Characteristics and Cryopreservation of Semen of Sex-Reversed Females of Salmonid Fish
Abstract
1. Introduction
1.1. Definition
1.2. The Use of SRFs in Aquaculture
1.3. Justification
2. Hormonal Induction of Sex Reversal
2.1. Historic
2.2. Masculinisation—Type of Hormones
2.3. Important Factors for Sex Reversal
2.4. Timing of Sexual Differentiation
2.5. Methods of Steroid Administration and Their Doses
2.6. Environmental Pollution Issues Related to Sex Reversal under Hatchery Conditions
2.7. Species-Specific Characteristics Important for the Efficacy of Sex Reversal
2.8. Identification of SRF
3. Morphology and Histology of the Reproductive Tract
4. Semen Characteristics
4.1. Spermatozoa Characteristics
4.1.1. Sperm Concentration
4.1.2. Sperm Motility Parameters
4.1.3. Sperm Viability
4.1.4. Mitochondrial Membrane Integrity and ATP
4.2. Seminal Plasma Characteristics
4.2.1. Seminal Plasma Composition
4.2.2. Seminal Plasma Osmolality
4.3. Biochemical Characteristics
4.3.1. Antitrypsin Activity and Protein Concentration
4.3.2. Lactate Dehydrogenase
4.3.3. Oxidative Stress in SRF Semen
4.3.4. ROS and Lipid Peroxidation
4.3.5. Total Antioxidant Capacity
4.3.6. Enzymatic Antioxidants
4.3.7. DNA Fragmentation
5. Factors Determining Semen Quality
5.1. Introduction
5.2. Season
5.3. Strain Effects
5.4. Other Factors
6. Maturation of Semen
6.1. In Vitro Maturation
6.2. Maturation during Equilibration before Freezing
6.3. The Mechanism of Maturation
7. Short-Term Semen Storage
8. Cryopreservation of SRF Sperm
8.1. Benefits of Cryopreservation
8.2. Cryopreservation Procedures
8.2.1. Procedures Based on the Dilution Ratio
8.2.2. Development of Standardised Procedure
Final Sperm Concentration in Straw
Final Glucose Concentration in the Straws
8.2.3. Post-Thaw Storage
Effects on Post-Thaw Sperm Motility
Effects on Fertilising Ability of Cryopreserved Sperm
8.2.4. Changes in Sperm Quality Parameters during Cryopreservation
8.2.5. Vitrification
8.2.6. Implementation of Cryopreserved Sperm into Hatchery Practices
Benefits of the Use of Cryopreserved Sperm in Hatcheries
Creation of Sperm Banks
Fertilisation of Eggs with the Use of Cryopreserved Sperm
9. Proteomics of SRF Sperm
9.1. Proteomic Comparison of Normal Male and SRF Testicular Semen of Rainbow Trout
9.2. Changes in the SRF Rainbow Trout Sperm Proteome after In Vitro Incubation in ASP
10. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAT1 | acetyl-CoA acetyltransferase, mitochondrial |
| ACO2 | aconitate hydratase, mitochondrial-like; |
| ALDH2 | aldehyde dehydrogenase, mitochondrial; |
| APOA1 | apolipoprotein A-I-1 precursor, apolipoprotein A-I-2 precursor; |
| APOA2 | apolipoprotein A-II precursor; |
| ATAD3A | ATPase family AAA domain-containing protein 3A; |
| ATP5F1A | ATP synthase subunit alpha, mitochondrial-like; |
| CCDC183 | coiled-coil domain-containing protein 183; |
| CCDC40 | coiled-coil domain-containing protein 40; |
| CCDC65 | coiled-coil domain-containing protein 65; |
| CCT2 | T-complex protein 1 subunit beta; |
| CCT7 | T-complex protein 1 subunit eta; |
| CFAP45 | cilia- and flagella-associated protein 45; |
| CFAP53 | cilia- and flagella-associated protein 53; |
| CFAP58 | cilia- and flagella-associated protein 58; |
| CKB | creatine kinase; |
| CNDP2 | cytosolic non-specific dipeptidase-like isoform X2; |
| DLD | dihydrolipoyl dehydrogenase, mitochondrial-like; |
| DNAI1 | dynein intermediate chain 1; |
| DNAJB13 | dnaJ homolog subfamily B member 13; |
| DRC1 | dynein regulatory complex protein 1; |
| EFHC1 | EF-hand domain-containing protein 1; |
| EFHC2 | EF-hand domain-containing family member C2; |
| ENKUR | enkurin isoform X1; |
| FH | fumarate hydratase, mitochondrial-like; |
| FKBP2 | peptidyl-prolyl cis-trans isomerase FKBP2; |
| GAS8 | growth arrest-specific protein 8; |
| GLUD1 | glutamate dehydrogenase, mitochondrial-like; |
| HSC70 | heat shock cognate 70 kDa protein-like isoform X1; |
| HSP90B1 | 94 kDa glucose-regulated protein precursor; |
| HSPA8 | heat shock cognate 70 kDa protein-like; |
| HSPA9 | stress-70 protein, mitochondrial-like; |
| HSPD1 | 60 kDa heat shock protein, mitochondrial precursor; |
| IDH2 | isocitrate dehydrogenase 2-1 (NADP+), mitochondrial; |
| IDH3B | isocitrate dehydrogenase [NAD] subunit beta, X2; |
| MCCC2 | methylcrotonoyl-CoA carboxylase beta chain, mitochondrial, partial; |
| MDH2 | malate dehydrogenase 2-1, NAD (mitochondrial); |
| MNS1 | meiosis-specific nuclear structural protein 1-like; |
| NME5 | nucleoside diphosphate kinase homolog 5 isoform X3; |
| NME7 | nucleoside diphosphate kinase 7; |
| OGDH | 2-oxoglutarate dehydrogenase, mitochondrial isoform X3; |
| PCCB | propionyl-CoA carboxylase beta chain, mitochondrial-like; |
| PDE7A | high affinity cAMP-specific 3’,5’-cyclic phosphodiesterase 7A isoform X2; |
| PDIA3 | disulfide-isomerase A3 precursor; |
| PSMC5 | 26S protease regulatory subunit 8 isoform X2; |
| PSMC6 | proteasome (prosome, macropain) 26S subunit, ATPase, 6, partial; |
| RAN | GTP-binding nuclear protein Ran; |
| RSPH6A | radial spoke head protein 6 homolog A-like; |
| SEPT11 | septin-11-like isoform X2; |
| SPAG6 | sperm-associated antigen 6; |
| SPATC1L | speriolin-like protein; |
| TEKT1 | tektin-1-like isoform X1; |
| TEKT2 | tektin-2-like isoform X1; |
| TEKT4 | tektin-4-like; |
| THOP1 | thimet oligopeptidase-like; |
| TKT | transketolase-like; |
| TTC25 | tetratricopeptide repeat protein 25-like; |
| TUBA1B | tubulin alpha chain; |
| TUBA3C | tubulin alpha chain, testis-specific; |
| TUBB4B | tubulin alpha, testis-specific. |
| NME5 | nucleoside diphosphate kinase homolog 5 isoform X3; |
| NME7 | nucleoside diphosphate kinase 7; |
References
- Donaldson, E.M. Manipulation of reproduction in farmed fish. Anim. Reprod. Sci. 1996, 42, 381–392. [Google Scholar] [CrossRef]
- Pandian, T.; Sheela, S. Hormonal induction of sex reversal in fish. Aquaculture 1995, 138, 1–22. [Google Scholar] [CrossRef]
