LytR-CpsA-Psr Glycopolymer Transferases: Essential Bricks in Gram-Positive Bacterial Cell Wall Assembly
Abstract
1. Introduction to the Review
2. Cell Wall Glycopolymers—Brief Insight into Composition and Biosynthesis
2.1. Peptidoglycan (PGN)—A General Perspective
2.2. Types of Glycopolymers Attached to PGN
2.2.1. General Considerations
2.2.2. Wall Teichoic Acids
2.2.3. Pyruvylated CWGPs
2.2.4. Capsules
2.2.5. Arabinogalactan
2.2.6. Rhamnose-Containing Cell Wall Glycopolymers
3. Lytr-CpsA-Psr (LCP) Enzymes—A General Perspective
4. LCP Enzymes According to Bacterial Species
4.1. Firmicutes—Order: Bacillales
4.1.1. Staphylococcus aureus
S. aureus LcpA, LcpB, LcpC at a Glance
S. aureus LcpA, LcpB, LcpC in CWGP Biosynthesis
S. aureus lcpA, lcpB, lcpC Genes and Physiological Effects
Crystal Structure of S. aureus LcpA
4.1.2. Bacillus subtilis
B. subtilis TagT, TagU, TagV, Genes and Physiological Effects
B. subtilis TagT, TagU, TagV in WTA Biosynthesis
Crystal Structure of B. subtilis TagT, TagU, TagV
4.1.3. Bacillus anthracis
B. anthracis LcpB1, LcpB2, LcpB3, LcpB4, LcpC, LcpD, Genes and Physiological Effects
B. anthracis LcpB1, LcpB2, LcpB3, LcpB4, LcpC, LcpD in S. aureus WTA Biosynthesis
4.2. Firmicutes—Order: Lactobacillales
4.2.1. Streptococcus pneumoniae
Sc. pneumoniae Cps2A, LytR, Psr, Genes and Physiological Effects
Crystal Structure of Sc. pneumoniae Cps2A
4.2.2. Streptococcus agalactiae
Sc. agalactiae CpsA, Gene and Physiological Effects
Sc. agalactiae CpsA in CPIII Biosynthesis
4.2.3. Streptococcus mutans
Sc. mutans BrpA and Psr, Genes and Physiological Effects
Sc. mutans BrpA and Psr in RhaCWGP Biosynthesis
4.2.4. Lactococcus lactis
Lc. lactis LcpA and LcpB, Genes and Physiological Implications
4.2.5. Lactobacillus plantarum
Lb. plantarum FlmA, FlmB and FlmC, Genes and Physiological Effects
Structural Model of Lb. plantarum FlmC
4.2.6. Enterococcus hirae
E. hirae LcpA, LcpB and LcpC
E. hirae LcpA Function
4.3. Actinobacteria—Order: Actinomycetales
4.3.1. Mycobacterium tuberculosis
M. tuberculosis Rv0822c, CpsA1, CpsA2 and Rv3840 Function
M. tuberculosis CpsA1
M. tuberculosis CpsA2
Additional M. tuberculosis Proteins Related to LCP Enzyme Function
4.3.2. Mycobacterium marinum
M. marinum MMAR_4858, MMAR_1274, MMAR_4966 (CpsA), and MMAR_5392
4.3.3. Corynebacterium glutamicum
C. glutamicum LcpA and LcpB
4.3.4. Streptomyces coelicolor
Sm. coelicolor PdtA and Ten Other LCP Proteins
4.3.5. Actinomyces oris
A. oris LcpA Participates in Protein Glycosylation
A. oris LcpB, LcpC and LcpD
Crystal Structure or A. oris LcpA
4.4. Cyanobacteria—Order: Nostocales
4.4.1. Anabena sp.
Anabena sp. ConR
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 4,6Pyr | 4,6-ketal-pyruvate |
| AATGalp | α-D-2-N-acetylamido-4-amino-galacto-pyranose |
| Ac | acetyl |
| A. oris | Actinomyces oris |
| AG | arabinogalactan |
| Ara | arabinose |
| B. anthracis | Bacillus anthracis |
| B. subtilis | Bacillus subtilis |
| C. glutamicum | Corynebacterium glutamicum |
| C6-OH | hydroxyl group at carbon 6 |
| CP2 | capsular polysaccharide serotype 2 |
| CP5 | capsular polysaccharide serotype 5 |
| CPS | capsular polysaccharide |
| CWGP | cell wall glycopolymer |
| E. hirae | Enterococcus hirae |
| f | furanosidic |
| Fuc | fucose |
| FucNAc | N-acetylfucosamine |
| Gal | galactose |
| GalNAc | N-acetylgalactosamine |
| GBS | group B Streptococcus |
| Glc | glucose |
| GlcNAc | N-acetylglucosamine |
| Gro-P | glycerolphosphate |
| Lb. plantarum | Lactobacillus plantarum |
| Lc. lactis | Lactococcus lactis |
| LCP protein | LytR-CpsA-Psr -glycopolymer transferase- |
| LPS | lipopolysaccharide |
| M. marinum | Mycobacterium marinum |
| M. tuberculosis | Mycobacterium tuberculosis |
| ManNAc | N-acetylmannosamine |
| ManNAcA | N-acetylmannosaminuronic acid |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| MurNAc | N-acetylmuramic acid |
| NDP | nucleosidediphosphate |
| NeuAc | N-acetylneuraminic acid |
| P | phosphate |
| p | pyranosidic |
| PDP | polydiglycosylphosphate |
| PGN | peptidoglycan |
| R | arginine |
| Rbo-P | ribitolphosphate |
| RGP | rhamnose-glucose polysaccharide |
| Rha | rhamnose |
| RhaCWGP | rhamnose-containing cell wall glycopolymer |
| RU | repeating unit |
| PBP5 | penicillin-binding protein 5 |
| PyrCWGP | pyruvylated cell wall glycopolymer |
| S. aureus | Staphylococcus aureus |
| Sc. mutans | Streptococcus mutans |
| Sc. pneumoniae | Streptococcus pneumoniae |
| Sc. agalactiae | Streptococcus agalactiae |
| S-layer | bacterial cell surface layer |
| SLH | S-layer homology |
| Sm. coelicolor | Streptomyces coelicolor |
| sp. | species |
| undp-P | undecaprenylphosphate |
| WTA | wall teichoic acid |
References
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef]
- Do, T.; Page, J.E.; Walker, S. Uncovering the Activities, Biological Roles, and Regulation of Bacterial Cell Wall Hydrolases and Tailoring Enzymes. J. Biol. Chem. 2020, 295, 3347–3361. [Google Scholar] [CrossRef]
- Vollmer, W.; Seligman, S.J. Architecture of Peptidoglycan: More Data and More Models. Trends Microbiol. 2010, 18, 59–66. [Google Scholar] [CrossRef]
- Höltje, J.V. Growth of the Stress-Bearing and Shape-Maintaining Murein Sacculus of Escherichia coli. Microbiol. Mol. Biol. Rev. 1998, 62, 181–203. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Kandler, O. Peptidoglycan Types of Bacterial Cell Walls and Their Taxonomic Implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef]
- Navarre, W.W.; Schneewind, O. Surface Proteins of Gram-Positive Bacteria and Mechanisms of Their Targeting to the Cell Wall Envelope. Microbiol. Mol. Biol. Rev. 1999, 63, 174–229. [Google Scholar] [CrossRef]
- Rajagopal, M.; Walker, S. Envelope Structures of Gram-Positive Bacteria. Curr. Top. Microbiol. Immunol. 2017, 404, 1–44. [Google Scholar]
- Kong, K.F.; Schneper, L.; Mathee, K. Beta-Lactam Antibiotics: From Antibiosis to Resistance and Bacteriology. Apmis 2010, 118, 1–36. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. Beta-Lactams and Beta-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. Synthesis of Essential Drugs—Antibiotics. In Synthesis of Essential Drugs; Vardanyan, R.S., Hruby, V.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 425–498. [Google Scholar]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef]
