Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis
Abstract
1. Introduction
2. Pathogenesis of DAH
3. Diagnosis of DAH
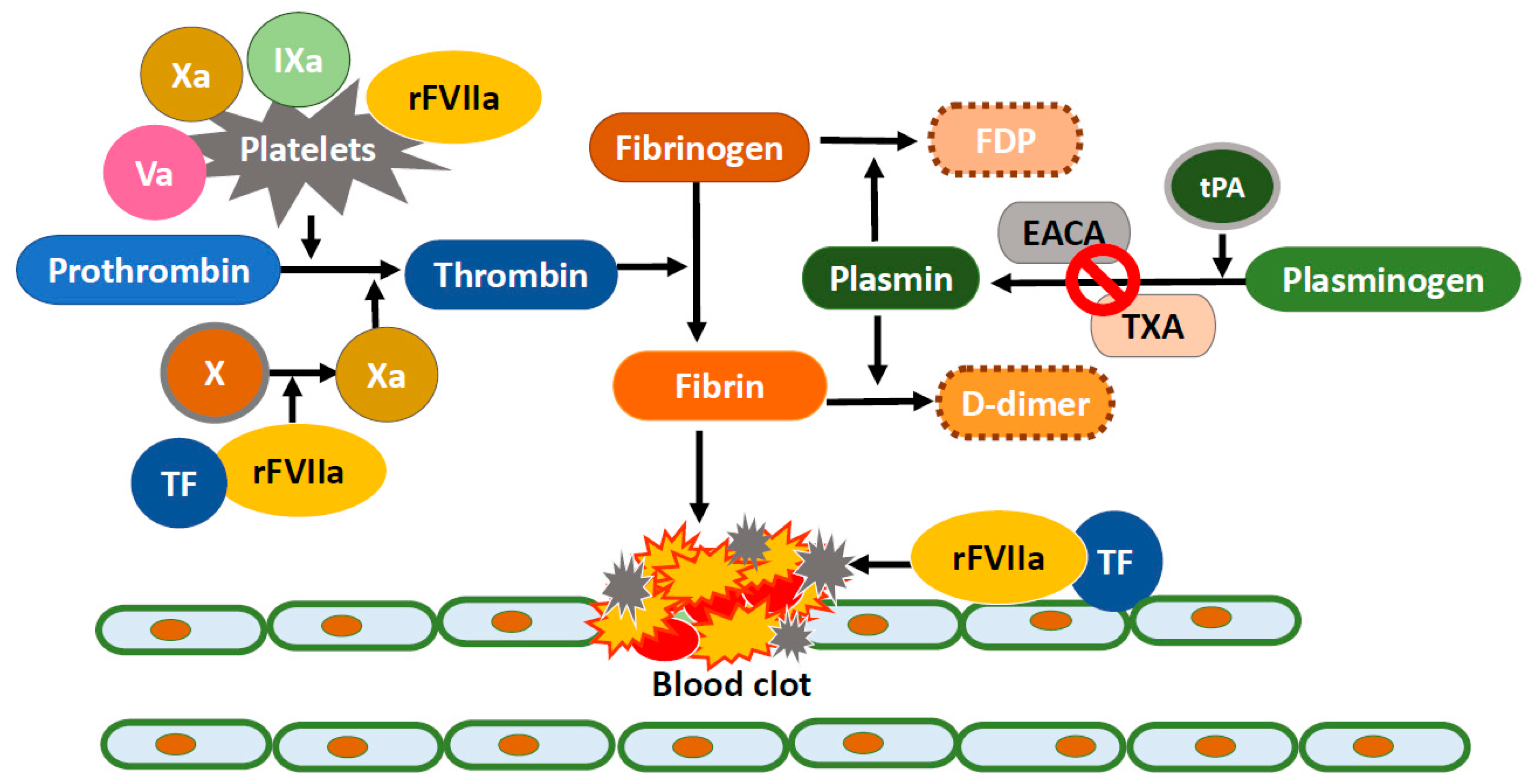
4. Treatment of DAH
5. Recombinant FVIIa Treatment for DAH
6. Recombinant FVIIa Treatment for DAH in Children
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | ANCA-associated vasculitis |
| ANA | anti-nuclear antibody |
| ANCA | anti-neutrophilic cytoplasmic antibody |
| APL | anti-phospholipid antibody |
| ARDS | Acute respiratory distress syndrome |
| BAL | bronchoalveolar lavage |
| β2GP | β-2glycoprotein1 |
| CL | anti-cardiolipin antibody |
| CR | complete response |
| CT | Chest computed tomography |
| DAH | Diffuse alveolar hemorrhage |
| DAMPs | danger-associated molecular patterns |
| EACA | Epsilon-amino-caproic acid |
| ESRD | end-stage renal disease |
| ETT | Endotracheal tube |
| GBM | Glomerular basement membrane |
| GVHD | graft-versus-host disease |
| HCT | Hematopoietic stem cell transplantation |
| HLH | Hemophagocytic lymphohistiocytosis |
| ICU | Intensive care unit |
| IPH | Idiopathic pulmonary hemosiderosis |
| NET | Neutrophil extra-cellular traps |
| NR | No response |
| PEEP | Positive end-expiratory pressure |
| PR | Partial response |
| RDS | Respiratory distress syndrome |
| FVIIa | Recombinant factor VIIa |
| RF | Rheumatoid factor |
| TF | Tissue factor |
| TFPI | Tissue factor pathway inhibitor |
| TXA | Tranexamic acid |
References
- Collard, H.R.; Schwarz, M.I. Diffuse alveolar hemorrhage. Clin. Chest Med. 2004, 25, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Blatny, J.; Mathew, P.; Monagle, P.; Ovesna, P.; Fiamoli, V. Safety and efficacy of recombinant activated factor VII in nonhemophilia children with severe or life-threatening bleeding: A report from the SeveNBleeP registry. Blood Coagul. Fibrinolysis 2014, 25, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Cetin, H.; Yalaz, M.; Akisu, M.; Karapinar, D.Y.; Kavakli, K.; Kultursay, N. The use of recombinant activated factor VII in the treatment of massive pulmonary hemorrhage in a preterm infant. Blood Coagul. Fibrinolysis 2006, 17, 213–216. [Google Scholar] [CrossRef]
- Pastores, S.M.; Papadopoulos, E.; Voigt, L.; Halpern, N.A. Diffuse alveolar hemorrhage after allogeneic hematopoietic stem-cell transplantation: Treatment with recombinant factor VIIa. Chest 2003, 124, 2400–2403. [Google Scholar] [CrossRef]
- Heslet, L.; Nielsen, J.D.; Levi, M.; Sengelov, H.; Johansson, P.I. Successful pulmonary administration of activated recombinant factor VII in diffuse alveolar hemorrhage. Crit. Care 2006, 10, R177. [Google Scholar] [CrossRef]
- Henke, D.; Falk, R.J.; Gabriel, D.A. Successful treatment of diffuse alveolar hemorrhage with activated factor VII. Ann. Intern. Med. 2004, 140, 493–494. [Google Scholar] [CrossRef]
- Heslet, L.; Nielsen, J.D.; Nepper-Christensen, S. Local pulmonary administration of factor VIIa (rFVIIa) in diffuse alveolar hemorrhage (DAH)—A review of a new treatment paradigm. Biologics 2012, 6, 37–46. [Google Scholar]
- Ioachimescu, O.C.; Stoller, J.K. Diffuse alveolar hemorrhage: Diagnosing it and finding the cause. Clevel. Clin. J. Med. 2008, 75, 258. [Google Scholar] [CrossRef]
- Lara, A.R.; Schwarz, M.I. Diffuse alveolar hemorrhage. Chest 2010, 137, 1164–1171. [Google Scholar] [CrossRef]
- Newsome, B.R.; Morales, J.E. Diffuse alveolar hemorrhage. South. Med. J. 2011, 104, 269–274. [Google Scholar] [CrossRef]
- Colby, T.V.; Fukuoka, J.; Ewaskow, S.P.; Helmers, R.; Leslie, K.O. Pathologic approach to pulmonary hemorrhage. Ann. Diagn. Pathol. 2001, 5, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Cordier, J.F.; Cottin, V. Alveolar hemorrhage in vasculitis: Primary and secondary. Semin. Respir. Crit. Care Med. 2011, 32, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, F.; Parambil, J.G.; Yi, E.S.; Decker, P.A.; Ryu, J.H. Haemosiderin-laden macrophages in the bronchoalveolar lavage fluid of patients with diffuse alveolar damage. Eur. Respir. J. 2009, 33, 1361–1366. [Google Scholar] [CrossRef]
- Castro, C.Y. ARDS and diffuse alveolar damage: A pathologist’s perspective. Semin. Thorac. Cardiovasc. Surg. 2006, 18, 13–19. [Google Scholar] [CrossRef]
