A Step-by-Step Approach to Improve Clinical Translation of Liposome-Based Nanomaterials, a Focus on Innate Immune and Inflammatory Responses
Abstract
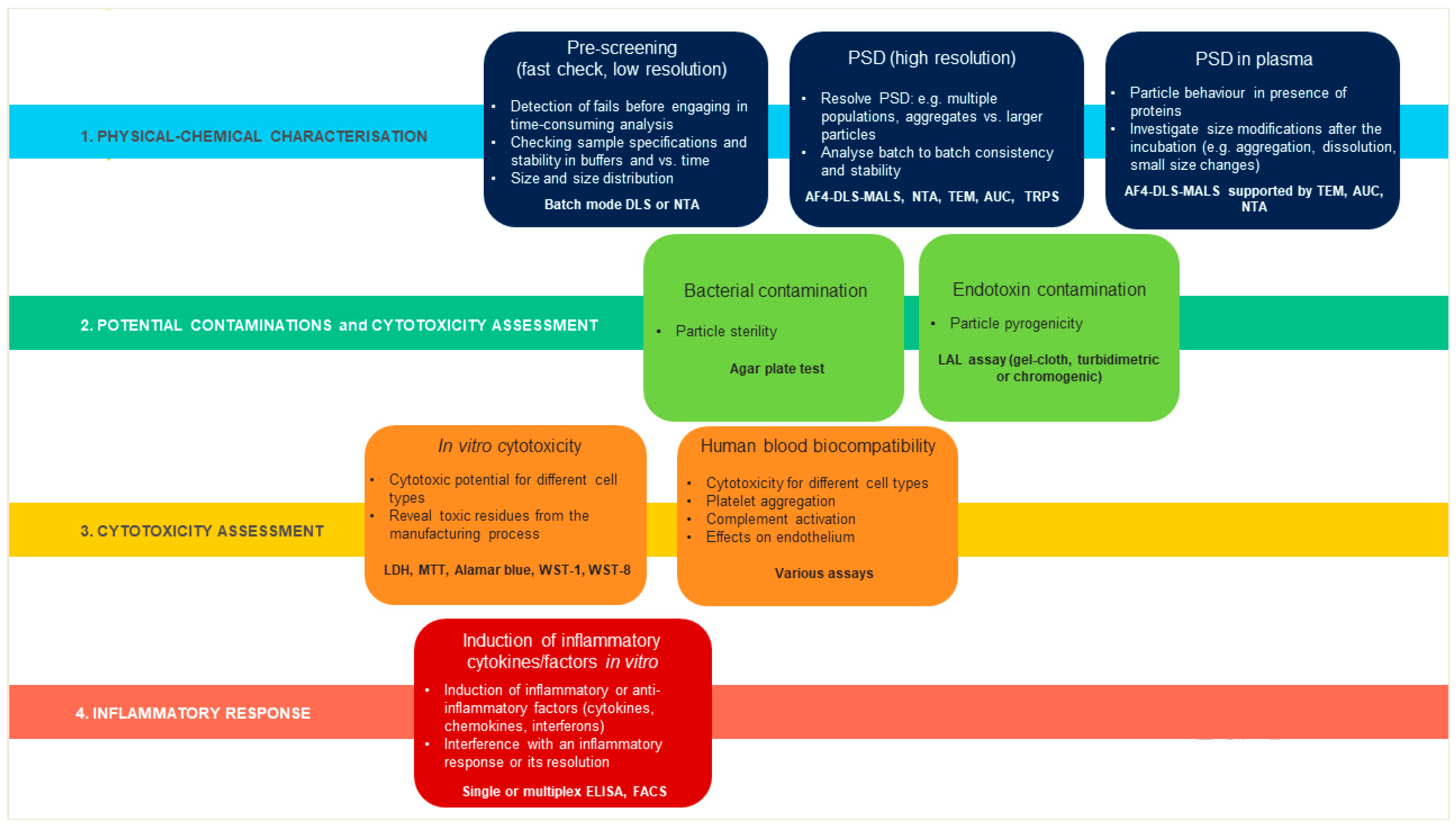
1. Introduction
2. Results
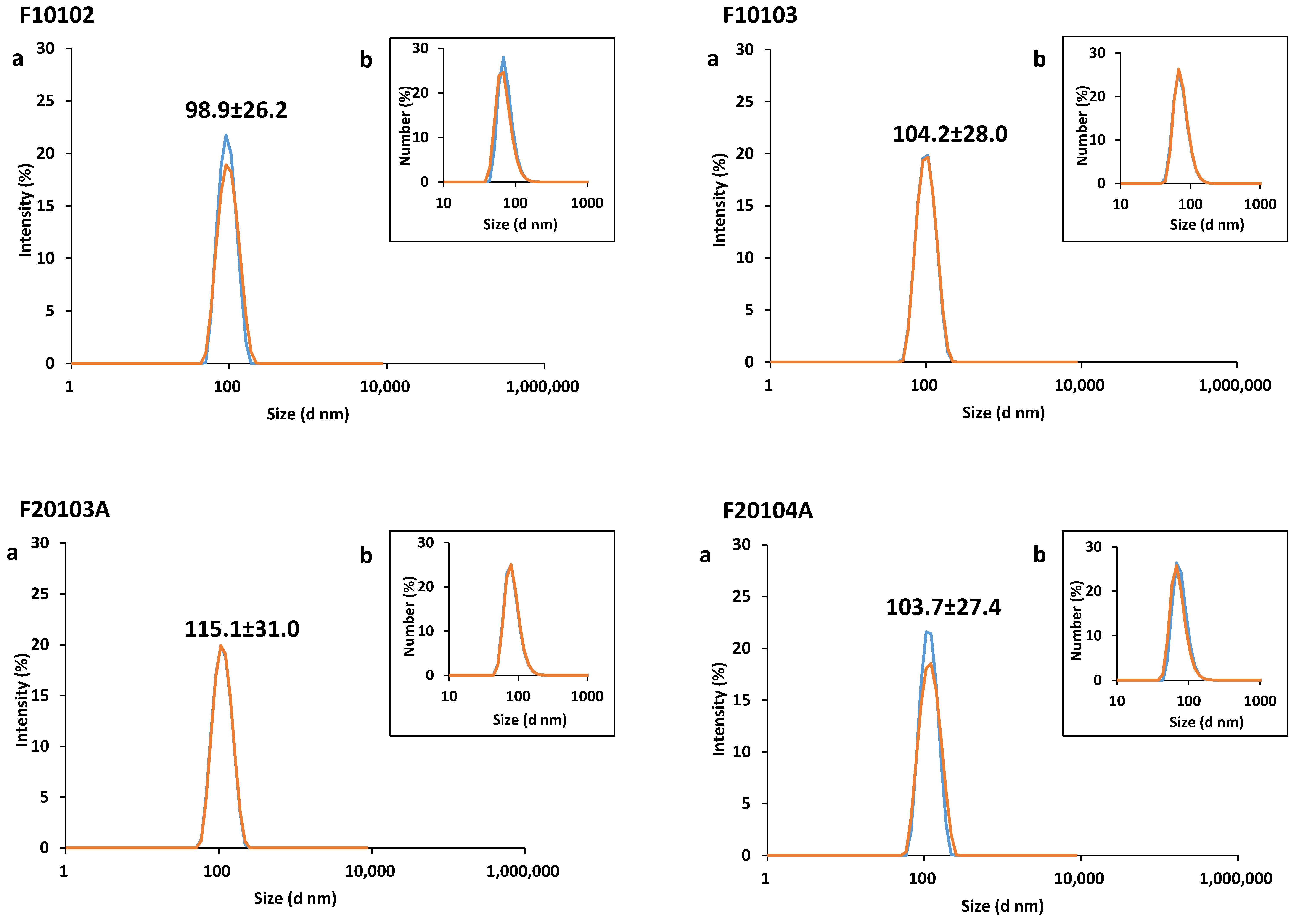
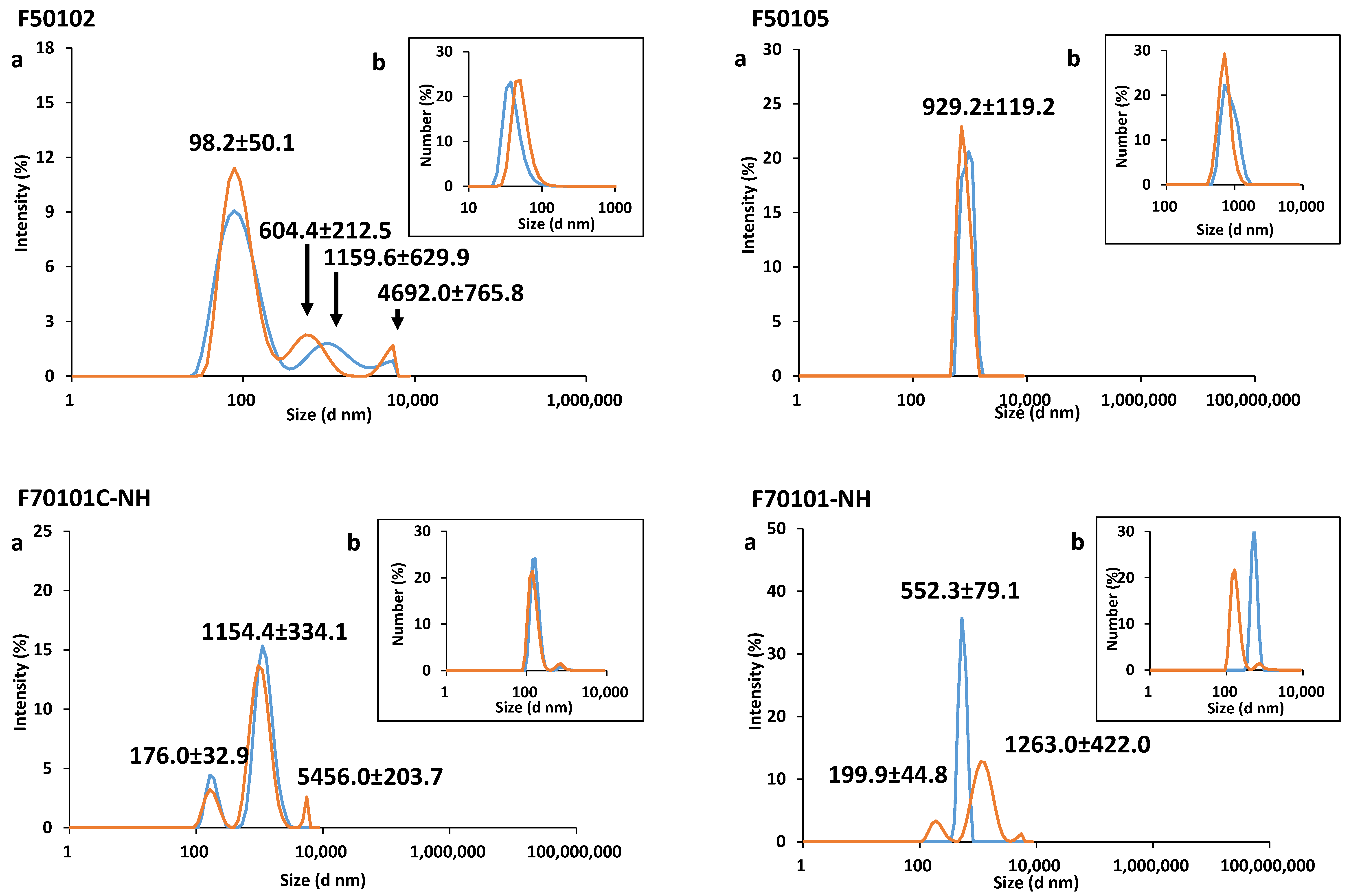
2.1. Physical-Chemical Characterization of Liposomes
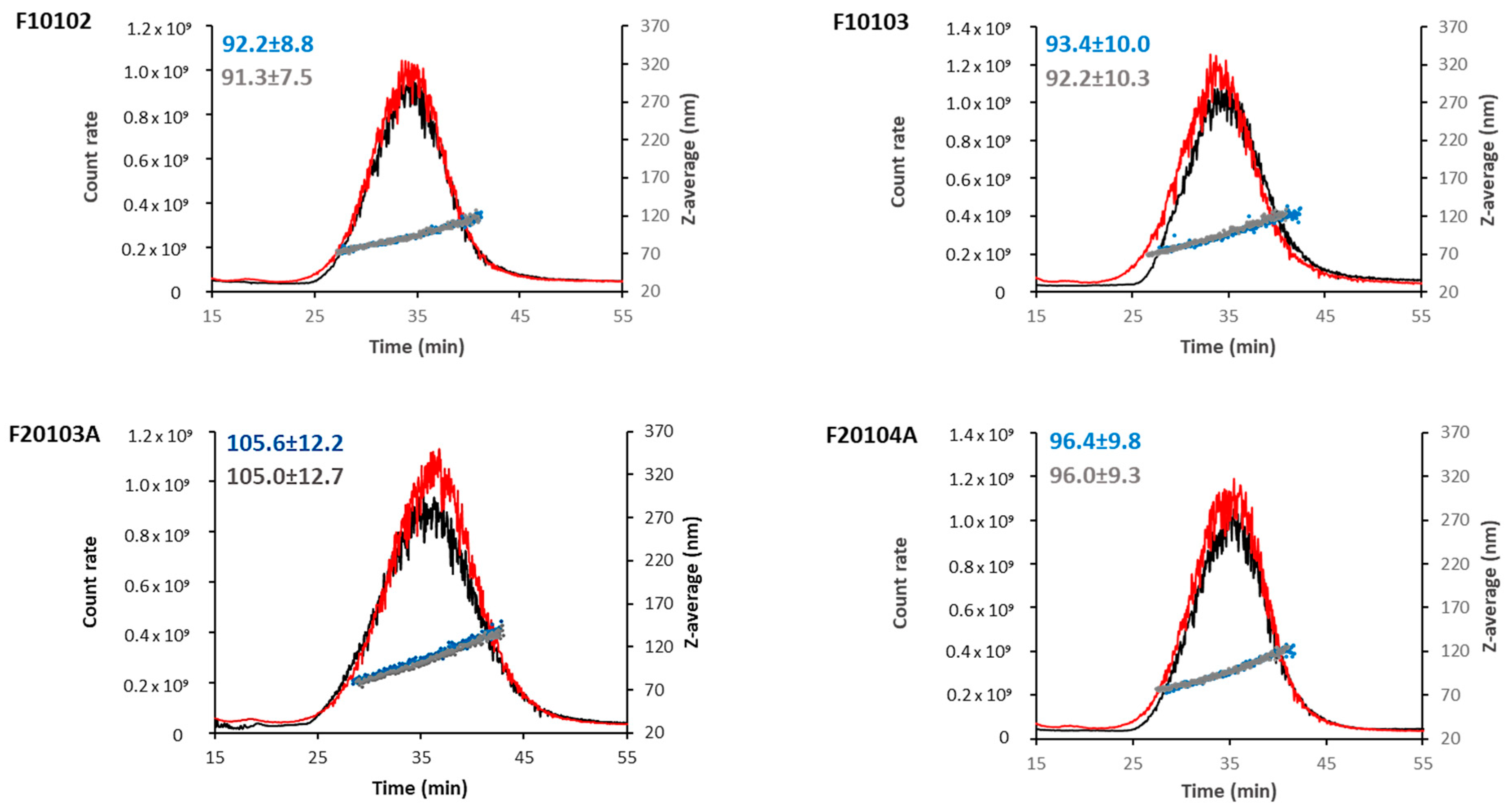
2.2. AF4-DLS Analysis
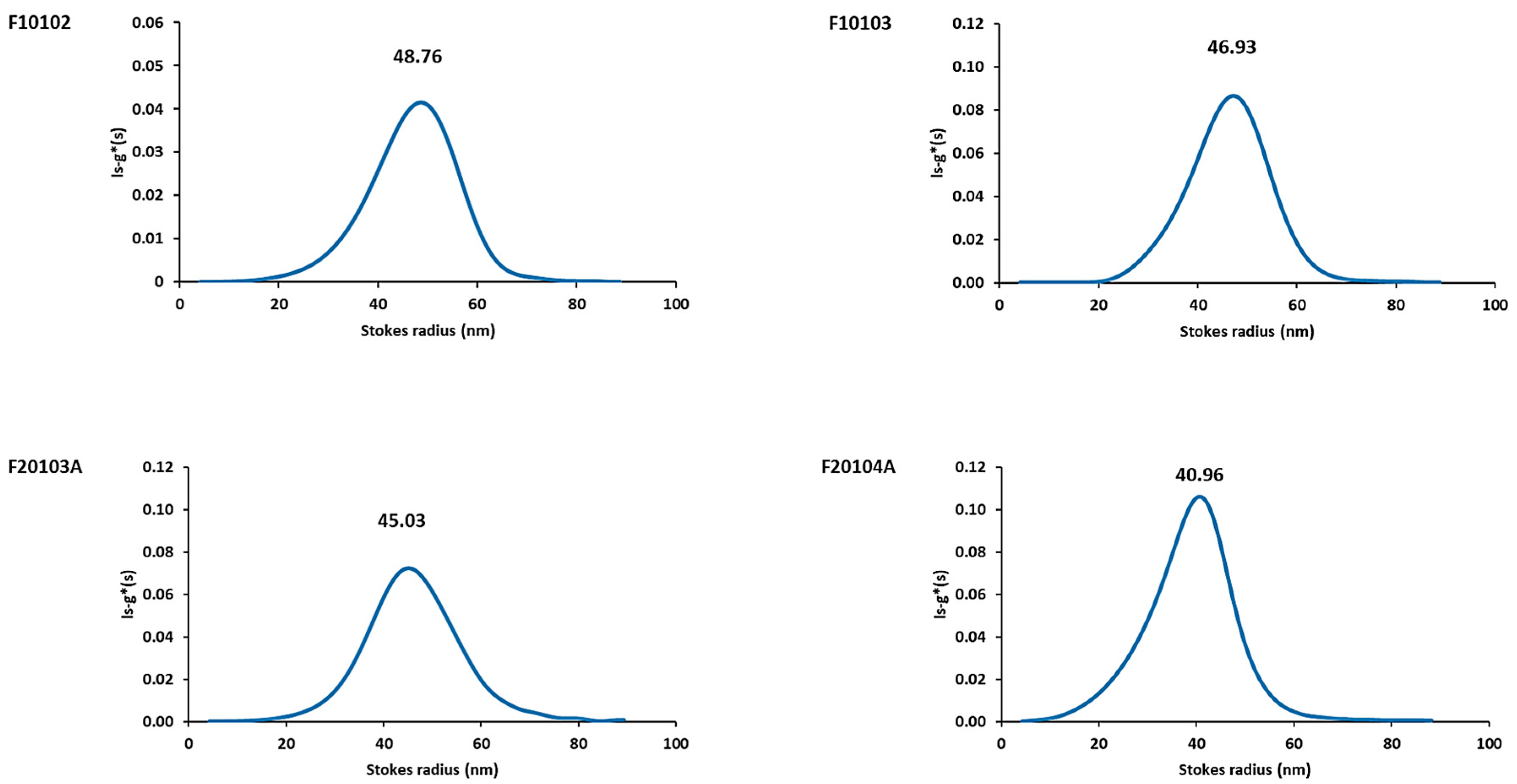
2.3. AUC Confirmed Results Obtained with DLS and AF4-DLS
2.4. Bacterial and Endotoxin Contamination
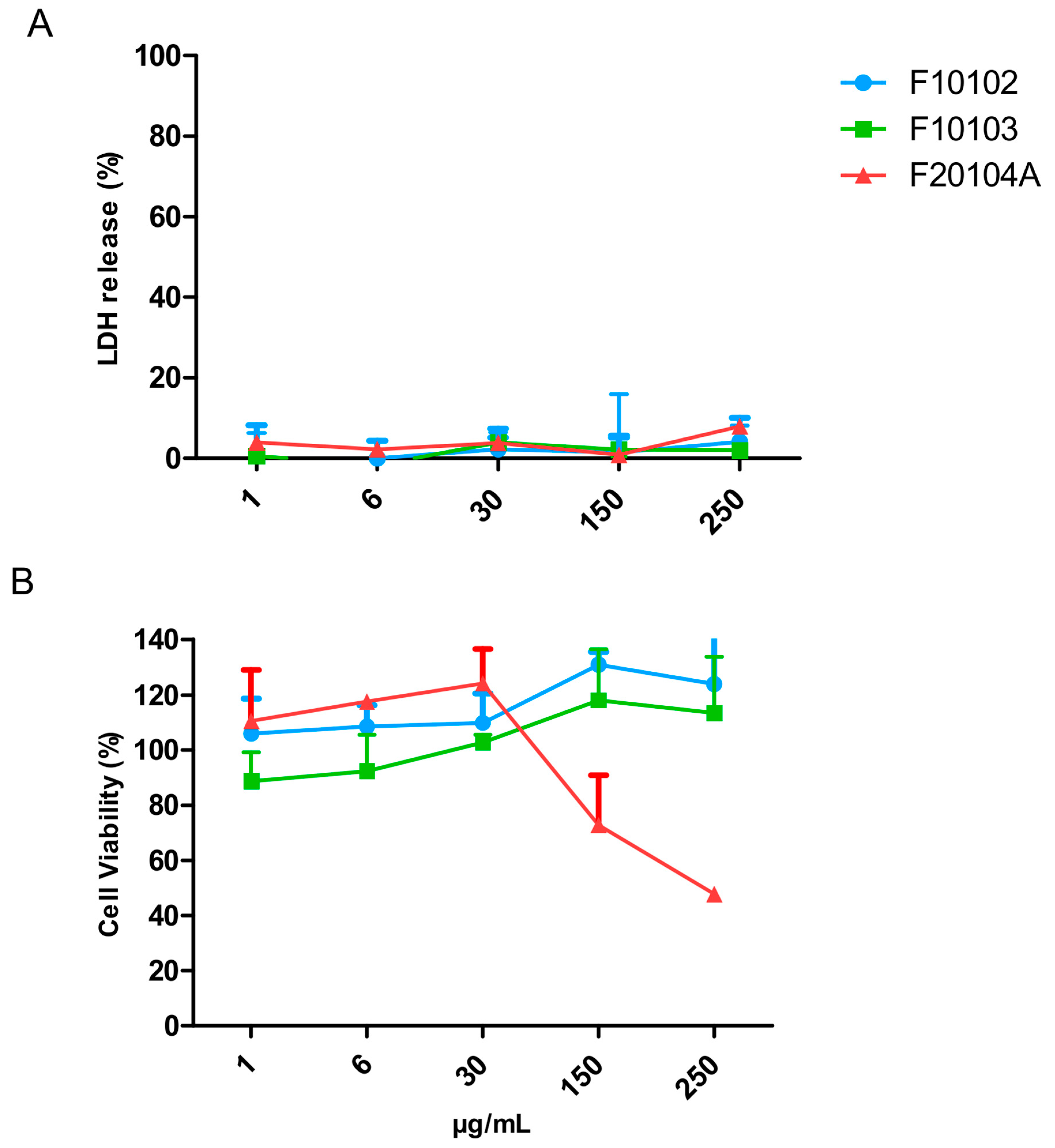
2.5. Assessment of Liposome Cytotoxicity
2.6. Effects of Liposomes on Complement Activation
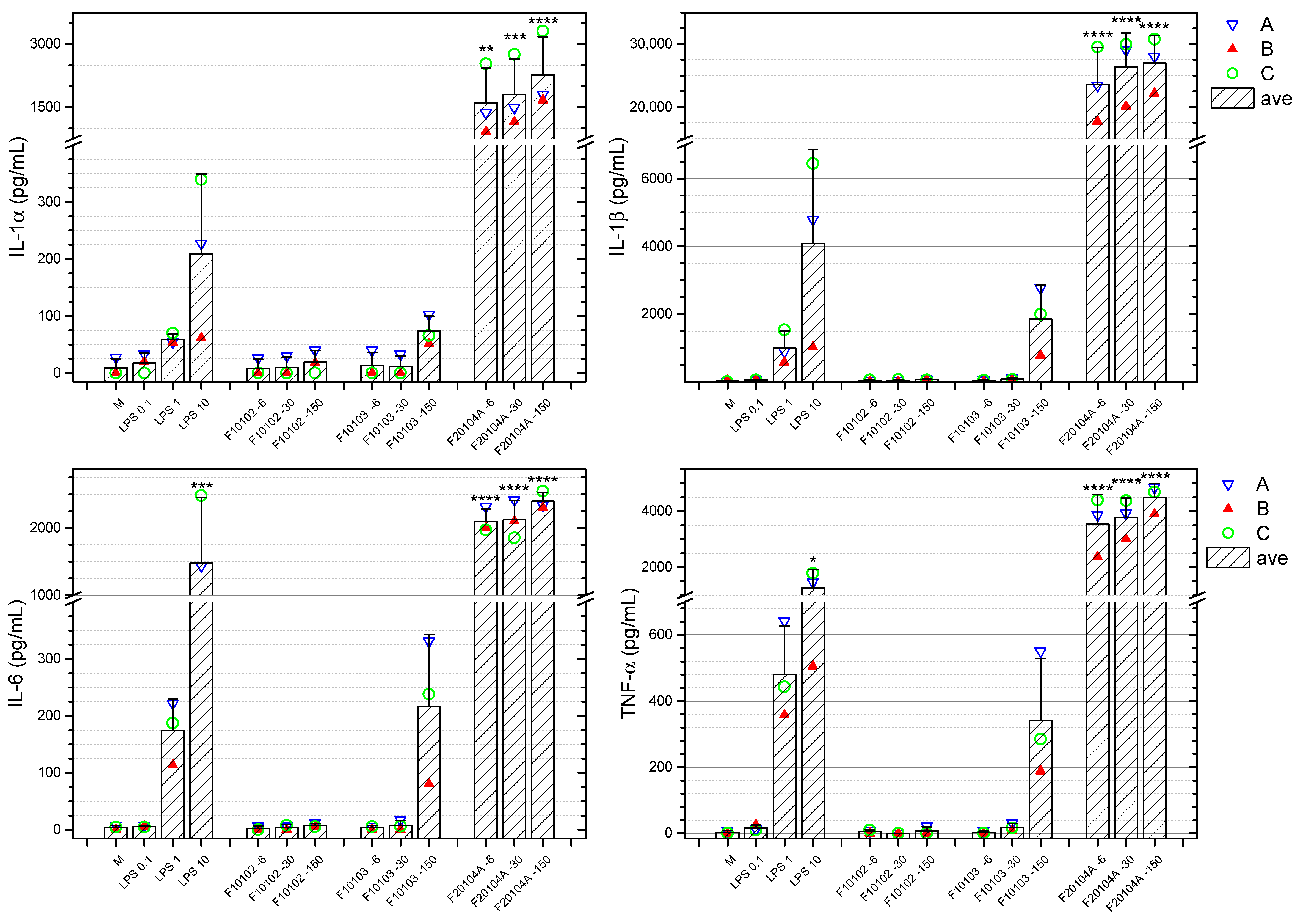
2.7. Effects of Liposomes on the Production of Inflammation-Related Cytokines by Human Blood Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Selection of Liposomes
5.2. Physical-Chemical Characterization
5.3. Bacterial and Endotoxin Contamination
5.3.1. Assessment of Bacterial Contamination
5.3.2. Assessment of Endotoxin Contamination
5.4. Cytotoxicity Evaluation
5.4.1. Hep G2 Cells
5.4.2. PBMC
5.5. Complement Activation Assays
5.6. Activation of Human Blood Leukocytes
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF4 | Asymmetrical Flow-field-flow-fractionation |
| ANOVA | Analysis of variance |
| AUC | Analytical Ultracentrifuge |
| CHOL | Cholesterol |
| DC-CHOL | 3b-(N-(N′,N′-dimethylaminoethane)-carbamoyl) cholesterol hydrochloride |
| DLS | Dynamic Light scattering |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOTAP | 1,2-dioleoyl-3-timethylamonium-propane |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| ELISA | Enzyme-linked immunosorbent assay |
| ELS | Electrophoretic Light Scattering |
| EMA | European Medicinal Agency |
