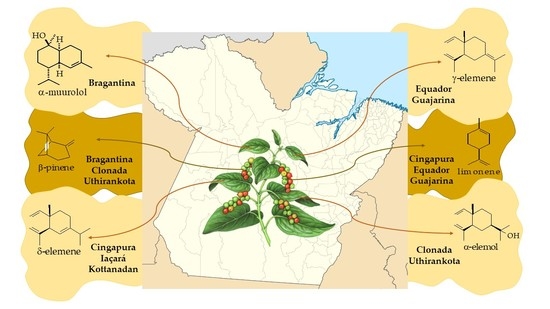
Secondary Metabolic Profile as a Tool for Distinction and Characterization of Cultivars of Black Pepper (Piper nigrum L.) Cultivated in Pará State, Brazil
Abstract
1. Introduction
2. Results and Discussion
2.1. Evaluation of Production and Identification of Secondary Metabolites from Cultivars of Black Pepper
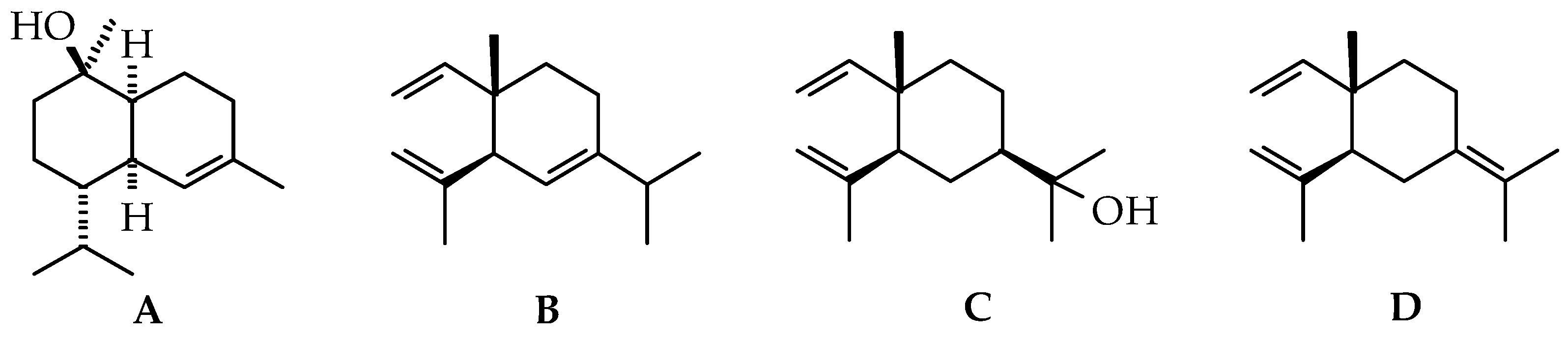
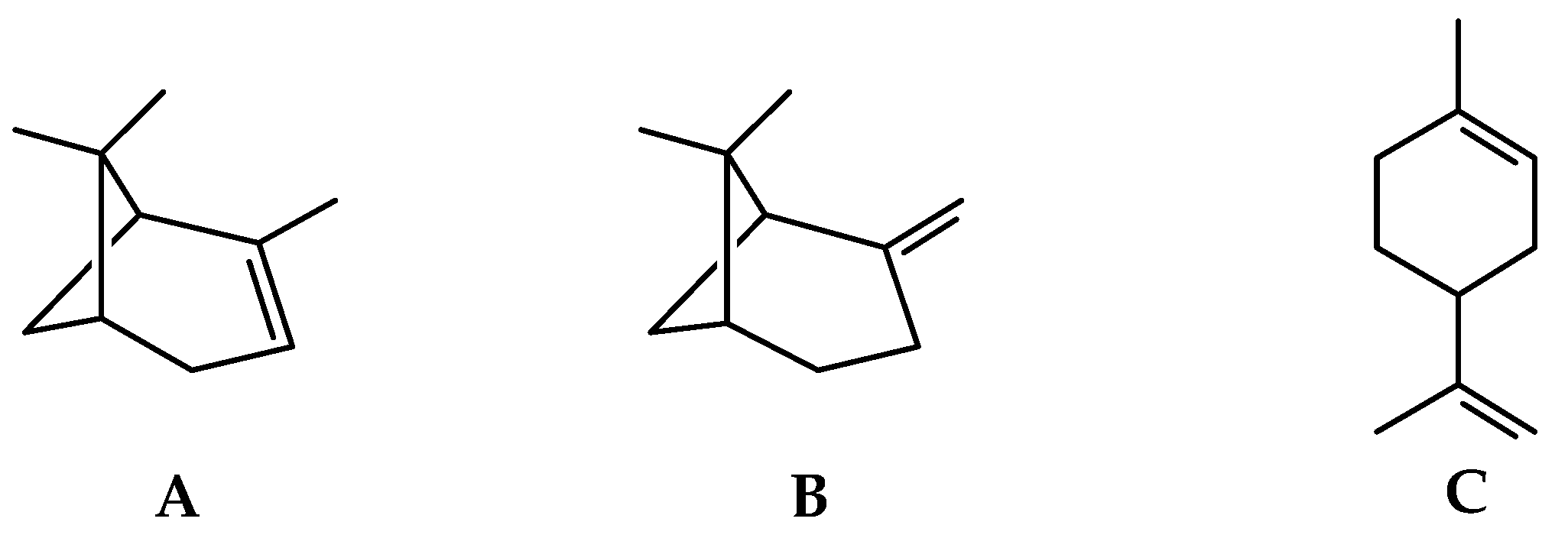
2.1.1. Volatile Compounds in the Leaves of Black Pepper
2.1.2. Volatile Compounds in the Fruits of Black Pepper
2.1.3. Hierarchical Cluster Analysis (HCA)
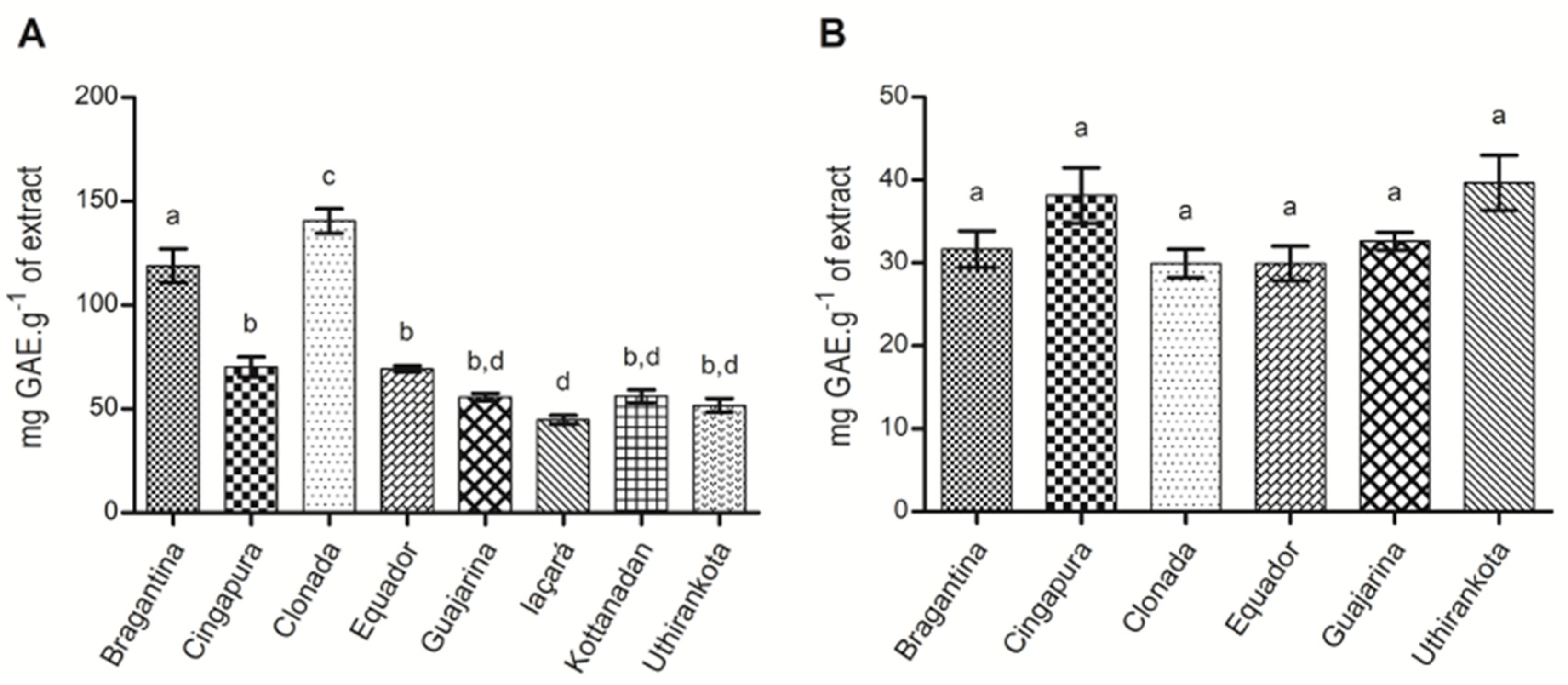
2.1.4. Total Phenolics Determination
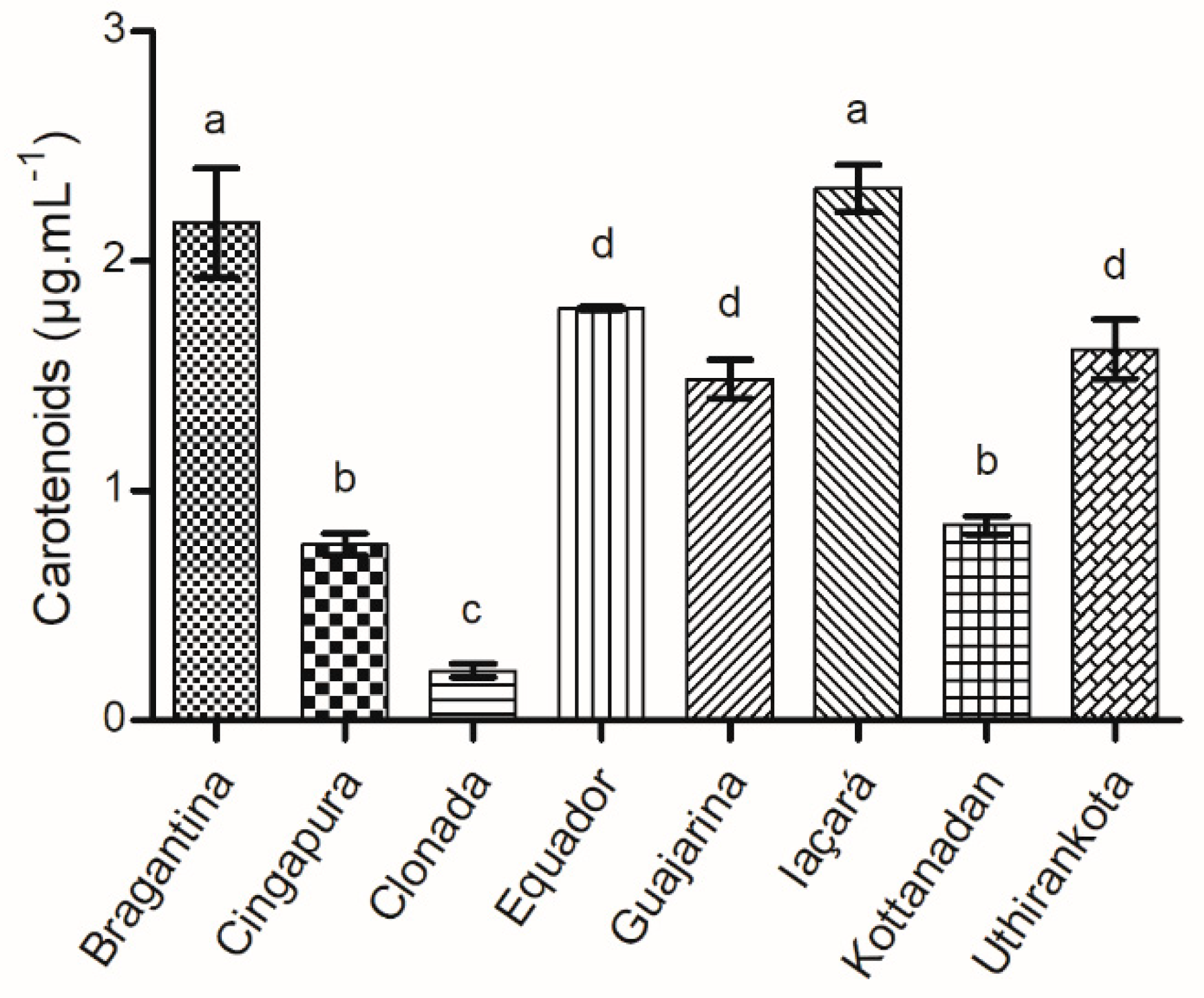
2.1.5. Total Carotenoids
2.2. Comparison of Enzymatic Activity In Vitro in the Leaves of Black Pepper Cultivars
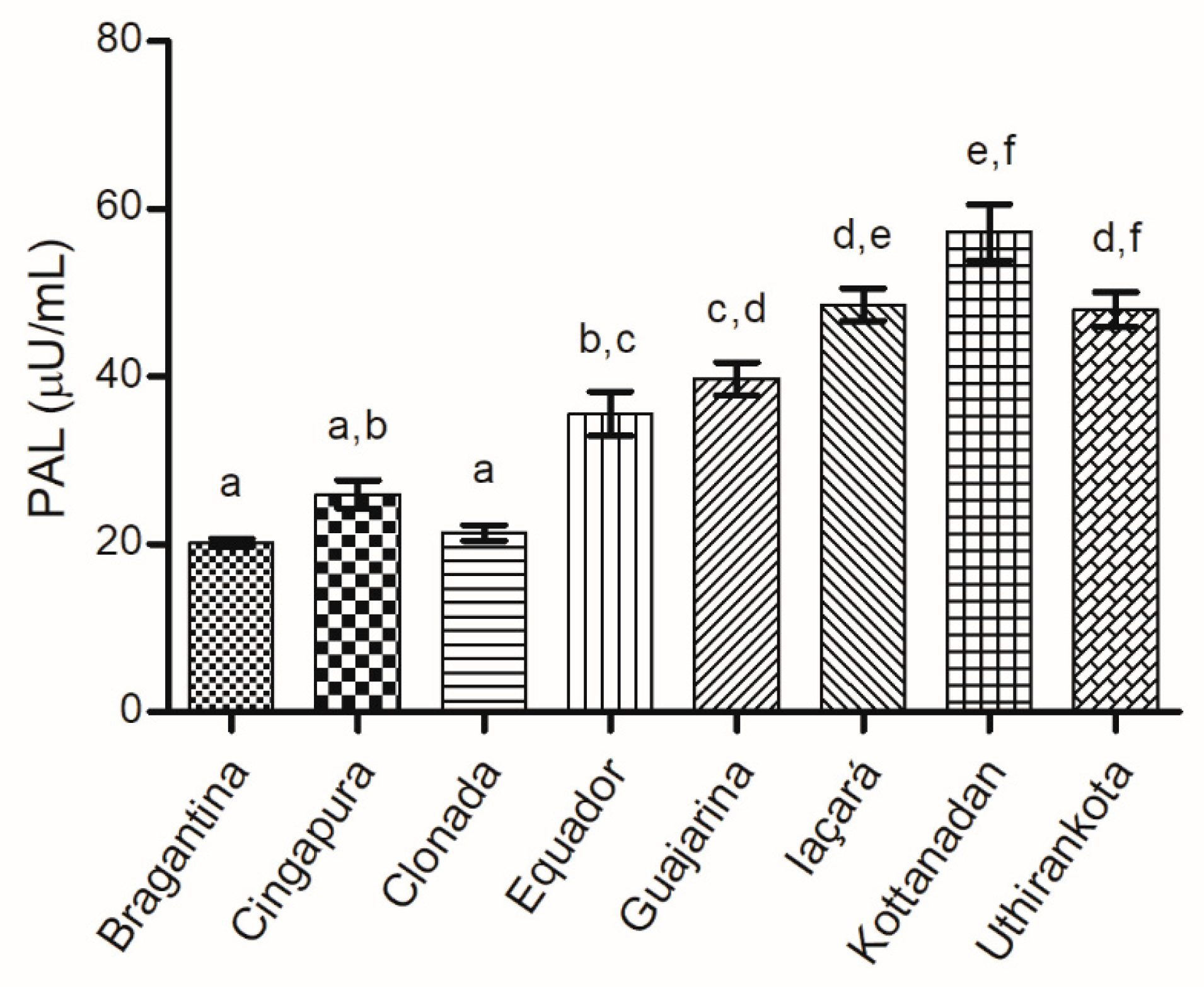
Phenylalanine Ammonia-Lyase (PAL) Activity