- Parambil, J.G.; Myers, J.L.; Aubry, M.C.; Ryu, J.H. Causes and prognosis of diffuse alveolar damage diagnosed on surgical lung biopsy. Chest 2007, 132, 50–57. [Google Scholar] [CrossRef]
- Park, M.S. Diffuse alveolar hemorrhage. Tuberc. Respir. Dis. 2013, 74, 151–162. [Google Scholar] [CrossRef]
- Aggarwal, N.R.; King, L.S.; D’Alessio, F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L709–L725. [Google Scholar] [CrossRef]
- Franks, T.J.; Koss, M.N. Pulmonary capillaritis. Curr. Opin. Pulm. Med. 2000, 6, 430–435. [Google Scholar] [CrossRef]
- Zhuang, H.; Han, S.; Lee, P.Y.; Khaybullin, R.; Shumyak, S.; Lu, L.; Chatha, A.; Afaneh, A.; Zhang, Y.; Xie, C.; et al. Pathogenesis of Diffuse Alveolar Hemorrhage in Murine Lupus. Arthritis Rheumatol. 2017, 69, 1280–1293. [Google Scholar] [CrossRef]
- Short, K.R.; Kroeze, E.; Fouchier, R.A.M.; Kuiken, T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect. Dis. 2014, 14, 57–69. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Mistry, P.; Carmona-Rivera, C.; Ombrello, A.K.; Hoffmann, P.; Seto, N.L.; Jones, A.; Stone, D.L.; Naz, F.; Carlucci, P.; Dell’Orso, S.; et al. Dysregulated neutrophil responses and neutrophil extracellular trap formation and degradation in PAPA syndrome. Ann. Rheum. Dis. 2018, 77, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kronbichler, A.; Park, D.D.; Park, Y.; Moon, H.; Kim, H.; Choi, J.H.; Choi, Y.; Shim, S.; Lyu, I.S.; et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun. Rev. 2017, 16, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Jarrot, P.A.; Tellier, E.; Plantureux, L.; Crescence, L.; Robert, S.; Chareyre, C.; Daniel, L.; Secq, V.; Garcia, S.; Dignat-George, F.; et al. Neutrophil extracellular traps are associated with the pathogenesis of diffuse alveolar hemorrhage in murine lupus. J. Autoimmun. 2019, 100, 120–130. [Google Scholar] [CrossRef]
- Liu, S.; Su, X.; Pan, P.; Zhang, L.; Hu, Y.; Tan, H.; Wu, D.; Liu, B.; Li, H.; Li, Y.; et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci. Rep. 2016, 6, 37252. [Google Scholar] [CrossRef]
- Afessa, B.; Tefferi, A.; Litzow, M.R.; Krowka, M.J.; Wylam, M.E.; Peters, S.G. Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am. J. Respir. Crit. Care Med. 2002, 166, 641–645. [Google Scholar] [CrossRef]
- Sisson, J.H.; Thompson, A.B.; Anderson, J.R.; Robbins, R.A.; Spurzem, J.R.; Spence, P.R.; Reed, E.C.; Armitage, J.O.; Vose, J.M.; Arneson, M.A. Airway inflammation predicts diffuse alveolar hemorrhage during bone marrow transplantation in patients with Hodgkin disease. Am. Rev. Respir. Dis. 1992, 146, 439–443. [Google Scholar] [CrossRef]
- Piguet, P.F.; Grau, G.E.; Collart, M.A.; Vassalli, P.; Kapanci, Y. Pneumopathies of the graft-versus-host reaction. Alveolitis associated with an increased level of tumor necrosis factor mRNA and chronic interstitial pneumonitis. Lab. Investig. 1989, 61, 37–45. [Google Scholar]
- Capizzi, S.A.; Kumar, S.; Huneke, N.E.; Gertz, M.A.; Inwards, D.J.; Litzow, M.R.; Lacy, M.Q.; Gastineau, D.A.; Prakash, U.B.; Tefferi, A. Peri-engraftment respiratory distress syndrome during autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001, 27, 1299–1303. [Google Scholar] [CrossRef]
- Srivastava, A.; Gottlieb, D.; Bradstock, K.F. Diffuse alveolar haemorrhage associated with microangiopathy after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1995, 15, 863–867. [Google Scholar] [PubMed]
- Agustí, C.; Ramirez, J.; Picado, C.; Xaubet, A.; Carreras, E.; Ballester, E.; Torres, A.; Battochia, C.; Rodriguez-Roisin, R. Diffuse alveolar hemorrhage in allogeneic bone marrow transplantation. A postmortem study. Am. J. Respir. Crit. Care Med. 1995, 151, 1006–1010. [Google Scholar] [PubMed]
- Roychowdhury, M.; Pambuccian, S.E.; Aslan, D.L.; Jessurun, J.; Rose, A.G.; Manivel, J.C.; Gulbahce, H.E. Pulmonary complications after bone marrow transplantation: An autopsy study from a large transplantation center. Arch. Pathol. Lab. Med. 2005, 129, 366–371. [Google Scholar] [PubMed]
