Targeting WASF3 Signaling in Metastatic Cancer
Abstract
1. Introduction
2. Genetic Dissection of WASF3-Related Signal Transduction Pathways
2.1. Regulation of WASF3 Transcription
2.1.1. Regulation by Signal Transducer and Activator of Transcription 3 (STAT3)
2.1.2. Regulation by Hypoxia-Inducible Factor 1-Alpha (HIF1A)
2.2. Regulation of WASF3 Protein Stability
2.2.1. Regulation by Heat Shock Protein 70 (HSP70)
2.2.2. Regulation by ATAD3A-Dependent GRP78
2.3. Regulation of WASF3 Protein Phosphorylation
2.3.1. Regulation by Phosphoinositide 3-Kinase (PI3K)
2.3.2. Regulation by JAK2
2.3.3. Regulation by Abelson Tyrosine Kinase (ABL)
2.3.4. Regulation by HSP90
2.3.5. Regulation by Human Epidermal Growth Factor Receptor 2/3 (HER2/HER3) Signaling Axis
3. Controlling Invasion and Metastasis through WASF3 and microRNA (miRNA or miR) Interactions
3.1. Regulation by chr1-miR-200s
3.2. Regulation by miR-217
3.3. Regulation by miR-31
3.4. Regulation by miR-93
4. The Critical Role of WASF3 in Facilitating the Invasion of Mutant RAS Expressing Cancer Cells
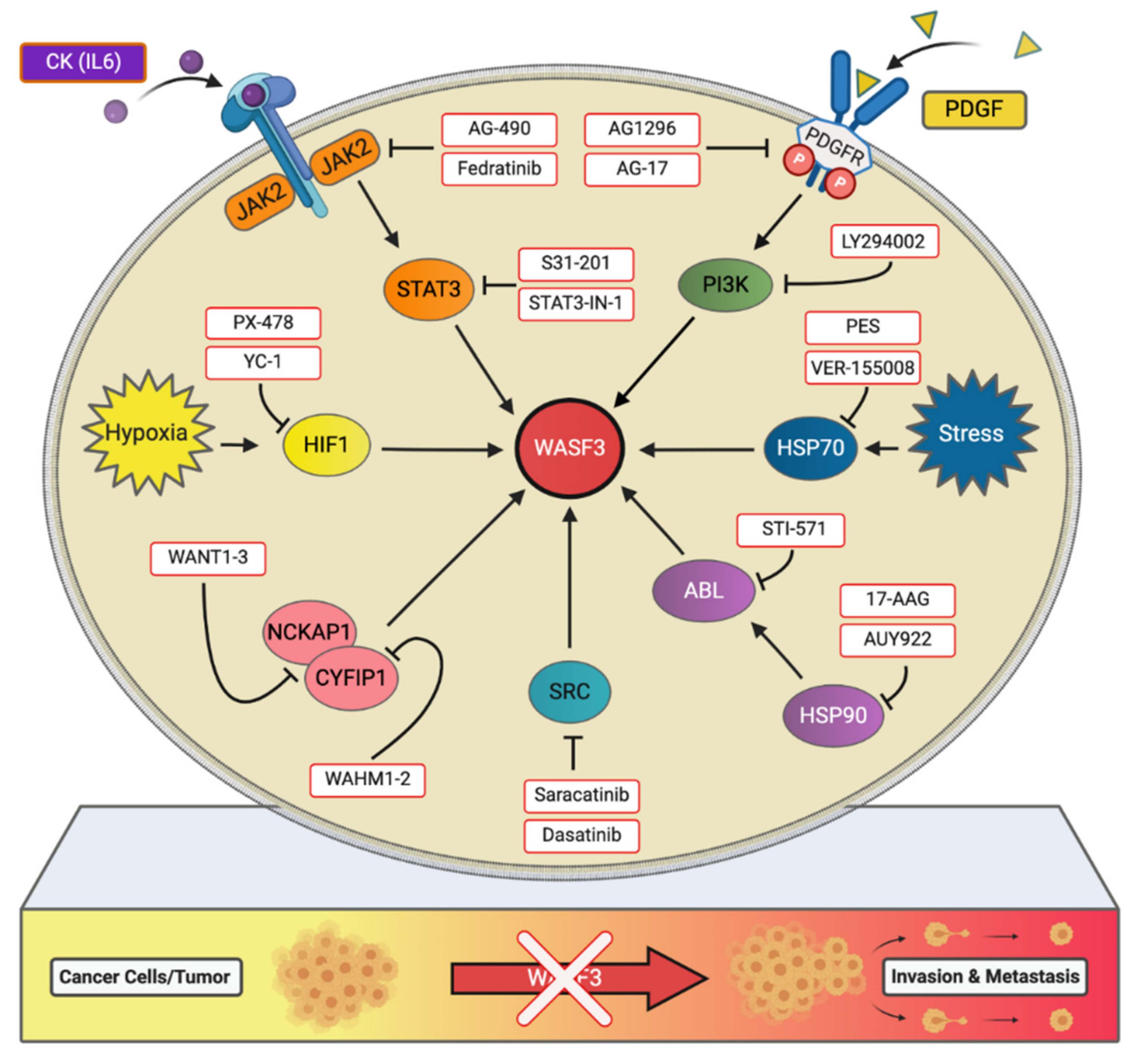
5. Targeting WASF3-Dependent Metastatic Pathways
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABL | Abelson tyrosine kinase |
| Arp | Actin-related protein |
| ATAD3A | AAA domain containing 3A |
| CYFIP1 | cytoplasmic FMR1 interacting protein 1 |
| EMT | epithelial-to-mesenchymal transition |
| ER | endoplasmic reticulum |
| HER2 | human epidermal growth factor receptor 2 |
| HER3 | human epidermal growth factor receptor 3 |
| HIF1A | hypoxia-inducible factor 1-alpha |
| HRE | hypoxia responsive element |
| HSP70 | heat shock protein 70 |
| HSP90 | heat shock protein 70 |
| IL-6 | interleukin 6 |
| IP | immunoprecipitation |
| JAK2 | Janus kinase 2 |
| miRNA or miR | microRNA |
| MMP | matrix metalloproteinase; |
| NCKAP1 | NCK associated protein 1 |
| PDGF | platelet-derived growth factor |
| PI3K | phosphoinositide 3-kinase |
| PyMT | the polyoma middle-T antigen |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| SH2 | SRC homology 2 |
| STAT3 | signal transducer and activator of transcription 3 |
| UTR | untranslated region |
| VCA | verprolin-cofilin-acidic |
| WASF3 | Wiskott-Aldrich syndrome protein family member 3 |
| WHD | WASF homology domain |
| WRC | WASF regulatory complex |
| ZEB1 | zinc finger E-box-binding homeobox 1 |
References
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Kurisu, S.; Takenawa, T. The WASP and WAVE family proteins. Genome Biol. 2009, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Schaks, M.; Singh, S.P.; Kage, F.; Thomason, P.; Klünemann, T.; Steffen, A.; Blankenfeldt, W.; Stradal, T.E.; Insall, R.H.; Rottner, K. Distinct Interaction Sites of Rac GTPase with WAVE Regulatory Complex Have Non-redundant Functions in Vivo. Curr. Biol. 2018, 28, 3674–3684.e6. [Google Scholar] [CrossRef] [PubMed]