| EUNCL | European Nanomedicine Characterization Laboratories |
| FDA | Food and Drug Administration |
| Hep G2 | Human Hepatocarcinoma G2 cell line |
| HSPC | Hydrogenated phosphatidylcholine |
| ICH-S8 | Guideline S8 (Immunotoxicology studies for human pharmaceuticals) of the EMA International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) |
| ISO | International Standards Organization |
| JRC | Joint Research Centre |
| LAL | Limulus Amoebocyte Lysate |
| LDH | Lactate dehydrogenase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H tetrazolium bromide |
| NM | Nanomaterial |
| PBMC | Peripheral blood mononuclear cells |
| PBS | Phosphate Buffer Saline |
| PC | Phosphatidylcholine |
| PDI | Polydispersity Index |
| PSD | Particle Size Distribution |
| SD | Standard Deviation |
| TLR4 | Toll-like-receptor 4 |
| USNCL | National Cancer Institute-Nanotechnology Characterization Laboratory |
References
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- FDA News Release. FDA Approves First Treatment for Certain Types of Poor-Prognosis Acute Myeloid Leukemia. 2017. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-certain-types-poor-prognosis-acute-myeloid-leukemia (accessed on 9 December 2020).
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D.; et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H. Immunological and toxicological considerations for the design of liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Duschl, A.; Boraschi, D.; Duschl, A. Nanoparticles and the Immune System Safety and Effects, 1st ed.; Boraschi, D., Duschl, A., Eds.; Academic Press: Cambridge, MA, USA, 2013; p. 138. [Google Scholar]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Singh, J. International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J. Pharmacol. Pharmacother. 2015, 6, 185–187. [Google Scholar] [CrossRef]
