An In Vivo Study of a Rat Fluid-Percussion-Induced Traumatic Brain Injury Model with [11C]PBR28 and [18F]flumazenil PET Imaging
Abstract
1. Introduction
2. Results
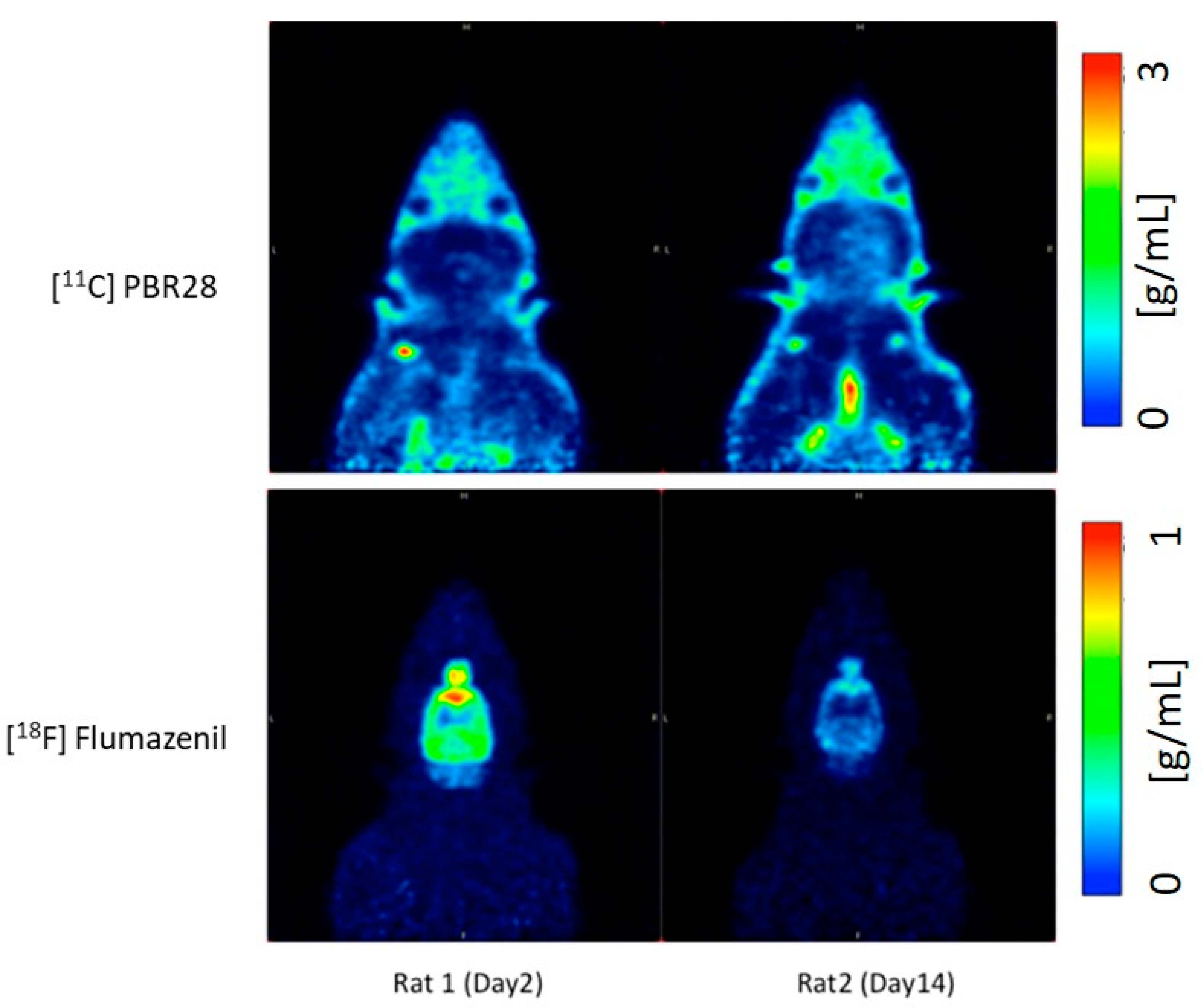
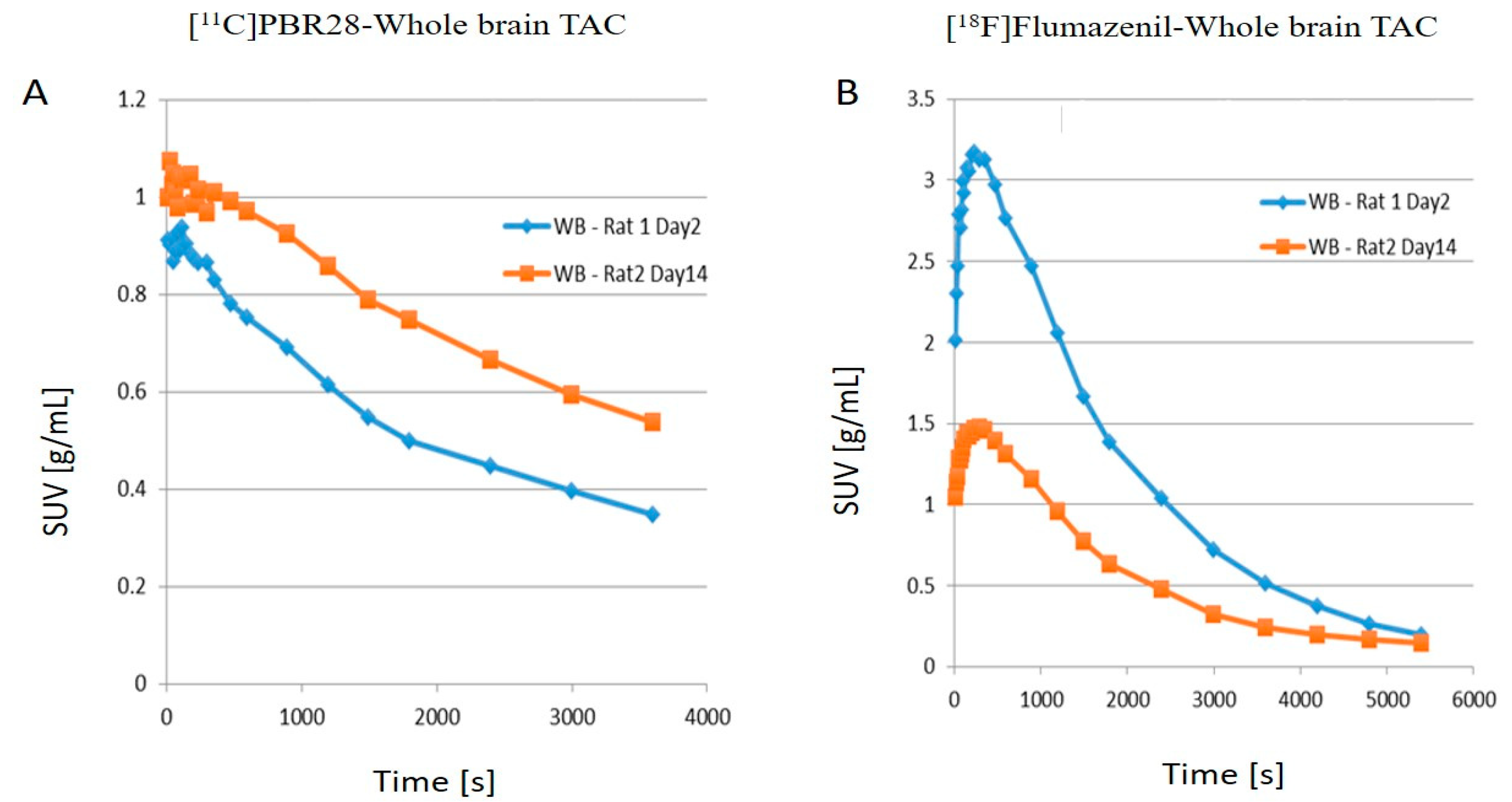
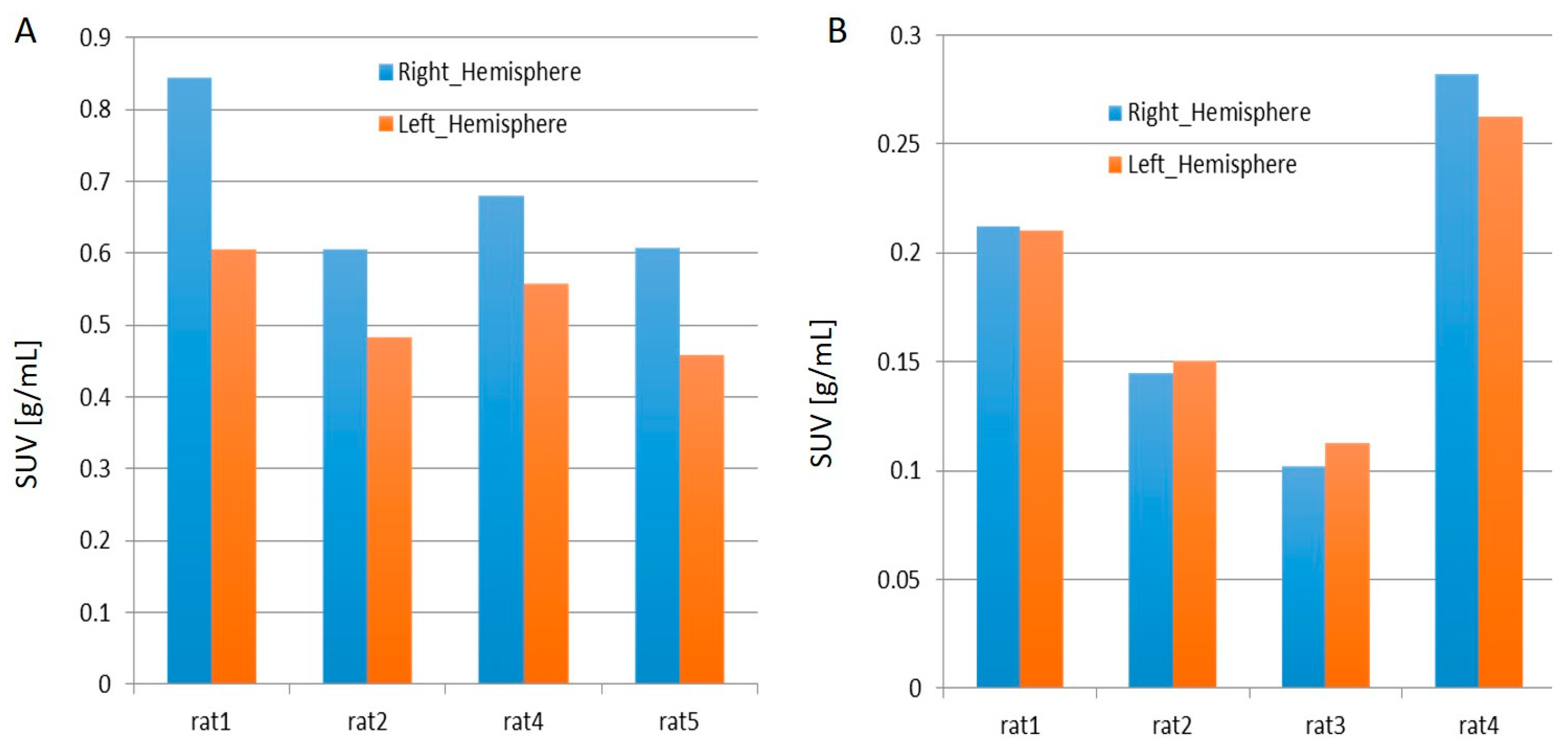
2.1. PET Imaging
2.2. Immunohistochemical Analyses
3. Materials and Methods
3.1. Preparation for Lateral Fluid Percussion
3.2. Post-FPI Monitoring Phase
3.3. Euthanasia of the Animals
3.4. Perfusion
3.5. Immunohistochemistry
3.6. Synthesis of [11C]PBR28
3.7. Synthesis of [18F]flumazenil
3.8. PET Imaging and Data Analysis
4. Future Direction and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 11C | carbon-11 |
| 18F | fluorine-18 |
| 18O | oxygen-18 |
| AUC | area-under-curve |
| CT | computed tomography |
| DAB | 3, 3′-diaminobenzidine tetrachloride |
| DMF | N,N-dimethylformamide |
| DMSO | dimethylsulfoxide |
| GABAA | γ-aminobutyric acid type A |
| HPLC | high-performance liquid chromatography |
| LFP | lateral fluid percussion |
| LFPI | LFP-induced |
| LNP | lipid nanoparticle |
| PBS | phosphate-buffered saline |
| PBS-T | PBS-triton |
| PET | positron emission tomography |
| SPECT | single-photon emission computed tomography |
| SUV | standardized uptake values |
| SV2 | synaptic vesicle proteins 2 |
| TAC | time activity curve |
| TBI | traumatic brain injury |
| TSPO | translocator protein |
References
- Faul, M.; Xu, L.; Wald, M.M.; Coronado, V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalisation and Deaths 2002–2006; Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA, USA, 2010; pp. 1–71.
- French, L.M.; Parkinson, G.W. Assessing and treating veterans with traumatic brain injury. J. Clin. Psychol. 2008, 64, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Hoge, C.W.; McGurk, D.; Thomas, J.L.; Cox, A.L.; Engel, C.C.; Castro, C.A. Mild traumatic brain injury in US soldiers returning from Iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef]