- Bompard, G.; Caron, E. Regulation of WASP/WAVE proteins: Making a long story short. J. Cell Biol. 2004, 166, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Frugtniet, B.; Jiang, W.G.; Martin, T.A. Role of the WASP and WAVE family proteins in breast cancer invasion and metastasis. Breast Cancer 2015, 7, 99–109. [Google Scholar] [PubMed]
- Smith, B.A.; Daugherty-Clarke, K.; Goode, B.L.; Gelles, J. Pathway of actin filament branch formation by Arp2/3 complex revealed by single-molecule imaging. Proc. Natl. Acad. Sci. USA 2013, 110, 1285–1290. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Lorenz, M.; Kempiak, S.; Sarmiento, C.; Coniglio, S.; Symons, M.; Segall, J.; Eddy, R.; Miki, H.; Takenawa, T.; et al. Molecular mechanisms of invadopodium formation: The role of the N-WASP-Arp2/3 complex pathway and cofilin. J. Cell Biol. 2005, 168, 441–452. [Google Scholar] [CrossRef]
- Kurisu, S.; Suetsugu, S.; Yamazaki, D.; Yamaguchi, H.; Takenawa, T. Rac-WAVE2 signaling is involved in the invasive and metastatic phenotypes of murine melanoma cells. Oncogene 2005, 24, 1309–1319. [Google Scholar] [CrossRef]
- Takahashi, K.; Suzuki, K. WAVE2, N-WASP, and Mena facilitate cell invasion via phosphatidylinositol 3-kinase-dependent local accumulation of actin filaments. J. Cell Biochem. 2011, 112, 3421–3429. [Google Scholar] [CrossRef]
- Taniuchi, K.; Furihata, M.; Naganuma, S.; Saibara, T. WAVE2 is associated with poor prognosis in pancreatic cancers and promotes cell motility and invasiveness via binding to ACTN4. Cancer Med. 2018, 7, 5733–5751. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Li, X.; Cowell, J.K. c-Abl-mediated phosphorylation of WAVE3 is required for lamellipodia formation and cell migration. J. Biol. Chem. 2007, 282, 26257–26265. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, G.C.; Chen, X.J.; Xue, Z.R. Expression of WASF3 in patients with non-small cell lung cancer: Correlation with clinicopathological features and prognosis. Oncol. Lett. 2014, 8, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, C.; Chen, W.; Liu, Y.; Yin, X.; Lai, J.; Liang, L.; Wang, Q.; Wang, A.; Zheng, C. WAVE3 promotes proliferation, migration and invasion via the AKT pathway in pancreatic cancer. Int. J. Oncol. 2018, 53, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Geng, J.; Ren, Z.; Xiong, C.; Li, Y.; Liu, H. WAVE3 upregulation in esophageal squamous cell carcinoma and its effect on the migration of human esophageal cancer cell lines in vitro. Mol. Med. Rep. 2020, 22, 465–473. [Google Scholar] [CrossRef]
