hauser, R. Urinary paraben concentrations and ovarian aging among women from a fertility center. Environ. Health Perspect. 2013, 121, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Ali, E.H.; Elgoly, A.H. Combined prenatal and postnatal butyl paraben exposureproduces autism-like symptoms in offspring: Comparison with valproic acid autistic model. Pharmacol. Biochem. Behav. 2013, 111, 102–110. [Google Scholar] [CrossRef]
- Lara-Valderrábano, L.; Galván, E.J.; Rocha, L. Propylparaben suppresses epileptiform activity in hippocampal CA1 pyramidal cells in vitro. Epilepsy Res. 2017, 136, 126–129. [Google Scholar] [CrossRef]
- Kodani, S.D.; Overby, H.B.; Morisseau, C.; Chen, J.; Zhao, L.; Hammock, B.D. Parabens inhibit fatty acid amide hydrolase: A potential role in paraben-enhanced 3T3-L1 adipocyte differentiation. Toxicol. Lett. 2016, 262, 92–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forner-Piquer, I.; Santangeli, S.; Maradonna, F.; Verde, R.; Piscitelli, F.; Di Marzo, V.; Habibi, H.R.; Carnevali, O. Role of Bisphenol A on the Endocannabinoid System at central and peripheral levels: Effects on adult female zebrafish. Chemosphere 2018, 205, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Forner-Piquer, I.; Mylonas, C.C.; Calduch-Giner, J.; Maradonna, F.; Gioacchini, G.; Allarà, M.; Piscitelli, F.; Di Marzo, V.; Pérez-Sánchez, J.; Carnevali, O. Endocrine disruptors in the diet of male Sparus aurata: Modulation of the endocannabinoid system at the hepatic and central level by Di-isononyl phthalate and Bisphenol A. Environ. Int. 2018, 119, 54–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocci, P.; Mozzicafreddo, M.; Angeletti, M.; Mosconi, G.; Palermo, F.A. Differential tissue regulation of peroxisome proliferator-activated receptor α (PPARα) and cannabinoid receptor 1 (CB1) gene transcription pathways by diethylene glycol dibenzoate (DEGB): Preliminary observations in a seabream (Sparus aurata) in vivo model. Environ. Toxicol. Pharmacol. 2017, 55, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, V.; Palermo, F.; Mosconi, G.; Cottone, E.; Cocci, P.; Nabissi, M.; Borgio, L.; Polzonetti-Magni, A.M.; Franzoni, M.F. Xenoestrogens elicit a modulation of endocannabinoid system and estrogen receptors in 4NP treated goldfish, Carassius auratus. Gen. Comp. Endocrinol. 2011, 174, 30–35. [Google Scholar] [CrossRef]
- Forner-Piquer, I.; Beato, S.; Piscitelli, F.; Santangeli, S.; Di Marzo, V.; Habibi, H.R.; Maradonna, F.; Carnevali, O. Effects of BPA on zebrafish gonads: Focus on the endocannabinoid system. Environ. Pollut. 2020, 264, 114710. [Google Scholar] [CrossRef] [PubMed]
- Forner-Piquer, I.; Fakriadis, I.; Mylonas, C.C.; Piscitelli, F.; Di Marzo, V.; Maradonna, F.; Calduch-Giner, J.; Pérez-Sánchez, J.; Carnevali, O. Effects of Dietary Bisphenol A on the Reproductive Function of Gilthead Sea Bream (Sparus aurata) Testes. Int. J. Mol. Sci. 2019, 20, 5003. [Google Scholar] [CrossRef] [Green Version]
- Forner-Piquer, I.; Mylonas, C.C.; Fakriadis, I.; Papadaki, M.; Piscitelli, F.; Di Marzo, V.; Calduch-Giner, J.; Pérez-Sánchez, J.; Carnevali, O. Effects of diisononyl phthalate (DiNP) on the endocannabinoid and reproductive systems of male gilthead sea bream (Sparus aurata) during the spawning season. Arch. Toxicol. 2019, 93, 727–741. [Google Scholar] [CrossRef] [Green Version]