- Dixon, C.E.; Lyeth, B.G.; Povlishock, J.T.; Findling, R.L.; Hamm, R.J.; Marmarou, A.; Young, H.F.; Hayes, R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987, 67, 110–119. [Google Scholar] [CrossRef]
- Thompson, H.J.; Lifshitz, J.; Marklund, N.; Grady, M.S.; Graham, D.I.; Hovda, D.A.; McIntosh, T.K. Lateral fluid percussion brain injury: A 15-year review and evaluation. J. Neurotrauma 2005, 22, 42–75. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.E.; Clifton, G.L.; Lighthall, J.W.; Yaghmai, A.A.; Hayes, R.L. A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 1991, 39, 253–262. [Google Scholar] [CrossRef]
- Lighthall, J.W. Controlled cortical impact: A new experimental brain injury model. J. Neurotrauma 1988, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Marmarou, A.; Foda, M.A.A.-E.; Van Den Brink, W.; Campbell, J.; Kita, H.; Demetriadou, K. A new model of diffuse brain injury in rats: Part I: Pathophysiology and biomechanics. J. Neurosurg. 1994, 80, 291–300. [Google Scholar] [CrossRef]
- Alder, J.; Fujioka, W.; Lifshitz, J.; Crockett, D.P.; Thakker-Varia, S. Lateral fluid percussion: Model of traumatic brain injury in mice. JoVE J. Vis. Exp. 2011, e3063. [Google Scholar] [CrossRef]
- Kabadi, S.V.; Hilton, G.D.; Stoica, B.A.; Zapple, D.N.; Faden, A.I. Fluid-percussion–induced traumatic brain injury model in rats. Nat. Protoc. 2010, 5, 1552. [Google Scholar] [CrossRef]
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef]
- Zhimin, W.; Yang, C.-T.; Ghosh, K.K.; Kumar, S.; Padmanabhan, P.; Halldin, C.; Gulyás, B. In vivo PET Imaging of the changes in a rat fluid-percussion-induced traumatic brain injury model with [11C] PBR28 and [18F] flumazenil: A preliminary study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, S450–S451. [Google Scholar]