- Geffen, A.J.; Evans, J. Sperm traits and fertilization success of male and sex-reversed female rainbow trout (Oncorhynchus mykiss). Aquaculture 2000, 182, 61–72. [Google Scholar] [CrossRef]
- Baroiller, J.-F.; D’Cotta, H. The reversible sex of gonochoristic fish: Insights and consequences. Sex. Dev. 2016, 10, 242–266. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, M.; Benfey, T.J. Gonadal differentiation and hormonal sex reversal in arctic charr (Salvelinus alpinus). J. Exp. Zool. Part A Ecol. Genet. Physiol. 2007, 307, 527–534. [Google Scholar] [CrossRef]
- Haffray, P.; Petit, V.; Guiguen, Y.; Quillet, E.; Rault, P.; Fostier, A. Successful production of monosex female brook trout Salvelinus fontinalis using gynogenetic sex reversed males by a combination of methyltestosterone immersion and oral treatments. Aquaculture 2009, 290, 47–52. [Google Scholar] [CrossRef]
- Fitzpatrick, J.L.; Henry, J.C.; Liley, N.R.; Devlin, R.H. Sperm characteristics and fertilization success of masculinized coho salmon (Oncorhynchus kisutch). Aquaculture 2005, 249, 459–468. [Google Scholar] [CrossRef]
- Budd, A.M.; Banh, Q.Q.T.; Domingos, J.A.; Jerry, D.R. Sex control in fish: Approaches, challenges and opportunities for aquaculture. J. Mar. Sci. Eng. 2015, 3, 329–355. [Google Scholar] [CrossRef]
- Migaud, H.; Bell, G.; Cabrita, E.; McAndrew, B.; Davie, A.; Bobe, J.; Herráez, M.; Carrillo, M. Gamete quality and broodstock management in temperate fish. Rev. Aquac. 2013, 5, S194–S223. [Google Scholar] [CrossRef]
- Weber, G.M.; Lee, C.-S. Current and future assisted reproductive technologies for fish species. In Current and Future Reproductive Technologies and World Food Production; Lamb, G.C., DiLorenzo, N., Eds.; Springer: New York, NY, USA, 2014; pp. 33–76. [Google Scholar]
- Donaldson, E.M.; Hunter, G.A. Sex control in fish with particular reference to salmonids. Can. J. Fish. Aquat. Sci. 1982, 39, 99–110. [Google Scholar] [CrossRef]
- Hunter, G.A.; Donaldson, E.M. 5 Hormonal sex control and its application to fish culture. Fish Physiol. 1983, 9, 223–303. [Google Scholar] [CrossRef]
- Piferrer, F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 2001, 197, 229–281. [Google Scholar] [CrossRef]
- Francis, R.C. Sexual lability in teleosts: Developmental factors. Q. Rev. Biol. 1992, 67, 1–18. [Google Scholar] [CrossRef]
- Padoa, E. Differenziazione e inversione sessuale (femminilizzazione) di avannotti di trota (Salmo irideus) trattati con ormone fol-licolare. Monit. Zool. Ital. 1937, 48, 195–203. [Google Scholar]
- Ashby, K.R. The effect of steroid hormones on the brown trout (Salmo trutta L.) during the period of gonadal differentiation. J. Embryol. Exp. Morphol. 1957, 5, 225–249. [Google Scholar]
- Hunter, G.A.; Donaldson, E.M.; Goetz, F.W.; Edgell, P.R. Production of all-female and sterile coho salmon, and experimental evidence for male heterogamety. Trans. Am. Fish. Soc. 1982, 111, 367–372. [Google Scholar] [CrossRef]
- Hunter, G.A.; Donaldson, E.M.; Stoss, J.; Baker, I. Production of monosex female groups of chinook salmon (Oncorhynchus tshawytscha) by the fertilization of normal ova with sperm from sex-reversed females. Aquaculture 1983, 33, 355–364. [Google Scholar] [CrossRef]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Piferrer, F.; Baker, I.J.; Donaldson, E.M. Effects of natural, synthetic, aromatizable, and nonaromatizable androgens in inducing male sex differentiation in genotypic female chinook salmon (Oncorhynchus tshawytscha). Gen. Comp. Endocrinol. 1993, 91, 59–65. [Google Scholar] [CrossRef]
- Guiguen, Y.; Bertho, S.; Herpin, A.; Fostier, A. Sex determination and sex control in salmonidae. In Sex Control in Aquaculture; Wang, H., Piferrer, F., Chen, S., Chen, S.-L., Shen, Z.-G., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 251–280. [Google Scholar]
- Demska-Zakęś, K.; Hliwa, P.; Matyjewicz, P.; Zakęś, Z. Effect of 17 alpha-methyltestosterone and 11 beta-hydroxyandrostenedione on the development of reproductive system in rainbow trout (Oncorhynchus mykiss Walbaum). Arch. Polish Fish. 1999, 7, 227–235. [Google Scholar]
- Kuźmiński, H.; Dobosz, S. Effect of sex reversal in rainbow trout (Oncorhynchus mykiss Walbaum) using 17α-methyltestosterone and 11β-hydroxyandrostenedione. Arch. Pol. Fish. 2010, 18, 45–49. [Google Scholar] [CrossRef]
- Feist, G.; Yeoh, C.-G.; Fitzpatrick, M.S.; Schreck, C.B. The production of functional sex-reversed male rainbow trout with 17α-methyltestosterone and 11 β-hydroxyandrostenedione. Aquaculture 1995, 131, 145–152. [Google Scholar] [CrossRef]
- Feist, G.; Schreck, C.B.; Gharrett, A.J. Controlling the Sex of Salmonids; Oregon Sea Grant, Oregon State University: Corvallis, OR, USA, 1996. [Google Scholar]
- Yossa, R.; Bardon-Albaret, A.; Chiasson, M.A.; Liu, Q.; Duston, J.; Manning, T.; Benfey, T.J. Controlling preharvest maturity in farmed Arctic char: A review from the Canadian perspective. J. World Aquac. Soc. 2019, 50, 894–907. [Google Scholar] [CrossRef]
- Hliwa, P.; Kuźmiński, H.; Dobosz, S.; Nynca, J.; Dietrich, G.J.; Ziomek, E.; Ciereszko, A. Gonad morphology and semen quality of rainbow trout (Oncorhynchus mykiss) neo-males. In New Species in Aquaculture—Reproduction, Breeding, Prophylaxis; Zakęś, Z., Demska-Zakęś, K., Kowalska, A., Eds.; IRŚ: Olsztyn, Poland, 2011; pp. 105–116. (In Polish) [Google Scholar]
- Fatima, S.; Adams, M.; Wilkinson, R. Sex reversal of brook trout (Salvelinus fontinalis) by 17α-methyltestosterone exposure: A serial experimental approach to determine optimal timing and delivery regimes. Anim. Reprod. Sci. 2016, 175, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Adams, M.B.; Wilkinson, R. Histological study of gonadal development and sex differentiation in Salvelinus fontinalis under Tasmanian climate conditions. Aust. J. Zool. 2011, 59, 321–331. [Google Scholar] [CrossRef]
- Valdivia, K.; Jouanno, E.; Volff, J.-N.; Galiana-Arnoux, D.; Guyomard, R.; Helary, L.; Mourot, B.; Fostier, A.; Quillet, E.; Guiguen, Y. High temperature increases the masculinization rate of the all-female (xx) rainbow trout “mal” population. PLoS ONE 2014, 9, e113355. [Google Scholar] [CrossRef]