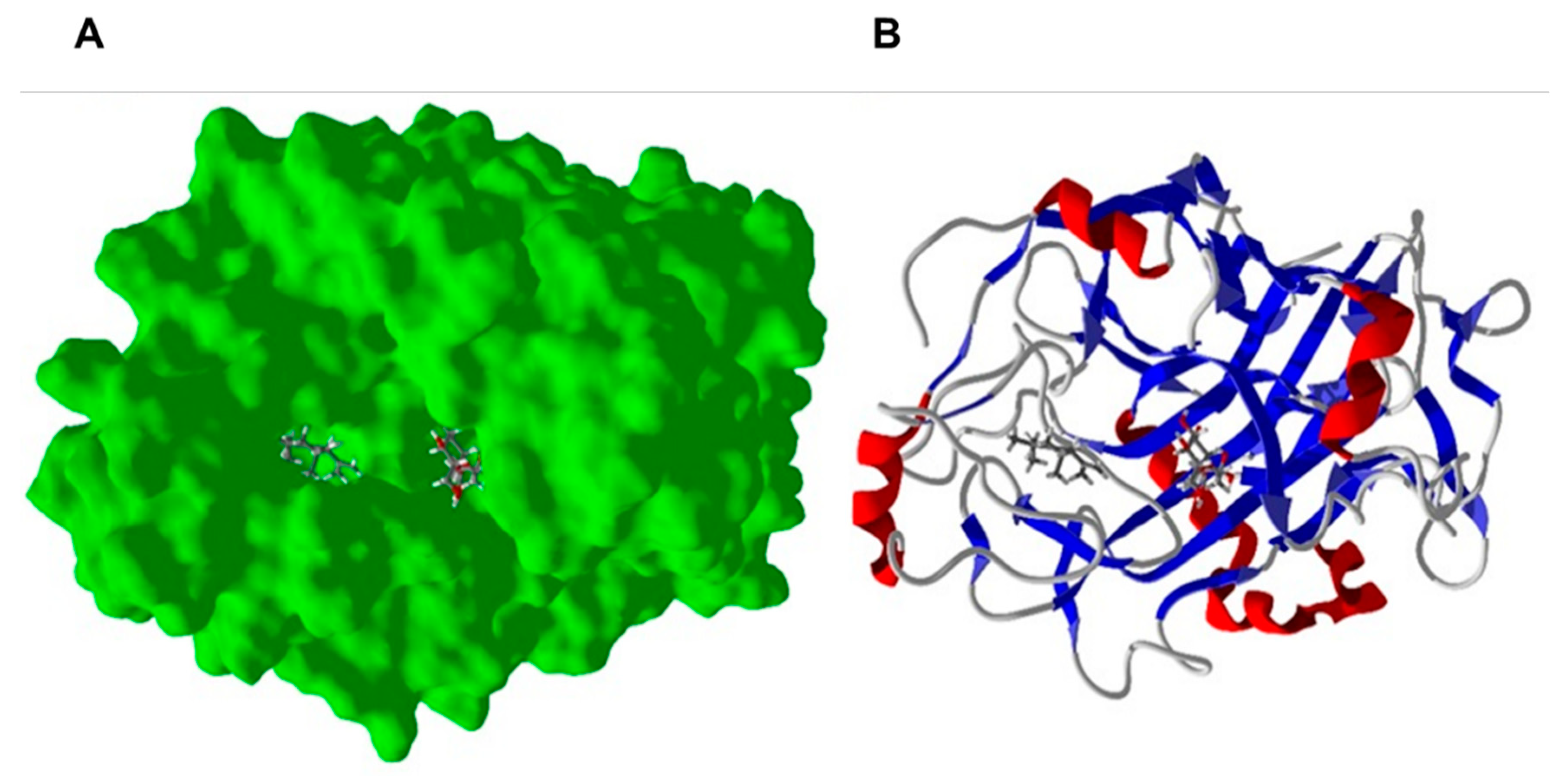
2.3. Homology Modeling and Molecular Docking to Fusarium
3. Materials and Methods
3.1. Plant Material
3.2. Identification of Secondary Metabolites from Cultivars of Black Pepper
3.2.1. Extraction and Analysis of Volatile Organic Compounds (VOCs)
3.2.2. Extracts Preparation
3.2.3. Total Phenolics Determination
3.2.4. Total Carotenoids
3.3. Determination of In Vitro Enzymatic Activity from Leaves of Black Pepper Cultivars
Phenylalanine Ammonia-Lyase (PAL) Activity
3.4. Computational Methods
3.4.1. Homology Modeling
3.4.2. Molecular Docking
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abs | Absorbance |
| AMF | Arbuscular mycorrhizal fungi |
| ANOVA | Analysis of variance |
| Cartotal | Total carotenoids |
| ChlA | Chlorophyll A |
| ChlB | Chlorophyll B |
| CLas | Candidatus Liberibacter asiaticus |
| DTT | Dithiothreitol |
| EDTA | Ethylenediaminetetraacetic acid |
| EIMS | Electron ionization mass spectrometry |
| EMBRAPA | Brazilian Agricultural Research Corporation |
| EO | Essential oil |
| GAE | Gallic acid equivalents |
| GC-MS | Gas chromatography–mass spectrometry |
| HCA | Hierarchical cluster analysis |
| NCBI | National Center for Biotechnology Information |
| PAL | Phenylalanine ammonia-lyase |
| PCA | Principal component analysis |
| PDB | Protein Data Bank |
| PVP | Polyvinylpolypyrrolidone |
| RI | Retention indices |
| VOC | Volatile organic compounds |
References
- Durant-Archibold, A.A.; Santana, A.I.; Gupta, M.P. Ethnomedical uses and pharmacological activities of most prevalent species of genus Piper in Panama: A review. J. Ethnopharmacol. 2018, 217, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Jardim Botânico do Rio de Janeiro Piperaceae in Flora do Brasil 2020: Em Construção. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB12735 (accessed on 25 May 2020).