- Teng, Y.; Mei, Y.; Hawthorn, L.; Cowell, J.K. WASF3 regulates miR-200 inactivation by ZEB1 through suppression of KISS1 leading to increased invasiveness in breast cancer cells. Oncogene 2014, 33, 203–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, W.; Kansakar, U.; Markovic, V.; Wang, B.; Sossey-Alaoui, K. WAVE3 phosphorylation regulates the interplay between PI3K, TGF-β, and EGF signaling pathways in breast cancer. Oncogenesis 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Nie, Y.; Hu, S.; Liu, S.; Fang, N.; Guo, F.; Yang, L.; Liang, X. WASF3 expression correlates with poor prognosis in gastric cancer patients. Future Oncol. 2019, 15, 1605–1615. [Google Scholar] [CrossRef]
- Ji, Y.; Li, B.; Zhu, Z.; Guo, X.; He, W.; Fan, Z.; Zhang, W. Overexpression of WAVE3 promotes tumor invasiveness and confers an unfavorable prognosis in human hepatocellular carcinoma. Biomed. Pharmacother. 2015, 69, 409–415. [Google Scholar] [CrossRef]
- Bledzka, K.; Schiemann, B.; Schiemann, W.P.; Fox, P.; Plow, E.F.; Sossey-Alaoui, K. The WAVE3-YB1 interaction regulates cancer stem cells activity in breast cancer. Oncotarget 2017, 8, 104072. [Google Scholar] [CrossRef]
- Park, G.B.; Kim, D. TLR5/7-mediated PI3K activation triggers epithelial-mesenchymal transition of ovarian cancer cells through WAVE3-dependent mesothelin or OCT4/SOX2 expression. Oncol. Rep. 2017, 38, 3167–3176. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Ranalli, T.A.; Li, X.; Bakin, A.V.; Cowell, J.K. WAVE3 promotes cell motility and invasion through the regulation of MMP-1, MMP-3, and MMP-9 expression. Exp. Cell Res. 2005, 308, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ghoshal, P.; Ngoka, L.; Mei, Y.; Cowell, J.K. Critical role of the WASF3 gene in JAK2/STAT3 regulation of cancer cell motility. Carcinogenesis 2013, 34, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, P.; Teng, Y.; Lesoon, L.A.; Cowell, J.K. HIF1A induces expression of the WASF3 metastasis-associated gene under hypoxic conditions. Int. J. Cancer 2012, 131, E905–E915. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ngoka, L.; Mei, Y.; Lesoon, L.; Cowell, J.K. HSP90 and HSP70 proteins are essential for stabilization and activation of WASF3 metastasis-promoting protein. J. Biol. Chem. 2012, 287, 10051–10059. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xie, Q.; Guo, X.; Xu, J.; Wang, A.; Li, J.; Zhu, J.; Wu, X.-R.; Huang, H.; Huang, C. p63α protein up-regulates heat shock protein 70 expression via E2F1 transcription factor 1, promoting Wasf3/Wave3/MMP9 signaling and bladder cancer invasion. J. Biol. Chem. 2017, 292, 15952–15963. [Google Scholar] [CrossRef]