- Hesketh, A.; Hill, C.; Mokhtar, J.; Novotna, G.; Tran, N.; Bibb, M.; Hong, H.J. Genome-Wide Dynamics of a Bacterial Response to Antibiotics That Target the Cell Envelope. BMC Genom. 2011, 12, 226. [Google Scholar] [CrossRef]
- Neuhaus, F.C.; Baddiley, J. A Continuum of Anionic Charge: Structures and Functions of D-alanyl-teichoic Acids in Gram-Positive Bacteria. Microbiol. Mol. Biol. Rev. 2003, 67, 686–723. [Google Scholar]
- Schäffer, C.; Messner, P. The Structure of Secondary Cell Wall Polymers: How Gram-Positive Bacteria Stick Their Cell Walls Together. Microbiology 2005, 151, 643–651. [Google Scholar] [CrossRef]
- Weidenmaier, C.; Peschel, A. Teichoic Acids and Related Cell-Wall Glycopolymers in Gram-Positive Physiology and Host Interactions. Nat. Rev. Microbiol. 2008, 6, 276–287. [Google Scholar] [CrossRef]
- Percy, M.G.; Gründling, A. Lipoteichoic Acid Synthesis and Function in Gram-Positive Bacteria. Annu. Rev. Microbiol. 2014, 68, 81–100. [Google Scholar] [CrossRef]
- Brown, S.; Santa Maria, J.P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2013, 67, 313–336. [Google Scholar] [CrossRef]
- Pasquina, L.W.; Santa Maria, J.P.; Walker, S. Teichoic Acid Biosynthesis as an Antibiotic Target. Curr. Opin. Microbiol. 2013, 16, 531–537. [Google Scholar] [CrossRef]
- Archibald, A.R.; Hancock, I.C.; Harwood, C.R. Cell Wall Structure, Synthesis and Turnover. In Bacillus Subtilis and Other Gram-Positive Bacteria; Sonenshein, A., Hoch, J.A., Losick, R., Eds.; ASM Press: Washington, DC, USA, 1993; pp. 381–410. [Google Scholar]
- Archibald, A.R.; Baddiley, J.; Heptinstall, S. The Distribution of the Glucosyl Substituents along the Chain of the Teichoic Acid in Walls of Lactobacillus buchneri N.C.I.B. 8007. Biochem. J. 1969, 111, 245–246. [Google Scholar] [CrossRef]
- Hancock, I.C.; Baddiley, J. Biosynthesis of the Bacterial Envelope Polymers Teichoic Acid and Teichuronic Acid. In The Enzymes of Biological Membranes; Martonosi, A.N., Ed.; Plenum Press: New York, NY, USA, 1985; Volume 2, pp. 279–307. [Google Scholar]
- Araki, Y.; Ito, E. Linkage Units in Cell Walls of Gram-Positive Bacteria. Crit. Rev. Microbiol. 1989, 17, 121–135. [Google Scholar] [CrossRef]
- Naumova, I.B.; Shashkov, A.S. Anionic Polymers in Cell Walls of Gram-Positive Bacteria. Biochemistry 1997, 62, 809–840. [Google Scholar] [PubMed]
- Whitfield, C.; Trent, M.S. Biosynthesis and Export of Bacterial Lipopolysaccharides. Annu. Rev. Biochem. 2014, 83, 99–128. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, L.; Kos, V.; Whitfield, C. ABC Transporters Involved in Export of Cell Surface Glycoconjugates. Microbiol. Mol. Biol. Rev. 2010, 74, 341–362. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.R.; Neely, M.N. Coordinate Regulation of Gram-Positive Cell Surface Components. Curr. Opin. Microbiol. 2012, 15, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Rausch, M.; Deisinger, J.P.; Ulm, H.; Müller, A.; Li, W.; Hardt, P.; Wang, X.; Li, X.; Sylvester, M.; Engeser, M.; et al. Coordination of Capsule Assembly and Cell Wall Biosynthesis in Staphylococcus aureus. Nat. Commun. 2019, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, K.; Matano, L.M.; Qiao, Y.; Kahne, D.; Walker, S. in vitro Reconstitution Demonstrates the Cell Wall Ligase Activity of LCP Proteins. Nat. Chem. Biol. 2017, 13, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Biswas, R.; Herbert, S.; Kulauzovic, E.; Weidenmaier, C.; Peschel, A.; Götz, F. Influence of Wall Teichoic Acid on Lysozyme Resistance in Staphylococcus aureus. J. Bacteriol. 2007, 189, 280. [Google Scholar] [CrossRef]
- Xia, G.; Kohler, T.; Peschel, A. The Wall Teichoic Acid and Lipoteichoic Acid Polymers of Staphylococcus aureus. Int. J. Med. Microbiol. 2010, 300, 148–154. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Ginsberg, C.; Zhang, Y.H.; Yuan, Y.; Walker, S. In vitro Reconstitution of Two Essential Steps in Wall Teichoic Acid Biosynthesis. ACS Chem. Biol. 2006, 1, 25–28. [Google Scholar] [CrossRef]
- Brown, S.; Meredith, T.; Swoboda, J.; Walker, S. Staphylococcus aureus and Bacillus subtilis W23 Make Polyribitol Wall Teichoic Acids Using Different Enzymatic Pathways. Chem. Biol. 2010, 17, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Weidenmaier, C.; Kokai-Kun, J.F.; Kristian, S.A.; Chanturiya, T.; Kalbacher, H.; Gross, M.; Nicholson, G.; Neumeister, B.; Mond, J.J.; Peschel, A. Role of Teichoic Acids in Staphylococcus aureus Nasal Colonization, a Major Risk Factor in Nosocomial Infections. Nat. Med. 2004, 10, 243–245. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, M.A.; Pereira, M.P.; Chung, Y.S.; Zhao, W.; Chau, A.; Kenney, T.J.; Sulavik, M.C.; Black, T.A.; Brown, E.D. Lesions in Teichoic Acid Biosynthesis in Staphylococcus aureus Lead to a Lethal Gain of Function in the Otherwise Dispensable Pathway. J. Bacteriol. 2006, 188, 4183–4189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Ginsberg, C.; Yuan, Y.; Walker, S. Acceptor Substrate Selectivity and Kinetic Mechanism of Bacillus subtilis TagA. Biochemistry 2006, 45, 10895–10904. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, S.; Zhang, Y.H.; Walker, S. A Revised Pathway Proposed for Staphylococcus aureus Wall Teichoic Acid Biosynthesis Based on In Vitro Reconstitution of the Intracellular Steps. Chem. Biol. 2008, 15, 12–21. [Google Scholar] [CrossRef]
- D’Elia, M.A.; Henderson, J.A.; Beveridge, T.J.; Heinrichs, D.E.; Brown, E.D. The N-acetylmannosamine Transferase Catalyzes the First Committed Step of Teichoic Acid Assembly in Bacillus subtilis and Staphylococcus aureus. J. Bacteriol. 2009, 191, 4030–4034. [Google Scholar] [CrossRef]
- Lazarevic, V.; Abellan, F.X.; Moller, S.B.; Karamata, D.; Mauel, C. Comparison of Ribitol and Glycerol Teichoic Acid Genes in Bacillus subtilis W23 and 168: Identical Function, Similar Divergent Organization, but Different Regulation. Microbiology 2002, 148, 815–824. [Google Scholar] [CrossRef]
- Swoboda, J.G.; Campbell, J.; Meredith, T.C.; Walker, S. Wall Teichoic Acid Function, Biosynthesis, and Inhibition. ChemBioChem 2010, 11, 35–45. [Google Scholar] [CrossRef]
- Kawai, F.; Grass, S.; Kim, Y.; Choi, K.-J.; St Geme, J.W., III; Yeo, H.-J. Structural Insights into the Glycosyltransferase Activity of the Actinobacillus pleuropneumoniae HMW1C-Like Protein. J. Biol. Chem. 2011, 286, 38546–38557. [Google Scholar] [CrossRef]