- Sen, S.; Gode, A.; Ramanujam, S.; Ravikanth, G.; Aravind, N.A. Modeling the impact of climate change on wild Piper nigrum (Black Pepper) in Western Ghats, India using ecological niche models. J. Plant Res. 2016, 129, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Grinevicius, V.M.A.S.; Andrade, K.S.; Ourique, F.; Micke, G.A.; Ferreira, S.R.S.; Pedrosa, R.C. Antitumor activity of conventional and supercritical extracts from Piper nigrum L. cultivar Bragantina through cell cycle arrest and apoptosis induction. J. Supercrit. Fluids 2017, 128, 94–101. [Google Scholar] [CrossRef]
- Bagheri, H.; Abdul Manap, M.Y.B.; Solati, Z. Antioxidant activity of Piper nigrum L. essential oil extracted by supercritical CO2 extraction and hydro-distillation. Talanta 2014, 121, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Swathy, J.S.; Mishra, P.; Thomas, J.; Mukherjee, A.; Chandrasekaran, N. Antimicrobial potency of high-energy emulsified black pepper oil nanoemulsion against aquaculture pathogen. Aquaculture 2018, 491, 210–220. [Google Scholar] [CrossRef]
- Duangjai, A.; Ingkaninan, K.; Praputbut, S.; Limpeanchob, N. Black pepper and piperine reduce cholesterol uptake and enhance translocation of cholesterol transporter proteins. J. Nat. Med. 2013, 67, 303–310. [Google Scholar] [CrossRef]
- Aziz, D.M.; Hama, J.R.; Alam, S.M. Synthesising a novel derivatives of piperine from black pepper (Piper nigrum L.). J. Food Meas. Charact. 2015, 9, 324–331. [Google Scholar] [CrossRef]
- Da Silva, J.K.; da Trindade, R.; Alves, N.S.; Figueiredo, P.L.; Maia, J.G.S.; Setzer, W.N. Essential Oils from Neotropical Piper Species and Their Biological Activities. Int. J. Mol. Sci. 2017, 18, 2571. [Google Scholar] [CrossRef]
- Abdulazeez, M.A.; Sani, I.; James, B.D.; Abdullahi, A.S. Black Pepper (Piper nigrum L.) Oils; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780124166448. [Google Scholar]
- Damanhouri, Z.A. A Review on Therapeutic Potential of Piper nigrum L. (Black Pepper): The King of Spices. Med. Aromat. Plants 2014, 3, 161. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Abdalla, W.E. Black pepper fruit (Piper nigrum L.) as antibacterial agent: A mini-review. J. Bacteriol. Mycol. Open Access 2018, 6, 141–145. [Google Scholar] [CrossRef]
- De Lemos, O.F.; Poltronieri, M.C.; Rodrigues, S.d.M.; de Menezes, I.C.; Mondin, M. Conservação e Melhoramento Genético da pimenteira-do-reino. Infoteca Cnptia Embrapa Br. 2011, 1, 45. [Google Scholar]
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Baskar, V.; Ramalingam, S. In vitro and in planta nematicidal activity of black pepper (Piper nigrum L.) leaf extracts. Crop Prot. 2017, 100, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.S.; Dayod, M.; Tawan, C.S. Phenetic Analysis of Cultivated Black Pepper (Piper nigrum L.) in Malaysia. Int. J. Agron. 2018, 2018, 3894924. [Google Scholar] [CrossRef]
- Jaramillo, M.A.; Manos, P.S.; Zimmer, E.A. Phylogenetic relationships of the perianthless Piperales: Reconstructing the evolution of floral development. Int. J. Plant Sci. 2004, 165, 403–416. [Google Scholar] [CrossRef]
- Carvalho Filgueiras, G.; Kingo, A.; Homma, O.; Souza, M.A.; Santos, D. Conjuntura do Mercado da Pimenta-do-Reino No Brasil e No Mundo; Embrapa Amazônia Oriental: Belém, Brazil, 2009. [Google Scholar]
- Dosoky, N.S.; Satyal, P.; Barata, L.M.; da Silva, J.K.R.; Setzer, W.N. Volatiles of Black Pepper Fruits (Piper nigrum L.). Molecules 2019, 24, 4244. [Google Scholar] [CrossRef]
- Zhu, F.; Mojel, R.; Li, G. Physicochemical properties of black pepper (Piper nigrum) starch. Carbohydr. Polym. 2018, 181, 986–993. [Google Scholar] [CrossRef]
- Khan, M.; Hanif, M.A.; Rehman, R.; Bhatti, I.A. Black Piper. Med. Plants South Asia 2020, 75–86. [Google Scholar] [CrossRef]
- Costa, M.R.T.d.R.; Homma, A.K.O.; Rebello, F.K.; Souza Filho, A.P.d.S.; Fernandes, G.L.d.C.; Baleixe, W. Atividade Agropecuária no Estado do Pará; Embrapa Amazônia Oriental: Belém, Brazil, 2017. [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística—IBGE Produção Agrícola Municipal. Available online: https://sidra.ibge.gov.br/tabela/1613 (accessed on 25 May 2020).
