Tailoring Pyro- and Orthophosphate Species to Enhance Stem Cell Adhesion to Phosphate Glasses
Abstract
1. Introduction
2. Results
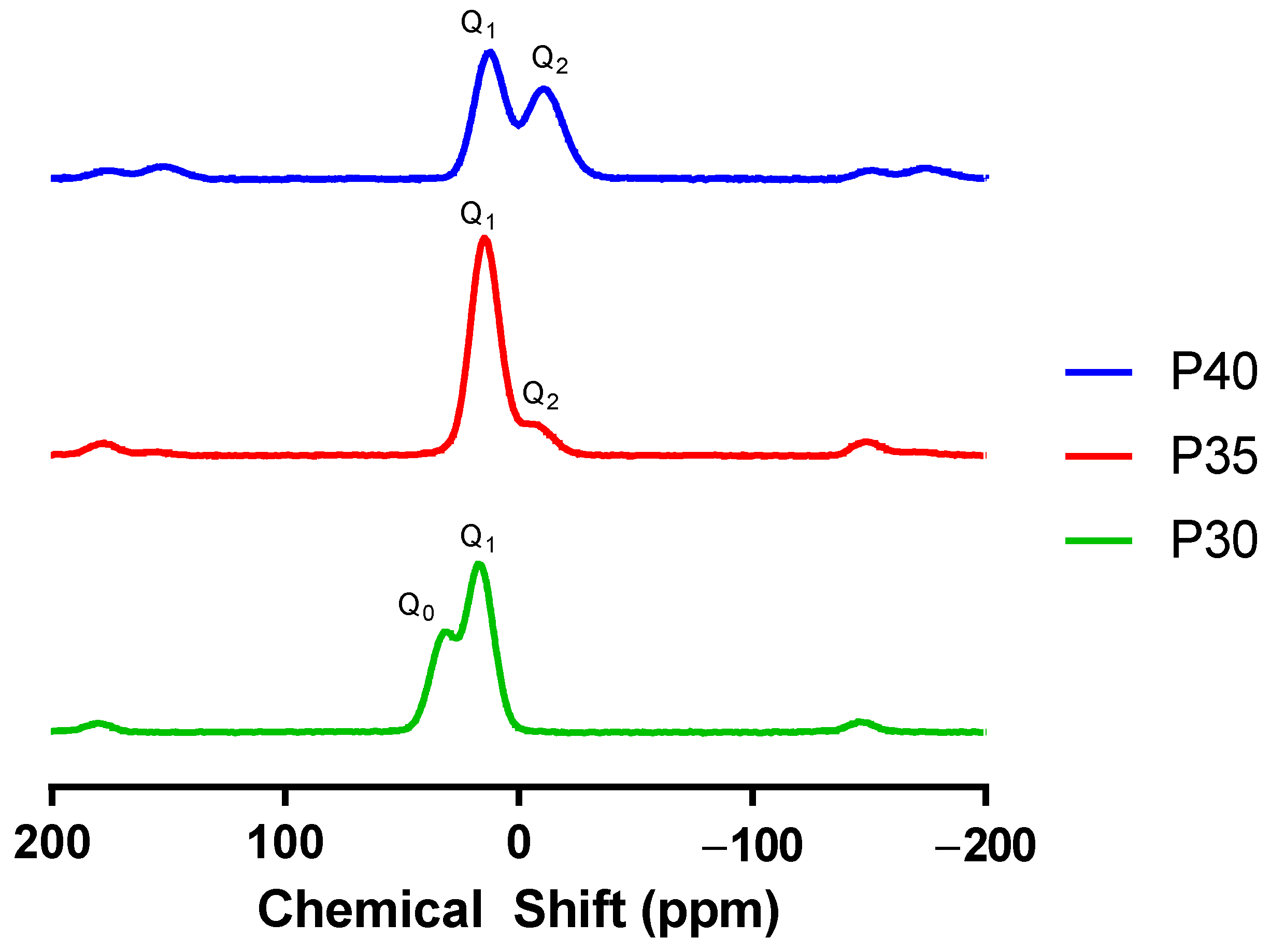
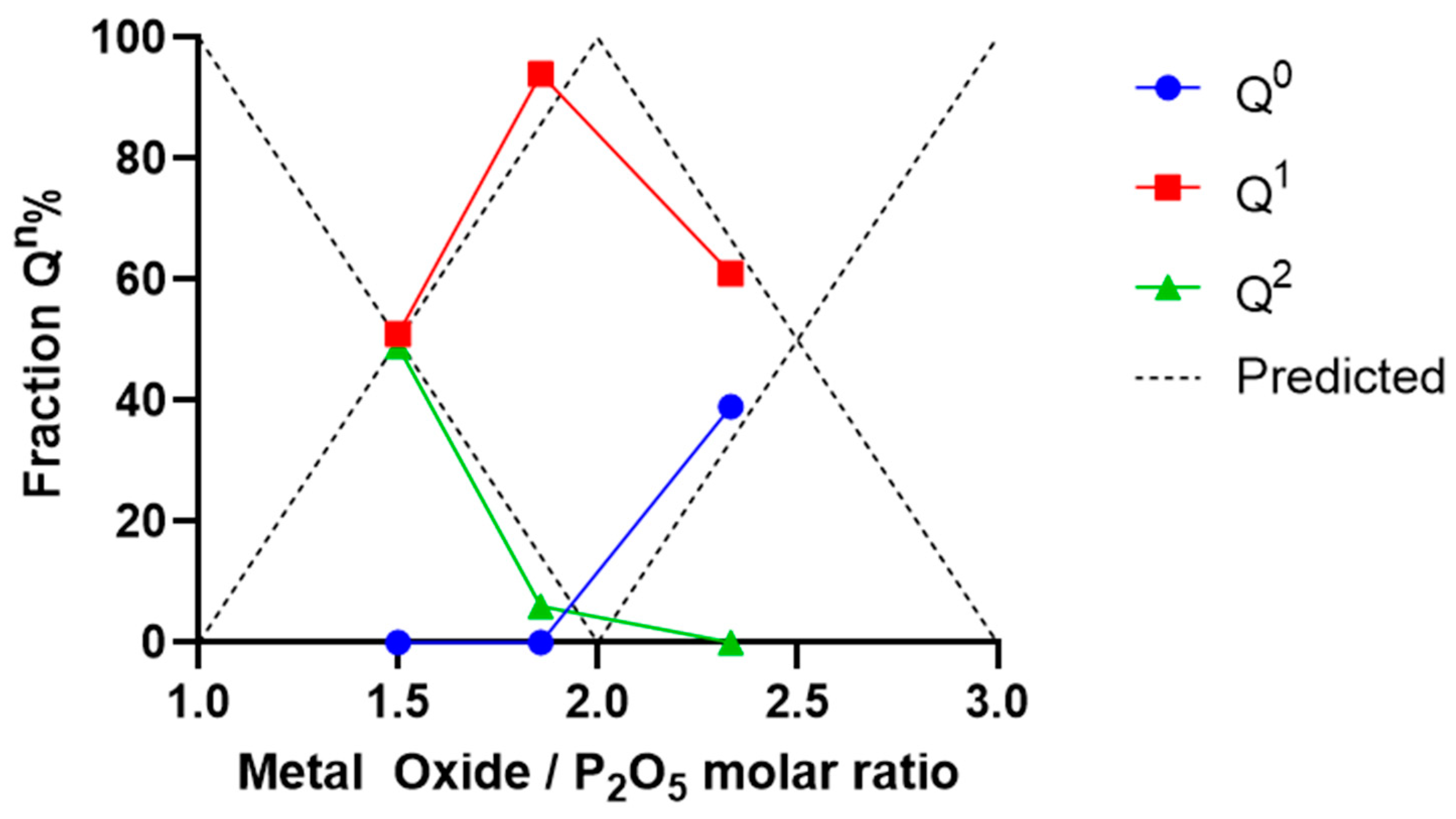
2.1. Glass Characterisation
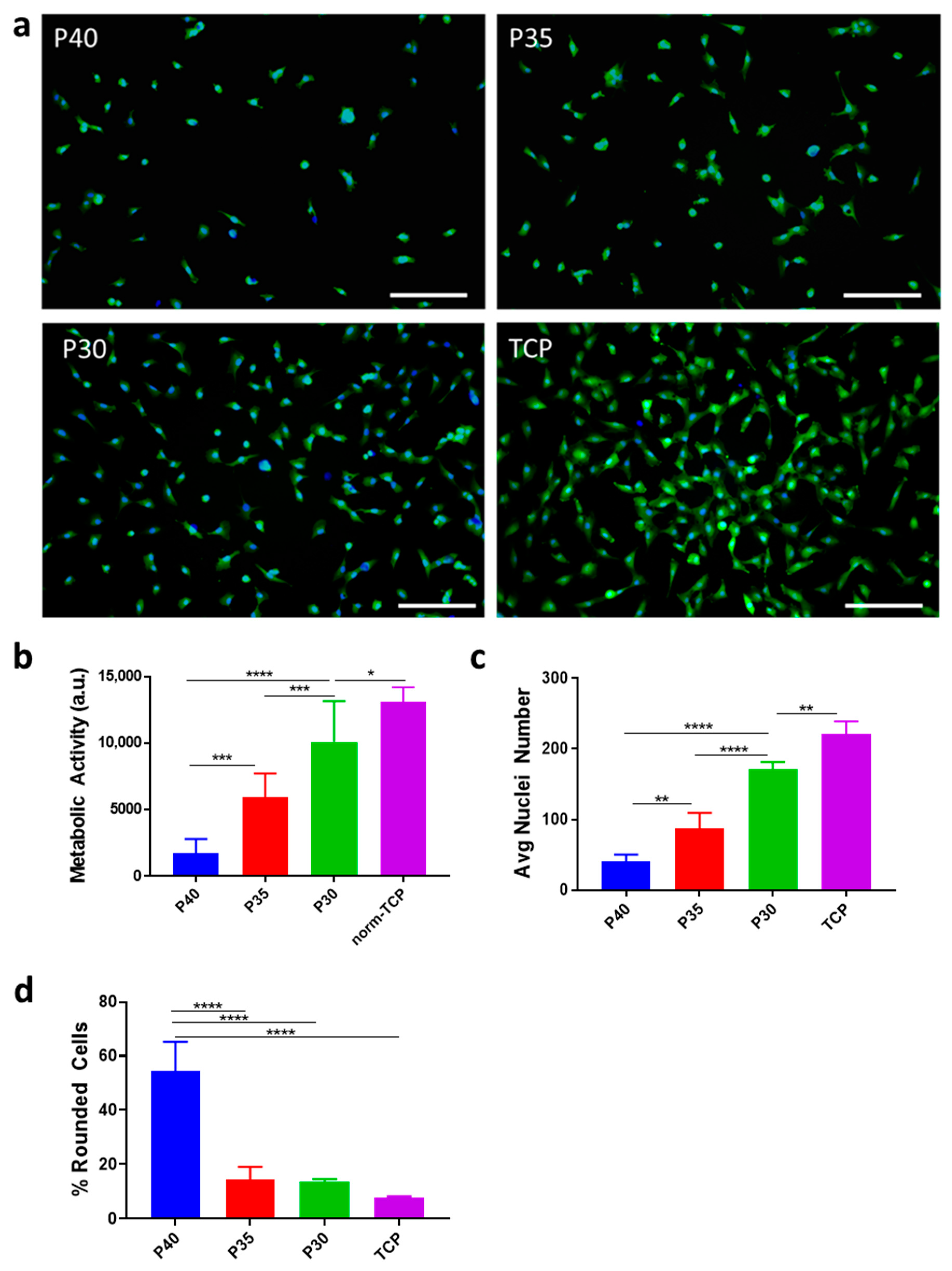
2.2. Cell Adhesion
3. Discussion
3.1. Material Characterisation
3.2. Cell Adhesion
3.3. Impact beyond Cell Adhesion
4. Materials and Methods
4.1. PBG Production
4.2. Energy-Dispersive X-ray Spectroscopy
4.3. Thermal Analysis
4.4. X-ray Powder Diffraction
4.5. Nuclear Magnetic Resonance
4.6. Cell Culture
4.7. Metabolic Activity Assay
4.8. Nuclear Staining and Imaging
4.9. Statistical Analysis
5. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| CaSR | Calcium-sensing receptor |
| DSC | Differential scanning calorimeter |
| FCS | Foetal calf serum |
| GFP | Green fluorescence protein |
| HA | Hydroxyapatite |
| MSC | Mesenchymal stem cell |
| NMR | Nuclear magnetic resonance |
| PBG | Phosphate based glass |
| PPi | Inorganic pyrophosphate |
| SEM | Standard error of the mean |
| Tc | Glass crystallisation temperature |
| TCP | Tissue culture plastic |
| Tg | Glass transition temperature |
| Tm | Glass melting temperature |
| TNAP | tissue non-specific alkaline phosphatases |
| XRD | X-ray powder diffraction |
References
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered Biomaterials for in Situ Tissue Regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Fiorillo, L.; D’Amico, C.; Turkina, A.Y.; Nicita, F.; Amoroso, G.; Risitano, G. Endo and Exoskeleton: New Technologies on Composite Materials. Prosthesis 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Delfi, M.; Ghomi, M.; Zarrabi, A.; Mohammadinejad, R.; Taraghdari, Z.B.; Ashrafizadeh, M.; Zare, E.N.; Agarwal, T.; Padil, V.V.T.; Mokhtari, B.; et al. Functionalization of Polymers and Nanomaterials for Biomedical Applications: Antimicrobial Platforms and Drug Carriers. Prosthesis 2020, 2, 117–139. [Google Scholar] [CrossRef]
- Varanko, A.; Saha, S.; Chilkoti, A. Recent Trends in Protein and Peptide-Based Biomaterials for Advanced Drug Delivery. Adv. Drug Deliv. Rev. 2020, 156, 133–187. [Google Scholar] [CrossRef]
- Kundu, R.; Payal, P. Antimicrobial Hydrogels: Promising Soft Biomaterials. ChemistrySelect 2020, 5, 14800–14810. [Google Scholar] [CrossRef]