- Baudier, J. ATAD3 proteins: Brokers of a mitochondria-endoplasmic reticulum connection in mammalian cells. Biol Rev Camb. Philos. Soc. 2018, 93, 827–844. [Google Scholar] [CrossRef]
- Lang, L.; Loveless, R.; Teng, Y. Emerging Links between Control of Mitochondrial Protein ATAD3A and Cancer. Int. J. Mol. Sci. 2020, 21, 7917. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, X.; Li, H.; Shull, A.; Kim, J.; Cowell, J.K. Mitochondrial ATAD3A combines with GRP78 to regulate the WASF3 metastasis-promoting protein. Oncogene 2016, 35, 333–343. [Google Scholar] [CrossRef]
- Sossey-Alaoui, K.; Li, X.; Ranalli, T.A.; Cowell, J.K. WAVE3-mediated cell migration and lamellipodia formation are regulated downstream of phosphatidylinositol 3-kinase. J. Biol Chem. 2005, 280, 21748–21755. [Google Scholar] [CrossRef]
- Taylor, M.A.; Davuluri, G.; Parvani, J.G.; Schiemann, B.J.; Wendt, M.K.; Plow, E.F.; Schiemann, W.P.; Sossey-Alaoui, K. Upregulated WAVE3 expression is essential for TGF-β-mediated EMT and metastasis of triple-negative breast cancer cells. Breast Cancer Res. Treat. 2013, 142, 341–353. [Google Scholar] [CrossRef]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemother. Res. Pract. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Han, A.; Polsdofer, E.; Liu, S.; Liu, B. Understanding the biology of HER3 receptor as a therapeutic target in human cancer. Acta Pharm. Sin. B 2018, 8, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Pi, W.; Wang, Y.; Cowell, J.K. WASF3 provides the conduit to facilitate invasion and metastasis in breast cancer cells through HER2/HER3 signaling. Oncogene 2016, 35, 4633–4640. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sossey-Alaoui, K.; Bialkowska, K.; Plow, E.F. The miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasion. J. Biol. Chem. 2009, 284, 33019–33029. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Ma, L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle 2015, 14, 481–487. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, W.; Yin, X.; Lai, J.; Wang, Q.; Liang, L.; Wang, W.; Wang, A.; Zheng, C. WAVE3 induces EMT and promotes migration and invasion in intrahepatic cholangiocarcinoma. Dig. Dis. Sci. 2016, 61, 1950–1960. [Google Scholar] [CrossRef]
- Davuluri, G.; Augoff, K.; Schiemann, W.P.; Plow, E.F. Sossey-Alaoui, K.; WAVE3-NFκB interplay is essential for the survival and invasion of cancer cells. PLoS ONE 2014, 9, e110627. [Google Scholar] [CrossRef][Green Version]