- Lee, P.; King, H.; Pankhurst, N. Preliminary Assessment of Sex Inversion of Farmed Atlantic Salmon by Dietary and Immersion Androgen Treatments. N. Am. J. Aquac. 2004, 66, 1–7. [Google Scholar] [CrossRef]
- Galbreath, P.F.; Adams, N.D.; Sherrill, L.W., III. Successful sex reversal of brook trout with 17α-methyldihydrotestosterone treatments. N. Am. J. Aquac. 2003, 65, 235–239. [Google Scholar] [CrossRef]
- Chevassus, B.; Krieg, F. Effect of the concentration and duration of methyltestosterone treatment on masculinization rate in the brown trout (Salmo trutta). Aquat. Living Resour. 1992, 5, 325–328. [Google Scholar] [CrossRef][Green Version]
- Heath, D.D.; Rankin, L.; Bryden, C.A.; Heath, J.W.; Shrimpton, J.M. Heritability and Y-chromosome influence in the jack male life history of chinook salmon (Oncorhynchus tshawytscha). Heredity 2002, 89, 311–317. [Google Scholar] [CrossRef]
- Baron, D.; Montfort, J.; Houlgatte, R.; Fostier, A.; Guiguen, Y. Androgen-induced masculinization in rainbow trout results in a marked dysregulation of early gonadal gene expression profiles. BMC Genom. 2007, 8, 357. [Google Scholar] [CrossRef]
- Baron, D.; Houlgatte, R.; Fostier, A.; Guiguen, Y. Expression profiling of candidate genes during ovary-to-testis trans-differentiation in rainbow trout masculinized by androgens. Gen. Comp. Endocrinol. 2008, 156, 369–378. [Google Scholar] [CrossRef]
- Atar, H.H.; Bekcan, S.; Dogankaya, L. Effects of different hormones on sex reversal of rainbow trout (Oncorhynchus mykissWalbaum) and production of all-female populations. Biotechnol. Biotechnol. Equip. 2009, 23, 1509–1514. [Google Scholar] [CrossRef]
- Ninhaus-Silveira, A.; Foresti, F.; Tabata, Y.A.; Rigolino, M.G.; Veríssimo-Silveira, R. Cryopreservation of semen from functional sex-reversed genotypic females of the rainbow trout, Oncorhynchus mykiss. Braz. Arch. Biol. Technol. 2006, 49, 73–77. [Google Scholar] [CrossRef]
- Weber, G.M.; Leeds, T.D.; Schneider, R.P. Sex reversal of female rainbow trout by immersion in 17α-methyltestosterone. Aquaculture 2020, 528, 735535. [Google Scholar] [CrossRef]
- Barry, T.P.; Marwah, A.; Marwah, P. Stability of 17α-methyltestosterone in fish feed. Aquaculture 2007, 271, 523–529. [Google Scholar] [CrossRef]
- Piferrer, F.; Donaldson, E.M. The comparative effectiveness of the natural and a synthetic estrogen for the direct feminization of chinook salmon (Oncorhynchus tshawytscha). Aquaculture 1992, 106, 183–193. [Google Scholar] [CrossRef]
- Johnstone, R.; MacLachlan, P. Further observations on the sex inversion of Atlantic salmon, Salmo salar L., Using 17α methyl testosterone. Aquac. Res. 1994, 25, 855–859. [Google Scholar] [CrossRef]
- Devaux, A.; Bony, S.; Plenet, S.; Sagnes, P.; Segura, S.; Suaire, R.; Novak, M.; Gilles, A.; Olivier, J.-M. Field evidence of reproduction impairment through sperm DNA damage in the fish nase (Chondrostoma nasus) in anthropized hydrosystems. Aquat. Toxicol. 2015, 169, 113–122. [Google Scholar] [CrossRef]
- Lehnert, S.J.; Heath, D.D.; Pitcher, T.E. Sperm trait differences between wild and farmed Chinook salmon (Oncorhynchus tshawytscha). Aquaculture 2012, 344–349, 242–247. [Google Scholar] [CrossRef]
- Cousin-Gerber, M.; Burger, G.; Boisseau, C.; Chevassus, B. Effect of methyltestosterone on sex differentiation and gonad morphogenesis in rainbow trout Oncorhynchus mykiss. Aquat. Living Resour. 1989, 2, 225–230. [Google Scholar] [CrossRef]
- Baynes, S. Fertilisation procedures for use in all-female brood production. In Trout News; CEFAS: Suffolk, UK, 1999; Volume 28, pp. 23–25. [Google Scholar]
- Hliwa, P.; Bah, M.M.; Kuźmiński, H.; Dobosz, S.; Ciereszko, A. Ultrasound evaluation of the gonadal structure in sex-reversed rainbow trout females. Aquac. Int. 2013, 22, 89–96. [Google Scholar] [CrossRef]
- Komen, H.; Thorgaard, G.H. Androgenesis, gynogenesis and the production of clones in fishes: A review. Aquaculture 2007, 269, 150–173. [Google Scholar] [CrossRef]
- Kowalski, R.K.; Sarosiek, B.; Demianowicz, W.; Judek, J.; Goryczko, K.; Dobosz, S.; Kuźmiński, H.; Demska-Zakeś, K.; Babiak, I.; Glogowski, J. Quantitative characteristics of rainbow trout, Oncorhynchus mykiss, neo-males (XX genotype) and super-males (YY genotype) sperm. World Acad. Sci. Eng. Technol. 2011, 5, 315–322. [Google Scholar] [CrossRef]
- Bye, V.J.; Lincoln, R.F. Commercial methods for the control of sexual maturation in rainbow trout (Salmo gairdneri R.). Aquaculture 1986, 57, 299–309. [Google Scholar] [CrossRef]
- Galas, J.F.; Hejmej, A.; Glogowski, J.; Bilińska, B. Morphological and functional alterations in testes and efferent ducts of homogametic rainbow trout Oncorhynchus mykiss walbaum. Ann. N. Y. Acad. Sci. 2009, 1163, 398–401. [Google Scholar] [CrossRef]
- de Castro, P.L.; Patil, J.G. Comparative gonad histology and semen quality of normal (XY) and neo-males (XX) of Atlantic salmon (Salmo salar). Aquac. Res. 2019, 50, 3171–3180. [Google Scholar] [CrossRef]
- Petersen, C.; Söder, O. The sertoli cell—a hormonal target and ‘super’ nurse for germ cells that determines testicular size. Horm. Res. Paediatr. 2006, 66, 153–161. [Google Scholar] [CrossRef]
- Nynca, J.; Kuźmiński, H.; Dietrich, G.; Hliwa, P.; Dobosz, S.; Liszewska, E.; Karol, H.; Ciereszko, A. Changes in sperm parameters of sex-reversed female rainbow trout during spawning season in relation to sperm parameters of normal males. Theriogenology 2012, 77, 1381–1389. [Google Scholar] [CrossRef]
- Judycka, S.; Słowińska, M.; Nynca, J.; Liszewska, E.; Dobosz, S.; Ciereszko, A. Oxidative stress in cryopreserved semen of sex-reversed female and normal male rainbow trout. Aquaculture 2020, 528, 735531. [Google Scholar] [CrossRef]