- Navarro, M.; Ginebra, M.-P.; Planell, J.A. Cellular Response to Calcium Phosphate Glasses with Controlled Solubility. J. Biomed. Mater. Res. A 2003, 67, 1009–1015. [Google Scholar] [CrossRef]
- Lapa, A.; Cresswell, M.; Jackson, P.; Boccaccini, A.R. Phosphate Glass Fibres with Therapeutic Ions Release Capability—A Review. Adv. Appl. Ceram. 2019, 119, 1–14. [Google Scholar] [CrossRef]
- Zachariasen, W.H. The Atomic Arrangement in Glass. J. Am. Chem. Soc. 1932, 54, 3841–3851. [Google Scholar] [CrossRef]
- Brow, R.K. Review: The Structure of Simple Phosphate Glasses. J. Non-Cryst. Solids 2000, 263–264, 1–28. [Google Scholar] [CrossRef]
- Kaseman, D.C.; Retsinas, A.; Kalampounias, A.G.; Papatheodorou, G.N.; Sen, S. Q-Speciation and Network Structure Evolution in Invert Calcium Silicate Glasses. J. Phys. Chem. B 2015, 119, 8440–8445. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Pickup, D.M.; Valappil, S.P.; Newport, R.J.; Knowles, J.C. Bioactive Functional Materials: A Perspective on Phosphate-Based Glasses. J. Mater. Chem. 2009, 19, 690–701. [Google Scholar] [CrossRef]
- Rao, K.J. Structural Chemistry of Glasses; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Walter, G.; Vogel, J.; Hoppe, U.; Hartmann, P. The Structure of CaO–Na2O–MgO–P2O5 Invert Glass. J. Non-Cryst. Solids 2001, 296, 212–223. [Google Scholar] [CrossRef]
- Lee, S.; Maeda, H.; Obata, A.; Ueda, K.; Narushima, T.; Kasuga, T. Structure and Dissolution Behavior of MgO–P2O5–TiO2/Nb2O5 (Mg/P ≥ 1) Invert Glasses. J. Ceram. Soc. Jpn. 2015, 123, 942–948. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.N.; Azari, F.; Sørensen, E.S.; Kaartinen, M.T.; McKee, M.D. Pyrophosphate Inhibits Mineralization of Osteoblast Cultures by Binding to Mineral, Up-Regulating Osteopontin, and Inhibiting Alkaline Phosphatase Activity. J. Biol. Chem. 2007, 282, 15872–15883. [Google Scholar] [CrossRef]
- Pujari-Palmer, M.; Pujari-Palmer, S.; Lu, X.; Lind, T.; Melhus, H.; Engstrand, T.; Karlsson-Ott, M.; Engqvist, H. Pyrophosphate Stimulates Differentiation, Matrix Gene Expression and Alkaline Phosphatase Activity in Osteoblasts. PLoS ONE 2016, 11, e0163530. [Google Scholar] [CrossRef]