- Koh, H.; Nakamae, H.; Koh, K.R.; Ohsawa, M.; Nakane, T.; Takeoka, Y.; Aimoto, R.; Aimoto, M.; Wada-Inoue, E.; Terada, Y.; et al. Serum cytokine profiles at the onset of severe, diffuse alveolar hemorrhage complicating allogeneic hematopoietic stem cell transplantation, treated successfully with pulse intravenous cyclophosphamide. Acta Haematol. 2010, 124, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.L.; Cartin-Ceba, R.; Specks, U.; Peikert, T. Update on diffuse alveolar hemorrhage and pulmonary vasculitis. Immunol. Allergy Clin. N. Am. 2012, 32, 587–600. [Google Scholar] [CrossRef] [PubMed]
- De Lassence, A.; Fleury-Feith, J.; Escudier, E.; Beaune, J.; Bernaudin, J.F.; Cordonnier, C. Alveolar hemorrhage. Diagnostic criteria and results in 194 immunocompromised hosts. Am. J. Respir. Crit. Care Med. 1995, 151, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Perez-Arellano, J.L.; Losa Garcia, J.E.; Garcia Macias, M.C.; Gomez Gomez, F.; Jimenez Lopez, A.; de Castro, S. Hemosiderin-laden macrophages in bronchoalveolar lavage fluid. Acta Cytol. 1992, 36, 26–30. [Google Scholar]
- Golde, D.W.; Drew, W.L.; Klein, H.Z.; Finley, T.N.; Cline, M.J. Occult pulmonary haemorrhage in leukaemia. Br. Med. J. 1975, 2, 166–168. [Google Scholar] [CrossRef]
- Travis, W.D.; Colby, T.V.; Lombard, C.; Carpenter, H.A. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am. J. Surg. Pathol. 1990, 14, 1112–1125. [Google Scholar] [CrossRef]
- Susarla, S.C.; Fan, L.L. Diffuse alveolar hemorrhage syndromes in children. Curr. Opin. Pediatr. 2007, 19, 314–320. [Google Scholar] [CrossRef]
- Park, J.A. Diffuse alveolar hemorrhage and recombinant factor VIIa treatment in pediatric patients. Korean J. Pediatr. 2016, 59, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kirby, R.R.; Downs, J.B.; Civetta, J.M.; Modell, J.H.; Dannemiller, F.J.; Klein, E.F.; Hodges, M. High level positive end expiratory pressure (PEEP) in acute respiratory insufficiency. Chest 1975, 67, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Collino, F.; Maiolo, G.; Rapetti, F.; Romitti, F.; Tonetti, T.; Vasques, F.; Quintel, M. Positive end-expiratory pressure: How to set it at the individual level. Ann. Transl. Med. 2017, 5, 288. [Google Scholar] [CrossRef]
- Heggen, J.; West, C.; Olson, E.; Olson, T.; Teague, G.; Fortenberry, J.; Yeager, A.M. Diffuse alveolar hemorrhage in pediatric hematopoietic cell transplant patients. Pediatrics 2002, 109, 965–971. [Google Scholar] [CrossRef]
- Raptis, A.; Mavroudis, D.; Suffredini, A.; Molldrem, J.; Rhee, F.V.; Childs, R.; Phang, S.; Barrett, A. High-dose corticosteroid therapy for diffuse alveolar hemorrhage in allogeneic bone marrow stem cell transplant recipients. Bone Marrow Transplant. 1999, 24, 879–883. [Google Scholar] [CrossRef]
- Majhail, N.S.; Parks, K.; Defor, T.E.; Weisdorf, D.J. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: Related and high-risk clinical syndromes. Biol. Blood Marrow Transplant. 2006, 12, 1038–1046. [Google Scholar] [CrossRef]
- Rathi, N.K.; Tanner, A.R.; Dinh, A.; Dong, W.; Feng, L.; Ensor, J.; Wallace, S.K.; Haque, S.A.; Rondon, G.; Price, K.J.; et al. Low-, medium- and high-dose steroids with or without aminocaproic acid in adult hematopoietic SCT patients with diffuse alveolar hemorrhage. Bone Marrow Transplant. 2015, 50, 420–426. [Google Scholar] [CrossRef]
- Walsh, M.; Merkel, P.A.; Peh, C.A.; Szpirt, W.M.; Puéchal, X.; Fujimoto, S.; Hawley, C.M.; Khalidi, N.; Floßmann, O.; Wald, R.; et al. Plasma Exchange and Glucocorticoids in Severe ANCA-Associated Vasculitis. N. Engl. J. Med. 2020, 382, 622–631. [Google Scholar] [CrossRef]
- Klemmer, P.J.; Chalermskulrat, W.; Reif, M.S.; Hogan, S.L.; Henke, D.C.; Falk, R.J. Plasmapheresis therapy for diffuse alveolar hemorrhage in patients with small-vessel vasculitis. Am. J. Kidney Dis. 2003, 42, 1149–1153. [Google Scholar] [CrossRef]
- Gallagher, H.; Kwan, J.T.; Jayne, D.R. Pulmonary renal syndrome: A 4-year, single-center experience. Am. J. Kidney Dis 2002, 39, 42–47. [Google Scholar] [CrossRef]