- Duarte, M.d.L.R.; Poltronieri, M.C.; Chu, E.Y.; Oliveira, R.F.d.; Lemos, O.F.; Benchimol, R.L.; da Conceição, H.E.O.; de Souza, G.F. Coleção Plantar: A Cultura da Pimenta-do-Reino, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brazil, 2006; ISBN 85-7383-380-7. [Google Scholar]
- De Castro, G.L.S.; De Lemos, O.F.; Tremacoldi, C.R.; Moraes, F.K.C.; Dos Santos, L.R.R.; Pinheiro, H.A. Susceptibility of in vitro black pepper plant to the filtrate from a Fusarium solani f. sp. piperis culture. Plant Cell. Tissue Organ Cult. 2016, 127, 263–268. [Google Scholar] [CrossRef]
- Verpoorte, R.; Alfermann, A.W. Metabolic Engineering of Plant Secondary Metabolism; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; ISBN 0792363604. [Google Scholar]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Raj Joshi, D.; Adhikari, N.; Raj Joshi, D.; Chandra Shrestha, A. A review on diversified use of the king of spices: Piper nigrum (black pepper). Artic. Int. J. Pharm. Sci. Res. 2018, 9, 4089–4101. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Arslan, U.; Ilhan, K.; Karabulut, O.A. Antifungal activity of aqueous extracts of spices against bean rust (Uromyces appendiculatus). Allelopath. J. 2009, 24, 207–213. [Google Scholar]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Alhadrami, H.A.A.; Bhandari, A. Quantification of total phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrum Linn fruits. Asian Pac. J. Trop. Biomed. 2015, 5, 101–107. [Google Scholar] [CrossRef]
- Gupta, N.; Parashar, P.; Mittal, M.; Mehra, V.; Khatri, M. Antibacterial potential of Elletaria cardamomum, Syzygium aromaticum and Piper nigrum, their synergistic effects and phytochemical determination. J. Pharm. Res. 2014, 8, 1091–1097. [Google Scholar]
- Li, Y.X.; Zhang, C.; Pan, S.; Chen, L.; Liu, M.; Yang, K.; Zeng, X.; Tian, J. Analysis of chemical components and biological activities of essential oils from black and white pepper (Piper nigrum L.) in five provinces of southern China. LWT—Food Sci. Technol. 2020, 117, 108644. [Google Scholar] [CrossRef]
- Davies, H.V.; Shepherd, L.V.T.; Stewart, D.; Frank, T.; Röhlig, R.M.; Engel, K.-H. Metabolome variability in crop plant species—When, where, how much and so what? Regul. Toxicol. Pharmacol. 2010, 58, S54–S61. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Silva, J.R.A.; Nascimento, S.B.; da Luz, S.F.M.; Meireles, E.N.; Alves, C.N.; Ramos, A.R.; Maia, J.G.S. Antifungal activity and computational study of constituents from Piper divaricatum essential oil against Fusarium infection in black pepper. Molecules 2014, 19, 17926–17942. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complementary Altern. Med. 2017, 17, 60. [Google Scholar] [CrossRef]
- Shao, Q.; Zhu, W. Exploring the Ligand Binding/Unbinding Pathway by Selectively Enhanced Sampling of Ligand in a Protein-Ligand Complex. J. Phys. Chem. B 2019, 123, 7974–7983. [Google Scholar] [CrossRef]
- Setzer, W.N.; Byler, K.G. In-silico Approaches to Natural Products Drug Discovery: A Review of the Recent Literature. In Natural Products and Drug Discovery: From Pharmacochemistry to Pharmacological Approaches; Diniz, M.d.F.F.M., Scotti, L., Scotti, M.T., Alves, M.F., Eds.; Editora UFPB: João Pessoa, PB, Brazil, 2018; pp. 11–82. [Google Scholar]
- Edward, E.J.; King, W.S.; Teck, S.L.C.; Jiwan, M.; Aziz, Z.F.A.; Kundat, F.R.; Ahmed, O.H.; Majid, N.M.A. Antagonistic activities of endophytic bacteria against Fusarium wilt of black pepper (Piper nigrum). Int. J. Agric. Biol. 2013, 15, 291–296. [Google Scholar]
- Lau, E.T.; Tani, A.; Khew, C.Y.; Chua, Y.Q.; Hwang, S.S. Plant growth-promoting bacteria as potential bio-inoculants and biocontrol agents to promote black pepper plant cultivation. Microbiol. Res. 2020, 240, 126549. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.T.; Khew, C.Y.; Hwang, S.S. Transcriptomic analysis of pepper plants provides insights into host responses to Fusarium solani infestation. J. Biotechnol. 2020, 314–315, 53–62. [Google Scholar] [CrossRef]
- El-Gawad, A.; Elshamy, A.; El Gendy, A.; Gaara, A.; Assaeed, A. Volatiles Profiling, Allelopathic Activity, and Antioxidant Potentiality of Xanthium strumarium Leaves Essential Oil from Egypt: Evidence from Chemometrics Analysis. Molecules 2019, 24, 584. [Google Scholar] [CrossRef] [PubMed]
- Da Trindade, R.; Almeida, L.; Xavier, L.; Lins, A.L.; Andrade, E.H.; Maia, J.G.; Mello, A.; Setzer, W.N.; Ramos, A.; Da Silva, J.K. Arbuscular mycorrhizal fungi colonization promotes changes in the volatile compounds and enzymatic activity of lipoxygenase and phenylalanine ammonia lyase in Piper nigrum L. “Bragantina”. Plants 2019, 8, 442. [Google Scholar] [CrossRef]
- Mota, J.d.S.; de Souza, D.S.; Boone, C.V.; Cardoso, C.A.L.; Caramão, E.B. Identification of the Volatile Compounds of Leaf, Flower, Root and Stem Oils of Piper amalago (Piperaceae). J. Essent. Oil-Bearing Plants 2013, 16, 11–16. [Google Scholar] [CrossRef]
- Rocha, D.S.; Da Silva, J.M.; Do Amaral Ferraz Navarro, D.M.; Camara, C.A.G.; De Lira, C.S.; Ramos, C.S. Potential antimicrobial and chemical composition of essential oils from Piper caldense tissues. J. Mex. Chem. Soc. 2016, 60, 148–151. [Google Scholar] [CrossRef]
- Bernuci, K.; Iwanaga, C.; Fernandez-Andrade, C.; Lorenzetti, F.; Torres-Santos, E.; Faiões, V.; Gonçalves, J.; do Amaral, W.; Deschamps, C.; Scodro, R.; et al. Evaluation of Chemical Composition and Antileishmanial and Antituberculosis Activities of Essential Oils of Piper Species. Molecules 2016, 21, 1698. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Ribeiro, A.F.; Guimarães, E.F.; Maia, J.G.S. Essential oil composition of Piper manausense yuncker. J. Essent. Oil-Bear. Plants 2005, 8, 295–299. [Google Scholar] [CrossRef]
- Setzer, W.N.; Park, G.; Agius, B.R.; Stokes, S.L.; Walker, T.M.; Haber, W.A. Chemical compositions and biological activities of leaf essential oils of twelve species of Piper from Monteverde, Costa Rica. Nat. Prod. Commun. 2008, 3, 1367–1374. [Google Scholar] [CrossRef]
- Da Luz, S.F.M.; Yamaguchi, L.F.; Kato, M.J.; de Lemos, O.F.; Xavier, L.P.; Maia, J.G.S.; Ramos, A.d.R.; Setzer, W.N.; da Silva, J.K.d.R. Secondary metabolic profiles of two cultivars of Piper nigrum (black pepper) resulting from infection by Fusarium solani f. sp. piperis. Int. J. Mol. Sci. 2017, 18, 2434. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.K.R.; Pinto, L.C.; Burbano, R.M.R.; Montenegro, R.C.; Andrade, E.H.A.; Maia, J.G.S. Composition and cytotoxic and antioxidant activities of the oil of Piper aequale Vahl. Lipids Health Dis. 2016, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.d.B.F.; Balbino, J.M.; Zini, C.A.; Ribeiro, V.L.S.; Bordignon, S.A.L.; Von Poser, G. Acaricidal activity and chemical composition of the essential oil from three Piper species. Parasitol. Res. 2010, 107, 243–248. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Pinto, L.C.; Burbano, R.M.R.; Montenegro, R.C.; Guimarães, E.F.; Andrade, E.H.A.; Maia, J.G.S. Essential oils of Amazon Piper species and their cytotoxic, antifungal, antioxidant and anti-cholinesterase activities. Ind. Crops Prod. 2014, 58, 55–60. [Google Scholar] [CrossRef]