- Zilla, M.; Lunderberg, J.; Schneewind, O.; Missiakas, D. Bacillus anthracis lcp Genes Support Vegetative Growth, Envelope Assembly, and Spore Formation. J. Bacteriol. 2015, 197, 3731–3741. [Google Scholar] [CrossRef]
- Hager, F.F.; Lopez-Guzman, A.; Krauter, S.; Blaukopf, M.; Polter, M.; Brockhausen, I.; Kosma, P.; Schäffer, C. Functional Characterization of Enzymatic Steps Involved in Pyruvylation of Bacterial Secondary Cell Wall Polymer Fragments. Front. Microbiol. 2018, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Lunderberg, J.M.; Chateau, A.; Schneewind, O.; Missiakas, D. Genes Required for Bacillus anthracis Secondary Cell Wall Polysaccharide Synthesis. J. Bacteriol. 2017, 199, e00613-16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, S.H.; Wang, H.; Labroli, M.; Koseoglu, S.; Zuck, P.; Mayhood, T.; Gill, C.; Mann, P.; Sher, X.; Ha, S.; et al. TarO-Specific Inhibitors of Wall Teichoic Acid Biosynthesis Restore β-Lactam Efficacy against Methicillin-Resistant Staphylococci. Sci. Transl. Med. 2016, 8, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Sewell, E.W.; Brown, E.D. Taking Aim at Wall Teichoic Acid Synthesis: New Biology and New Leads for Antibiotics. J. Antibiot. 2014, 67, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mesnage, S.; Fontaine, T.; Mignot, T.; Delepierre, M.; Mock, M.; Fouet, A. Bacterial SLH Domain Proteins Are Non-Covalently Anchored to the Cell Surface via a Conserved Mechanism Involving Wall Polysaccharide Pyruvylation. EMBO J. 2000, 19, 4473–4484. [Google Scholar] [CrossRef]
- Hübscher, J.; Lüthy, L.; Berger-Bächi, B.; Stutzmann Meier, P. Phylogenetic Distribution and Membrane Topology of the LytR-CpsA-Psr Protein Family. BMC Genom. 2008, 9, 617. [Google Scholar] [CrossRef]
- Fouet, A. The Surface of Bacillus anthracis. Mol. Asp. Med. 2009, 30, 374–385. [Google Scholar] [CrossRef]
- Choudhury, B.; Leoff, C.; Saile, E.; Wilkins, P.; Quinn, C.P.; Kannenberg, E.L.; Carlson, R.W. The Structure of the Major Cell Wall Polysaccharide of Bacillus anthracis Is Species-Specific. J. Biol. Chem. 2006, 281, 27932–27941. [Google Scholar] [CrossRef]
- Blackler, R.J.; López-Guzmán, A.; Hager, F.F.; Janesch, B.; Martinz, G.; Gagnon, S.M.L.; Haji-Ghassemi, O.; Kosma, P.; Messner, P.; Schäffer, C.; et al. Structural Basis of Cell Wall Anchoring by SLH Domains in Paenibacillus alvei. Nat. Commun. 2018, 9, 3120. [Google Scholar] [CrossRef]
- Sychantha, D.; Chapman, R.N.; Bamford, N.C.; Boons, G.J.; Howell, P.L.; Clarke, A.J. Molecular Basis for the Attachment of S-Layer Proteins to the Cell Wall of Bacillus anthracis. Biochemistry 2018, 57, 1949–1953. [Google Scholar] [CrossRef]
- Messner, P.; Schäffer, C.; Egelseer, E.M.; Sleytr, U.B. Occurrence, Structure, Chemistry, Genetics, Morphogenesis and Functions of S-Layers. In Prokaryotic Cell Wall Compounds—Structure and Biochemistry; König, H., Claus, H., Varma, A., Eds.; Springer: Berlin, Germany, 2010; pp. 53–109. [Google Scholar]
- Chung, S.; Shin, S.H.; Bertozzi, C.R.; De Yoreo, J.J. Self-Catalyzed Growth of S Layers via an Amorphous-to-Crystalline Transition Limited by Folding Kinetics. Proc. Natl. Acad. Sci. USA 2010, 107, 16536–16541. [Google Scholar] [PubMed]
- Messner, P.; Allmaier, G.; Schäffer, C.; Wugeditsch, T.; Lortal, S.; König, H.; Niemetz, R.; Dorner, M. Biochemistry of S-Layers. FEMS Microbiol. Rev. 1997, 20, 25–46. [Google Scholar] [PubMed]
- Kern, J.; Ryan, C.; Faull, K.; Schneewind, O. Bacillus anthracis Surface-Layer Proteins Assemble by Binding to the Secondary Cell Wall Polysaccharide in a Manner That Requires csaB and tagO. J. Mol. Biol. 2010, 401, 757–775. [Google Scholar] [PubMed]
- Forsberg, L.S.; Abshire, T.G.; Friedlander, A.; Quinn, C.P.; Kannenberg, E.L.; Carlson, R.W. Localization and Structural Analysis of a Conserved Pyruvylated Epitope in Bacillus anthracis Secondary Cell Wall Polysaccharides and Characterization of the Galactose-Deficient Wall Polysaccharide from Avirulent B. anthracis CDC 684. Glycobiology 2012, 22, 1103–1117. [Google Scholar]
- Forsberg, L.S.; Choudhury, B.; Leoff, C.; Marston, C.K.; Hoffmaster, A.R.; Saile, E.; Quinn, C.P.; Kannenberg, E.L.; Carlson, R.W. Secondary Cell Wall Polysaccharides from Bacillus cereus Strains G9241, 03BB87 and 03BB102 Causing Fatal Pneumonia Share Similar Glycosyl Structures with the Polysaccharides from Bacillus anthracis. Glycobiology 2011, 21, 934–948. [Google Scholar]
- Schäffer, C.; Müller, N.; Mandal, P.K.; Christian, R.; Zayni, S.; Messner, P. A Pyrophosphate Bridge Links the Pyruvate-Containing Secondary Cell Wall Polymer of Paenibacillus alvei CCM 2051 to Muramic Acid. Glycoconj. J. 2000, 17, 681–690. [Google Scholar]
- Janesch, B.; Messner, P.; Schäffer, C. Are the Surface Layer Homology Domains Essential for Cell Surface Display and Glycosylation of the S-Layer Protein from Paenibacillus alvei CCM 2051T? J. Bacteriol. 2013, 195, 565–575. [Google Scholar]
- Whitfield, C. Biosynthesis and Assembly of Capsular Polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar]
- Whitfield, C.; Wear, S.S.; Sande, C. Assembly of Bacterial Capsular Polysaccharides and Exopolysaccharides. Annu. Rev. Microbiol. 2020, 74, 521–543. [Google Scholar] [PubMed]
- Yother, J. Capsules of Streptococcus pneumoniae and Other Bacteria: Paradigms for Polysaccharide Biosynthesis and Regulation. Annu. Rev. Microbiol. 2011, 65, 563–581. [Google Scholar]
- Grzegorzewicz, A.E.; de Sousa-d’Auria, C.; McNeil, M.R.; Huc-Claustre, E.; Jones, V.; Petit, C.; Angala, S.K.; Zemanová, J.; Wang, Q.; Belardinelli, J.M.; et al. Assembling of the Mycobacterium tuberculosis Cell Wall Core. J. Biol. Chem. 2016, 291, 18867–18879. [Google Scholar]
- Ballister, E.R.; Samanovic, M.I.; Darwin, K.H. Mycobacterium tuberculosis Rv2700 Contributes to Cell Envelope Integrity and Virulence. J. Bacteriol. 2019, 201, e00228-19. [Google Scholar] [CrossRef]
- Barry, C.E.; Crick, D.C.; McNeil, M.R. Targeting the Formation of the Cell Wall Core of M. tuberculosis. Infect. Dis. Drug Targets 2007, 7, 182–202. [Google Scholar]
- Jackson, M.; McNeil, M.R.; Brennan, P.J. Progress in Targeting Cell Envelope Biogenesis in Mycobacterium tuberculosis. Future Microbiol. 2013, 8, 855–875. [Google Scholar]