- Rossi, G.; Dufrusine, B.; Lizzi, A.R.; Luzi, C.; Piccoli, A.; Fezza, F.; Iorio, R.; D’Andrea, G.; Dainese, E.; Cecconi, S.; et al. Bisphenol A Deranges the Endocannabinoid System of Primary Sertoli Cells with an Impact on Inhibin B Production. Int. J. Mol. Sci. 2020, 21, 8986. [Google Scholar] [CrossRef]
- Zbucka-Kretowska, M.; Zbucki, R.; Parfieniuk, E.; Maslyk, M.; Lazarek, U.; Miltyk, W.; Czerniecki, J.; Wolczynski, S.; Kretowski, A.; Ciborowski, M. Evaluation of Bisphenol A influence on endocannabinoid system in pregnant women. Chemosphere 2018, 203, 387–392. [Google Scholar] [CrossRef]
- Zamkowska, D.; Karwacka, A.; Jurewicz, J.; Radwan, M. Environmental exposure to non persistent endocrine disrupting chemicals and semen quality: An overview of the current epidemiological evidence. Int. J. Occup. Med. Environ. Health 2018, 31, 377–414. [Google Scholar] [CrossRef] [PubMed]
- Den Hond, E.; Tournaye, H.; De Sutter, P.; Ombelet, W.; Baeyens, W.; Covaci, A.; Cox, B.; Nawrot, T.S.; Van Larebeke, N.; D’Hooghe, T. Human exposure to endocrine disrupting chemicals and fertility: A case-control study in male subfertility patients. Environ. Int. 2015, 84, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Jellyman, J.K.; Ross, M.G. Epigenomics, gestational programming and risk of metabolic syndrome. Int. J. Obes. 2015, 39, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Gillette, R.; Son, M.J.; Ton, L.; Gore, A.C.; Crews, D. Passing experiences on to future generations: Endocrine disruptors and transgenerational inheritance of epimutations in brain and sperm. Epigenetics 2018, 13, 1106–1126. [Google Scholar] [CrossRef] [Green Version]
- Maamar, M.B.; King, S.E.; Nilsson, E.; Beck, D.; Skinner, M.K. Epigenetic transgenerational inheritance of parent-of-origin allelic transmission of outcross pathology and sperm epimutations. Dev. Biol. 2020, 458, 106–119. [Google Scholar] [CrossRef]
- Lee, S.Y.; Oh, S.M.; Chung, K.H. Estrogenic effects of marijuana smoke condensate and cannabinoid compounds. Toxicol. Appl. Pharmacol. 2006, 214, 270–278. [Google Scholar] [CrossRef]
- Zufferey, F.; Donzé, N.; Rahban, R.; Senn, A.; Stettler, E.; Rudaz, S.; Nef, S.; Rossier, M.F. Semen endocannabinoids are correlated to sperm quality in a cohort of 200 young Swiss men. Andrology 2020, 8, 1126–1135. [Google Scholar] [CrossRef]
- Ambrosini, A.; Zolese, G.; Ambrosi, S.; Ragni, L.; Tiano, L.; Littarru, G.; Bertoli, E.; Mantero, F.; Boscaro, M.; Balercia, G. Oleoylethanolamide protects human sperm cells from oxidation stress: Studies on cases of idiopathic infertility. Biol. Reprod. 2006, 74, 659–665. [Google Scholar] [CrossRef]
- Bar, J.; Lahav, J.; Hod, M.; Ben-Rafael, Z.; Weinberger, I.; Brosens, J. Regulation of platelet aggregation and adenosinetriphosphate release in vitro by 17beta-estradiol and medroxyprogesterone acetate in postmenopausal women. Thromb. Haemost. 2000, 84, 695–700. [Google Scholar]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Sachais, B.S. Platelet–endothelial interactions in atherosclerosis. Curr. Atheroscler. Rep. 2001, 3, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Prevot, V.; Beauvillain, J.C.; Cadet, P.; Fimiani, C.; Welters, I.; Fricchione, G.L.; Breton, C.; Lassalle, P.; Salzet, M.; et al. Cell-surface estrogen receptors mediate calcium-dependent nitric oxide release in human endothelia. Circulation 2000, 101, 1594–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarrone, M.; Bari, M.; Battista, N.; Finazzi-Agrò, A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood 2002, 100, 4040–4048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunos, G.; Jarai, Z.; Batkai, S.; Goparaju, S.K.; Ishac, E.J.; Liu, J.; Wang, L.; Wagner, J.A. Endocannabinoids as cardiovascular modulators. Chem. Phys. Lipids 2000, 108, 159–168. [Google Scholar] [CrossRef]