- Gao, X.; Chen, J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. Exp. Neurol. 2011, 70, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, S.J.; Acosta, S.; Lozano, D. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ. 2017, 3, 135–142. [Google Scholar] [PubMed]
- Schain, M.; Kreisl, W.C. Neuroinflammation in neurodegenerative disorders—A review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Venneti, S.; Lopresti, B.J.; Wiley, C.A. The peripheral benzodiazepine receptor (translocator protein 18 kDa) in microglia: From pathology to imaging. Prog. Neurobiol. 2006, 80, 308–322. [Google Scholar] [CrossRef]
- Venneti, S.; Lopresti, B.J.; Wiley, C.A. Molecular imaging of microglia/macrophages in the brain. Glia 2013, 61, 10–23. [Google Scholar] [CrossRef]
- Owen, D.R.; Howell, O.W.; Tang, S.-P.; Wells, L.A.; Bennacef, I.; Bergstrom, M.; Gunn, R.N.; Rabiner, E.A.; Wilkins, M.R.; Reynolds, R. Two binding sites for [3H] PBR28 in human brain: Implications for TSPO PET imaging of neuroinflammation. J. Cereb. Blood Flow Metab. 2010, 30, 1608–1618. [Google Scholar] [CrossRef]
- Walker, M.D.; Dinelle, K.; Kornelsen, R.; Lee, N.V.; Miao, Q.; Adam, M.; Takhar, C.; Mak, E.; Schulzer, M.; Farrer, M.J. [11C] PBR28 PET imaging is sensitive to neuroinflammation in the aged rat. J. Cereb. Blood Flow Metab. 2015, 35, 1331–1338. [Google Scholar] [CrossRef]
- Zanotti-Fregonara, P.; Pascual, B.; Rizzo, G.; Yu, M.; Pal, N.; Beers, D.; Carter, R.; Appel, S.H.; Atassi, N.; Masdeu, J.C. Head-to-Head Comparison of 11C-PBR28 and 18F-GE180 for Quantification of the Translocator Protein in the Human Brain. J. Nucl. Med. 2018, 59, 1260–1266. [Google Scholar] [CrossRef]
- Pascual, B.; Prieto, E.; Arbizu, J.; Marti-Climent, J.M.; Peñuelas, I.; Quincoces, G.; Zarauza, R.; Pappatà, S.; Masdeu, J.C. Decreased carbon-11-flumazenil binding in early Alzheimer’s disease. Brain 2012, 135, 2817–2825. [Google Scholar] [CrossRef][Green Version]