- Avella, E.; Rios-Motta, J. Main constituents and cytotoxic activity of the essential oil of Piper artanthe. Chem. Nat. Compd. 2010, 46, 651–653. [Google Scholar] [CrossRef]
- Andrade, E.H.A.; Alves, C.N.; Guimarães, E.F.; Carreira, L.M.M.; Maia, J.G.S. Variability in essential oil composition of Piper dilatatum L.C. Rich. Biochem. Syst. Ecol. 2011, 39, 669–675. [Google Scholar] [CrossRef]
- Da Luz, S.F.M.; Reis, L.d.A.; de Lemos, O.F.; Maia, J.G.S.; de Mello, A.H.; Ramos, A.R.; da Silva, J.K.R. Effect of arbuscular mycorrhizal fungi on the essential oil composition and antioxidant activity of black pepper (Piper nigrum L.). Int. J. Appl. Res. Nat. Prod. 2016, 9, 10–17. [Google Scholar]
- Torquilho, H.S.; Pinto, A.C.; Godoy, R.L.D.O.; Guimarães, E.F. Essential oil of Piper cernum Vell. var. cernum yuncker from Rio de Janeiro, Brazil. J. Essent. Oil Res. 2000, 12, 443–444. [Google Scholar] [CrossRef]
- De Abreu, A.M.; Brighente, I.M.C.; Deaguiar, E.M.; Rebelo, R.A. Volatile constituents of Piperaceae from Santa Catarina, Brazil—Essential oil composition of Piper cernuum Vell. and Peperomia emarginella (Sw.) C. DC. J. Essent. Oil Res. 2005, 17, 286–288. [Google Scholar] [CrossRef]
- Gasparetto, A.; Bella Cruz, A.; Wagner, T.M.; Bonomini, T.J.; Correa, R.; Malheiros, A. Seasonal variation in the chemical composition, antimicrobial and mutagenic potential of essential oils from Piper cernuum. Ind. Crops Prod. 2017, 95, 256–263. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Bello, A.; Urquiola, A. Composition of the essential oil of Piper hispidum sw. from Cuba. J. Essent. Oil Res. 2004, 16, 459–460. [Google Scholar] [CrossRef]
- Autran, E.S.; Neves, I.A.; da Silva, C.S.B.; Santos, G.K.N.; Câmara, C.A.G.d.; Navarro, D.M.A.F. Chemical composition, oviposition deterrent and larvicidal activities against Aedes aegypti of essential oils from Piper marginatum Jacq. (Piperaceae). Bioresour. Technol. 2009, 100, 2284–2288. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.K.R.; Silva, N.N.S.; Santana, J.F.S.; Andrade, E.H.A.; Maia, J.G.S.; Setzer, W.N. Phenylpropanoid-rich essential oils of Piper species from the Amazon and their antifungal and anti-cholinesterase activities. Nat. Prod. Commun. 2016, 11, 1907–1911. [Google Scholar] [CrossRef] [PubMed]
- Assis, A.; Brito, V.; Bittencourt, M.; Silva, L.; Oliveira, F.; Oliveira, R. Essential oils composition of four Piper species from Brazil. J. Essent. Oil Res. 2013, 25, 203–209. [Google Scholar] [CrossRef]
- De Souza, S.P.; Valverde, S.S.; Costa, N.F.; Calheiros, A.S.; Lima, K.S.C.; Frutuoso, V.S.; Lima, A.L.S. Chemical composition and antinociceptive activity of the essential oil of Piper mollicomum and Piper rivinoides. J. Med. Plants Res. 2014, 8, 788–793. [Google Scholar] [CrossRef]
- Ramos, L.S.; da Silva, M.L.; Luz, A.I.R.; Zoghbi, M.G.B.; Maia, J.G.S. Essential Oil of Piper marginatum. J. Nat. Prod. 1986, 49, 712–713. [Google Scholar] [CrossRef]
- Hoff Brait, D.R.; Mattos Vaz, M.S.; da Silva Arrigo, J.; Borges de Carvalho, L.N.; Souza de Araújo, F.H.; Vani, J.M.; da Silva Mota, J.; Cardoso, C.A.L.; Oliveira, R.J.; Negrão, F.J.; et al. Toxicological analysis and anti-inflammatory effects of essential oil from Piper vicosanum leaves. Regul. Toxicol. Pharmacol. 2015, 73, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Papa, F.; Ricciutelli, M.; Cianfaglione, K.; Maggi, F. Isofuranodiene is the main volatile constituent of Smyrnium perfoliatum L. subsp. perfoliatum growing in central Italy. Nat. Prod. Res. 2016, 30, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Kaldre, D.; Gleason, J.L. An organocatalytic Cope rearrangement. Angew. Chem.-Int. Ed. 2016, 55, 11557–11561. [Google Scholar] [CrossRef]
- Setzer, W.N. Ab initio analysis of the Cope rearrangement of germacrane sesquiterpenoids. J. Mol. Model. 2008, 14, 335–342. [Google Scholar] [CrossRef]
- Benitez, N.P.; Meléndez León, E.M.; Stashenko, E.E. Essential oil composition from two species of Piperaceae family grown in Colombia. J. Chromatogr. Sci. 2009, 47, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.F.S.; Rocha, J.E.; Bezerra, C.F.; do Nascimento Silva, M.K.; de Matos, Y.M.L.S.; de Freitas, T.S.; dos Santos, A.T.L.; da Cruz, R.P.; Machado, A.J.T.; Rodrigues, T.H.S.; et al. Chemical composition, antifungal activity and potential anti-virulence evaluation of the Eugenia uniflora essential oil against Candida spp. Food Chem. 2018, 261, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, J.S.; Barroso, A.S.; Mourão, R.H.V.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Seasonal and antioxidant evaluation of essential oil from Eugenia uniflora L., curzerene-rich, thermally produced in situ. Biomolecules 2020, 10, 328. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, T.J.; Safeer, A.L.; Jayarajan, K.; Leela, N.K.; Vipin, T.M.; Saji, K.V.; Shiva, K.N.; Parthasarathy, V.A.; Mammootty, K.P. Correlation of metabolites in the leaf and berries of selected black pepper varieties. Sci. Hortic. (Amst.) 2010, 123, 418–422. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B.; Geissler, M. Aroma compound analysis of Piper nigrum and Piper guineense essential oils from Cameroon using solid-phase microextraction-gas chromatography, solid-phase microextraction-gas chromatography-mass spectrometry and olfactometry. J. Chromatogr. A 2002, 976, 265–275. [Google Scholar] [CrossRef]
- Abukawsar, M.M.; Saleh-e-In, M.M.; Ahsan, M.A.; Rahim, M.M.; Bhuiyan, M.N.H.; Roy, S.K.; Ghosh, A.; Naher, S. Chemical, pharmacological and nutritional quality assessment of black pepper (Piper nigrum L.) seed cultivars. J. Food Biochem. 2018, 42, e12590. [Google Scholar] [CrossRef]
- Menon, A.N.; Padmakumari, K.P. Studies on essential oil composition of cultivars of black pepper (Piper nigrum L.)—V. J. Essent. Oil Res. 2005, 17, 153–155. [Google Scholar] [CrossRef]
- François, T.; Michel, J.D.P.; Lambert, S.M.; Ndifor, F.; Vyry, W.N.A.; Henri, A.Z.P.; Chantal, M. Comparative essential oils composition and insecticidal effect of different tissues of Piper capense L., Piper guineense schum. et thonn., Piper nigrum L. and Piper umbellatum L. grown in Cameroon. Afr. J. Biotechnol. 2009, 8, 424–431. [Google Scholar] [CrossRef]
- Martins, A.P.; Salgueiro, L.; Vila, R.; Tomi, F.; Cañigueral, S.; Casanova, J.; Proença Da Cunha, A.; Adzet, T. Essential oils from four Piper species. Phytochemistry 1998, 49, 2019–2023. [Google Scholar] [CrossRef]
- Edelmann, A.; Diewok, J.; Schuster, K.C.; Lendl, B. Rapid method for the discrimination of red wine cultivars based on mid-infrared spectroscopy of phenolic wine extracts. J. Agric. Food Chem. 2001, 49, 1139–1145. [Google Scholar] [CrossRef]