- Hannon, G.; Lysaght, J.; Liptrott, N.J.; Prina-Mello, A. Immunotoxicity considerations for next generation cancer nanomedicines. Adv. Sci. 2019, 6, 1900133. [Google Scholar] [CrossRef]
- Davis, M.M. A prescription for human immunology. Immunity 2008, 29, 835–838. [Google Scholar] [CrossRef]
- Webb, D.R. Animal models of human disease: Inflammation. Biochem. Pharmacol. 2014, 87, 121–130. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. Model validity in nanoimmunosafety: Advantages and disadvantages of in vivo vs in vitro models, and human vs animal models. Curr. Bionanotechnol. 2016, 2, 71–76. [Google Scholar]
- Brodin, P.; Davis, M.M. Human immune system variation. Nat. Rev. Immunol. 2017, 17, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Mishra, V.; Mehra, N.K. Targeted drug delivery to macrophages. Expert Opin. Drug Deliv. 2013, 10, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yang, X.; Zhang, R.-X.; Luo, Y.-X.; Li, J.-L.; Hou, J.; Zhang, C.; Li, Y.-J.; Shi, J.; Lu, L.; et al. Monocyte mediated brain targeting delivery of macromolecular drug for the therapy of depression. Nanomedicine 2015, 11, 391–400. [Google Scholar] [CrossRef]
- Klauber, T.C.B.; Laursen, J.M.; Zucker, D.; Brix, S.; Jensen, S.S.; Andresen, T.L. Delivery of TLR7 agonist to monocytes and dendritic cells by DCIR targeted liposomes induces robust production of anti-cancer cytokines. Acta Biomater. 2017, 53, 367–377. [Google Scholar] [CrossRef]
- Gregoriadis, G. Liposomes for drugs and vaccines. Trends Biotechnol. 1985, 3, 235–241. [Google Scholar] [CrossRef]
- Kedmi, R.; Ben-Arie, N.; Peer, D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials 2010, 31, 6867–6875. [Google Scholar] [CrossRef]