- Heiss, W.-D.; Grond, M.; Thiel, A.; Ghaemi, M.; Sobesky, J.; Rudolf, J.; Bauer, B.; Wienhard, K. Permanent cortical damage detected by flumazenil positron emission tomography in acute stroke. Stroke 1998, 29, 454–461. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Odano, I.; Halldin, C.; Karlsson, P.; Varrone, A.; Airaksinen, A.J.; Krasikova, R.N.; Farde, L. [18F] Flumazenil binding to central benzodiazepine receptor studies by PET:–Quantitative analysis and comparisons with [11C] flumazenil–. Neuroimage 2009, 45, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Rodnick, M.E.; Hockley, B.G.; Sherman, P.; Quesada, C.; Battle, M.R.; Jackson, A.; Linder, K.E.; Macholl, S.; Trigg, W.J.; Kilbourn, M.R. Novel fluorine-18 PET radiotracers based on flumazenil for GABAA imaging in the brain. Nucl. Med. Biol. 2013, 40, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Bădan, M.-I.; Bonci, E.-A.; Piciu, D. A review on immunohistochemical and histopathologic validation in PET-CT findings with consideration to microRNAs. Med. Pharm. Rep. 2019, 92, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Arenth, P.M.; Russell, K.C.; Scanlon, J.M.; Kessler, L.J.; Ricker, J.H. Corpus callosum integrity and neuropsychological performance after traumatic brain injury: A diffusion tensor imaging study. J. Head Trauma Rehabil. 2014, 29, E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W.; Weber, M.T.; Cullen, D.K.; Siman, R.; Smith, D.H. SNTF immunostaining reveals previously undetected axonal pathology in traumatic brain injury. Acta Neuropathol. 2016, 131, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Loane, D.J.; Kumar, A.; Stoica, B.A.; Cabatbat, R.; Faden, A.I. Progressive neurodegeneration after experimental brain trauma: Association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 2014, 73, 14–29. [Google Scholar] [CrossRef]