- Golub, E.E.; Boesze-Battaglia, K. The Role of Alkaline Phosphatase in Mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Lakhkar, N.J.; Lee, I.-H.; Kim, H.-W.; Salih, V.; Wall, I.B.; Knowles, J.C. Bone Formation Controlled by Biologically Relevant Inorganic Ions: Role and Controlled Delivery from Phosphate-Based Glasses. Adv. Drug Deliv. Rev. 2013, 65, 405–420. [Google Scholar] [CrossRef]
- Grover, L.M.; Wright, A.J.; Gbureck, U.; Bolarinwa, A.; Song, J.; Liu, Y.; Farrar, D.F.; Howling, G.; Rose, J.; Barralet, J.E. The Effect of Amorphous Pyrophosphate on Calcium Phosphate Cement Resorption and Bone Generation. Biomaterials 2013, 34, 6631–6637. [Google Scholar] [CrossRef]
- Murphy, W.L.; McDevitt, T.C.; Engler, A.J. Materials as Stem Cell Regulators. Nat. Mater. 2014, 13, 547–557. [Google Scholar] [CrossRef]
- Pi, M.; Faber, P.; Ekema, G.; Jackson, P.D.; Ting, A.; Wang, N.; Fontilla-Poole, M.; Mays, R.W.; Brunden, K.R.; Harrington, J.J.; et al. Identification of a Novel Extracellular Cation-Sensing G-Protein-Coupled Receptor. J. Biol. Chem. 2005, 280, 40201–40209. [Google Scholar] [CrossRef] [PubMed]
- Tharmalingam, S.; Hampson, D.R. The Calcium-Sensing Receptor and Integrins in Cellular Differentiation and Migration. Front. Physiol. 2016, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.E. Structure and Mechanism of Alkaline Phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1992, 21, 441–483. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.M.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High Concentrations of Magnesium Modulate Vascular Endothelial Cell Behaviour in Vitro. Biochim. Biophys. Acta—Mol. Basis Dis. 2004, 1689, 6–12. [Google Scholar] [CrossRef]
- Matsunaga, T.; Weihrauch, D.W.; Moniz, M.C.; Tessmer, J.; Warltier, D.C.; Chilian, W.M. Angiostatin Inhibits Coronary Angiogenesis During Impaired Production of Nitric Oxide. Circulation 2002, 105, 2185–2191. [Google Scholar] [CrossRef]
- Patel, U.; Macri-Pellizzeri, L.; Zakir Hossain, K.M.; Scammell, B.E.; Grant, D.M.; Scotchford, C.A.; Hannon, A.C.; Kennedy, A.R.; Barney, E.R.; Ahmed, I.; et al. In Vitro Cellular Testing of Strontium/Calcium Substituted Phosphate Glass Discs and Microspheres Shows Potential for Bone Regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 396–405. [Google Scholar] [CrossRef]