- Tabernero, J.; Shapiro, G.I.; Lorusso, P.M.; Cervantes, A.; Schwartz, G.K.; Weiss, G.J.; Paz-Ares, L.; Cho, D.C.; Infante, J.R.; Alsina, M.; et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013, 3, 406–417. [Google Scholar] [CrossRef]
- Mehn, D.; Iavicoli, P.; Cabaleiro, N.; Borgos, S.E.; Caputo, F.; Geiss, O.; Calzolai, L.; Rossi, F.; Gilliland, D. Analytical ultracentrifugation for analysis of doxorubicin loaded liposomes. Int. J. Pharm. 2017, 523, 320–326. [Google Scholar] [CrossRef]
- Schuck, P.; Rossmanith, P. Determination of the sedimentation coefficient distribution by least-squares boundary modeling. Biopolymers 2000, 54, 328–341. [Google Scholar] [CrossRef]
- Mittal, V.; Völkel, A.; Cölfen, H. Analytical ultracentrifugation of model nanoparticles: Comparison of different analysis methods. Macromol. Biosci. 2010, 10, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Wohlleben, W. Validity range of centrifuges for the regulation of nanomaterials: From classification to as-tested coronas. J. Nanoparticle Res. 2012, 14, 1–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control. Release 2019, 299, 31–43. [Google Scholar] [CrossRef]
- Mehn, D.; Caputo, F.; Rösslein, M.; Calzolai, L.; Saint-Antonin, F.; Courant, T.; Wick, P.; Gilliland, D. Larger or more? Nanoparticle characterisation methods for recognition of dimers. RSC Adv. 2017, 7, 27747–27754. [Google Scholar] [CrossRef]
- Kim, K.-M.; Song, J.H.; Kim, M.-K.; Chung, S.-T.; Jeong, J.; Yang, J.-Y.; Choi, A.-J.; Choi, H.-J.; Oh, J.-M. Physicochemical analysis methods for nanomaterials considering their toxicological evaluations. Mol. Cell. Toxicol. 2014, 10, 347–360. [Google Scholar] [CrossRef]
- Iavicoli, P.; Urbán, P.; Bella, A.; Ryadnov, M.G.; Rossi, F.; Calzolai, L. Application of asymmetric flow field-flow fractionation hyphenations for liposome-antimicrobial peptide interaction. J. Chromatogr. A 2015, 1422, 260–269. [Google Scholar] [CrossRef] [PubMed]