- McNeil, M.; Daffe, M.; Brennan, P.J. Evidence for the Nature of the Link between the Arabinogalactan and Peptidoglycan of Mycobacterial Cell Walls. J. Biol. Chem. 1990, 265, 18200–18206. [Google Scholar]
- Mills, J.A.; Motichka, K.; Jucker, M.; Wu, H.P.; Uhlik, B.C.; Stern, R.J.; Scherman, M.S.; Vissa, V.D.; Pan, F.; Kundu, M.; et al. Inactivation of the Mycobacterial Rhamnosyltransferase, Which Is Needed for the Formation of the Arabinogalactan-Peptidoglycan Linker, Leads to Irreversible Loss of Viability. J. Biol. Chem. 2004, 279, 43540–43546. [Google Scholar] [CrossRef]
- Yagi, T.; Mahapatra, S.; Mikusova, K.; Crick, D.C.; Brennan, P.J. Polymerization of Mycobacterial Arabinogalactan and Ligation to Peptidoglycan. J. Biol. Chem. 2003, 278, 26497–26504. [Google Scholar] [CrossRef]
- Mikusová, K.; Mikus, M.; Besra, G.S.; Hancock, I.; Brennan, P.J. Biosynthesis of the Linkage Region of the Mycobacterial Cell Wall. J. Biol. Chem. 1996, 271, 7820–7828. [Google Scholar] [CrossRef]
- Kremer, L.; Dover, L.G.; Morehouse, C.; Hitchin, P.; Everett, M.; Morris, H.R.; Dell, A.; Brennan, P.J.; McNeil, M.R.; Flaherty, C.; et al. Galactan Biosynthesis in Mycobacterium tuberculosis. Identification of a Bifunctional UDP-galactofuranosyltransferase. J. Biol. Chem. 2001, 276, 26430–26440. [Google Scholar]
- Dianišková, P.; Korduláková, J.; Skovierová, H.; Kaur, D.; Jackson, M.; Brennan, P.J.; Mikušová, K. Investigation of ABC Transporter from Mycobacterial Arabinogalactan Biosynthetic Cluster. Gen. Physiol. Biophys. 2011, 30, 239–250. [Google Scholar]
- Hancock, I.C.; Carman, S.; Besra, G.S.; Brennan, P.J.; Waite, E. Ligation of Arabinogalactan to Peptidoglycan in the Cell Wall of Mycobacterium smegmatis Requires Concomitant Synthesis of the Two Wall Polymers. Microbiology 2002, 148, 3059–3067. [Google Scholar] [CrossRef]
- Mistou, M.Y.; Sutcliffe, I.C.; van Sorge, N.M. Bacterial Glycobiology: Rhamnose-Containing Cell Wall Polysaccharides in Gram-Positive Bacteria. FEMS Microbiol. Rev. 2016, 40, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Chapot-Chartier, M.-P.; Vinogradov, E.; Sadovskaya, I.; Andre, G.; Mistou, M.-Y.; Trieu-Cuot, P.; Furlan, S.; Bidnenko, E.; Courtin, P.; Péchoux, C.; et al. Cell Surface of Lactococcus lactis Is Covered by a Protective Polysaccharide Pellicle. J. Biol. Chem. 2010, 285, 10464–10471. [Google Scholar] [PubMed]
- Swanson, J.; Gotschlich, E.C. Electron Microscopic Studies on Streptococci. II. Group A Carbohydrate. J. Exp. Med. 1973, 138, 245–258. [Google Scholar] [PubMed]
- Kovacs, C.J.; Faustoferri, R.C.; Bischer, A.P.; Quivey, R.G., Jr. Streptococcus mutans Requires Mature Rhamnose-Glucose Polysaccharides for Proper Pathophysiology, Morphogenesis and Cellular Division. Mol. Microbiol. 2019, 112, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Mäki, M.; Renkonen, R. Biosynthesis of 6-deoxyhexose glycans in bacteria. Glycobiology 2004, 14, 1r–15r. [Google Scholar] [CrossRef]
- Boehm, M.; Hoy, B.; Rohde, M.; Tegtmeyer, N.; Baek, K.T.; Oyarzabal, O.A.; Brondsted, L.; Wessler, S.; Backert, S. Rapid Paracellular Transmigration of Campylobacter jejuni across Polarized Epithelial Cells without Affecting TER: Role of Proteolytic-active HtrA Cleaving E-cadherin but Not Fibronectin. Gut Pathog. 2012, 4, 3. [Google Scholar] [CrossRef]
- Kawai, Y.; Marles-Wright, J.; Cleverley, R.M.; Emmins, R.; Ishikawa, S.; Kuwano, M.; Heinz, N.; Bui, N.K.; Hoyland, C.N.; Ogasawara, N.; et al. A Widespread Family of Bacterial Cell Wall Assembly Proteins. EMBO J. 2011, 30, 4931–4941. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus Infections. New Engl. J. Med. 1998, 339, 520–532. [Google Scholar]
- Solis, N.; Parker, B.L.; Kwong, S.M.; Robinson, G.; Firth, N.; Cordwell, S.J. Staphylococcus aureus Surface Proteins Involved in Adaptation to Oxacillin Identified Using a Novel Cell Shaving Approach. J. Proteome Res. 2014, 13, 2954–2972. [Google Scholar] [CrossRef]
- David, M.Z.; Daum, R.S. Community-Associated Methicillin-Resistant Staphylococcus aureus: Epidemiology and Clinical Consequences of an Emerging Epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of Methicillin-Resistant Staphylococcus aureus Infection. Clin. Infect. Dis. 2008, 46 (Suppl. 5), S350–S359. [Google Scholar] [PubMed]
- Yokoyama, K.; Miyashita, T.; Araki, Y.; Ito, E. Structure and Functions of Linkage Unit Intermediates in the Biosynthesis of Ribitol Teichoic Acids in Staphylococcus aureus H and Bacillus subtilis W23. Eur. J. Biochem. 1986, 161, 479–489. [Google Scholar] [CrossRef]
- Thakker, M.; Park, J.S.; Carey, V.; Lee, J.C. Staphylococcus aureus Serotype 5 Capsular Polysaccharide Is Antiphagocytic and Enhances Bacterial Virulence in a Murine Bacteremia Model. Infect. Immun. 1998, 66, 5183–5189. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.G.; Frankel, M.B.; Dengler, V.; Schneewind, O.; Missiakas, D. Staphylococcus aureus Mutants Lacking the LytR-CpsA-Psr Family of Enzymes Release Cell Wall Teichoic Acids into the Extracellular Medium. J. Bacteriol. 2013, 195, 4650–4659. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.G.; Kim, H.K.; Schneewind, O.; Missiakas, D. The Capsular Polysaccharide of Staphylococcus aureus Is Attached to Peptidoglycan by the LytR-CpsA-Psr (LCP) Family of Enzymes. J. Biol. Chem. 2014, 289, 15680–15690. [Google Scholar]
- Over, B.; Heusser, R.; McCallum, N.; Schulthess, B.; Kupferschmied, P.; Gaiani, J.M.; Sifri, C.D.; Berger-Bächi, B.; Stutzmann Meier, P. LytR-CpsA-Psr Proteins in Staphylococcus aureus Display Partial Functional Redundancy and the Deletion of All Three Severely Impairs Septum Placement and Cell Separation. FEMS Microbiol. Lett. 2011, 320, 142–151. [Google Scholar] [CrossRef]
- Hübscher, J.; McCallum, N.; Sifri, C.D.; Majcherczyk, P.A.; Entenza, J.M.; Heusser, R.; Berger-Bächi, B.; Stutzmann Meier, P. MsrR Contributes to Cell Surface Characteristics and Virulence in Staphylococcus aureus. FEMS Microbiol. Lett. 2009, 295, 251–260. [Google Scholar]
- Dengler, V.; Meier, P.S.; Heusser, R.; Kupferschmied, P.; Fazekas, J.; Friebe, S.; Staufer, S.B.; Majcherczyk, P.A.; Moreillon, P.; Berger-Bachi, B.; et al. Deletion of Hypothetical Wall Teichoic Acid Ligases in Staphylococcus aureus Activates the Cell Wall Stress Response. FEMS Microbiol. Lett. 2012, 333, 109–120. [Google Scholar] [CrossRef]