- Nynca, J.; Kuźmiński, H.; Dietrich, G.J.; Hliwa, P.; Dobosz, S.; Liszewska, E.; Karol, H.; Ciereszko, A. Biochemical and physiological characteristics of semen of sex-reversed female rainbow trout (Oncorhynchus mykiss, Walbaum). Theriogenology 2012, 77, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Inanan, B.E.; Yılmaz, F. Seasonal and age-related changes in semen characteristics and composition of seminal plasma in sex-reverse female rainbow trout (Oncorhynchus mykiss) in comparison with normal males. Anim. Reprod. Sci. 2018, 196, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Judycka, S.; Nynca, J.; Liszewska, E.; Mostek, A.; Ciereszko, A. Comparative analysis of sperm freezability of sex-reversed female brook trout and sex-reversed female rainbow trout semen. Aquaculture 2019, 498, 201–207. [Google Scholar] [CrossRef]
- Nynca, J.; Słowińska, M.; Judycka, S.; Dobosz, S.; Ciereszko, A. Acquiring the potential for motility is accompanied by profound changes in the testicular sperm proteome of sex-reversed female and normal male rainbow trout. Aquaculture 2020, 521, 735033. [Google Scholar] [CrossRef]
- Nynca, J.; Adamek, M.; Ciereszko, A. Identification of differentially expressed proteins in testicular semen of sex-reversed female (XX) and normal male (XY) rainbow trout1. J. Anim. Sci. 2017, 95, 3173–3183. [Google Scholar] [CrossRef]
- Judycka, S.; Ciereszko, A.; Dobosz, S.; Zalewski, T.; Dietrich, G.J. Effect of dilution in sperm maturation media and time of storage on sperm motility and fertilizing capacity of cryopreserved semen of sex-reversed female rainbow trout. Gen. Comp. Endocrinol. 2017, 245, 89–93. [Google Scholar] [CrossRef]
- Nynca, J.; Judycka, S.; Liszewska, E.; Dobosz, S.; Grudniewska, J.; Arai, K.; Fujimoto, T.; Ciereszko, A. Utility of different sugar extenders for cryopreservation and post-thaw storage of sperm from Salmonidae species. Aquaculture 2016, 464, 340–348. [Google Scholar] [CrossRef]
- Judycka, S.; Nynca, J.; Liszewska, E.; Dobosz, S.; Zalewski, T.; Ciereszko, A. Potassium ions in extender differentially influence the post-thaw sperm motility of salmonid fish. Cryobiology 2016, 73, 248–256. [Google Scholar] [CrossRef]
- Ciereszko, A.; Dietrich, G.; Nynca, J.; Krom, J.; Dobosz, S. Semen from sex-reversed rainbow trout of spring strain can be successfully cryopreserved and used for fertilization of elevated number of eggs. Aquaculture 2015, 448, 564–568. [Google Scholar] [CrossRef]
- Ciereszko, A.; Dietrich, G.J.; Nynca, J.; Dobosz, S.; Krom, J. Maturation of spermatozoa from rainbow trout (Oncorhynchus mykiss) sex-reversed females using artificial seminal plasma or glucose–methanol extender. Theriogenology 2015, 83, 1213–1218. [Google Scholar] [CrossRef]
- Dietrich, G.; Nynca, J.; Dobosz, S.; Zalewski, T.; Ciereszko, A. Application of glucose–methanol extender to cryopreservation of semen of sex-reversed females rainbow trout results in high post-thaw sperm motility and fertilizing ability. Aquaculture 2014, 434, 27–32. [Google Scholar] [CrossRef]
- Figueroa, E.; Risopatrón, J.; Sánchez, R.; Isachenko, V.; Merino, O.; Isachenko, V.; Valdebenito, I. Spermatozoa vitrification of sex-reversed rainbow trout (Oncorhynchus mykiss): Effect of seminal plasma on physiological parameters. Aquaculture 2013, 372–375, 119–126. [Google Scholar] [CrossRef]
- Haffray, P.; Sambroni, E.; Enright, W.J.; Driancourt, M.A.; Mikolajczyk, T.; Rault, P.; Breton, B. Efficiency of GonazonTM in rainbow trout, the first officially approved inducer of ovulation in the EU. Cybium 2008, 32, 312–313. [Google Scholar]
- Dong, Q.; Huang, C.; Tiersch, T.R. Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: Evidence from sperm agglutination in oysters. Cryobiology. 2007, 54, 87–98. [Google Scholar] [CrossRef]
- Rurangwa, E.; Kime, D.; Ollevier, F.; Nash, J. The measurement of sperm motility and factors affecting sperm quality in cultured fish. Aquaculture 2004, 234, 1–28. [Google Scholar] [CrossRef]
- Gallego, V.; Asturiano, J.F. Fish sperm motility assessment as a tool for aquaculture research: A historical approach. Rev. Aquac. 2019, 11, 697–724. [Google Scholar] [CrossRef]
- Boryshpolets, S.; Kowalski, R.K.; Dietrich, G.; Dzyuba, B.; Ciereszko, A. Different computer-assisted sperm analysis (CASA) systems highly influence sperm motility parameters. Theriogenology 2013, 80, 758–765. [Google Scholar] [CrossRef]
- Hossain, M.S.; Johannisson, A.; Wallgren, M.; Nagy, S.; Siqueira, A.P.; Rodriguez-Martinez, H. Flow cytometry for the assessment of animal sperm integrity and functionality: State of the art. Asian J. Androl. 2011, 13, 406–419. [Google Scholar] [CrossRef]
- Jamieson, B.G.M. Avian spermatozoa: Structure and phylogeny. In Reproductive Biology and Phylogeny of Birds, Part A: Phy-logeny, Morphology, Hormones and Fertilization; CRC Press, Taylor & Francis Group: Abingdon, UK, 2006; pp. 349–398. [Google Scholar]
- Agnihotri, S.K.; Agrawal, A.K.; Hakim, B.A.; Vishwakarma, A.L.; Narender, T.; Sachan, R.; Sachdev, M. Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 953–960. [Google Scholar] [CrossRef]
- Ogier De Baulny, B.; Le Vern, Y.; Kerboeuf, D.; Maisse, G. Flow cytometric evaluation of mitochondrial activity and membrane integrity in fresh and cryopreserved rainbow trout (Oncorhynchus mykiss) spermatozoa. Cryobiology 1997, 34, 141–149. [Google Scholar] [CrossRef]
- Trigo, P.; Merino, O.; Figueroa, E.; Valdebenito, I.; Sánchez, R.; Risopatrón, J. Effect of short-term semen storage in salmon (Oncorhynchus mykiss) on sperm functional parameters evaluated by flow cytometry. Andrologia 2015, 47, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.; Ronot, X.; Dadoune, J.P. Human sperm mitochondrial function related to motility: A flow and image cytometric assessment. J. Androl. 1989, 10, 439–448. [Google Scholar] [CrossRef]
- Folgerø, T.; Bertheussen, K.; Lindal, S.; Torbergsen, T.; Øian, P. Andrology: Mitochondrial disease and reduced sperm motility. Hum. Reprod. 1993, 8, 1863–1868. [Google Scholar] [CrossRef]
- Ciereszko, A.; Dabrowski, K. Effect of ascorbic acid supplement in vitro on rainbow trout sperm viability. Aquac. Int. 2000, 8, 1–8. [Google Scholar] [CrossRef]