- Nishimura, K.; Waki, D.; Kadoba, K.; Mukoyama, H.; Yokota, T.; Murabe, H. Efficacy of Plasma Exchange in Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis. Ther. Apher. Dial. 2019, 23, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Casian, A.; Flossmann, O.; Westman, K.; Höglund, P.; Pusey, C.; Jayne, D.R. Long-term follow-up of patients with severe ANCA-associated vasculitis comparing plasma exchange to intravenous methylprednisolone treatment is unclear. Kidney Int. 2013, 84, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Szczepiorkowski, Z.M.; Winters, J.L.; Bandarenko, N.; Kim, H.C.; Linenberger, M.L.; Marques, M.B.; Sarode, R.; Schwartz, J.; Weinstein, R.; Shaz, B.H.; et al. Guidelines on the use of therapeutic apheresis in clinical practice--evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J. Clin. Apher. 2010, 25, 83–177. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Jayne, D. Rituximab in the treatment of anti-neutrophil cytoplasm antibody associated vasculitis and systemic lupus erythematosus: Past, present and future. Kidney Int. 2007, 72, 676–682. [Google Scholar] [CrossRef][Green Version]
- Jones, R.B.; Ferraro, A.J.; Chaudhry, A.N.; Brogan, P.; Salama, A.D.; Smith, K.G.; Savage, C.O.; Jayne, D.R. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2009, 60, 2156–2168. [Google Scholar] [CrossRef]
- Keogh, K.A.; Wylam, M.E.; Stone, J.H.; Specks, U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005, 52, 262–268. [Google Scholar] [CrossRef]
- Stone, J.H.; Merkel, P.A.; Spiera, R.; Seo, P.; Langford, C.A.; Hoffman, G.S.; Kallenberg, C.G.; St Clair, E.W.; Turkiewicz, A.; Tchao, N.K.; et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 2010, 363, 221–232. [Google Scholar] [CrossRef]
- Keogh, K.A.; Ytterberg, S.R.; Fervenza, F.C.; Carlson, K.A.; Schroeder, D.R.; Specks, U. Rituximab for refractory Wegener’s granulomatosis: Report of a prospective, open-label pilot trial. Am. J. Respir. Crit. Care Med. 2006, 173, 180–187. [Google Scholar] [CrossRef]
- Cartin-Ceba, R.; Fervenza, F.C.; Specks, U. Treatment of antineutrophil cytoplasmic antibody-associated vasculitis with rituximab. Curr. Opin. Rheumatol. 2012, 24, 15–23. [Google Scholar] [CrossRef]
- Jones, R.B.; Tervaert, J.W.; Hauser, T.; Luqmani, R.; Morgan, M.D.; Peh, C.A.; Savage, C.O.; Segelmark, M.; Tesar, V.; van Paassen, P.; et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N. Engl. J. Med. 2010, 363, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, P.M. Hemostatic drugs. N. Engl. J. Med. 1998, 339, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Solomonov, A.; Fruchter, O.; Zuckerman, T.; Brenner, B.; Yigla, M. Pulmonary hemorrhage: A novel mode of therapy. Respir. Med. 2009, 103, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, E.R.; Schmees, L.R.; Resendiz, K.; Justino, H.; Anders, M.M. Inhaled Tranexamic Acid as a Novel Treatment for Pulmonary Hemorrhage in Critically Ill Pediatric Patients: An Observational Study. Crit. Care Explor. 2020, 2, e0075. [Google Scholar] [CrossRef] [PubMed]
- Bafaqih, H.; Chehab, M.; Almohaimeed, S.; Thabet, F.; Alhejaily, A.; AlShahrani, M.; Zolaly, M.A.; Abdelmoneim, A.A.; Abd, E.S. Pilot trial of a novel two-step therapy protocol using nebulized tranexamic acid and recombinant factor VIIa in children with intractable diffuse alveolar hemorrhage. Ann. Saudi Med. 2015, 35, 231–239. [Google Scholar] [CrossRef]
- Sanz, M.A.; Montesinos, P. Open issues on bleeding and thrombosis in acute promyelocytic leukemia. Thromb. Res. 2010, 125 (Suppl. 2), S51–S54. [Google Scholar] [CrossRef]
- Sander, M.; Spies, C.D.; Martiny, V.; Rosenthal, C.; Wernecke, K.D.; von Heymann, C. Mortality associated with administration of high-dose tranexamic acid and aprotinin in primary open-heart procedures: A retrospective analysis. Crit. Care 2010, 14, R148. [Google Scholar] [CrossRef]
- Marshall, A.; Li, A.; Drucker, A.; Dzik, W. Aminocaproic acid use in hospitalized patients with hematological malignancy: A case series. Hematol. Oncol. 2016, 34, 147–153. [Google Scholar] [CrossRef]
- Wanko, S.O.; Broadwater, G.; Folz, R.J.; Chao, N.J. Diffuse alveolar hemorrhage: Retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol. Blood Marrow Transplant. 2006, 12, 949–953. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Sasaki, H.; Nakamura, H. Treatment of hemoptysis patients by thrombin and fibrinogen-thrombin infusion therapy using a fiberoptic bronchoscope. Chest 1989, 96, 473–476. [Google Scholar] [CrossRef]