- Atilano, M.L.; Pereira, P.M.; Yates, J.; Reed, P.; Veiga, H.; Pinho, M.G.; Filipe, S.R. Teichoic Acids Are Temporal and Spatial Regulators of Peptidoglycan Cross-Linking in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2010, 107, 18991–18996. [Google Scholar] [CrossRef]
- Campbell, J.; Singh, A.K.; Santa Maria, J.P., Jr.; Kim, Y.; Brown, S.; Swoboda, J.G.; Mylonakis, E.; Wilkinson, B.J.; Walker, S. Synthetic Lethal Compound Combinations Reveal a Fundamental Connection between Wall Teichoic Acid and Peptidoglycan Biosyntheses in Staphylococcus aureus. ACS Chem. Biol. 2011, 6, 106–116. [Google Scholar] [CrossRef]
- Farha, M.A.; Leung, A.; Sewell, E.W.; D’Elia, M.A.; Allison, S.E.; Ejim, L.; Pereira, P.M.; Pinho, M.G.; Wright, G.D.; Brown, E.D. Inhibition of WTA Synthesis Blocks the Cooperative Action of PBPs and Sensitizes MRSA to Beta-Lactams. ACS Chem. Biol. 2013, 8, 226–233. [Google Scholar] [CrossRef]
- Eberhardt, A.; Hoyland, C.N.; Vollmer, D.; Bisle, S.; Cleverley, R.M.; Johnsborg, O.; Havarstein, L.S.; Lewis, R.J.; Vollmer, W. Attachment of Capsular Polysaccharide to the Cell Wall in Streptococcus pneumoniae. Microb. Drug Resist. 2012, 18, 240–255. [Google Scholar] [CrossRef]
- Schaefer, K.; Owens, T.W.; Kahne, D.; Walker, S. Substrate Preferences Establish the Order of Cell Wall Assembly in Staphylococcus aureus. J. Am. Chem. Soc. 2018, 140, 2442–2445. [Google Scholar] [CrossRef]
- Hanson, B.R.; Runft, D.L.; Streeter, C.; Kumar, A.; Carion, T.W.; Neely, M.N. Functional Analysis of the CpsA Protein of Streptococcus agalactiae. J. Bacteriol. 2012, 194, 1668–1678. [Google Scholar] [CrossRef]
- Toniolo, C.; Balducci, E.; Romano, M.R.; Proietti, D.; Ferlenghi, I.; Grandi, G.; Berti, F.; Ros, I.M.; Janulczyk, R. Streptococcus agalactiae Capsule Polymer Length and Attachment Is Determined by the Proteins CpsABCD. J. Biol. Chem. 2015, 290, 9521–9532. [Google Scholar] [CrossRef]
- Horzempa, J.; Dean, C.R.; Goldberg, J.B.; Castric, P. Pseudomonas aeruginosa 1244 Pilin Glycosylation: Glycan Substrate Recognition. J. Bacteriol. 2006, 188, 4244–4252. [Google Scholar] [CrossRef]
- D’Elia, M.A.; Millar, K.E.; Beveridge, T.J.; Brown, E.D. Wall Teichoic Acid Polymers Are Dispensable for Cell Viability in Bacillus subtilis. J. Bacteriol. 2006, 188, 8313–8316. [Google Scholar] [CrossRef]
- Rossi, J.; Bischoff, M.; Wada, A.; Berger-Bächi, B. MsrR, a Putative Cell Envelope-Associated Element Involved in Staphylococcus aureus sarA Attenuation. Antimicrob. Agents Chemother. 2003, 47, 2558–2564. [Google Scholar] [CrossRef]
- Li, F.; Zhai, D.; Wu, Z.; Zhao, Y.; Qiao, D.; Zhao, X. Impairment of the Cell Wall Ligase, LytR-CpsA-Psr Protein (LcpC), in Methicillin Resistant Staphylococcus aureus Reduces Is Resistance to Antibiotics and Infection in a Mouse Model of Sepsis. Front. Microbiol. 2020, 11, 557. [Google Scholar] [CrossRef]
- Katayama, Y.; Sekine, M.; Hishinuma, T.; Aiba, Y.; Hiramatsu, K. Complete Reconstitution of the Vancomycin-Intermediate Staphylococcus aureus Phenotype of Strain Mu50 in Vancomycin-Susceptible S. aureus. Antimicrob. Agents Chemother. 2016, 60, 3730–3742. [Google Scholar] [CrossRef] [PubMed]
- Li, F.K.K.; Rosell, F.I.; Gale, R.T.; Simorre, J.P.; Brown, E.D.; Strynadka, N.C.J. Crystallographic Analysis of Staphylococcus aureus LcpA, the Primary Wall Teichoic Acid Ligase. J. Biol. Chem. 2020, 295, 2629–2639. [Google Scholar] [CrossRef] [PubMed]
- Morales Angeles, D.; Scheffers, D.J. The Cell Wall of Bacillus subtilis. Curr. Issues Mol. Biol. 2020, 41, 539–596. [Google Scholar] [CrossRef] [PubMed]
- Schirner, K.; Stone, L.K.; Walker, S. ABC Transporters Required for Export of Wall Teichoic Acids Do Not Discriminate between Different Main Chain Polymers. ACS Chem. Biol. 2011, 6, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.T.; Li, F.K.K.; Sun, T.; Strynadka, N.C.J.; Brown, E.D. B. subtilis LytR-CpsA-Psr Enzymes Transfer Wall Teichoic Acids from Authentic Lipid-Linked Substrates to Mature Peptidoglycan In Vitro. Cell Chem. Biol. 2017, 24, 1537–1546 e4. [Google Scholar] [CrossRef]
- Mock, M.; Fouet, A. Anthrax. Ann. Rev. Microbiol. 2001, 55, 647–671. [Google Scholar] [CrossRef]
- Ruthel, G.; Ribot, W.J.; Bavari, S.; Hoover, T.A. Time-Lapse Confocal Imaging of Development of Bacillus anthracis in Macrophages. J. Infect. Dis. 2004, 189, 1313–1316. [Google Scholar] [CrossRef][Green Version]
- Garufi, G.; Hendrickx, A.P.; Beeri, K.; Kern, J.W.; Sharma, A.; Richter, S.G.; Schneewind, O.; Missiakas, D. Synthesis of Lipoteichoic Acids in Bacillus anthracis. J. Bacteriol. 2012, 194, 4312–4321. [Google Scholar] [CrossRef]
- Richter, S.; Anderson, V.J.; Garufi, G.; Lu, L.; Budzik, J.M.; Joachimiak, A.; He, C.; Schneewind, O.; Missiakas, D. Capsule Anchoring in Bacillus anthracis Occurs by a Transpeptidation Reaction That Is Inhibited by Capsidin. Mol. Microbiol. 2009, 71, 404–420. [Google Scholar] [CrossRef]
- Fagan, R.P.; Fairweather, N.F. Biogenesis and Functions of Bacterial S-Layers. Nat. Rev. Microbiol. 2014, 12, 211–222. [Google Scholar] [CrossRef]
- Liziewski Zilla, M.; Chan, Y.G.Y.; Lunderberg, J.M.; Schneewind, O.; Missiakas, D. LytR-CpsA-Psr Enzymes as Determinants of Bacillus anthracis Secondary Cell Wall Polysaccharide Assembly. J. Bacteriol. 2015, 197, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, S.; Winer, B.Y.; Jaganathan, A.; Patel, J.; Sgobba, M.; Schuch, R.; Gupta, Y.K.; Haider, S.; Wang, R.; Fischetti, V.A. Anthrax SET Protein: A Potential Virulence Determinant That Epigenetically Represses NF-kappaB Activation in Infected Macrophages. J. Biol. Chem. 2013, 288, 23458–23472. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.; Schneewind, O. BslA, the S-Layer Adhesin of B. anthracis, Is a Virulence Factor for Anthrax Pathogenesis. Mol. Microbiol. 2010, 75, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Lunderberg, J.M.; Liszewski Zilla, M.; Missiakas, D.; Schneewind, O. Bacillus anthracis tagO Is Required for Vegetative Growth and Secondary Cell Wall Polysaccharide Synthesis. J. Bacteriol. 2015, 197, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Missiakas, D.; Schneewind, O. GneZ, a UDP-GlcNAc 2-Epimerase, Is Required for S-Layer Assembly and Vegetative Growth of Bacillus anthracis. J. Bacteriol. 2014, 196, 2969–2978. [Google Scholar] [CrossRef][Green Version]