- ISO 22412:2017 Particle Size Analysis—Dynamic Light Scattering (DLS). Available online: https://www.iso.org/standard/65410.html (accessed on 21 September 2020).
- ISO 9276-1:1998 Representation of Results of Particle Size Analysis—Part 1: Graphical Representation. Available online: https://www.iso.org/standard/25860.html (accessed on 21 September 2020).
- Gioria, S.; Caputo, F.; Urbán, P.; Maguire, C.M.; Bremer-Hoffmann, S.; Prina-Mello, A.; Calzolai, L.; Mehn, D. Are existing standard methods suitable for the evaluation of nanomedicines: Some case studies. Nanomedicine 2018, 13, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Mehn, D.; Clogston, J.; Rösslein, M.; Prina-Mello, A.; Borgos, S.; Gioria, S.; Calzolai, L. Asymmetric-flow field-flow fractionation for measuring particle size, drug loading and (in)stability of nanopharmaceuticals. The joint view of European Union Nanomedicine Characterization Laboratory and National Cancer Institute-Nanotechnology Character. J. Chromatogr. A 2020, 1635, 461767. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Simberg, D.; Skotland, T.; Yaghmur, A.; Hunter, A.C. The interplay between blood proteins, complement, and macrophages on nanomedicine performance and responses. J. Pharmacol. Exp. Ther. 2019, 370, 581–592. [Google Scholar] [CrossRef]
- Clogston, J.D.; Hackley, V.A.; Prina-Mello, A.; Puri, S.; Sonzini, S.; Soo, P.L. Sizing up the next generation of nanomedicines. Pharm. Res. 2020, 37, 6. [Google Scholar] [CrossRef]
- Mehn, D.; Capomaccio, R.; Gioria, S.; Gilliland, D.; Calzolai, L. Analytical ultracentrifugation for measuring drug distribution of doxorubicin loaded liposomes in human serum. J. Nanoparticle Res. 2020, 22, 158. [Google Scholar] [CrossRef]