- Morisawa, M.; Suzuki, K.; Morisawa, S. Effect of potassium and osmolarity on spermatozoa motility of salmonid fishes. J. Exp. Biol. 1983, 107, 105–113. [Google Scholar]
- Marshall, W.S.; Bryson, S.E.; Idler, D.R. Gonadotropin stimulation of K+ secretion and Na+ absorption by brook trout (Salvelinus fontinalis) sperm duct epithelium. Gen. Comp. Endocrinol. 1989, 75, 118–128. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Patzner, R.A.; Welsmann, T. The spermatic ducts of salmonid fishes (Salmonidae, Teleostei). Morphology, histochemistry and composition of the secretion. J. Fish Biol. 1993, 42, 79–93. [Google Scholar] [CrossRef]
- Schulz, R.W.; Miura, T. Spermatogenesis and its endocrine regulation. Fish Physiol. Biochem. 2002, 26, 43–56. [Google Scholar] [CrossRef]
- Billard, R. Spermatogenesis and spermatology of some teleost fish species. Reprod. Nutr. Dev. 1986, 26, 877–920. [Google Scholar] [CrossRef]
- Glogowski, J.; Kwasnik, M.; Piros, B.; Dabrowski, K.; Goryczko, K.; Dobosz, S.; Kuzminski, H.; Ciereszko, A. Characterization of rainbow trout milt collected with a catheter: Semen parameters and cryopreservation success. Aquac. Res. 2000, 31, 289–296. [Google Scholar] [CrossRef]
- Król, J.; Żarski, D.; Bernáth, G.; Palińska-Żarska, K.; Krejszeff, S.; Długoński, A.; Horvath, A. Effect of urine contamination on semen quality variables in Eurasian perch Perca fluviatilis L. Anim. Reprod. Sci. 2018, 197, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, K.; Ciereszko, A. Proteinase inhibitor(s) in seminal plasma of teleost fish. J. Fish Biol. 1994, 45, 801–809. [Google Scholar] [CrossRef]
- Ciereszko, A.; Piros, B.; Dabrowski, K.; Kucharczyk, D.; Łuczyński, M.J.; Dobosz, S.; Glogowski, J. Serine proteinase inhibitors of seminal plasma of teleost fish: Distribution of activity, electrophoretic profiles and relation to proteinase inhibitors of blood. J. Fish Biol. 1998, 53, 1292–1305. [Google Scholar] [CrossRef]
- Wojtczak, M.; Całka, J.; Glogowski, J.; Ciereszko, A. Isolation and characterization of α1-proteinase inhibitor from common carp (Cyprinus carpio) seminal plasma. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 264–276. [Google Scholar] [CrossRef]
- Inanan, B.; Öğretmen, F.; Inanan, T.; Yılmaz, F. Total antioxidant capacity, catalase activity, and lipid peroxidation changes in seminal plasma of sex-reversed female and male rainbow trout (Oncorhynchus mykiss) during spawning season. Theriogenology 2016, 86, 1975–1982. [Google Scholar] [CrossRef]
- Ciereszko, A.; Dabrowski, K. Relationship between biochemical constituents of fish semen and fertility: The effect of short-term storage. Fish Physiol. Biochem. 1994, 12, 357–367. [Google Scholar] [CrossRef]
- Aitken, R.J.; Krausz, C. Oxidative stress, DNA damage and the Y chromosome. Reproduction 2001, 122, 497–506. [Google Scholar] [CrossRef]
- Bennetts, L.E.; Aitken, R.J. A comparative study of oxidative DNA damage in mammalian spermatozoa. Mol. Reprod. Dev. 2005, 71, 77–87. [Google Scholar] [CrossRef]
- Agarwal, A.; Prabakaran, S.A.; Said, T.M. Prevention of oxidative stress injury to sperm. J. Androl. 2005, 26, 654–660. [Google Scholar] [CrossRef]
- Cabrita, E.; Martínez-Páramo, S.; Gavaia, P.J.; Riesco, M.F.; Valcarce, D.G.; Sarasquete, C.; Herráez, M.P.; Robles, V. Factors enhancing fish sperm quality and emerging tools for sperm analysis. Aquaculture 2014, 432, 389–401. [Google Scholar] [CrossRef]
- Pini, T.; Leahy, T.; De Graaf, S.P. Sublethal sperm freezing damage: Manifestations and solutions. Theriogenology 2018, 118, 172–181. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Mansour, N. A comparative study on antioxidant systems in semen of species of the Percidae, Salmonidae, Cyprinidae, and Lotidae for improving semen storage techniques. Aquaculture 2010, 307, 130–140. [Google Scholar] [CrossRef]
- Twigg, J.P.; Irvine, D.S.; Aitken, R.J. Oxidative damage to DNA in human spermatozoa does not preclude pronucleus formation at intracytoplasmic sperm injection. Hum. Reprod. 1998, 13, 1864–1871. [Google Scholar] [CrossRef]
- Labbe, C.; Martoriati, A.; Devaux, A.; Maisse, G. Effect of sperm cryopreservation on sperm DNA stability and progeny development in rainbow trout. Mol. Reprod. Dev. 2001, 60, 397–404. [Google Scholar] [CrossRef]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function—in sickness and in health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Martínez-Páramo, S.; Cabrita, E.; Martínez-Pastor, F.; De Paz, P.; Herráez, M. Evaluation of oxidative DNA damage promoted by storage in sperm from sex-reversed rainbow trout. Theriogenology 2009, 71, 605–613. [Google Scholar] [CrossRef]
- Cabrita, E.; Robles, V.; Rebordinos, L.; Sarasquete, C.; Herráez, M.P. Evaluation of DNA damage in rainbow trout (Oncorhynchus mykiss) and gilthead sea bream (Sparus aurata) cryopreserved sperm. Cryobiology 2005, 50, 144–153. [Google Scholar] [CrossRef]
- Dietrich, G.; Szpyrka, A.; Wojtczak, M.; Dobosz, S.; Goryczko, K.; Żakowski, Ł.; Ciereszko, A.; Dietrich, M.A. Effects of UV irradiation and hydrogen peroxide on DNA fragmentation, motility and fertilizing ability of rainbow trout (Oncorhynchus mykiss) spermatozoa. Theriogenology 2005, 64, 1809–1822. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Martínez-Páramo, S.; Beirão, J.; Herráez, M.P. Fertilization capacity with rainbow trout DNA-damaged sperm and embryo developmental success. Reproduction 2010, 139, 989–997. [Google Scholar] [CrossRef]
- Robles, V.; Cabrita, E.; Cuñado, S.; Herráez, M.P. Sperm cryopreservation of sex-reversed rainbow trout (Oncorhynchus mykiss): Parameters that affect its ability for freezing. Aquaculture 2003, 224, 203–212. [Google Scholar] [CrossRef]
- Judycka, S.; Nynca, J.; Ciereszko, A. Opportunities and challenges related to the implementation of sperm cryopreservation into breeding of salmonid fishes. Theriogenology 2019, 132, 12–21. [Google Scholar] [CrossRef]
- Bromage, N.; Porter, M.; Randall, C. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 2001, 197, 63–98. [Google Scholar] [CrossRef]