- de Gracia, J.; de la Rosa, D.; Catalán, E.; Alvarez, A.; Bravo, C.; Morell, F. Use of endoscopic fibrinogen-thrombin in the treatment of severe hemoptysis. Respir. Med. 2003, 97, 790–795. [Google Scholar] [CrossRef]
- Lee, J.; Rhee, C.K.; Kim, S.C.; Kim, Y.K.; Kim, H.J.; Lee, S.; Cho, S.G.; Lee, J.W. Use of intrapulmonary administration of thrombin in hematological malignancy patients with alveolar haemorrhage: A case series. Medicine 2020, 99, e20284. [Google Scholar] [CrossRef]
- Göbel, K.; Eichler, S.; Wiendl, H.; Chavakis, T.; Kleinschnitz, C.; Meuth, S.G. The Coagulation Factors Fibrinogen, Thrombin, and Factor XII in Inflammatory Disorders-A Systematic Review. Front. Immunol. 2018, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Lew, W.K.; Weaver, F.A. Clinical use of topical thrombin as a surgical hemostat. Biologics 2008, 2, 593–599. [Google Scholar]
- Baker, M.S.; Diab, K.J.; Carlos, W.G.; Mathur, P. Intrapulmonary Recombinant Factor VII as an Effective Treatment for Diffuse Alveolar Hemorrhage: A Case Series. J. Bronchol. Interv. Pulmonol. 2016, 23, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Haitsma, J.J.; Zhang, H.; Slutsky, A.S. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia—A review. Crit. Care Med. 2006, 34, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Millo, J.; Levi, M.; Hack, C.E.; Weverling, G.J.; Garrard, C.S.; van der Poll, T. Local activation of coagulation and inhibition of fibrinolysis in the lung during ventilator associated pneumonia. Thorax 2004, 59, 130–135. [Google Scholar] [CrossRef]
- Levi, M.; Schultz, M.J.; Rijneveld, A.W.; van der Poll, T. Bronchoalveolar coagulation and fibrinolysis in endotoxemia and pneumonia. Crit. Care Med. 2003, 31 (Suppl. 4), S238–S242. [Google Scholar] [CrossRef]
- Levi, M.; Levy, J.H.; Andersen, H.F.; Truloff, D. Safety of recombinant activated factor VII in randomized clinical trials. N. Engl. J. Med. 2010, 363, 1791–1800. [Google Scholar] [CrossRef]
- Stanworth, S.J.; Birchall, J.; Doree, C.J.; Hyde, C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst. Rev. 2007, CD005011. [Google Scholar] [CrossRef]
- Park, J.A.; Kim, B.J. Intrapulmonary recombinant factor VIIa for diffuse alveolar hemorrhage in children. Pediatrics 2015, 135, e216–e220. [Google Scholar] [CrossRef] [PubMed]
- Esper, R.C.; Estrada, I.E.; de la Torre Leon, T.; Gutierrez, A.O.; Lopez, J.A. Treatment of diffuse alveolar hemorrhage secondary to lupus erythematosus with recombinant activated factor VII administered with a jet nebulizer. J. Intensive Care 2014, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Ellery, P.E.; Adams, M.J. Tissue factor pathway inhibitor: Then and now. Semin. Thromb. Hemost. 2014, 40, 881–886. [Google Scholar] [CrossRef]
- Wood, J.P.; Ellery, P.E.; Maroney, S.A.; Mast, A.E. Biology of tissue factor pathway inhibitor. Blood 2014, 123, 2934–2943. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.; Killander, A.; Hippe, E.; Helleberg, C.; Ellegard, J.; Holm, M.; Kutti, J.; Mellqvist, U.H.; Johansson, J.E.; Glazer, S.; et al. Clinical experience with recombinant factor VIIa in patients with thrombocytopenia. Haemostasis 1996, 26 (Suppl. 1), 159–164. [Google Scholar] [PubMed]
- Al Hammadi, A.M.; Sallah, S. Efficacy and safety of recombinant factor VIIa in the treatment of bleeding episodes in patients with aplastic anemia. J. Thromb. Haemost. 2007, 5, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Farah, R.A.; Hamod, D.; Melick, N.; Giansily-Blaizot, M.; Sallah, S. Successful prophylaxis against intracranial hemorrhage using weekly administration of activated recombinant factor VII in a newborn with severe factor VII deficiency. J. Thromb. Haemost. 2007, 5, 433–434. [Google Scholar] [CrossRef]
- Tengborn, L.; Petruson, B. A patient with Glanzmann thrombasthenia and epistaxis successfully treated with recombinant factor VIIa. Thromb. Haemost. 1996, 75, 981–982. [Google Scholar] [PubMed]
- Kenet, G.; Walden, R.; Eldad, A.; Martinowitz, U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet 1999, 354, 1879. [Google Scholar] [CrossRef]