- Cole, J.L.; Lary, J.W.; PMoody, T.; Laue, T.M. Analytical ultracentrifugation: Sedimentation velocity and sedimentation equilibrium. Methods Cell Biol. 2008, 84, 143–179. [Google Scholar] [PubMed]
- Brooks, R.A.; Wimhurst, J.A.; Rushton, N. Endotoxin contamination of particles produces misleading inflammatory cytokine responses from macrophages in vitro. J. Bone Jt. Surg. Ser. B 2002, 84, 295–299. [Google Scholar] [CrossRef]
- Galanos, C.; Freudenberg, M.; Katschinski, T.; Salomoa, R. MHKY. Tumor necrosis factor and host response to endotoxin. Bact. Endotoxic Lipopolysaccharides 1992, 2, 75–102. [Google Scholar]
- Dinarello, C.A. Interleukin-1 and interleukin-1 antagonism. Blood 1991, 77, 1627–1652. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Cerami, A. The biology of cachectin/TNF-a primary mediator of the host response. Annu. Rev. Immunol. 1989, 7, 625–655. [Google Scholar] [CrossRef] [PubMed]
- Loppnow, H. LPS, recIL1 and smooth muscle cell-IL1 activate vascular cells by specific mechanisms. Prog. Clin. Biol. Res. 1994, 388, 309–321. [Google Scholar]
- Mattern, T.; Thanhäuser, A.; Reiling, N.; Toellner, K.M.; Duchrow, M.; Kusumoto, S.; Rietschel, E.T.; Ernst, M.; Brade, H.; Flad, H.D. Endotoxin and lipid A stimulate proliferation of human T cells in the presence of autologous monocytes. J. Immunol. 1994, 153, 2996–3004. [Google Scholar]
- Haziot, A.; Tsuberi, B.Z.; Goyert, S.M. Neutrophil CD14: Biochemical properties and role in the secretion of tumor necrosis factor-alpha in response to lipopolysaccharide. J. Immunol. 1993, 150, 5556–5565. [Google Scholar]
- Dobrovolskaia, M.A.; McNeil, S.E. Handbook of Immunological Properties of Engineered Nanomaterials, 2nd ed.; World Scientific Pub Co. Inc.: Singapore, 2016; Volume 6. [Google Scholar]
- EUNCL European Nanomedicine Characterisation Laboratory. 2017. Available online: http://www.euncl.eu/ (accessed on 29 July 2020).