- Labbe, C.; Maisse, G. Influence of rainbow trout thermal acclimation on sperm cryopreservation: Relation to change in the lipid composition of the plasma membrane. Aquaculture 1996, 145, 281–294. [Google Scholar] [CrossRef]
- Fenkes, M.; Fitzpatrick, J.L.; Ozolina, K.; Shiels, H.A.; Nudds, R.L. Sperm in hot water: Direct and indirect thermal challenges interact to impact on brown trout sperm quality. J. Exp. Biol. 2017, 220, 2513–2520. [Google Scholar] [CrossRef]
- Wolf, K. Physiological salines for fresh-water teleosts. Progress. Fish-Cult. 1963, 25, 135–140. [Google Scholar] [CrossRef]
- Morisawa, S.; Morisawa, M. Induction of potential for sperm motility by bicarbonate and pH in rainbow trout and chum salmon. J. Exp. Biol. 1988, 136, 13–22. [Google Scholar]
- Kobayashi, T.; Fushiki, S.; Ueno, K. Improvement of sperm motility of sex-reversed male rainbow trout, Oncorhynchus mykiss, by incubation in high-ph artificial seminal plasma. Environ. Boil. Fishes 2004, 69, 419–425. [Google Scholar] [CrossRef]
- Itoh, A.; Inaba, K.; Ohtake, H.; Fujinoki, M.; Morisawa, M. Characterization of a cAMP-dependent protein kinase catalytic subunit from rainbow trout spermatozoa. Biochem. Biophys. Res. Commun. 2003, 305, 855–861. [Google Scholar] [CrossRef]
- Alavi, S.M.H.; Cosson, J.; Bondarenko, O.; Linhart, O. Sperm motility in fishes: (III) diversity of regulatory signals from membrane to the axoneme. Theriogenology 2019, 136, 143–165. [Google Scholar] [CrossRef]
- Tresguerres, M.; Barott, K.L.; Barron, M.E.; Roa, J.N. Established and potential physiological roles of bicarbonate-sensing soluble adenylyl cyclase (sAC) in aquatic animals. J. Exp. Biol. 2014, 217, 663–672. [Google Scholar] [CrossRef]
- McNiven, M.; Gallant, R.; Richardson, G. Fresh storage of rainbow trout (Oncorhynchus mykiss) semen using a non-aqueous medium. Aquaculture 1993, 109, 71–82. [Google Scholar] [CrossRef]
- Contreras, P.; Dumorné, K.; Ulloa-Rodríguez, P.; Merino, O.; Figueroa, E.; Farías, J.G.; Valdebenito, I.; Risopatrón, J. Effects of short-term storage on sperm function in fish semen: A review. Rev. Aquac. 2019. [Google Scholar] [CrossRef]
- Cabrita, E.; Robles, V.; Herraez, P. Chilled storage of sperm and eggs. In Methods in Reproductive Aquaculture, Marine and Freshwater Species; Bobe, J., Labbe, C., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 219–231. [Google Scholar]
- Cabrita, E.; Sarasquete, C.; Martínez-Páramo, S.; Robles, V.; Beirão, J.; Pérez-Cerezales, S.; Herráez, M.P. Cryopreservation of fish sperm: Applications and perspectives. J. Appl. Ichthyol. 2010, 26, 623–635. [Google Scholar] [CrossRef]
- McAndrew, B.J.; Rana, K.L.; Penman, D.J. Conservation and preservation of genetic variation in aquatic organisms. In Recent Advances in Aquaculture; Muir, J.F., Roberts, R.J., Eds.; Blackwell Scientific Publications: Oxford, UK, 1993; Volume 4, pp. 295–336. [Google Scholar]
- Lubzens, E.; Rothbard, S.; Hadani, A. Cryopreservation and viability of spermatozoa from the ornamental Japanese carp. Isr. J. Aquac. 1993, 45, 169–174. [Google Scholar]
- Erdahl, D.A.; Graham, F.F. Preservation of spermatozoa of brook trout and rainbow trout. CryoLetters 1980, 1, 203–208. [Google Scholar]
- Nynca, J.; Judycka, S.; Liszewska, E.; Dobosz, S.; Ciereszko, A. Standardization of spermatozoa concentration for cryopreservation of rainbow trout semen using a glucose-methanol extender. Aquaculture 2017, 477, 23–27. [Google Scholar] [CrossRef]
- Judycka, S.; Nynca, J.; Liszewska, E.; Dobosz, S.; Grudniewska, J.; Ciereszko, A. Optimal sperm concentration in straws and final glucose concentration in extender are crucial for improving the cryopreservation protocol of salmonid spermatozoa. Aquaculture 2018, 486, 90–97. [Google Scholar] [CrossRef]
- Judycka, S.; Żarski, D.; Dietrich, M.A.; Palińska-Żarska, K.; Karol, H.; Ciereszko, A. Standardized cryopreservation protocol of European perch (Perca fluviatilis) semen allows to obtain high fertilization rates with the use of frozen/thawed semen. Aquaculture 2019, 498, 208–216. [Google Scholar] [CrossRef]
- Horvath, A.; Labbé, C.; Jesensek, D.; Hoitsy, G.; Bernáth, G.; Kaczkó, D.; Bokor, Z.; Urbányi, B. Post-thaw storage of sperm from various salmonid species. J. Appl. Ichthyol. 2015, 31, 119–124. [Google Scholar] [CrossRef]
- Nynca, J.; Dietrich, G.; Dobosz, S.; Zalewski, T.; Ciereszko, A. Effect of postthaw storage time and sperm-to-egg ratio on fertility of cryopreserved brook trout sperm. Theriogenology 2015, 83, 253–256. [Google Scholar] [CrossRef]
- Billard, R. Reproduction in rainbow trout: Sex differentiation, dynamics of gametogenesis, biology and preservation of gametes. Aquaculture 1992, 100, 263–298. [Google Scholar] [CrossRef]
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon. J. Androl. 2000, 21, 1–7. [Google Scholar] [PubMed]
- Watson, P. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef]
- Li, P.; Hulak, M.; Koubek, P.; Sulc, M.; Dzyuba, B.; Boryshpolets, S.; Rodina, M.; Gela, D.; Maňásková, P.; Pěknicová, J.; et al. Ice-age endurance: The effects of cryopreservation on proteins of sperm of common carp, Cyprinus carpio L. Theriogenology 2010, 74, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, H.; Welch, G. Effects of reactive oxygen species on sperm function. Theriogenology 2012, 78, 1700–1708. [Google Scholar] [CrossRef]
- Figueroa, E.; Merino, O.; Risopatrón, J.; Isachenko, V.; Sánchez, R.; Effer, B.; Isachenko, E.; Farias, J.; Valdebenito, I. Effect of seminal plasma on Atlantic salmon (Salmo salar) sperm vitrification. Theriogenology 2015, 83, 238–245.e2. [Google Scholar] [CrossRef]
- Merino, O.; Risopatrón, J.; Sanchez, R.; Isachenko, E.; Figueroa, E.; Valdebenito, I.; Isachenko, V. Fish (Oncorhynchus mykiss) spermatozoa cryoprotectant-free vitrification: Stability of mitochondrion as criterion of effectiveness. Anim. Reprod. Sci. 2011, 124, 125–131. [Google Scholar] [CrossRef]