- Pihusch, M.; Bacigalupo, A.; Szer, J.; von Depka Prondzinski, M.; Gaspar-Blaudschun, B.; Hyveled, L.; Brenner, B. Recombinant activated factor VII in treatment of bleeding complications following hematopoietic stem cell transplantation. J. Thromb. Haemost. 2005, 3, 1935–1944. [Google Scholar] [CrossRef]
- Yadav, S.P.; Sachdeva, A.; Bhat, S.; Katewa, S. Successful control of massive gastrointestinal bleeding following umbilical cord blood transplantation (UCBT) by use of recombinant activated factor VII (rFVIIa) and octreotide infusion. Pediatr. Hematol. Oncol. 2010, 27, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Harkensee, C.; Vasdev, N.; Gennery, A.R.; Willetts, I.E.; Taylor, C. Prevention and management of BK-virus associated haemorrhagic cystitis in children following haematopoietic stem cell transplantation--a systematic review and evidence-based guidance for clinical management. Br. J. Haematol. 2008, 142, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.; Lin, Y.; Stanworth, S.; Birchall, J.; Doree, C.; Hyde, C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst. Rev. 2012, CD005011. [Google Scholar] [CrossRef]
- Brady, K.M.; Easley, R.B.; Tobias, J.D. Recombinant activated factor VII (rFVIIa) treatment in infants with hemorrhage. Paediatr. Anaesth. 2006, 16, 1042–1046. [Google Scholar] [CrossRef]
- Bhat, S.; Yadav, S.P.; Anjan, M.; Dinand, V.; Sachdeva, A. Recombinant activated factor VII usage in life threatening hemorrhage: A pediatric experience. Indian J. Pediatr. 2011, 78, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Elinoff, J.M.; Bagci, U.; Moriyama, B.; Dreiling, J.L.; Foster, B.; Gormley, N.J.; Salit, R.B.; Cai, R.; Sun, J.; Beri, A.; et al. Recombinant human factor VIIa for alveolar hemorrhage following allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2014, 20, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Dabar, G.; Harmouche, C.; Jammal, M. Efficacy of recombinant activated factor VII in diffuse alveolar haemorrhage. Rev. Mal. Respir. 2011, 28, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Estella, A.; Jareno, A.; Perez-Bello Fontaina, L. Intrapulmonary administration of recombinant activated factor VII in diffuse alveolar haemorrhage: A report of two case stories. Cases J. 2008, 1, 150. [Google Scholar] [CrossRef]
- Mandal, S.K.; Sagar, G.; Sahoo, M.; Jasuja, S. Recombinant activated factor VII for diffuse alveolar hemorrhage in microscopic polyangiitis. Indian J. Nephrol. 2012, 22, 130–132. [Google Scholar]
- Alabed, I.B. Treatment of diffuse alveolar hemorrhage in systemic lupus erythematosus patient with local pulmonary administration of factor VIIa (rFVIIa): A case report. Medicine 2014, 93, e72. [Google Scholar] [CrossRef]
- Khoulani, D.; Rao, B.; Khanshour, A.; Kuriakose, P.; Yessayan, L. Failure of Recombinant Activated Factor VII in Treatment of Diffuse Alveolar Hemorrhage due to Cryoglobulinemic Vasculitis. Case Rep. Hematol. 2014, 2014, 283086. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Kuhn, J.; Gabriel, D.; Barrow, J.; Jennette, J.C.; Henke, D.C. Use of Activated Factor VII in Patients with Diffuse Alveolar Hemorrhage: A 10 Years Institutional Experience. Lung 2015, 193, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.; Almeida, P.; Alvarez, M.; Ferrer, G.; Hernandez, F. Life-Threatening Pulmonary Hemorrhage Responds to Recombinant Factor VIIa: A Case Series in South Florida Hospitals. Cureus 2019, 11, e6202. [Google Scholar] [CrossRef] [PubMed]
- Meijer, K.; de Graaff, W.E.; Daenen, S.M.; van der Meer, J. Successful treatment of massive hemoptysis in acute leukemia with recombinant factor VIIa. Arch. Intern. Med. 2000, 160, 2216–2217. [Google Scholar] [CrossRef]
- White, B.; Martin, M.; Kelleher, S.; Browne, P.; McCann, S.R.; Smith, O.P. Successful use of recombinant FVIIa (Novoseven) in the management of pulmonary haemorrhage secondary to Aspergillus infection in a patient with leukaemia and acquired FVII deficiency. Br. J. Haematol. 1999, 106, 254–255. [Google Scholar] [CrossRef]
- Hicks, K.; Peng, D.; Gajewski, J.L. Treatment of diffuse alveolar hemorrhage after allogeneic bone marrow transplant with recombinant factor VIIa. Bone Marrow Transplant. 2002, 30, 975–978. [Google Scholar] [CrossRef]