- Alqaysi, M.; Aldaadaa, A.; Mordan, N.; Shah, R.; Knowles, J.C. Zinc and Strontium Based Phosphate Glass Beads: A Novel Material for Bone Tissue Engineering. Biomed. Mater. 2017, 12, 065011. [Google Scholar] [CrossRef]
- Lee, M.-J.; Seo, Y.-B.; Seo, J.-Y.; Ryu, J.-H.; Ahn, H.-J.; Kim, K.-M.; Kwon, J.-S.; Choi, S.-H. Development of a Bioactive Flowable Resin Composite Containing a Zinc-Doped Phosphate-Based Glass. Nanomaterials 2020, 10, 2311. [Google Scholar] [CrossRef]
- Lin, X.; Chen, Q.; Xiao, Y.; Gao, Y.; Ahmed, I.; Li, M.; Li, H.; Zhang, K.; Qiu, W.; Liu, X.; et al. Phosphate Glass Fibers Facilitate Proliferation and Osteogenesis through Runx2 Transcription in Murine Osteoblastic Cells. J. Biomed. Mater. Res. Part A 2020, 108, 316–326. [Google Scholar] [CrossRef]
- Foroutan, F.; Nikolaou, A.; Kyffin, B.A.; Elliott, R.M.; Felipe-Sotelo, M.; Gutierrez-Merino, J.; Carta, D. Multifunctional Phosphate-Based Glass Fibres Prepared via Electrospinning of Coacervate Precursors: Controlled Delivery, Biocompatibility and Antibacterial Activity. Materialia 2020, 14, 100939. [Google Scholar] [CrossRef]
- Sghayyar, H.N.M.; Lim, S.S.; Ahmed, I.; Lai, J.Y.; Cheong, X.Y.; Chong, Z.W.; Lim, A.F.X.; Loh, H.-S. Fish Biowaste Gelatin Coated Phosphate-Glass Fibres for Wound-Healing Application. Eur. Polym. J. 2020, 122, 109386. [Google Scholar] [CrossRef]
- Ahmed, I.; Ready, D.; Wilson, M.; Knowles, J.C. Antimicrobial Effect of Silver-Doped Phosphate-Based Glasses. J. Biomed. Mater. Res. Part A 2006, 79, 618–626. [Google Scholar] [CrossRef]
- McLaren, J.S.; Macri-Pellizzeri, L.; Hossain, K.M.Z.; Patel, U.; Grant, D.M.; Scammell, B.E.; Ahmed, I.; Sottile, V. Porous Phosphate-Based Glass Microspheres Show Biocompatibility, Tissue Infiltration, and Osteogenic Onset in an Ovine Bone Defect Model. ACS Appl. Mater. Interfaces 2019, 11, 15436–15446. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Schlesinger, P.H.; Huang, C.L.-H.; Zaidi, M. Calcium signalling and calcium transport in bone disease. Subcell. Biochem. 2007, 45, 539–562. [Google Scholar] [PubMed]