- Magee, A.D.; Yother, J. Requirement for Capsule in Colonization by Streptococcus pneumoniae. Infect. Immun. 2001, 69, 3755–3761. [Google Scholar] [CrossRef]
- Van der Poll, T.; Opal, S.M. Pathogenesis, Treatment, and Prevention of Pneumococcal Pneumonia. Lancet 2009, 374, 1543–1556. [Google Scholar] [CrossRef]
- Larson, T.R.; Yother, J. Streptococcus pneumoniae Capsular Polysaccharide Is Linked to Peptidoglycan via a Direct Glycosidic Bond to β-D-N-acetylglucosamine. Proc. Natl. Acad. Sci. USA 2017, 114, 5695–5700. [Google Scholar] [CrossRef]
- Schneewind, O.; Missiakas, D. Lipoteichoic Acids, Phosphate-Containing Polymers in the Envelope of Gram-Positive Bacteria. J. Bacteriol. 2014, 196, 1133–1142. [Google Scholar] [CrossRef]
- Denapaite, D.; Brückner, R.; Hakenbeck, R.; Vollmer, W. Biosynthesis of Teichoic Acids in Streptococcus pneumoniae and Closely Related Species: Lessons from Genomes. Microb. Drug Resist. 2012, 18, 344–358. [Google Scholar] [CrossRef]
- Heß, N.; Waldow, F.; Kohler, T.P.; Rohde, M.; Kreikemeyer, B.; Gómez-Mejia, A.; Hain, T.; Schwudke, D.; Vollmer, W.; Hammerschmidt, S.; et al. Lipoteichoic Acid Deficiency Permits Normal Growth but Impairs Virulence of Streptococcus pneumoniae. Nat. Commun. 2017, 8, 2093. [Google Scholar] [CrossRef] [PubMed]
- Johnsborg, O.; Havarstein, L.S. Pneumococcal LytR, a Protein from the LytR-CpsA-Psr Family, Is Essential for Normal Septum Formation in Streptococcus pneumoniae. J. Bacteriol. 2009, 191, 5859–5864. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murayama, S.Y.; Seki, C.; Sakata, H.; Sunaoshi, K.; Nakayama, E.; Iwata, S.; Sunakawa, K.; Ubukata, K.; Invasive Streptococcal Disease Working Group. Capsular Type and Antibiotic Resistance in Streptococcus agalactiae Isolates from Patients, Ranging from Newborns to the Elderly, with Invasive Infections. Antimicrob. Agents Chemother. 2009, 53, 2650–2653. [Google Scholar] [CrossRef][Green Version]
- Cieslewicz, M.J.; Chaffin, D.; Glusman, G.; Kasper, D.; Madan, A.; Rodrigues, S.; Fahey, J.; Wessels, M.R.; Rubens, C.E. Structural and Genetic Diversity of Group B Streptococcus Capsular Polysaccharides. Infect. Immun. 2005, 73, 3096–3103. [Google Scholar] [CrossRef]
- Michon, F.; Brisson, J.R.; Dell, A.; Kasper, D.L.; Jennings, H.J. Multiantennary Group-Specific Polysaccharide of Group B Streptococcus. Biochemistry 1988, 27, 5341–5351. [Google Scholar] [CrossRef]
- Deng, L.; Kasper, D.L.; Krick, T.P.; Wessels, M.R. Characterization of the Linkage between the Type III Capsular Polysaccharide and the Bacterial Cell Wall of Group B Streptococcus. J. Biol. Chem. 2000, 275, 7497–7504. [Google Scholar] [CrossRef]
- Rowe, H.M.; Hanson, B.R.; Runft, D.L.; Lin, Q.; Firestine, S.M.; Neely, M.N. Modification of the CpsA Protein Reveals a Role in Alteration of the Streptococcus agalactiae Cell Envelope. Infect. Immun. 2015, 83, 1497–1506. [Google Scholar] [CrossRef]
- Shibata, Y.; Yamashita, Y.; van der Ploeg, J.R. The Serotype-Specific Glucose Side Chain of Rhamnose-Glucose Polysaccharides Is Essential for Adsorption of Bacteriophage M102 to Streptococcus mutans. FEMS Microbiol. Lett. 2009, 294, 68–73. [Google Scholar] [CrossRef]
- De, A.; Liao, S.; Bitoun, J.P.; Roth, R.; Beatty, W.L.; Wu, H.; Wen, Z.T. Deficiency of RgpG Causes Major Defects in Cell Division and Biofilm Formation, and Deficiency of LytR-CpsA-Psr Family Proteins Leads to Accumulation of Cell Wall Antigens in Culture Medium by Streptococcus mutans. Appl. Environ. Microbiol. 2017, 83, e00928-17. [Google Scholar] [CrossRef]
- Nakano, K.; Ooshima, T. Serotype Classification of Streptococcus mutans and Its Detection outside the Oral Cavity. Future Microbiol. 2009, 4, 891–902. [Google Scholar] [CrossRef]
- Kovacs, C.J.; Faustoferri, R.C.; Quivey, R.G., Jr. RgpF Is Required for Maintenance of Stress Tolerance and Virulence in Streptococcus mutans. J. Bacteriol. 2017, 199, e00497-17. [Google Scholar] [CrossRef] [PubMed]
- Bitoun, J.P.; Liao, S.; McKey, B.A.; Yao, X.; Fan, Y.; Abranches, J.; Beatty, W.L.; Wen, Z.T. Psr Is Involved in Regulation of Glucan Production, and Double Deficiency of BrpA and Psr Is Lethal in Streptococcus mutans. Microbiology 2013, 159, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Bitoun, J.P.; Liao, S.; Yao, X.; Ahn, S.J.; Isoda, R.; Nguyen, A.H.; Brady, L.J.; Burne, R.A.; Abranches, J.; Wen, Z.T. BrpA Is Involved in Regulation of Cell Envelope Stress Responses in Streptococcus mutans. Appl. Eniviron. Microbiol. 2012, 78, 2914–2922. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.T.; Baker, H.V.; Burne, R.A. Influence of BrpA on Critical Virulence Attributes of Streptococcus mutans. J. Bacteriol. 2006, 188, 2983–2992. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Shibata, Y.; Nakano, Y.; Tsuda, H.; Kido, N.; Ohta, M.; Koga, T. A Novel Gene Required for Rhamnose-Glucose Polysaccharide Synthesis in Streptococcus mutans. J. Bacteriol. 1999, 181, 6556–6559. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Yamashita, Y.; Ozaki, K.; Nakano, Y.; Koga, T. Expression and Characterization of Streptococcal rgp Genes Required for Rhamnan Synthesis in Escherichia coli. Infect. Immun. 2002, 70, 2891–2898. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Mercenier, A. Mucosal Delivery of Therapeutic and Prophylactic Molecules Using Lactic Acid Bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Aubry, C.; Motta, J.P.; Deraison, C.; Steidler, L.; Vergnolle, N.; Chatel, J.M.; Langella, P. Engineering Lactococci and Lactobacilli for Human Health. Curr. Opin. Microbiol. 2013, 16, 278–283. [Google Scholar] [CrossRef]
- Farenc, C.; Spinelli, S.; Vinogradov, E.; Tremblay, D.; Blangy, S.; Sadovskaya, I.; Moineau, S.; Cambillau, C. Molecular Insights on the Recognition of a Lactococcus lactis Cell Wall Pellicle by the Phage 1358 Receptor Binding Protein. J. Virol. 2014, 88, 7005–7015. [Google Scholar] [CrossRef]
- Chapot-Chartier, M.P.; Kulakauskas, S. Cell Wall Structure and Function in Lactic Acid Bacteria. Microb. Cell Fact. 2014, 13, S9. [Google Scholar] [CrossRef]
- Sadovskaya, I.; Vinogradov, E.; Courtin, P.; Armalyte, J.; Meyrand, M.; Giaouris, E.; Palussière, S.; Furlan, S.; Péchoux, C.; Ainsworth, S.; et al. Another Brick in the Wall: A Rhamnan Polysaccharide Trapped inside Peptidoglycan of Lactococcus lactis. mBio 2017, 8, e01303-17. [Google Scholar] [CrossRef] [PubMed]