- Merino, O.; Sánchez, R.; Risopatrón, J.; Isachenko, V.; Katkov, I.; Figueroa, E.; Valdebenito, I.; Mallmann, P.; Isachenko, V. Cryoprotectant-free vitrification of fish (Oncorhynchus mykiss) spermatozoa: First report. Andrologia 2012, 44, 390–395. [Google Scholar] [CrossRef]
- Kása, E.; Lujić, J.; Marinović, Z.; Kollár, T.; Bernáth, G.; Bokor, Z.; Urbányi, B.; Lefler, K.K.; Jesenšek, D.; Horváth, Á. Development of sperm vitrification protocols for two endangered salmonid species: The Adriatic grayling, Thymallus thymallus, and the marble trout, Salmo marmoratus. Fish Physiol. Biochem. 2018, 44, 1499–1507. [Google Scholar] [CrossRef]
- Fahy, G.; Macfarlane, D.; Angell, C.; Meryman, H. Vitrification as an approach to cryopreservation. Cryobiology 1984, 21, 407–426. [Google Scholar] [CrossRef]
- Truscott, B.; Idler, D.R.; Hoyle, R.J.; Freeman, H.C. Sub-zero preservation of atlantic salmon sperm. J. Fish. Res. Board Can. 1968, 25, 363–372. [Google Scholar] [CrossRef]
- Martínez-Páramo, S.; Horvath, A.; Labbé, C.; Zhang, T.; Robles, V.; Herráez, P.; Suquet, M.; Adams, S.; Viveiros, A.T.M.; Tiersch, T.R.; et al. Cryobanking of aquatic species. Aquaculture 2017, 472, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.A.; Nynca, J.; Ciereszko, A. Proteomic and metabolomic insights into the functions of the male reproductive system in fishes. Theriogenology 2019, 132, 182–200. [Google Scholar] [CrossRef] [PubMed]
- Leeds, T.D.; Vallejo, R.L.; Weber, G.M.; Gonzalez-Pena, D.; Silverstein, J.T. Response to five generations of selection for growth performance traits in rainbow trout (Oncorhynchus mykiss). Aquaculture 2016, 465, 341–351. [Google Scholar] [CrossRef]

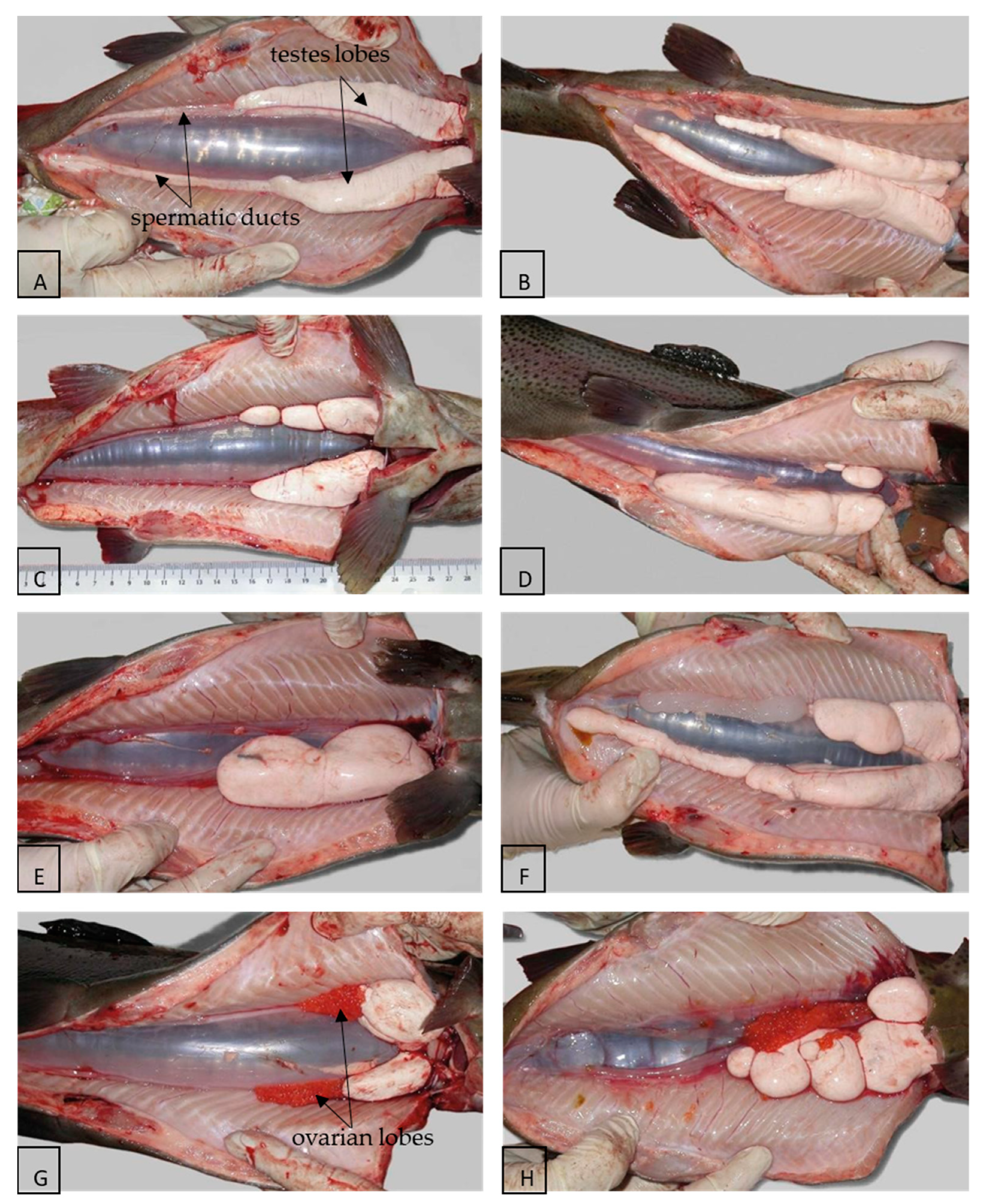
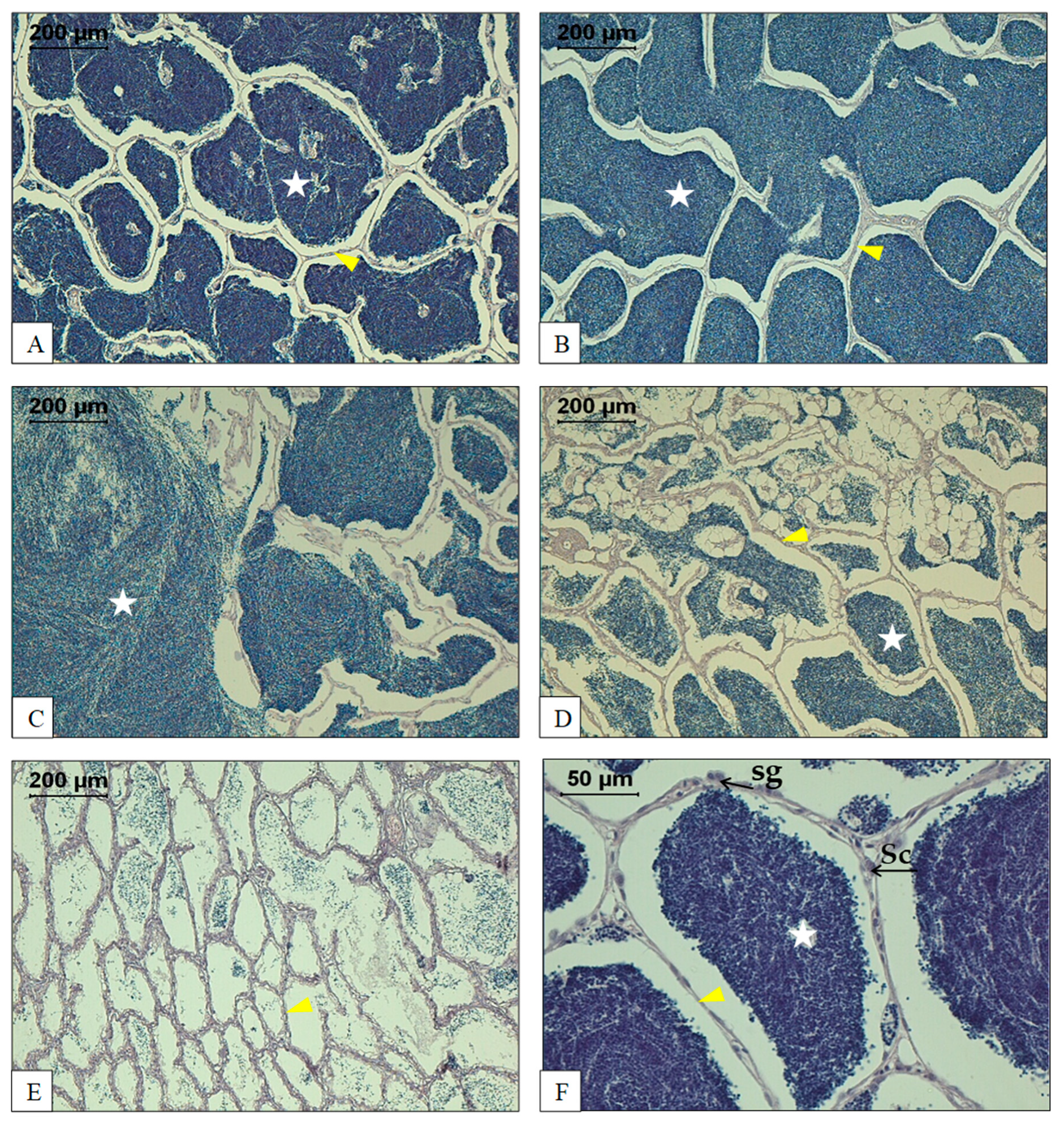
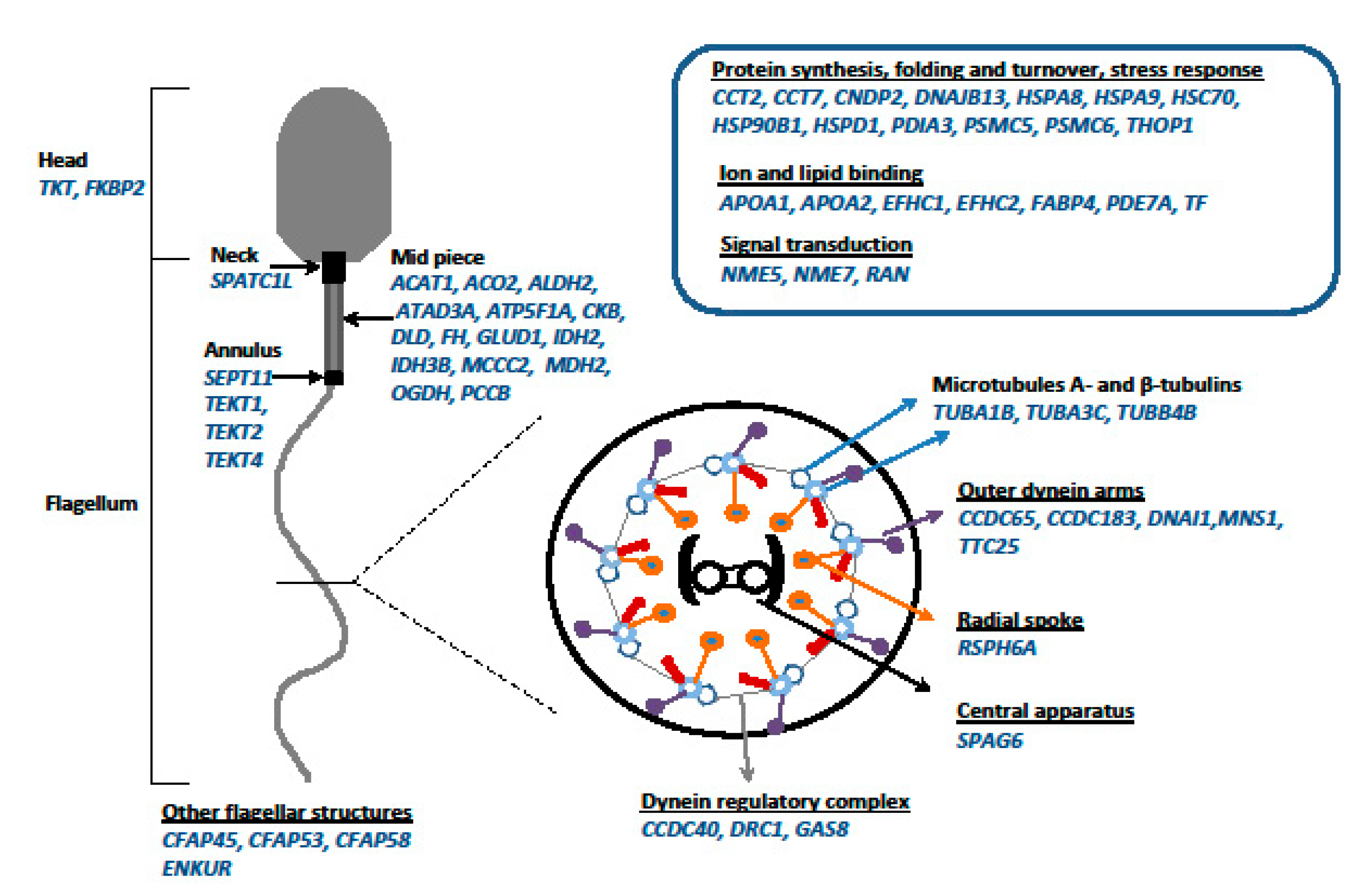
| Species | Hormone/Method of Application | Functional Male (%) | Dysfunctional Male (%) | Female (%) | Bisexual (%) | Sterile/ Immature (%) | Reference |
|---|---|---|---|---|---|---|---|
| Rainbow trout (Oncorhynchus mykiss) | MT/(6 ppm) feed | 5.3 | 54.4 | 36.7 | 1.8 | 1.8 | [23] |
| OHA/(20 ppm) feed | 1.6 | 93.4 | 1.7 | 1.6 | 1.7 | ||
| OHA/(20 ppm) feed | 3.0 | 89.6 | – | 7.4 | – | [27] | |
| Brook trout (Salvelinus fontinalis) | MT/(400 µg·L−1) immersion | 17.0 | – | – | – | 83.0 | [28] |
| MT/(400 µg·L−1) immersion and (3 mg·kg−1) feed | 31.0 | – | – | 8.0 | 61.0 | ||
| MT/(400 µg·L−1) immersion and (3 mg·kg−1) feed | 14.0 | 1.0 | 85.0 | [6] |
| Species | Hormone/Dose | Treatment | Main Outcome | Reference |
|---|---|---|---|---|
| Arctic charr (Salvelinus alpinus) | MDHT/10 mg L−1 | Weekly immersion 140 °C-days post-hatch | 90% males | [5] |
| MDHT/0.5 mg kg−1 | Feeding 140–600 °C-days post-hatch | 100% males | ||
| Atlantic salmon (Salmo salar) | MT/1 or 3 mg kg−1 | Feeding 800 °C-days | 100% males | [31] |
| MDHT/1 mg kg−1 | Feeding 800 °C-days | 100% males | ||
| MDHT/0.4 mg L−1 | Immersion 7 and 14 d after hatch | 100% males | ||
| Brook trout (Salvelinus fontinalis) | MDHT/0.5 mg L−1 | Immersion 10 d after hatch | up to 45% males | [32] |
| MDHT/0.5 mg kg−1 | Feeding 60 d beginning at first feeding | 100% fem. progeny | ||
| MT/0.4 mg L−1 | Immersion 4 weekly starting 1 week | 100% males | [6] | |
| MT/3 mg kg−1 | before hatching and feeding 800 °C-days from the first feeding | |||
| MT/0.4 mg L−1 | Immersion on 6th and 4th day pre hatching | 75% males | [28] | |
| Brown trout (Salmo trutta) | MT/3 mg kg−1 | Feeding for 800 °C-days | >90% males | [33] |
| Chinook salmon (Oncorhynchus tshawytscha) | MDHT/0.4 mg L−1 | Immersion on 3 days after 50% hatch | 100% males | [20] |
| MT/0.4 mg L−1 | Immersion on 520 °C-days and 620 °C-days after hatching | 71% functional males | [34] | |
| Coho salmon (Oncorhynchus kisutch) | MT/0.2 mg L−1 | Two immersions weekly, starting during final hatching | 46% functional males | [7] |
| Rainbow trout (Oncorhynchus mykiss) | OHA/20 mg kg−1 | 60 days from the first feeding | 96.6% males | [23] |
| OHA/0.4 mg L−1 | Immersion 20 dpf for 2 h and | 100% functional males | [24] | |
| OHA/3 mg kg−1 | 60 days from the first feeding | |||
| OHA/10 mg kg−1 | 3 months from the first feeding | 100% functional males | [35,36] | |
| MT/3 mg kg−1 | 60 days from the first feeding | 87% males | [37] | |
| MT/0.25 mg kg−1 | 80 days from the first feeding 1024 °C-days | functional males | [38] | |
| MT/0.4 mg L−1 | 2 h at 1 week post-hatching and weekly for 5 weeks, starting at the onset of first feeding | ~50% functional males | [39] |
| Species/ Spawning | Type of Semen | Sperm Concentration (×109 spz mL−1) or Spermatocrit (%) | Sperm Motility (%) | VCL (μm s−1) | VSL (μm s−1) | VAP (μm s−1) | ALH (μm) | LIN (%) | Viability (%) | Seminal Plasma Osmolality (mOsm kg−1) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atlantic salmon (Salmo salar) | |||||||||||
| Winter | testicular | 38.4 ± 1.1 | 57.2 ± 36.5 | 155.3 ± 48.6 | 87.5 ± 31.0 | 139.4 ± 41.5 | 63.9 ± 12.8 | 71.6 ± 7.6 | [52] | ||
| Brook trout (Salvelinus fontinalis) | |||||||||||
| November | testicular | 29.0 ± 7.0 | 59.0 ± 8.0 | 170.0 ± 17.0 | 20.0 ± 6.0 | 68.0 ± 6.0 | 20.0 ± 2.0 | 13.0 ± 4.0 | 307 ± 9 | [58] | |
| 30.0 ± 4.0 | 53.0 ± 7.0 | 155.0 ± 13.0 | 25.0 ± 7.0 | 73.0 ± 12.0 | 20.0 ± 3.0 | 18.0 ± 5.0 | 302 ± 4 | ||||
| Chinook salmon (Oncorhynchus tshawytscha) | |||||||||||
| October | stripped | 0.005 | 80 | 110 | [44] | ||||||
| Coho salmon (Oncorhynchus kisutch) | |||||||||||
| Fall | stripped | 20% | >30 | 60 | [7] | ||||||
| testicular | 80% | >10 | 35 | ||||||||
| Rainbow trout (Oncorhynchus mykiss) | |||||||||||
| April | testicular | 32.6 ± 2.9 | 26 | ~110 | ~14 | ~60 | ~11 | ~14 | ~90 | 287 ± 8 | [59] |
| January | testicular | 31.8 ± 3.2 | 77.3 ± 6.9 | 227.8 ± 30.2 | 87.5 ± 1.0 | 299 ± 8 | [55] | ||||
| November | testicular | 28.0 ± 3.0 | 59.0 ± 17.0 | 142.0 ± 22.0 | 40.0 ± 15.0 | 86.0 ± 21.0 | 15.0 ± 2.0 | 27.0 ± 6.0 | 303 ± 3 | [58] | |
| 26.0 ± 4.0 | 69.0 ± 11.0 | 123.0 ± 14.0 | 20.0 ± 5.0 | 61.0 ± 11.0 | 16.0 ± 3.0 | 17.0 ± 2.0 | 304 ± 4 | ||||
| 31.0 ± 9.0 | 56.0 ± 10.0 | 135.0 ± 31.0 | 17.0 ± 6.0 | 51.0 ± 13.0 | 16.0 ± 4.0 | 13.0 ± 4.0 | 305 ± 4 | ||||