- Matamoros-Veloza, A.; Hossain, K.M.Z.; Scammell, B.E.; Ahmed, I.; Hall, R.; Kapur, N. Formulating Injectable Pastes of Porous Calcium Phosphate Glass Microspheres for Bone Regeneration Applications. J. Mech. Behav. Biomed. Mater. 2020, 102, 103489. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Zhang, Y.; Yeh, Y.-C.; Wooten, A.L.; Xia, Y. Biodegradable Porous Beads and Their Potential Applications in Regenerative Medicine. J. Mater. Chem. 2012, 22, 11442–11451. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Patel, U.; Ahmed, I. Development of Microspheres for Biomedical Applications: A Review. Prog. Biomater. 2015, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.Y.; Ding, J.Y.; Lee, S.Y. 31P MAS-NMR and FTIR Analyses on the Structure of CuO-Containing Sodium Poly- and Meta-Phosphate Glasses. Mater. Chem. Phys. 2003, 80, 391–396. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H.; Sakurai, Y. Mechanism of Cell Detachment from Temperature-Modulated, Hydrophilic-Hydrophobic Polymer Surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Patel, U.; Kennedy, A.R.; Macri-Pellizzeri, L.; Sottile, V.; Grant, D.M.; Scammell, B.E.; Ahmed, I. Porous Calcium Phosphate Glass Microspheres for Orthobiologic Applications. Acta Biomater. 2018, 72, 396–406. [Google Scholar] [CrossRef]
- Hum, J.; Boccaccini, A.R. Collagen as Coating Material for 45S5 Bioactive Glass-Based Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1807. [Google Scholar] [CrossRef] [PubMed]
- Skelton, K.L.; Glenn, J.V.; Clarke, S.A.; Georgiou, G.; Valappil, S.P.; Knowles, J.C.; Nazhat, S.N.; Jordan, G.R. Effect of Ternary Phosphate-Based Glass Compositions on Osteoblast and Osteoblast-like Proliferation, Differentiation and Death in Vitro. Acta Biomater. 2007, 3, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H. The Mechanism of Mineralization and the Role of Alkaline Phosphatase in Health and Disease. J. Nippon. Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Orriss, I.R.; Arnett, T.R.; Russell, R.G.G. Pyrophosphate: A Key Inhibitor of Mineralisation. Curr. Opin. Pharmacol. 2016, 28, 57–68. [Google Scholar] [CrossRef]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millan, J.L. Tissue-Nonspecific Alkaline Phosphatase and Plasma Cell Membrane Glycoprotein-1 Are Central Antagonistic Regulators of Bone Mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef]