- Siezen, R.J.; van Hylckama Vlieg, J.E.T. Genomic Diversity and Versatility of Lactobacillus plantarum, a Natural Metabolic Engineer. Microb. Cell Fact. 2011, 10 (Suppl. 1), S3. [Google Scholar] [CrossRef] [PubMed]
- D’Abrosca, G.; Paladino, A.; Cuoco, E.; Marasco, R.; Pacifico, S.; Piccolella, S.; Vastano, V.; Sacco, M.; Isernia, C.; Muscariello, L.; et al. Structural Characterization of the Lactobacillus plantarum FlmC Protein Involved in Biofilm Formation. Molecules 2018, 23, 2252. [Google Scholar] [CrossRef]
- Tomita, S.; Furihata, K.; Nukada, T.; Satoh, E.; Uchimura, T.; Okada, S. Structures of Two Monomeric Units of Teichoic Acid Prepared from the Cell Wall of Lactobacillus plantarum NRIC 1068. Biosci. Biotechnol. Biochem. 2009, 73, 530–535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muscariello, L.; Marino, C.; Capri, U.; Vastano, V.; Marasco, R.; Sacco, M. CcpA and Three Newly Identified Proteins Are Involved in Biofilm Development in Lactobacillus plantarum. J. Basic Microbiol. 2013, 53, 62–71. [Google Scholar] [CrossRef]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-Binding Proteins and Beta-Lactam Resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef]
- Maréchal, M.; Amoroso, A.; Morlot, C.; Vernet, T.; Coyette, J.; Joris, B. Enterococcus hirae LcpA (Psr), a New Peptidoglycan-Binding Protein Localized at the Division Site. BMC Microbiol. 2016, 16, 239. [Google Scholar] [CrossRef]
- Ligozzi, M.; Pittaluga, F.; Fontana, R. Identification of a Genetic Element (psr) Which Negatively Controls Expression of Enterococcus hirae Penicillin-Binding Protein 5. J. Bacteriol. 1993, 175, 2046–2051. [Google Scholar] [CrossRef]
- Massidda, O.; Kariyama, R.; Daneo-Moore, L.; Shockman, G.D. Evidence that the PBP 5 Synthesis Repressor (psr) of Enterococcus hirae Is Also Involved in the Regulation of Cell Wall Composition and Other Cell Wall-Related Properties. J. Bacteriol. 1996, 178, 5272–5278. [Google Scholar] [CrossRef][Green Version]
- Russell, D.G. Mycobacterium tuberculosis: Here Today, and Here Tomorrow. Nat. Rev. Mol. Cell Biol. 2001, 2, 569–577. [Google Scholar] [CrossRef]
- Harrison, J.; Lloyd, G.; Joe, M.; Lowary, T.L.; Reynolds, E.; Walters-Morgan, H.; Bhatt, A.; Lovering, A.; Besra, G.S.; Alderwick, L.J. Lcp1 Is Phosphotransferase Responsible for Ligating Arabinogalactan to Peptidoglycan in Mycobacterium tuberculosis. mBio 2016, 7, e00972-16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an Exopolysaccharide Produced by Lactobacillus plantarum YW11 Isolated from Tibet Kefir. Carbohydr. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Malm, S.; Maaß, S.; Schaible, U.E.; Ehlers, S.; Niemann, S. In Vivo Virulence of Mycobacterium tuberculosis Depends on a Single Homologue of the LytR-CpsA-Psr Proteins. Sci. Rep. 2018, 8, 3936. [Google Scholar] [CrossRef] [PubMed]
- Hugonnet, J.E.; Tremblay, L.W.; Boshoff, H.I.; Barry, C.E., III; Blanchard, J.S. Meropenem-Clavulanate Is Effective against Extensively drug-Resistant Mycobacterium tuberculosis. Science 2009, 323, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.D.; North, R.J.; LaCourse, R.; Mudgett, J.S.; Shah, S.K.; Nathan, C.F. Identification of Nitric Oxide Synthase as a Protective Locus against Tuberculosis. Proc. Natl. Acad. Sci. USA 1997, 94, 5243–5248. [Google Scholar] [CrossRef]
- Tuan, J.; Spichler-Moffarah, A.; Ogbuagu, O. Mycobacterium marinum: Nodular Hand Lesions after a Fishing Expedition. BMJ Case Rep. 2020, 13, e238835. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, L.; Jones, V.; Wang, C.; Hua, Y.; Shi, X.; Feng, X.; Jackson, M.; Niu, C.; Gao, Q. CpsA, a LytR-CpsA-Psr Family Protein in Mycobacterium marinum, Is Required for Cell Wall Integrity and Virulence. Infect. Immun. 2015, 83, 2844–2854. [Google Scholar] [CrossRef]
- Donovan, C.; Bramkamp, M. Cell Division in Corynebacterineae. Front. Microbiol. 2014, 5, 132. [Google Scholar] [CrossRef]
- Eggeling, L.; Bott, M. A Giant Market and a Powerful Metabolism: L-Lysine Provided by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2015, 99, 3387–3394. [Google Scholar] [CrossRef]
- Baumgart, M.; Schubert, K.; Bramkamp, M.; Frunzke, J. Impact of LytR-CpsA-Psr Proteins on Cell Wall Biosynthesis in Corynebacterium glutamicum. J. Bacteriol. 2016, 198, 3045–3059. [Google Scholar] [CrossRef]
- Bentley, S.D.; Chater, K.F.; Cerdeño-Tárraga, A.M.; Challis, G.L.; Thomson, N.R.; James, K.D.; Harris, D.E.; Quail, M.A.; Kieser, H.; Harper, D.; et al. Complete Genome Sequence of the Model Actinomycete Streptomyces coelicolor A3(2). Nature 2002, 417, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Flärdh, K. Growth Polarity and Cell Division in Streptomyces. Curr. Opin. Microbiol. 2003, 6, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, E.-M.; Latus, A.; Sigle, S.; Maldener, I.; Wohlleben, W.; Muth, G. Genetic Analysis of SCO2997, Encoding a TagF Homologue, Indicates a Role for Wall Teichoicacids in Sporulation of Streptomyces coelicolor A3(2). J. Bacteriol. 2011, 193, 6080–6085. [Google Scholar] [CrossRef] [PubMed]
- Shashkov, A.S.; Ostash, B.E.; Fedorenko, V.A.; Streshinskaya, G.M.; Tul’skaya, E.M.; Senchenkova, S.N.; Baryshnikova, L.M.; Evtushenko, L.I. Novel Teichulosonic Acid from Cell Wall of Streptomyces coelicolor M145. Carbohydr. Res. 2012, 359, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Sigle, S.; Steblau, N.; Wohlleben, W.; Muth, G. Polydiglycosylphosphate Transferase PdtA (SCO2578) of Streptomyces coelicolor A3(2) Is Crucial for Proper Sporulation and Apical Tip Extension under Stress Conditions. Appl. Environ. Microbiol. 2016, 82, 5661–5672. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, E.M.; Heichlinger, A.; Schirner, K.; Winkler, J.; Latus, A.; Maldener, I.; Wohlleben, W.; Muth, G. Proteins Encoded by the mre Gene Cluster in Streptomyces coelicolor A3(2) Cooperate in Spore Wall Synthesis. Mol. Microbiol. 2011, 79, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.A. Cellular Impermeability and Uptake of Biocides and Antibiotics in Gram-Positive Bacteria and Mycobacteria. J Appl. Microbiol. 2002, 92, 46S–54S. [Google Scholar] [CrossRef]
- Tang, G.; Yip, H.K.; Samaranayake, L.P.; Luo, G.; Lo, E.C.M.; Teo, C.S. Actinomyces spp. in Supragingival Plaque of Ethnic Chinese Preschool Children with and without Active Dental Caries. Caries Res. 2003, 37, 381–390. [Google Scholar] [CrossRef]