- Cano, A.; Medina, A.; Bermejo, A. Bioactive compounds in different citrus varieties. Discrimination among cultivars. J. Food Compos. Anal. 2008, 21, 377–381. [Google Scholar] [CrossRef]
- Islam, M.A.; Ryu, K.Y.; Khan, N.; Song, O.Y.; Jeong, J.Y.; Son, J.H.; Jamila, N.; Kim, K.S. Determination of the Volatile Compounds in Five Varieties of Piper betle L. from Bangladesh Using Simultaneous Distillation Extraction and Gas Chromatography/Mass Spectrometry (SDE-GC/MS). Anal. Lett. 2020, 53, 2413–2430. [Google Scholar] [CrossRef]
- Hijaz, F.; Nehela, Y.; Killiny, N. Possible role of plant volatiles in tolerance against huanglongbing in citrus. Plant Signal. Behav. 2016, 11, e1138193. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.d.L.R.; De Albuquerque, F.C.; Poltronieri, M.C. Phytosanitary conditions of black pepper cultivars crop in Brazil. Int. Pepper News Bull. 2002, 1, 16–22. [Google Scholar]
- Poltronieri, M.C.; de Lemos, O.F. Pimenta-do-Reino: Cultivares; Embrapa Amazônia Oriental: Belém, Brazil, 2014. [Google Scholar]
- Cao, Y.; Fang, S.; Fu, X.; Shang, X.; Yang, W. Seasonal Variation in Phenolic Compounds and Antioxidant Activity in Leaves of Cyclocarya paliurus (Batal.) Iljinskaja. Forests 2019, 10, 624. [Google Scholar] [CrossRef]
- Gu, F.; Huang, F.; Wu, G.; Zhu, H. Contribution of polyphenol oxidation, chlorophyll and vitamin C degradation to the blackening of Piper nigrum L. Molecules 2018, 23, 370. [Google Scholar] [CrossRef] [PubMed]
- Ranilla, L.G.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol. 2010, 101, 4676–4689. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, D.; John Zachariah, T. In vitro antioxidant activity and cytotoxicity of sequential extracts from selected black pepper (Piper nigrum L.) varieties and Piper species. Int. Food Res. J. 2017, 24, 75–85. [Google Scholar]
- Tang, C.-S.; Cai, W.-F.; Kohl, K.; Nishimoto, R.K. Plant Stress and Allelopathy. In Allelopathy; Inderjit, Dakshini, K.M.M., Einhellig, F.A., Eds.; American Chemical Society: Washington, DC, USA, 1994; pp. 142–157. ISBN 9780841230613. [Google Scholar]
- Misharina, T.A. Antiradical properties of essential oils and extracts from coriander, cardamom, white, red, and black peppers. Appl. Biochem. Microbiol. 2016, 52, 79–86. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoid oxidation products as stress signals in plants. Plant J. 2013, 79, 597–606. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Puthur, J.T. Drought stress responses in tolerant and sensitive varieties of black pepper (Piper nigrum L.). J. Plant. Crop. 2014, 42, 78–85. [Google Scholar]
- Muthalagu, A.; Ankegowda, S.J.; Peeran, M.F.; Jagannath, H.; Akshitha, G.; Rajkumar, B.; Chaudhary, N. Effect of Natural Growth Enhancer on Growth, Physiological and Biochemical Attributes in Black Pepper (Piper nigrum L.). Int. J. Curr. Microbiol. App. Sci. 2018, Special Issue 6, 2857–2866. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Yrjönen, T.; Vuorela, H.; Holm, Y. Bioactive compounds from medicinal plants: Focus on Piper species. S. Afr. J. Bot. 2017, 112, 54–69. [Google Scholar] [CrossRef]
- Niu, M.; Xu, X.; Shen, Y.; Yao, Y.; Qiao, J.; Zhu, F.; Zeng, L.; Liu, X.; Xu, K. Piperlongumine is a novel nuclear export inhibitor with potent anticancer activity. Chem. Biol. Interact. 2015, 237, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, G.; Iqbal, A.; Mahboob, A.; M. Farhat, D.; Ahmed, T. Memory Enhancing Effect of Black Pepper in the AlCl3 Induced Neurotoxicity Mouse Model is Mediated through Its Active Component Chavicine. Curr. Pharm. Biotechnol. 2016, 17, 962–973. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic Compounds; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128132784. [Google Scholar]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid biosynthetic pathways in plants: Versatile targets for metabolic engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef]
- Yang, L.; Han, Y.; Li, P.; Li, F.; Ali, S.; Hou, M. Silicon amendment is involved in the induction of plant defense responses to a phloem feeder. Sci. Rep. 2017, 7, 4232. [Google Scholar] [CrossRef]
- Anh, T.T.; Linh, T.B.; Phong, N.V.; Bien, T.L.T.; Tram, T.H.N.; Don, L.D. Expression of Proteins Related to Phytophthora capsici Tolerance in Black Pepper (Piper nigrum L.). Int. J. Agric. Innov. Res. 2018, 6, 75–79. [Google Scholar]
- Shobha, M.S.; Lakshmi Devi, N.; Mahadeva Murthy, S. Induction of Systemic Resistance by Rhizobacterial and Endophytic Fungi against Foot Rot Disease of Piper nigrum L. by Increasing Enzyme Defense Activity. Int. J. Environ. Agric. Biotechnol. 2019, 4, 86–98. [Google Scholar] [CrossRef][Green Version]
- Sulzenbacher, G.; Schülein, M.; Davies, G.J. Structure of the endoglucanase I from Fusarium oxysporum: Native, cellobiose, and 3,4-epoxybutyl β-D-cellobioside-inhibited forms, at 2.3 Å resolution. Biochemistry 1997, 36, 5902–5911. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Dec. Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds, 3rd ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-1-119-06984-3. [Google Scholar]
- Sousa, C.M.D.M.; Silva, H.R.E.; Vieira, G.M.; Ayres, M.C.C.; Da Costa, C.L.S.; Araújo, D.S.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.D.M.; Brandão, M.S.; et al. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim. Nova 2007, 30, 351–355. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. In Handbook of Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., Sporns, P., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 2005; pp. F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Miazek, K.; Ledakowicz, S. Chlorophyll extraction from leaves, needles and microalgae: A kinetic approach. Int. J. Agric. Biol. Eng. 2013, 6, 107–115. [Google Scholar] [CrossRef]
- Dickerson, D.P.; Pascholati, S.F.; Hagerman, A.E.; Butler, L.G.; Nicholson, R.L. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol. Plant Pathol. 1984, 25, 111–123. [Google Scholar] [CrossRef]
- Vaganan, M.M.; Ravi, I.; Nandakumar, A.; Sarumathi, S.; Sundararaju, P.; Mustaffa, M.M. Phenylpropanoid enzymes, phenolic polymers and metabolites as chemical defenses to infection of Pratylenchus coffeae in roots of resistant and susceptible bananas (Musa spp.). Indian J. Exp. Biol. 2014, 52, 252–260. [Google Scholar] [PubMed]
- Gerber, P.R.; Müller, K. MAB, a generally applicable molecular force field for structure modelling in medicinal chemistry. J. Comput. Aided. Mol. Des. 1995, 9, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cieplak, P.; Kollman, P.A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2000, 21, 1049–1074. [Google Scholar] [CrossRef]
- Case, D.A.; Darden, T.A.; Cheatham, T.E.; Simmerling, C.L.; Wang, J.; Duke, R.E.; Luo, R.; Crowley, M.; Walker, R.C.; Zhang, W.; et al. Amber 10; University of California: San Francisco, CA, USA, 2008. [Google Scholar]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
 Susceptible;
Susceptible;  Medium tolerance;
Medium tolerance;  Tolerant.