- Potter, T.M.; Neun, B.W.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Detection of bacterial contamination in nanoparticle formulations by agar plate test. Methods Mol. Biol. 2018, 1682, 19–22. [Google Scholar]
- Li, Y.; Italiani, P.; Casals, E.; Tran, N.; Puntes, V.F.; Boraschi, D. Optimising the use of commercial LAL assays for the analysis of endotoxin contamination in metal colloids and metal oxide nanoparticles. Nanotoxicology 2015, 9, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Boraschi, D. Endotoxin contamination: A key element in the interpretation of nanosafety studies. Nanomedicine 2016, 11, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fujita, M.; Boraschi, D. Endotoxin contamination in nanomaterials leads to the misinterpretation of immunosafety results. Front. Immunol. 2017, 8, 472. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Neun, B.W.; Clogston, J.D.; Grossman, J.H. McNeil SE. Choice of method for endotoxin detection depends on nanoformulation. Nanomedicine 2014, 9, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- ISO 29701:2010(en). Nanotechnologies—Endotoxin Test on Nanomaterial Samples for In Vitro Systems—Limulus Amebocyte Lysate (LAL) Test. 2010. Available online: https://www.iso.org/standard/45640.html (accessed on 7 June 2020).
- Bacterial Endotoxins|USP. 2005. Available online: https://www.usp.org/harmonization-standards/pdg/general-methods/bacterial-endotoxins (accessed on 7 June 2020).
- Oostingh, G.J.; Casals, E.; Italiani, P.; Colognato, R.; Stritzinger, R.; Ponti, J.; Pfaller, T.; Kohl, Y.; Ooms, D.; Favilli, F.; et al. Problems and challenges in the development and validation of human cell-based assays to determine nanoparticle-induced immunomodulatory effects. Part. Fibre Toxicol. 2011, 8, 8. [Google Scholar] [CrossRef]
- Simões, S.; Filipe, A.; Faneca, H.; Mano, M.; Penacho, N.; Duzgunes, N.; De Lima, M.P. Cationic liposomes for gene delivery. Expert Opin. Drug Deliv. 2005, 2, 237–254. [Google Scholar] [CrossRef]
- Christensen, D.; Korsholm, K.S.; Andersen, P.; Agger, E.M. Cationic liposomes as vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 513–521. [Google Scholar] [CrossRef]
- Heuts, J.; Varypataki, E.M.; Van Der Maaden, K.; Romeijn, S.; Drijfhout, J.W.; Van Scheltinga, A.T.; Ossendorp, F.; Jiskoot, W. Cationic liposomes: A flexible vaccine delivery system for physicochemically diverse antigenic peptides. Pharm. Res. 2018, 35, 207. [Google Scholar] [CrossRef]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and innate immunity: New perspectives on host defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef]
- Haile, L.A.; Polumuri, S.K.; Rao, R.; Kelley-Baker, L.; Kryndushkin, D.; Rajaiah, R.; Israely, T.; Rao, V.A.; Verthelyi, D. Cell based assay identifies TLR2 and TLR4 stimulating impurities in Interferon beta. Sci. Rep. 2017, 7, 10490. [Google Scholar] [CrossRef]
- Anchordoquy, T.J.; Barenholz, Y.; Boraschi, D.; Chorny, M.; Decuzzi, P.; Dobrovolskaia, M.A.; Farhangrazi, Z.S.; Farrell, D.; Gabizon, A.; Ghandehari, H.; et al. Mechanisms and barriers in cancer nanomedicine: Addressing challenges, looking for solutions. ACS Nano 2017, 11, 12–18. [Google Scholar] [CrossRef] [PubMed]
- ASTM E2526-08(2013). Standard Test Method for Evaluation of Cytotoxicity of Nanoparticulate Materials in Porcine Kidney Cells and Human Hepatocarcinoma Cells. 2013. Available online: https://www.astm.org/Standards/E2526.htm (accessed on 28 July 2020).
- ISO/TR 10993-22:2017 Biological Evaluation of Medical Devices—Part 22: Guidance on Nanomaterials. Available online: https://www.iso.org/standard/65918.html (accessed on 30 July 2020).
- Szebeni, J.; Moghimi, S.M. Liposome triggering of innate immune responses: A perspective on benefits and adverse reactions. J. Liposome Res. 2009, 19, 85–90. [Google Scholar] [CrossRef] [PubMed]
- DeJager, W.; Bourcier, K.; Rijkers, G.T.; Prakken, B.J.; Seyfert-Margolis, V. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol. 2008, 10, 52. [Google Scholar]
- ISO/TS 21362:2018-Nanotechnologies—Analysis of Nano-Objects Using Asymmetrical-Flow and Centrifugal Field-Flow Fractionation. Available online: https://www.iso.org/standard/70761.html (accessed on 21 September 2020).
- Caputo, F.; Arnould, A.; Bacia, M.; Ling, W.L.; Rustique, E.; Texier, I.; Mello, A.P.; Couffin, A.-C. Measuring particle size distribution by asymmetric flow field flow fractionation: A powerful method for the preclinical characterization of lipid-based nanoparticles. Mol. Pharm. 2019, 16, 756–767. [Google Scholar] [CrossRef]
- Munson, T.E. Guideline for validation of the LAL test as an end-product endotoxin test for human and biological drug products. Prog. Clin. Biol. Res. 1985, 189, 211–220. [Google Scholar]
- FDA. 0027-Guidance for Industry Pyrogen and Endotoxins Testing: Questions and Answers. 2012; pp. 1–10. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-pyrogen-and-endotoxins-testing-questions-and-answers (accessed on 10 June 2020).
- EUNCL_Assay-Cascade 2016. Available online: http://www.euncl.eu/about-us/assay-cascade/PDFs/Toxicology/EUNCL_GTA_002.pdf?m=1468937871& (accessed on 29 July 2020).