- Patel, U.; Moss, R.M.; Hossain, K.M.Z.; Kennedy, A.R.; Barney, E.R.; Ahmed, I.; Hannon, A.C. Structural and Physico-Chemical Analysis of Calcium/Strontium Substituted, near-Invert Phosphate Based Glasses for Biomedical Applications. Acta Biomater. 2017, 60, 109–127. [Google Scholar] [CrossRef]
- Brauer, D.S.; Rüssel, C.; Li, W.; Habelitz, S. Effect of Degradation Rates of Resorbable Phosphate Invert Glasses on in Vitro Osteoblast Proliferation. J. Biomed. Mater. Res. A 2006, 77, 213–219. [Google Scholar] [CrossRef]
- Brauer, D.S. Bioactive Glasses—Structure and Properties. Angew. Chem. Int. Ed. Engl. 2015, 54, 4160–4181. [Google Scholar] [CrossRef]
- Ahmed, I.; Lewis, M.; Olsen, I.; Knowles, J.C. Phosphate Glasses for Tissue Engineering: Part 1. Processing and Characterisation of a Ternary-Based P2O5–CaO–Na2O Glass System. Biomaterials 2004, 25, 491–499. [Google Scholar] [CrossRef]
- Okura, T.; Miyachi, T.; Monma, H. Properties and Vibrational Spectra of Magnesium Phosphate Glasses for Nuclear Waste Immobilization. J. Eur. Ceram. Soc. 2006, 26, 831–836. [Google Scholar] [CrossRef]
- Uo, M.; Mizuno, M.; Kuboki, Y.; Makishima, A.; Watari, F. Properties and Cytotoxicity of Water Soluble Na2O–CaO–P2O5 Glasses. Biomaterials 1998, 19, 2277–2284. [Google Scholar] [CrossRef]
- Franks, K.; Abrahams, I.; Georgiou, G.; Knowles, J.C. Investigation of Thermal Parameters and Crytallisation in a Ternary CaO–Na2O–P2O5-Based Glass System. Biomaterials 2001, 22, 497–501. [Google Scholar] [CrossRef]
- Ahmed, I.; Parsons, A.J.; Palmer, G.; Knowles, J.C.; Walker, G.S.; Rudd, C.D. Weight Loss, Ion Release and Initial Mechanical Properties of a Binary Calcium Phosphate Glass Fibre/PCL Composite. Acta Biomater. 2008, 4, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, T.; Abe, Y. Calcium Phosphate Invert Glasses with Soda and Titania. J. Non-Cryst. Solids 1999, 243, 70–74. [Google Scholar] [CrossRef]
- Wazer, J.; Fluck, E. Principles of Phosphorus Chemistry. VI. Reorganization of Quadruply Connected Monophosphorus Compounds. Part B. The Chlorophosphoric Acids. J. Am. Chem. Soc. 1959, 81, 6360–6363. [Google Scholar] [CrossRef]
- Tharmalingam, S.; Daulat, A.M.; Antflick, J.E.; Ahmed, S.M.; Nemeth, E.F.; Angers, S.; Conigrave, A.D.; Hampson, D.R. Calcium-Sensing Receptor Modulates Cell Adhesion and Migration via Integrins. J. Biol. Chem. 2011, 286, 40922–40933. [Google Scholar] [CrossRef]