- Maccarrone, M.; Finazzi-Agrò, A. Endocannabinoids and their actions. Vitam. Horm. 2002, 65, 225–255. [Google Scholar] [PubMed]
- Maccarrone, M.; Bari, M.; Di Rienzo, M.; Finazzi-Agrò, A.; Rossi, A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J. Biol. Chem. 2003, 278, 32726–32732. [Google Scholar] [CrossRef] [Green Version]
- Ramer, R.; Schwarz, R.; Hinz, B. Modulation of the Endocannabinoid System as a Potential Anticancer Strategy. Front. Pharmacol. 2019, 10, 430. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Liu, Y.; Huang, S.; Liu, G.; Xie, C.; Zhou, J.; Fan, W.; Li, Q.; Wang, Q.; Zhong, D.; et al. Overexpression of cannabinoid receptors CB1 and CB2 correlates with improved prognosis of patients with hepatocellular carcinoma. Cancer Genet. Cytogenet. 2006, 171, 31–38. [Google Scholar] [CrossRef]
- Melck, D.; De Petrocellis, L.; Orlando, P.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology 2000, 141, 118–126. [Google Scholar] [CrossRef]
- Mimeault, M.; Pommery, N.; Wattez, N.; Bailly, C.; Henichart, J.P. Antiproliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: Implication of epidermal growth factor receptor downregulation and ceramide production. Prostate 2003, 56, 1–12. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Ligresti, A.; Schiano Moriello, A.; Iappelli, M.; Verde, R.; Stott, C.G.; Cristino, L.; Orlando, P.; Di Marzo, V. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 2013, 168, 79–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.C.; Hammarsten, P.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; Mancini, G.; Lutz, B.; Bergh, A.; Fowler, C.J. A high cannabinoid CB 1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer 2009, 45, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Czifra, G.; Varga, A.; Nyeste, K.; Marincsák, R.; Tóth, B.I.; Kovács, I.; Kovács, L.; Bíró, T. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J. Cancer Res. Clin. Oncol. 2009, 135, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Caffarel, M.M.; Andradas, C.; Mira, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Flores, J.M.; García-Real, I.; Palacios, J.; Mañes, S.; et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer 2010, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Melck, D.; Palmisano, A.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 8375–8380. [Google Scholar] [CrossRef] [Green Version]
- Laezza, C.; D’Alessandro, A.; Paladino, S.; Malfitano, A.M.; Proto, C.M.; Gazzerro, P.; Pisanti, S.; Santoro, A.; Ciaglia, E.; Bifulco, M. Anandamide inhibits the Wnt/β-catenin signalling pathway in human breast cancer MDA MB 231 cells. Eur. J. Cancer 2012, 48, 3112–3122. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef]
- Hasenoehrl, C.; Feuersinger, D.; Sturm, E.M.; Bärnthaler, T.; Heitzer, E.; Graf, R.; Grill, M.; Pichler, M.; Beck, S.; Butcher, L.; et al. G protein-coupled receptor GPR55 promotes colorectal cancer and has opposing effects to cannabinoid receptor 1. Int. J. Cancer 2018, 142, 121–132. [Google Scholar] [CrossRef]