- Girgis, F.; Pace, J.; Sweet, J.; Miller, J.P. Hippocampal neurophysiologic changes after mild traumatic brain injury and potential neuromodulation treatment approaches. Front. Syst. Neurosci. 2016, 10, 8. [Google Scholar] [CrossRef]
- Kaur, C.; Ling, E. Activation and re-expression of surface antigen in microglia following an epidural application of kainic acid in the rat brain. J. Anat. 1992, 180, 333–342. [Google Scholar]
- Kanwar, J.R.; Sun, X.; Punj, V.; Sriramoju, B.; Mohan, R.R.; Zhou, S.-F.; Chauhan, A.; Kanwar, R.K. Nanoparticles in the treatment and diagnosis of neurological disorders: Untamed dragon with fire power to heal. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 399–414. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Palanivel, M.; Kumar, A.; Máthé, D.; Radda, G.K.; Lim, K.-L.; Gulyás, B. Nanotheranostic agents for neurodegenerative diseases. Emerg. Top. Life Sci. 2020, 4, 645–675. [Google Scholar]
- Sharma, M.; Dube, T.; Chibh, S.; Kour, A.; Mishra, J.; Panda, J.J. Nanotheranostics, a future remedy of neurological disorders. Expert Opin. Drug Ddelivery 2019, 16, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; England, C.G.; Chen, F.; Cai, W. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv. Drug Deliv. Rev. 2017, 113, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Waarde, A.v. Measuring receptor occupancy with PET. Curr. Pharm. Des. 2000, 6, 1593–1610. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Hong, H.; Cai, W. Positron emission tomography image-guided drug delivery: Current status and future perspectives. Mol. Pharm. 2014, 11, 3777–3797. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Song, S.; Zhao, J.; Tian, M.; Li, C. Theranostic CuS nanoparticles targeting folate receptors for PET image-guided photothermal therapy. J. Mater. Chem. B 2015, 3, 8939–8948. [Google Scholar] [CrossRef]