- Yildirim, H.; Ucgun, I.; Yalcin, A.U.; Gulbas, Z.; Sahin, G.; Acikalin, M.F.; Metintas, M.; Ak, G. Recombinant factor VIIa treatment for life-threatening haemoptysis. Respirology 2006, 11, 652–654. [Google Scholar] [CrossRef]
- Macdonald, J.A.; Fraser, J.F.; Foot, C.L.; Tran, K. Successful use of recombinant factor VII in massive hemoptysis due to community-acquired pneumonia. Chest 2006, 130, 577–579. [Google Scholar] [CrossRef]
- Shenoy, A.; Savani, B.N.; Barrett, A.J. Recombinant factor VIIa to treat diffuse alveolar hemorrhage following allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2007, 13, 622–623. [Google Scholar] [CrossRef][Green Version]
- Gupta, S.; Jain, A.; Warneke, C.L.; Gupta, A.; Shannon, V.R.; Morice, R.C.; Onn, A.; Jimenez, C.A.; Bashoura, L.; Giralt, S.A.; et al. Outcome of alveolar hemorrhage in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007, 40, 71–78. [Google Scholar] [CrossRef]
- Lau, E.M.T.; Yozghatlian, V.; Kosky, C.; Moriarty, C.; Dentice, R.; Waugh, R.; Torzillo, P.J.; Bye, P.T. Recombinant activated factor VII for massive hemoptysis in patients with cystic fibrosis. Chest 2009, 136, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Tatopoulos, A.; Herbain, D.; Kazmierczak, C.; Bollaert, P.E.; Gibot, S. Parenteral use of recombinant activated factor VII during diffuse alveolar hemorrhage secondary to leptospirosis. Intensive Care Med. 2010, 36, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Tsuchiya, K.; Fujisawa, N. Risk factors of diffuse alveolar hemorrhage after acute ischemic stroke treated with tissue-type plasminogen activator. The effectiveness of activated recombinant factor VII treatment. Surg. Neurol. Int. 2020, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Witmer, C.M.; Huang, Y.S.; Lynch, K.; Raffini, L.J.; Shah, S.S. Off-label recombinant factor VIIa use and thrombosis in children: A multi-center cohort study. J. Pediatr. 2011, 158, 820–825.e1. [Google Scholar] [CrossRef]
- McQuilten, Z.K.; Barnes, C.; Zatta, A.; Phillips, L.E. Off-label use of recombinant factor VIIa in pediatric patients. Pediatrics 2012, 129, e1533–e1540. [Google Scholar] [CrossRef]
- MacLaren, R.; Weber, L.A.; Brake, H.; Gardner, M.A.; Tanzi, M. A multicenter assessment of recombinant factor VIIa off-label usage: Clinical experiences and associated outcomes. Transfusion 2005, 45, 1434–1442. [Google Scholar] [CrossRef]
- O’Connell, K.A.; Wood, J.J.; Wise, R.P.; Lozier, J.N.; Braun, M.M. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA 2006, 295, 293–298. [Google Scholar] [CrossRef]
- Young, G.; Wicklund, B.; Neff, P.; Johnson, C.; Nugent, D.J. Off-label use of rFVIIa in children with excessive bleeding: A consecutive study of 153 off-label uses in 139 children. Pediatr. Blood Cancer 2009, 53, 179–183. [Google Scholar] [CrossRef]
- Fekete, M.; Nemeth, A. Neonatal pulmonary haemorrhage, birthweight, gestational age and intrauterine growth. Acta Paediatr. Hung. 1985, 26, 65–73. [Google Scholar] [PubMed]
- Larcombe, P.J.; Kapur, N.; Fraser, C.J.; Coulthard, M.G.; Schlapbach, L.J. Intrabronchial administration of activated recombinant factor VII in a young child with diffuse alveolar hemorrhage. Pediatr. Blood Cancer 2014, 61, 570–571. [Google Scholar] [CrossRef] [PubMed]
- Veldman, A.; Fischer, D.; Voigt, B.; Beyer, P.A.; Schlosser, R.; Allendorf, A.; Kreuz, W. Life-threatening hemorrhage in neonates: Management with recombinant activated factor VII. Intensive Care Med. 2002, 28, 1635–1637. [Google Scholar] [CrossRef] [PubMed]
- Olomu, N.; Kulkarni, R.; Manco-Johnson, M. Treatment of severe pulmonary hemorrhage with activated recombinant factor VII (rFVIIa) in very low birth weight infants. J. Perinatol. 2002, 22, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Leibovitch, L.; Kenet, G.; Mazor, K.; Matok, I.; Vardi, A.; Barzilay, Z.; Paret, G. Recombinant activated factor VII for life-threatening pulmonary hemorrhage after pediatric cardiac surgery. Pediatr. Crit. Care Med. 2003, 4, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Blatt, J.; Gold, S.H.; Wiley, J.M.; Monahan, P.E.; Cooper, H.C.; Harvey, D. Off-label use of recombinant factor VIIa in patients following bone marrow transplantation. Bone Marrow Transplant. 2001, 28, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Grizelj, R.; Vukovic, J.; Filipovic-Grcic, B.; Saric, D.; Luetic, T. Successful use of recombinant activated FVII and aminocaproic acid in four neonates with life-threatening hemorrhage. Blood Coagul. Fibrinolysis 2006, 17, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Colin, A.A.; Shafieian, M.; Andreansky, M. Bronchoscopic instillation of activated recombinant factor VII to treat diffuse alveolar hemorrhage in a child. Pediatr. Pulmonol. 2010, 45, 411. [Google Scholar] [CrossRef]