- Xiong, J.-P.; Stehle, T.; Zhang, R.; Joachimiak, A.; Frech, M.; Goodman, S.L.; Arnaout, M.A. Crystal Structure of the Extracellular Segment of Integrin ΑVβ3 in Complex with an Arg-Gly-Asp Ligand. Science 2002, 296, 151–155. [Google Scholar] [CrossRef]
- Mould, A.P.; Akiyama, S.K.; Humphries, M.J. Regulation of Integrin Α5β1-Fibronectin Interactions by Divalent Cations evidence for distinct classes of binding sites for Mn2+, Mg2+, AND Ca2+. J. Biol. Chem. 1995, 270, 26270–26277. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Mizoguchi, T.; Ito, M.; Bitar, M.; Salih, V.; Knowles, J.C. In Vitro Bioactivity and Gene Expression by Cells Cultured on Titanium Dioxide Doped Phosphate-Based Glasses. Biomaterials 2007, 28, 2967–2977. [Google Scholar] [CrossRef]
- Bitar, M.; Salih, V.; Mudera, V.; Knowles, J.C.; Lewis, M.P. Soluble Phosphate Glasses: In Vitro Studies Using Human Cells of Hard and Soft Tissue Origin. Biomaterials 2004, 25, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, T.; Hosoi, Y.; Nogami, M.; Niinomi, M. Apatite Formation on Calcium Phosphate Invert Glasses in Simulated Body Fluid. J. Am. Ceram. Soc. 2001, 84, 450–452. [Google Scholar] [CrossRef]
- Tošić, M.; Nikolić, J.; Grujić, S.; Zivanovic, V.; Zildžović, S.N.; Matijašević, S.D.; Smiljanić, S. Dissolution Behavior of a Polyphosphate Glass into an Aqueous Solution under Static Leaching Conditions. J. Non-Cryst. Solids 2013, 362, 185–194. [Google Scholar] [CrossRef]
- Islam, M.T.; Felfel, R.M.; Neel, E.A.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive Calcium Phosphate–Based Glasses and Ceramics and Their Biomedical Applications: A Review. J. Tissue Eng. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-S.; Huang, Y.-C.; Lin, F.-H.; Chen, L.-T. The Effect of Sintered Dicalcium Pyrophosphate on Osteoclast Metabolism: An Ultrastructural Study. J. Biomed. Mater. Res. A 2003, 64, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Functionalized Calcium Orthophosphates (CaPO4) and Their Biomedical Applications. J. Mater. Chem. B 2019, 7, 7471–7489. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphate-Containing Biocomposites and Hybrid Biomaterials for Biomedical Applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef]