- Act MH. A Guiding Principles and the Fundamental Principle of Consent Code of Practice. 1983. Available online: https://www.hta.gov.uk/sites/default/files/HTA Code A_1.pdf (accessed on 12 June 2020).
- Li, Y.; Italiani, P.; Casals, E.; Valkenborg, D.; Mertens, I.; Baggerman, G.; Nelissen, I.; Puntes, V.F.; Boraschi, D. Assessing the immunosafety of engineered nanoparticles with a novel in vitro model based on human primary monocytes. ACS Appl. Mater. Interfaces 2016, 8, 28437–28447. [Google Scholar] [CrossRef]
- Noss, I.; Doekes, G.; Thorne, P.S.; Heederik, D.J.J.; Wouters, I.M. Comparison of the potency of a variety of β-glucans to induce cytokine production in human whole blood. Innate. Immun. 2013, 19, 10–19. [Google Scholar] [CrossRef]


| Liposome Description | Lipid Composition | Bulk Buffer Solution | Catalogue nr. | Lipid Stock Concentration (mg/mL) |
|---|---|---|---|---|
| Plain DPPC/CHOL liposomes | DPPC:CHOL (55:55 mol/mol) | 10% sucrose, 20 mM HEPES, <10% ethanol, pH 7.4 | F10102 | 34.6 |
| Plain DSPC/CHOL liposomes | DSPC:CHOL (55:55 mol/mol) | 10% sucrose, 20 mM HEPES, pH 7.4 | F10103 | 36.5 |
| DSPC/CHOL liposomes with ammonium sulfate gradient | DSPC:CHOL (55:55 mol/mol) | 10% sucrose, 20 mM NaPO4, pH 6.5 | F20103A | 42.2 |
| HSPC/CHOL liposomes with ammonium sulfate gradient | HSPC:CHOL (55:55 mol/mol) | 10% sucrose, 20 mM NaPO4, pH 6.5 | F20104A | 36.3 |
| DOTAP/DOPE cationic liposomes | DOTAP:DOPE (50:50 mol/mol) | Nuclease-free water, 10% ethanol, pH 5.5 | F50102 | 3.6 |
| DC-CHOL/DOPE cationic liposomes | DC-CHOL:DOPE (30:70 mol/mol) | Nuclease-free water, pH 5.5 | F50105 | 3.4 |
| Clophosome—high potency clodronate liposomes (neutral) | PC:CHOL (* mol/mol) | PBS (0.9% NaCl, 20 mM NaPO4), pH 7.5 | F70101C-NH | 20.0 |
| Control empty liposomes for Clophosome—high potency (neutral) | PC:CHOL (* mol/mol) | PBS (0.9% NaCl, 20 mM NaPO4), pH 7.4 | F70101-NH | 20.0 |
| Liposome | Diameter (nm) | PDI | Zeta-Potential (mV) | Mobility (µm cm/Vs) | ||
|---|---|---|---|---|---|---|
| Dh | Dh * | DS | ||||
| F10102 | 98.9 (±26.2) | 92.2 (±8.8) | 96.5 | 0.05 | −5.90 (±1.92) | −0.40 (±0.14) |
| F10103 | 104.2 (±28.0) | 93.4 (±10.0) | 93.9 | 0.06 | −11.90 (±2.20) | −0.90 (±0.16) |
| F20103A | 115.1 (±31.0) | 105.6 (±12.2) | 90.1 | 0.06 | −16.60 (±6.35) | −1.20 (±0.07) |
| F20104A | 103.7 (±27.4) | 96.4 (±9.8) | 93.9 | 0.05 | −11.80 (±2.09) | −0.80 (±0.15) |
| F50102 | 98.2 (±50.1) | ND | ND | 0.36 | +4.10 (±0.39) | +0.30 (±0.03) |
| F50105 | 929.2 (±119.3) | ND | ND | 0.49 | −8.10 (±3.40) | −0.60 (±0.26) |
| F70101C-NH | 552.3 (±79.1) | ND | ND | 0.59 | −7.50 (±0.01) | −0.50 (±0.01) |
| F70101-NH | 1154.4 (±334.2) | ND | ND | 0.54 | −7.20 (±0.72) | −0.50 (±0.05) |
| Liposomes | Kinetic LAL Test (OD 405 nm) | End-Point LAL Test (OD 540 nm) | ||
|---|---|---|---|---|
| Recovery Rate (%) | Endotoxin (EU/mg) | Recovery Rate (%) | Endotoxin (EU/mg) | |
| F10102 | 121 | 1.2 | 99 | 0.8 |
| F10103 | 135 | 14.5 | 93 | 8.4 |
| F20103A | NM | NM | 112 | 100.6 |
| F20104A | 131 | 0.7 | 110 | 1.0 |
| F50102 | NM | NM | NM | NM |
| F50105 | NM | NM | NM | NM |
| F70101C-NH | 111 | 660.0 | 107 | 273.3 |
| F70101-NH | 97 | 4.7 | 87 | 6.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Camera, G.; Lipsa, D.; Mehn, D.; Italiani, P.; Boraschi, D.; Gioria, S. A Step-by-Step Approach to Improve Clinical Translation of Liposome-Based Nanomaterials, a Focus on Innate Immune and Inflammatory Responses. Int. J. Mol. Sci. 2021, 22, 820. https://doi.org/10.3390/ijms22020820
Della Camera G, Lipsa D, Mehn D, Italiani P, Boraschi D, Gioria S. A Step-by-Step Approach to Improve Clinical Translation of Liposome-Based Nanomaterials, a Focus on Innate Immune and Inflammatory Responses. International Journal of Molecular Sciences. 2021; 22(2):820. https://doi.org/10.3390/ijms22020820
Chicago/Turabian StyleDella Camera, Giacomo, Dorelia Lipsa, Dora Mehn, Paola Italiani, Diana Boraschi, and Sabrina Gioria. 2021. "A Step-by-Step Approach to Improve Clinical Translation of Liposome-Based Nanomaterials, a Focus on Innate Immune and Inflammatory Responses" International Journal of Molecular Sciences 22, no. 2: 820. https://doi.org/10.3390/ijms22020820
APA StyleDella Camera, G., Lipsa, D., Mehn, D., Italiani, P., Boraschi, D., & Gioria, S. (2021). A Step-by-Step Approach to Improve Clinical Translation of Liposome-Based Nanomaterials, a Focus on Innate Immune and Inflammatory Responses. International Journal of Molecular Sciences, 22(2), 820. https://doi.org/10.3390/ijms22020820