- Marcos-Contreras, O.A.; Greineder, C.F.; Kiseleva, R.Y.; Parhiz, H.; Walsh, L.R.; Zuluaga-Ramirez, V.; Myerson, J.W.; Hood, E.D.; Villa, C.H.; Tombacz, I. Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood–brain barrier. Proc. Natl. Acad. Sci. USA 2020, 117, 3405–3414. [Google Scholar] [CrossRef]
- Ling, G.S.; Lee, E.Y.; Kalehua, A.N. Traumatic brain injury in the rat using the fluid-percussion model. Curr. Protoc. Neurosci. 2004, 28, 9.2.1–9.2.11. [Google Scholar] [CrossRef]
- Zacny, J.P.; Conley, K.; Galinkin, J. Comparing the subjective, psychomotor and physiological effects of intravenous buprenorphine and morphine in healthy volunteers. J. Pharmacol. Exp. Ther. 1997, 282, 1187–1197. [Google Scholar]
- Tóth, M.; Doorduin, J.; Häggkvist, J.; Varrone, A.; Amini, N.; Halldin, C.; Gulyás, B. Positron emission tomography studies with [11 C] PBR28 in the healthy rodent brain: Validating SUV as an outcome measure of neuroinflammation. PLoS ONE 2015, 10, e0125917. [Google Scholar] [CrossRef]
- Ryzhikov, N.N.; Seneca, N.; Krasikova, R.N.; Gomzina, N.A.; Shchukin, E.; Fedorova, O.S.; Vassiliev, D.A.; Gulyás, B.; Hall, H.; Savic, I.; et al. Preparation of highly specific radioactivity [18F] flumazenil and its evaluation in cynomolgus monkey by positron emission tomography. Nucl. Med. Biol. 2005, 32, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Tóth, M.; Major, P.; Patay, G.; Egri, G.; Häggkvist, J.; Varrone, A.; Farde, L.; Halldin, C.; Gulyás, B. Performance evaluation of the small-animal nanoScan PET/MRI system. J. Nucl. Med. 2013, 54, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, W.K.; Mirrione, M.M.; Biegon, A.; Alexoff, D.L.; Patel, V.; Dewey, S.L. Serial microPET measures of the metabolic reaction to a microdialysis probe implant. J. Neurosci. Methods 2006, 155, 272–284. [Google Scholar] [CrossRef]