- Santoro, A.; Pisanti, S.; Grimaldi, C.; Izzo, A.A.; Borrelli, F.; Proto, M.C.; Malfitano, A.M.; Gazzerro, P.; Laezza, C.; Bifulco, M. Rimonabant inhibits human colon cancer cell growth and reduces the formation of precancerous lesions in the mouse colon. Int. J. Cancer 2009, 125, 996–1003. [Google Scholar] [CrossRef]
- Ligresti, A.; Bisogno, T.; Matias, I.; De Petrocellis, L.; Cascio, M.G.; Cosenza, V.; D’argenio, G.; Scaglione, G.; Bifulco, M.; Sorrentini, I.; et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 2003, 125, 677–687. [Google Scholar] [CrossRef]
- Jacenik, D.; Beswick, E.J.; Krajewska, W.M.; Prossnitz, E.R. G protein-coupled estrogen receptor in colon function, immune regulation and carcinogenesis. World J. Gastroenterol. 2019, 25, 4092–4104. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; DiLeo, A.; Niv, Y.; Gustafsson, J.A. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016, 372, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisanti, S.; Malfitano, A.M.; Grimaldi, C.; Santoro, A.; Gazzerro, P.; Laezza, C.; Bifulco, M. Use of cannabinoid receptor agonists in cancer therapy as palliative and curative agents. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; De Petrocellis, L.; Di Marzo, V. From Phytocannabinoids to Cannabinoid Receptors and Endocannabinoids: Pleiotropic Physiological and Pathological Roles through Complex Pharmacology. Physiol. Rev. 2016, 96, 1593–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [Green Version]
- Sarnataro, D.; Pisanti, S.; Santoro, A.; Gazzerro, P.; Malfitano, A.M.; Laezza, C.; Bifulco, M. The cannabinoid CB1 receptor antagonist rimonabant (SR141716) inhibits human breast cancer cell proliferation through a lipid raft-mediated mechanism. Mol. Pharmacol. 2006, 70, 1298–1306. [Google Scholar] [CrossRef] [Green Version]
- Notarnicola, M.; Messa, C.; Orlando, A.; Bifulco, M.; Laezza, C.; Gazzerro, P.; Caruso, M.G. Estrogenicinduction of cannabinoid CB1 receptor in human colon cancercell lines. Scand. J. Gastroenterol. 2008, 43, 66–72. [Google Scholar] [CrossRef]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Tavares, C.B.; Gomes-Braga, F.; Costa-Silva, D.R.; Escórcio-Dourado, C.S.; Borges, U.S.; Conde-Junior, A.M.; Barros-Oliveira, M.C.; Sousa, E.B.; Barros, L.R.; Martins, L.M.; et al. Expression of estrogen and progesterone receptors in astrocytomas: A literature review. Clinics 2016, 71, 481–486. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Cheng, H.; Zhang, J.; Zhu, Y.; Chen, H.; Chen, L.; Qi, H.; Ren, G.; Tang, J.; et al. The Increased Expression of Estrogen-Related Receptor α Correlates with Wnt5a and Poor Prognosis in Patients with Glioma. Mol. Cancer Ther. 2019, 18, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Han, L.; Zhang, X.; Li, L.; Jiang, C.; Qiu, Y.; Huang, R.; Xie, B.; Lin, Z.; Ren, J.; et al. Alteration of endocannabinoid system in human gliomas. J. Neurochem. 2012, 120, 842–849. [Google Scholar] [CrossRef] [PubMed]
| Hormone-Dependent Cancer | Type of Study/Experimental Model | Main Results | Reference |
|---|---|---|---|
| Hepatocellular carcinoma (HCC) | HCC samples from patients at different stages of carcinogenesis | Overexpression of CB1 and CB2 receptor mRNA and protein expression levels. | [199] |
| Human prostate cancer (PCC) | PRL responsive DU-145 cells | AEA and 2-AG inhibited PRL—induced DU-145 cell proliferation. | [200] |