- Chen, D.-l.; Zhang, D.-s.; Lu, Y.-x.; Chen, L.-z.; Zeng, Z.-l.; He, M.-m.; Wang, F.-h.; Li, Y.-h.; Zhang, H.-z.; Pelicano, H.; et al. microRNA-217 inhibits tumor progression and metastasis by downregulating EZH2 and predicts favorable prognosis in gastric cancer. Oncotarget 2015, 6, 10868. [Google Scholar] [CrossRef]
- Shen, L.; Wang, P.; Yang, J.; Li, X. MicroRNA-217 regulates WASF3 expression and suppresses tumor growth and metastasis in osteosarcoma. PLoS ONE 2014, 9, e109138. [Google Scholar] [CrossRef]
- Wang, G.; Fu, Y.; Liu, G.; Ye, Y.; Zhang, X. miR-218 inhibits proliferation, migration, and EMT of gastric cancer cells by targeting WASF3. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017, 25, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Sossey-Alaoui, K.; Downs-Kelly, E.; Das, M.; Izem, L.; Tubbs, R.; Plow, E.F. WAVE3, an actin remodeling protein, is regulated by the metastasis suppressor microRNA, miR-31, during the invasion-metastasis cascade. Int. J. Cancer 2011, 129, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Kakeji, Y.; Shimono, Y. MicroRNA-93 targets WASF3 and functions as a metastasis suppressor in breast cancer. Cancer Sci. 2020, 111, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ngoka, L.; Cowell, J.K. Promotion of invasion by mutant RAS is dependent on activation of the WASF3 metastasis promoter gene. Geneschromosomes Cancer 2017, 56, 493–500. [Google Scholar] [CrossRef]
- Teng, Y.; Bahassan, A.; Dong, D.; Hanold, L.E.; Ren, X.; Kennedy, E.J.; Cowell, J.K. Targeting the WASF3-CYFIP1 Complex Using Stapled Peptides Suppresses Cancer Cell Invasion. Cancer Res. 2016, 76, 965–973. [Google Scholar] [CrossRef]
- Teng, Y.; Qin, H.; Bahassan, A.; Bendzunas, N.G.; Kennedy, E.J.; Cowell, J.K. The WASF3–NCKAP1–CYFIP1 complex is essential for breast cancer metastasis. Cancer Res. 2016, 76, 5133–5142. [Google Scholar] [CrossRef]
- Qin, H.; Lu, S.; Thangaraju, M.; Cowell, J.K. Wasf3 Deficiency Reveals Involvement in Metastasis in a Mouse Model of Breast Cancer. Am. J. Pathol. 2019, 189, 2450–2458. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loveless, R.; Teng, Y. Targeting WASF3 Signaling in Metastatic Cancer. Int. J. Mol. Sci. 2021, 22, 836. https://doi.org/10.3390/ijms22020836
Loveless R, Teng Y. Targeting WASF3 Signaling in Metastatic Cancer. International Journal of Molecular Sciences. 2021; 22(2):836. https://doi.org/10.3390/ijms22020836
Chicago/Turabian StyleLoveless, Reid, and Yong Teng. 2021. "Targeting WASF3 Signaling in Metastatic Cancer" International Journal of Molecular Sciences 22, no. 2: 836. https://doi.org/10.3390/ijms22020836
APA StyleLoveless, R., & Teng, Y. (2021). Targeting WASF3 Signaling in Metastatic Cancer. International Journal of Molecular Sciences, 22(2), 836. https://doi.org/10.3390/ijms22020836