- Li, S.; Cai, Z.; Zhang, W.; Holden, D.; Lin, S.-F.; Finnema, S.J.; Shirali, A.; Ropchan, J.; Carre, S.; Mercier, J. Synthesis and in vivo evaluation of [18 F] UCB-J for PET imaging of synaptic vesicle glycoprotein 2A (SV2A). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1952–1965. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.C.; Tresse, C.; Zheng, M.; Gouasmat, A.; Carroll, V.M.; Mistico, L.; Alagille, D.; Sandiego, C.M.; Papin, C.; Marek, K. Development and in vivo preclinical imaging of fluorine-18-labeled synaptic vesicle protein 2A (SV2A) PET tracers. Mol. Imaging Biol. 2019, 21, 509–518. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, K.K.; Padmanabhan, P.; Yang, C.-T.; Wang, Z.; Palanivel, M.; Ng, K.C.; Lu, J.; Carlstedt-Duke, J.; Halldin, C.; Gulyás, B. An In Vivo Study of a Rat Fluid-Percussion-Induced Traumatic Brain Injury Model with [11C]PBR28 and [18F]flumazenil PET Imaging. Int. J. Mol. Sci. 2021, 22, 951. https://doi.org/10.3390/ijms22020951
Ghosh KK, Padmanabhan P, Yang C-T, Wang Z, Palanivel M, Ng KC, Lu J, Carlstedt-Duke J, Halldin C, Gulyás B. An In Vivo Study of a Rat Fluid-Percussion-Induced Traumatic Brain Injury Model with [11C]PBR28 and [18F]flumazenil PET Imaging. International Journal of Molecular Sciences. 2021; 22(2):951. https://doi.org/10.3390/ijms22020951
Chicago/Turabian StyleGhosh, Krishna Kanta, Parasuraman Padmanabhan, Chang-Tong Yang, Zhimin Wang, Mathangi Palanivel, Kian Chye Ng, Jia Lu, Jan Carlstedt-Duke, Christer Halldin, and Balázs Gulyás. 2021. "An In Vivo Study of a Rat Fluid-Percussion-Induced Traumatic Brain Injury Model with [11C]PBR28 and [18F]flumazenil PET Imaging" International Journal of Molecular Sciences 22, no. 2: 951. https://doi.org/10.3390/ijms22020951
APA StyleGhosh, K. K., Padmanabhan, P., Yang, C.-T., Wang, Z., Palanivel, M., Ng, K. C., Lu, J., Carlstedt-Duke, J., Halldin, C., & Gulyás, B. (2021). An In Vivo Study of a Rat Fluid-Percussion-Induced Traumatic Brain Injury Model with [11C]PBR28 and [18F]flumazenil PET Imaging. International Journal of Molecular Sciences, 22(2), 951. https://doi.org/10.3390/ijms22020951