| AR-positive (LNCaP and 22RV1) and negative (DU-145 and PC-3) cells. Mouse model of xenograft tumor. | AEA and Cannabidiol (CBD) inhibited PCC cell proliferation and potentiated the effects of bicalutamide and docetaxel against LNCaP and DU-145 xenograft tumors. | [201,202] | |
| Human PCC tissues and normal (healthy) prostate tissues | CB1 receptor and TRPV1 mRNAs and protein levels were higher in PCC. TRPV1 correlated with increasing PCC tumor grades, whilst CB1 receptor levels were not. | [203,204] | |
| Breast cancer (BC) | MMTV-neu mouse model of ErbB2-driven metastatic breast cancer | Δ9-THC, marijuana and JWH-133 (CB2 receptor agonist) reduced cancer cell proliferation, impaired tumor angiogenesis, and reduced lung metastases. | [205] |
| Human ErbB2 BC samples | Overexpression of CB2 receptor correlated with ErbB2 expression. | ||
| MCF-7, MDA-MB231 and EFM-19 and T47D human BC cells | CB1 receptor stimulation by AEA reduced adhesion and migration of BC cells and AEA and 2-AG inhibited the nerve growth factor (NGF)−; inhibition of PRLr levels via CB1 receptor modulation of PRL responsive BC cells. AEA inhibited epithelial-mesenchymal transition of BC cells. | [22,200,206,207] | |
| Endometrial cancer (EC) | Hec50co and Ishikawa cells | eCBs and CBD reduced cell viability inducing apoptosis. | [208] |
| Colorectal cancer (CRC) | Azoxymethane (AOM)- and dextran sulfate sodium (DSS)-induced CRC mouse models. Cnr1−/− and Cnr1−/−/GPR55−/− double knockout mice | The putative GPR55 receptor acted as an oncogene and the CB1 receptor as a tumor suppressor. | [209] |
| Caco2, DLD-1, and Sw620 CRC cell lines; AOM-induced CRC mouse model | The CB1 receptor antagonist/inverse agonist SR141716 inhibited cell growth and reduced precancerous lesions in the mouse colon. Increased AEA levels also reduced CRC cell proliferation. The inhibitory effect of AEA was reached by 17β estradiol driven up-regulation of the CB1 receptor. | [6,210] | |
| CRC patients | Hypermethylation of the CNR1 gene in CRC patients and upregulation of CB1 receptor in normal tissues. | ||
| Colon biopsy by patients. CaCo-2 and DLD-1 cell lines | AEA and 2-AG levels were elevated in adenomas and CRCs. AEA and 2-AG treatment inhibited CRC cell proliferation in a CB1 and CB2 receptors dependent manner. | [211] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, A.; Mele, E.; Marino, M.; Viggiano, A.; Nori, S.L.; Meccariello, R. The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. Int. J. Mol. Sci. 2021, 22, 972. https://doi.org/10.3390/ijms22020972
Santoro A, Mele E, Marino M, Viggiano A, Nori SL, Meccariello R. The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. International Journal of Molecular Sciences. 2021; 22(2):972. https://doi.org/10.3390/ijms22020972
Chicago/Turabian StyleSantoro, Antonietta, Elena Mele, Marianna Marino, Andrea Viggiano, Stefania Lucia Nori, and Rosaria Meccariello. 2021. "The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery" International Journal of Molecular Sciences 22, no. 2: 972. https://doi.org/10.3390/ijms22020972
APA StyleSantoro, A., Mele, E., Marino, M., Viggiano, A., Nori, S. L., & Meccariello, R. (2021). The Complex Interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and Periphery. International Journal of Molecular Sciences, 22(2), 972. https://doi.org/10.3390/ijms22020972