Tolerant.
 Susceptible;
Susceptible;  Medium tolerance;
Medium tolerance;  Tolerant.
Tolerant.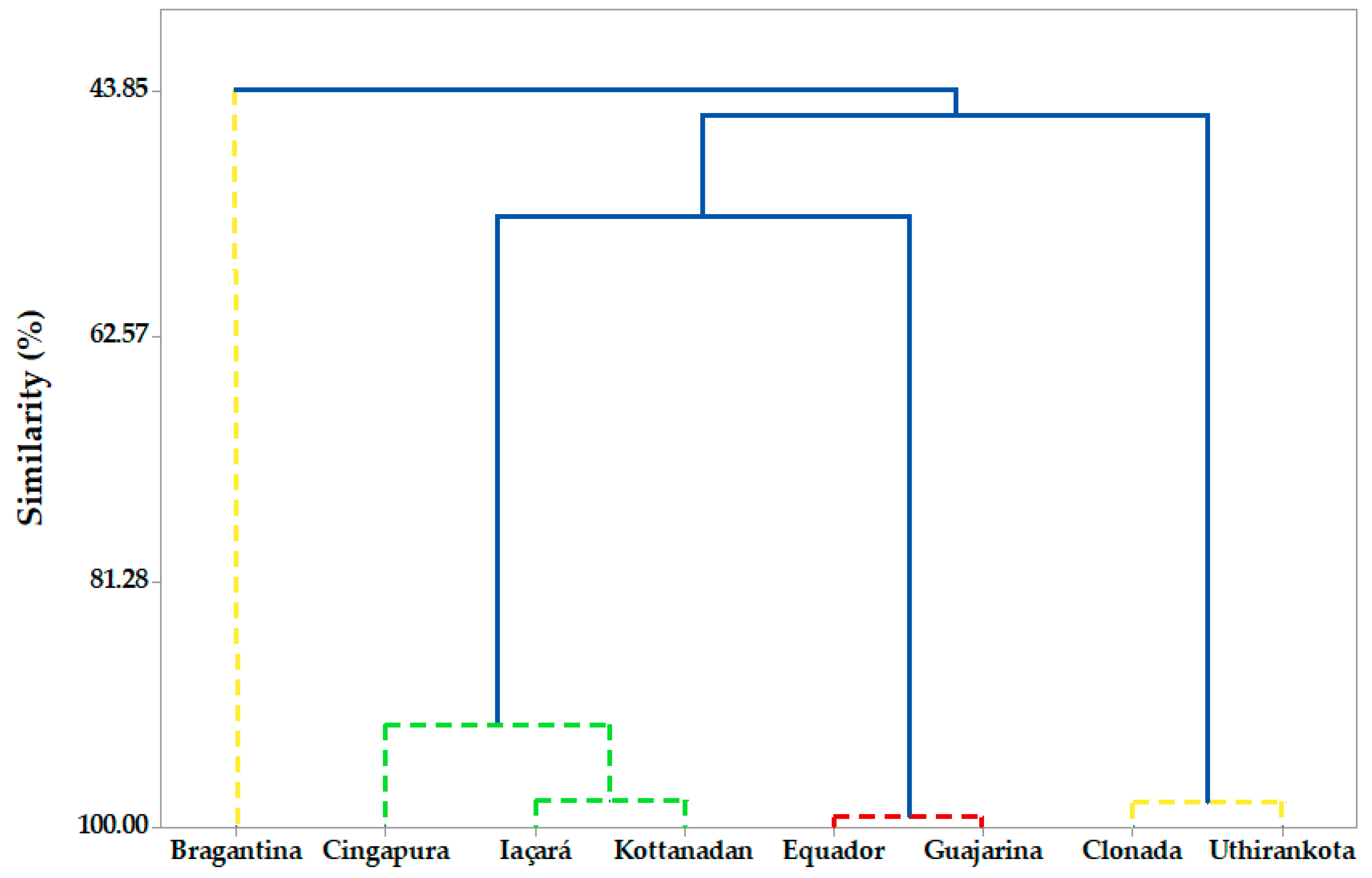
| Target | GenBank Accession Number | PDB Entry | Sequence Identity | Expectation Value |
|---|---|---|---|---|
| F. solani ornithine decarboxylase | ABC47117.1 | 4ZGY | 51.23% | 4 × 10−108 |
| F. oxysporum glucosamine fructose-6-phosphate aminotransferase | EWY94476 | 6R4E | 61.11% | 0.0 |
| F. odoratissimum NRRL 54006 β-glucosidase | XP_031065848 | 5NBS | 65.47% | 0.0 |
| F. vanettenii thiamine thiazole synthase | C7Z8P6.1 | 3JSK | 77.4% | 1 × 10−167 |
| F. oxysporum guanine nucleotide-binding protein, beta subunit | RKL24731 | 6CMO | 66.57% | 2 × 10−165 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barata, L.M.; Andrade, E.H.; Ramos, A.R.; de Lemos, O.F.; Setzer, W.N.; Byler, K.G.; Maia, J.G.S.; da Silva, J.K.R. Secondary Metabolic Profile as a Tool for Distinction and Characterization of Cultivars of Black Pepper (Piper nigrum L.) Cultivated in Pará State, Brazil. Int. J. Mol. Sci. 2021, 22, 890. https://doi.org/10.3390/ijms22020890
Barata LM, Andrade EH, Ramos AR, de Lemos OF, Setzer WN, Byler KG, Maia JGS, da Silva JKR. Secondary Metabolic Profile as a Tool for Distinction and Characterization of Cultivars of Black Pepper (Piper nigrum L.) Cultivated in Pará State, Brazil. International Journal of Molecular Sciences. 2021; 22(2):890. https://doi.org/10.3390/ijms22020890
Chicago/Turabian StyleBarata, Luccas M., Eloísa H. Andrade, Alessandra R. Ramos, Oriel F. de Lemos, William N. Setzer, Kendall G. Byler, José Guilherme S. Maia, and Joyce Kelly R. da Silva. 2021. "Secondary Metabolic Profile as a Tool for Distinction and Characterization of Cultivars of Black Pepper (Piper nigrum L.) Cultivated in Pará State, Brazil" International Journal of Molecular Sciences 22, no. 2: 890. https://doi.org/10.3390/ijms22020890
APA StyleBarata, L. M., Andrade, E. H., Ramos, A. R., de Lemos, O. F., Setzer, W. N., Byler, K. G., Maia, J. G. S., & da Silva, J. K. R. (2021). Secondary Metabolic Profile as a Tool for Distinction and Characterization of Cultivars of Black Pepper (Piper nigrum L.) Cultivated in Pará State, Brazil. International Journal of Molecular Sciences, 22(2), 890. https://doi.org/10.3390/ijms22020890