- Gosschalk, J.E.; Chang, C.; Sue, C.K.; Siegel, S.D.; Wu, C.; Kattke, M.D.; Yi, S.W.; Damoiseaux, R.; Jung, M.E.; Ton-That, H.; et al. A Cell-Based Screen in Actinomyces oris to Identify Sortase Inhibitors. Sci. Rep. 2020, 10, 8520. [Google Scholar] [CrossRef]
- Siegel, S.D.; Amer, B.R.; Wu, C.; Sawaya, M.R.; Gosschalk, J.E.; Clubb, R.T.; Ton-That, H. Structure and Mechanism of LcpA, a Phosphotransferase That Mediates Glycosylation of a Gram-Positive Bacterial Cell Wall-anchored Protein. mBio 2019, 10, e01580-18. [Google Scholar] [CrossRef]
- Amer, B.R.; Clubb, R.T. A Sweet New Role for LCP Enzymes in Protein Glycosylation. Mol. Microbiol. 2014, 94, 1197–1200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, C.; Huang, I.H.; Chang, C.; Reardon-Robinson, M.E.; Das, A.; Ton-That, H. Lethality of Sortase Depletion in Actinomyces oris Caused by Excessive Membrane Accumulation of a Surface Glycoprotein. Mol. Microbiol. 2014, 94, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, S.N.; Foster, T.J. Molecular Analysis of the tagF Gene, Encoding CDP-Glycerol:Poly(glycerophosphate) Glycerophosphotransferase of Staphylococcus epidermidis ATCC 14990. J. Bacteriol. 2000, 182, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Stebegg, R.; Wurzinger, B.; Mikulic, M.; Schmetterer, G. Chemoheterotrophic Growth of the Cyanobacterium Anabaena sp. Strain PCC 7120 Dependent on a Functional Cytochrome c Oxidase. J. Bacteriol. 2012, 194, 4601–4607. [Google Scholar] [CrossRef] [PubMed]
- Mella-Herrera, R.A.; Neunuebel, M.R.; Golden, J.W. Anabaena sp. Strain PCC 7120 conR Contains a LytR-CpsA-Psr Domain, Is Developmentally Regulated, and Is Essential for Diazotrophic Growth and Heterocyst Morphogenesis. Microbiology 2011, 157, 617–626. [Google Scholar] [CrossRef][Green Version]
- Huang, G.; Fan, Q.; Lechno-Yossef, S.; Wojciuch, E.; Wolk, C.P.; Kaneko, T.; Tabata, S. Clustered Genes Required for the Synthesis of Heterocyst Envelope Polysaccharide in Anabaena sp. Strain PCC 7120. J. Bacteriol. 2005, 187, 1114–1123. [Google Scholar] [CrossRef]
- Wang, Y.; Lechno-Yossef, S.; Gong, Y.; Fan, Q.; Wolk, C.P.; Xu, X. Predicted Glycosyl Transferase Genes Located Outside the HEP Island Are Required for Formation of Heterocyst Envelope Polysaccharide in Anabaena sp. Strain PCC 7120. J. Bacteriol. 2007, 189, 5372–5378. [Google Scholar] [CrossRef]
- Fan, Q.; Lechno-Yossef, S.; Ehira, S.; Kaneko, T.; Ohmori, M.; Sato, N.; Tabata, S.; Wolk, C.P. Signal Transduction Genes Required for Heterocyst Maturation in Anabaena sp. Strain PCC 7120. J. Bacteriol. 2006, 188, 6688–6693. [Google Scholar] [CrossRef]
- Bensing, B.A.; Khedri, Z.; Deng, L.; Yu, H.; Prakobphol, A.; Fisher, S.J.; Chen, X.; Iverson, T.M.; Varki, A.; Sullam, P.M. Novel Aspects of Sialoglycan Recognition by the Siglec-Like Domains of Streptococcal SRR Glycoproteins. Glycobiology 2016, 26, 1222–1234. [Google Scholar] [CrossRef]



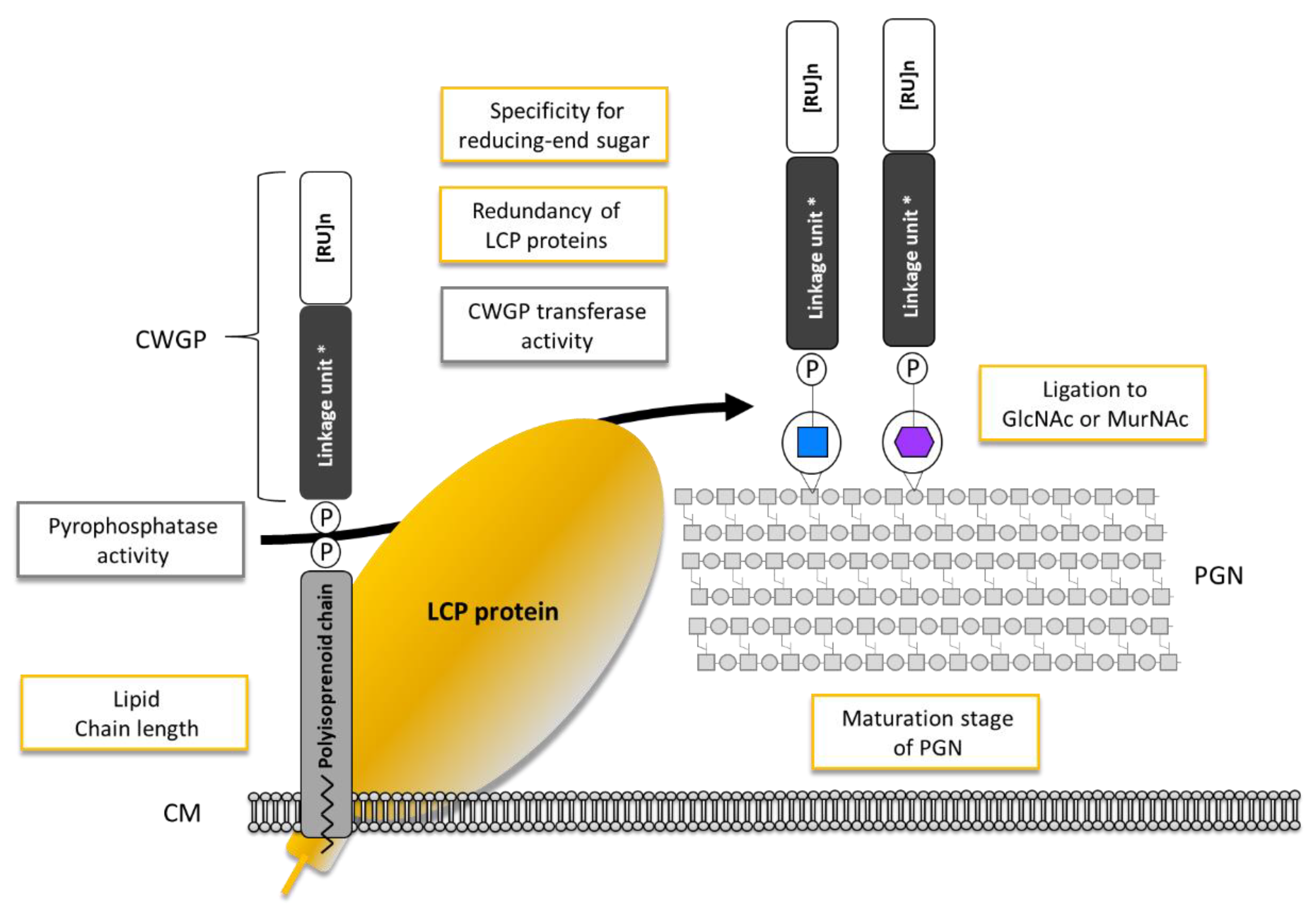

| Bacterium | LCP Name | Accession No. | Transferred CWGP | Linker | Acceptor | In Vitro Assay | References | |
|---|---|---|---|---|---|---|---|---|
| Substrate | Acceptor | |||||||
| A. oris | LcpA | AXE84_RS08820 | Unknown saccharide | n.d. | GspA protein | [174] | ||
| Anabena sp. | ConR | all0187 | Unknown PS | n.d. | n.d. | [179] | ||
| B. anthracis | LcpB1 LcpB2 LcpB3 LcpB4 LcpC LcpD | BAS1830, BAS0572, BAS0746, BAS3381, BAS5115, BAS5047 | PyrCWGP | β-D-ManpNAc-(1→4)-α-D-GlcpNAc-(1→P | MurNAc/PGN | [43] | ||
| B. subtilis | TagT TagU TagV | BSU_35840, BSU_35650, BSU_35520 | WTA | β-D-ManpNAc-(1→4)-α-D-GlcpNAc-(1→P | MurNAc/PGN | Lipid β | mature PGN | [109] |
| C. glutamicum | LcpA LcpB | Cg0847/NCgl0708 Cg3210/NCgl2802 | AG | α-L-Rhap-(1→3)-α-D-GlcpNAc-(1→P | MurNAc/PGN | [164] | ||
| E. hirae | LcpA LcpB LcpC | EHR_11445 EHR_11995 EHR_14364 | Unknown WTA | n.d. | PGN | [151] | ||
| Lb. plantarum | FlmA FlmB FlmC | LP_RS02565 LP_RS01195 LP_RS04280 | WTA | β-D-ManpNAc-(1→4)-α-D-GlcpNAc-(1→P | MurNAc/PGN | [147,149] | ||
| Lc. lactis | LcpA | llnz_02385 | RhaCWGPpolyrhamnan | β-D-ManpNAc-(1→4)-α-D-GlcpNAc-(1→P β-D-ManpNAc-(1→4)-α-D-GlcpNAc-(1→P | MurNAc/PGN | [145] | ||
| M. marinum | CpsA | MMAR_4966 MMAR_4858 MMAR_1274 MMAR_5392X | AG | α-L-Rhap-(1→3)-α-D-GlcpNAc-(1→P | MurNAc/PGN | [161] | ||
| M. tuberculosis | Rv0822c CpsA1/Lcp1 CpsA/CpsA2 Rv3840 | Rv0822c Rv3267 Rv3484 Rv3840 | AG | α-L-Rhap-(1→3)-α-D-GlcpNAc-(1→P | MurNAc/PGN | Galf2-Rha-GlcNAc-O-C8 Geranyl-PP | Un-crosslinked PGN | [65,155] |
| S. aureus | LcpA LcpB LcpC | MsrR SA0908 SA2103 | WTA CP5 | β-D-ManpNAc-(1→4)-α-D-GlcpNAc-(1→P RU β-D-FucpNAc-(1→P | MurNAc/PGN | C30-MLU Lipid Icap | Un-crosslinked PGN Lipid II PGN | [90,98] |
| Sc. agalactiae | CpsA | SAG_RS08600 | CPIII | OS-(1→P | GlcNAc/PGN | [131] | ||
| Sc. mutans | BrpA Psr | SMU_RS01995 SMU_787 | RhaCWGP | GlcpNAc-(1→P | PGN | [136,137,138] | ||
| Sc. pneumoniae | Cps2A LytR Psr | SPD_0315 SPD_1741 SPD_1202 | CP2 WTA | β-D-Glcp-(1→ RU AATGalp | GlcNAc/PGN | [97,126] | ||
| Sm. coelicolor | PdtA | SCO2578 SCO3042 SCO3043 SCO3044 SCO3045 SCO3046 SCO3047 SCO3048 SCO4755 SCO5358 SCO6020 | PDP | RU α-D-GlcpNAc-(1→P | MurNAc/PGN | [183] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanović, C.; Hager, F.F.; Schäffer, C. LytR-CpsA-Psr Glycopolymer Transferases: Essential Bricks in Gram-Positive Bacterial Cell Wall Assembly. Int. J. Mol. Sci. 2021, 22, 908. https://doi.org/10.3390/ijms22020908
Stefanović C, Hager FF, Schäffer C. LytR-CpsA-Psr Glycopolymer Transferases: Essential Bricks in Gram-Positive Bacterial Cell Wall Assembly. International Journal of Molecular Sciences. 2021; 22(2):908. https://doi.org/10.3390/ijms22020908
Chicago/Turabian StyleStefanović, Cordula, Fiona F. Hager, and Christina Schäffer. 2021. "LytR-CpsA-Psr Glycopolymer Transferases: Essential Bricks in Gram-Positive Bacterial Cell Wall Assembly" International Journal of Molecular Sciences 22, no. 2: 908. https://doi.org/10.3390/ijms22020908
APA StyleStefanović, C., Hager, F. F., & Schäffer, C. (2021). LytR-CpsA-Psr Glycopolymer Transferases: Essential Bricks in Gram-Positive Bacterial Cell Wall Assembly. International Journal of Molecular Sciences, 22(2), 908. https://doi.org/10.3390/ijms22020908