| P2O5 (mol%) | CaO (mol%) | MgO (mol%) | NaO (mol%) | ||
|---|---|---|---|---|---|
| P40 | Target | 40 | 16 | 24 | 20 |
| Measured | 39.4 ± 0.7 | 16.6 ± 0.6 | 24.0 ± 0.6 | 20.0 ± 0.7 | |
| P35 | Target | 35 | 21 | 24 | 20 |
| Measured | 34.0 ± 0.4 | 25.5 ± 1.7 | 21.9 ± 1.0 | 18.7 ± 0.7 | |
| P30 | Target | 30 | 26 | 24 | 20 |
| Measured | 28.2 ± 0.3 | 24.3 ± 0.7 | 26.0 ± 0.5 | 21.6 ± 0.5 |
| Tg Onset | Tc Onset | Tc 1 | Tc 2 | Tm 1 | Tm 2 | Window Tconset − Tg | |
|---|---|---|---|---|---|---|---|
| P40 | 445 | 563 | 592 | 761 | 838 | 118 | |
| P35 | 476 | 533 | 557 | 637 | 805 | 853 | 57 |
| P30 | 485 | 553 | 578 | 654 | 790 | 825 | 68 |
| Q0 | Q1 | Q2 | |
|---|---|---|---|
| P40 | 0 | 51 | 49 |
| P35 | 0 | 94 | 6 |
| P30 | 39 | 61 | 0 |
| Glass Code | P (mol%) | Ca (mol%) | Mg (mol%) | Na (mol%) |
|---|---|---|---|---|
| P40 | 40 | 16 | 24 | 20 |
| P35 | 35 | 21 | 24 | 20 |
| P30 | 30 | 26 | 24 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Melo, N.; Murrell, L.; Islam, M.T.; Titman, J.J.; Macri-Pellizzeri, L.; Ahmed, I.; Sottile, V. Tailoring Pyro- and Orthophosphate Species to Enhance Stem Cell Adhesion to Phosphate Glasses. Int. J. Mol. Sci. 2021, 22, 837. https://doi.org/10.3390/ijms22020837
De Melo N, Murrell L, Islam MT, Titman JJ, Macri-Pellizzeri L, Ahmed I, Sottile V. Tailoring Pyro- and Orthophosphate Species to Enhance Stem Cell Adhesion to Phosphate Glasses. International Journal of Molecular Sciences. 2021; 22(2):837. https://doi.org/10.3390/ijms22020837
Chicago/Turabian StyleDe Melo, Nigel, Lauren Murrell, Md Towhidul Islam, Jeremy J. Titman, Laura Macri-Pellizzeri, Ifty Ahmed, and Virginie Sottile. 2021. "Tailoring Pyro- and Orthophosphate Species to Enhance Stem Cell Adhesion to Phosphate Glasses" International Journal of Molecular Sciences 22, no. 2: 837. https://doi.org/10.3390/ijms22020837
APA StyleDe Melo, N., Murrell, L., Islam, M. T., Titman, J. J., Macri-Pellizzeri, L., Ahmed, I., & Sottile, V. (2021). Tailoring Pyro- and Orthophosphate Species to Enhance Stem Cell Adhesion to Phosphate Glasses. International Journal of Molecular Sciences, 22(2), 837. https://doi.org/10.3390/ijms22020837