| First spawning | testicular | [57] | |||||||||
| December | 22.77 ± 1.05 | 335 ± 8 | |||||||||
| January | 28.11 ± 1.5 | ||||||||||
| February | 30.44 ± 1.75 | 310 ± 3 | |||||||||
| Second spawning | |||||||||||
| December | 25.15 ± 3.01 | 342 ± 8 | |||||||||
| January | 36.83 ± 2.94 | ||||||||||
| February | 37.09 ± 3.30 | 311 ± 4 | |||||||||
| April | testicular | 44.3 ± 7.0 | 36.2 ± 16.1 | 112.5 ± 19.5 | 28.7 ± 11.8 | 63.1 ± 7.0 | 10.5 ± 3.3 | 24.7 ± 8.9 | 95.7 ± 2.7 | 305 ± 12 | [60] |
| April | testicular | 31.22 ± 7.55 | 52.8 ± 9.4 | 173.3 ± 25.4 | 46.5 ± 11.6 | 97.4 ± 18.9 | 21.4 ± 4.1 | 25.8 ± 6.0 | [61] | ||
| December | testicular | 32.2 ± 7.0 | 75 | 140 | 25 | 70 | 19 | 19 | 314 ± 12 | [62] | |
| December | testicular | 31.6 ± 6.2 | 65 | 150 | 45 | 80 | 16 | 25 | 283 ± 37 | [63] | |
| December | testicular | 32.7 ± 3.4 | [64] | ||||||||
| May | testicular | 30.1 ± 5.0 | 49.4 ± 7.2 | 140 | 22 | 60 | 17 | 16 | 290 ± 7 | [65] | |
| December | testicular | 42.8 ± 4.2 | 50 | 135 | 30 | 70 | 16 | 21 | 300 ± 8 | [66] | |
| testicular | 26.4 ± 2.4 | 64.0 ± 10.0 | [47] | ||||||||
| 35.1 ± 6.5 | 71.0 ± 11.0 | ||||||||||
| testicular | 12.0 ± 1.4 | >80% | 99.1 ± 5.1 | [67] | |||||||
| February motility: | testicular | [54] | |||||||||
| <25% | 37.5 ± 2.5 | 17.7 ± 6.5 | 130.0 ± 19.3 | 23.6 ± 7.6 | 16.9 ± 3.6 | 21.3 ± 7.7 | ~90.0 | 326.3 ± 10.9 | |||
| 25–50% | 36.6 ± 6.2 | 41.6 ± 6.4 | 122.5 ± 14.3 | 24.9 ± 6.6 | 15.3 ± 3.0 | 23.3 ± 6.6 | ~90.0 | 323.5 ± 11.8 | |||
| >50% | 32.5 ± 5.0 | 64.7 ± 11.9 | 149.2 ± 15.8 | 31.5 ± 8.2 | 8.9 ± 3.0 | 21.9 ± 5.1 | ~90.0 | 320.6 ± 8.4 | |||
| February-beginning | testicular | 35.5 ± 5.7 | 41.1 ± 20.2 | 133.2 ± 20.1 | 26.5 ± 8.2 | 17.0 ± 3.5 | 22.3 ± 6.9 | 323.6 ± 10.9 | [56] | ||
| April-middle | 32.9 ± 5.5 | 59.5 ± 18.1 | 151.4 ± 18.1 | 30.9 ± 12.9 | 19.1 ± 2.5 | 20.7 ± 6.6 | 316.4 ± 15.9 | ||||
| April-end of the spawning season | 38.8 ± 9.4 | 37.2 ± 22.2 | 114.6 ± 23.1 | 28.0 ± 8.7 | 15.5 ± 4.7 | 28.3 ± 9.5 | 308.9 ± 9.9 | ||||
| testicular | 21.6 ± 2.7 | 73.1 | 127.5 | 34.6 | 74.1 | 15.0 | 26.9 | 308 ± 20 | [49] | ||
| testicular | 13.6 ± 19.3 | [68] | |||||||||
| December and January | testicular | 22.5 ± 8.3 | [3] | ||||||||
| Spawning | Protein Concentration (mg mL−1) | Antitrypsin Activity (U L−1) | Lactate Dehydrogenase Activity (U L−1) | Reference |
|---|---|---|---|---|
| First spawning | [57] | |||
| December | 7.8 ± 1.9 | |||
| January | 6.1 ± 2.0 | |||
| February | 4.0 ± 1.6 | |||
| Second spawning | ||||
| December | 8.2 ± 1.3 | |||
| January | 5.7 ± 1.4 | |||
| February | 3.4 ± 1.4 | |||
| April | 27.3 ± 3.0 | [60] | ||
| February | [54] | |||
| Motility: | ||||
| <25% | 5.4 ± 1.9 | 1453 ± 496 | 1709 ± 658 | |
| 25–50% | 4.5 ± 1.4 | 1235 ± 544 | 1479 ± 632 | |
| >50% | 3.5 ± 1.3 | 849 ± 210 | 1152 ± 457 | |
| February-beginning | 4.3 ± 1.8 | 1117 ± 437 | 1408 ± 636 | [56] |
| April-middle | 2.9 ± 1.1 | 794 ± 270 | 1169 ± 513 | |
| April-end of the spawning season | 3.6 ± 1.9 | 748 ± 416 | 2897 ± 607 | |
| 7.7 ± 5.1 | [49] |
| Procedure Based on the Dilution Ratio | |||||||
|---|---|---|---|---|---|---|---|
| SRF Species | Extender Composition | Cryopreservation Procedure | Post-Thaw Motility (%) | Post-Thaw Viability (%) | Fertilising Ability (%) | References | |
| Dilution Ratio | Straw Size | ||||||
| Rainbow trout (Oncorhynchus mykiss) | 5.4% glucose, 9% DMSO, 10% egg yolk | 1:3 | 1.7 mL microfuge tube | 0–62% of control | [24] | ||
| 7% DMSO, 10% egg yolk, 7.5 mg mL−1 Dan Pro S760 in the mineral solution #6 from Erdahl and Graham (1980; 0.7 mM CaCl2 × 2H2O, 1.08 mM MgCl2 × 6H2O, 1.49 mM Na2HPO4, 34.3 mM KCl, 100 mM NaCl, 0.52 mM citric acid, 55.5 mM glucose, 20 mL KOH solution 226 mM, 20 mL bicine solution 324 mM, 323 mOsm kg−1, pH 7.4) | 1:3 | 0.5 mL | spring strain 1–6% | 72–78 | [106] | ||
| winter strain 18–29% | 74–84 | ||||||
| 80% of 5.4% glucose, 10% egg yolk, 10% DMSO | 1:3 | 0.5 mL | 56 ± 18 | [38] | |||
| #6 Erdahl and Graham (1980; see above) and 7% DMSO | 1:3 | 0.5 mL | 69 ± 4 | [102] | |||
| 0.18 M glucose, 9% methanol | 1:9 | 0.25 mL | 55 | 56 | 80 | [66] | |
| 57 ± 7 | 91 ± 2–94 ± 2 | [64] | |||||
| ~30 | [63] | ||||||
| 57 ± 9 | 72–87 | [61] | |||||
| 0.18 M glucose, 9% methanol | 1:9 | 0.25 mL | ~40 | [62] | |||
| 0.18 M sucrose, 9% methanol | ~40 | ||||||
| 0.18 M trehalose, 9% methanol | ~40 | ||||||
| Chinook salmon (Oncorhynchus tshawytscha) | 0.3 M glucose, 10% DMSO | 1:3 | pellets | 76 ± 8 | [18] | ||
| Standardized Procedure | |||||||
| SRF Species | Final Concentration of Cryoprotectants | Final Spermatozoa Concentration in Straw (×109 mL−1) | Straw Size | Post-Thaw Motility (%) | Post-Thaw Viability (%) | Fertilising Ability (%) | References |
| Rainbow trout (Oncorhynchus mykiss) | 0.15 M glucose, 7.5% methanol | 3 | 0.5 mL | 49 | 81–90 | [58] | |
| ~60 | ~70 | [55] | |||||
| Brook trout (Salvelinus fontinalis) | 0.19 M glucose, 7.5% methanol | 3 | 0.5 mL | 55 | 33–41 | [58] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judycka, S.; Nynca, J.; Hliwa, P.; Ciereszko, A. Characteristics and Cryopreservation of Semen of Sex-Reversed Females of Salmonid Fish. Int. J. Mol. Sci. 2021, 22, 964. https://doi.org/10.3390/ijms22020964
Judycka S, Nynca J, Hliwa P, Ciereszko A. Characteristics and Cryopreservation of Semen of Sex-Reversed Females of Salmonid Fish. International Journal of Molecular Sciences. 2021; 22(2):964. https://doi.org/10.3390/ijms22020964
Chicago/Turabian StyleJudycka, Sylwia, Joanna Nynca, Piotr Hliwa, and Andrzej Ciereszko. 2021. "Characteristics and Cryopreservation of Semen of Sex-Reversed Females of Salmonid Fish" International Journal of Molecular Sciences 22, no. 2: 964. https://doi.org/10.3390/ijms22020964
APA StyleJudycka, S., Nynca, J., Hliwa, P., & Ciereszko, A. (2021). Characteristics and Cryopreservation of Semen of Sex-Reversed Females of Salmonid Fish. International Journal of Molecular Sciences, 22(2), 964. https://doi.org/10.3390/ijms22020964