| Classification | Etiology | Nonspecific Findings | Specific Findings | Treatment | - | |
|---|---|---|---|---|---|---|
| Immune-mediated | ANCA-associated vasculitis | Granulomatosis with polyangiitis (Wegener granulomatosis) | Prolonged PT/aPTT/PT INR | c-ANCA, | Immunosuppressants | Corticosteroids |
| Microscopic polyangiitis | Thrombocytonia | p-ANCA (MPO-antibody) | Cyclophosphamide | |||
| Eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) | Leukocytosis | Rituximab | ||||
| Anti-phospholipid antibody syndrome | Eosinophilia | APL, lupus anticoagulant, anti-CL, anti-β2GP1 antibody | Azathioprine | |||
| SLE | Increased ESR | ANA, anti-dsDNA, anti-SM, anti-histone antibody | MMF | |||
| RA | Hematuria | RF | IVIG | |||
| Inflammatory myopathies | Patch diffuse alveolar infiltrates or | |||||
| Henoch-Schönlein purpura | Anti-CL IgA antibody | Removal of autoantibodies | Plasmapheresis | |||
| Ig A nephropathy | Air bronchogram on chest radiograph | |||||
| Anti-GBM antibody syndrome (Goodpasture’s syndrome) | anti-GBM antibody | Procoagulation | Fresh frozen plasma | |||
| Cryoglobulinemia | Platelet transfusion | |||||
| Behçet’s disease | Cryoprecipitates | |||||
| Hypocomplementemic urticarial vasculitis (anti-C1q vasculitis) | Vitamin K supplement | |||||
| Lung transplant rejection | TXA | |||||
| Drug-induced vasculitis | ACA | |||||
| Medications: warfarin, aspirin, amiodarone, phenytoin | FVIIa | |||||
| Idiopathic pulmonary capillaritis | Thrombin | |||||
| Non-immune mediated | Coagulopathy | |||||
| Non-cardiovascular disease | Withdrawal of offending medications | |||||
| Infection | Pseudomonas aeruginosa | |||||
| Aspergillus spp. | Broad-spectrum antibiotics | |||||
| CMV and herpes pneumonitis | ||||||
| Diffuse alveolar damage | Radiation | Antifungal agents | ||||
| Cytotoxic drugs | ||||||
| Acute respiratory distress syndrome | Antiviral therapy | |||||
| Hematopoietic stem cell transplantation | ||||||
| Idiopathic pulmonary hemosiderosis | Diuresis | |||||
| Cardiovascular disease | Pulmonary SOS | Kerley B lines on chest radiograph | ||||
| Mitral stenosis | Cardiac medical optimization | |||||
| Arteriovenous malformation | ||||||
| Pulmonary lymphangiomyomatosis | ||||||
| Pulmonary hypertension | ||||||
| Pulmonary capillary hemangiomatosis | ||||||
| Left ventricular dysfunction | ||||||
| References | Year | Patients (Sex/Age) | Previous History | Route | Dose of FVIIa | Additional Therapies | Outcome | TE Complication | Case of Death |
|---|---|---|---|---|---|---|---|---|---|
| Immune related | |||||||||
| Henke et al. [6] | 2004 | M/53 | ANCA-vasculitis (n = 2), viral infection | IV | 120 µg/kg × 3 doses | CS, CPM, MMF, plasmapheresis | CR | none | 0 |
| M/25 | SLE, APS, nephritis, pleural effusion | IV | 90 µg/kg × 3 doses | CS | CR | none | 0 | ||
| Heslet et al. [5]. | 2006 | M/63 | Sarcoidosis, septic shock | IP | 50 µg/kg × 1 dose | TXA, aprotinin, desmopressin | CR | none | 1 |
| F/34 | Wegener’s granulomatosis, Churg-Strauss vasculitis | IP | 50 µg/kg × 1doses | TXA, aprotinin | CR | none | 0 | ||
| Dabar et al. [97] | 2011 | NA | ANCA-vasculitis | NA | 90 µg/kg × 1 dose | none | CR | none | 0 |
| Mandal et al. [99] | 2012 | F/23 | Microscopic polyangitis (pulmonary renal vasculitis), necrotizing glomerulonephritis | IV | 90 µg/kg × 2 doses | MMF, plasmaphersis | CR | none | 0 |
| Esper et al. [82] | 2014 | F/37 | SLE, Sjögren syndrome | IP | 50 µg/kg × 1dose | CS, RTX | CR | none | 0 |
| Alabed et al. [100] | 2014 | F/37 | SLE, lupus nephritis | IP | 75 µg/kg × 1 dose | CS, CPM | CR | none | 0 |
| Khoulani et al. [101] | 2014 | F/51 | NHL, lupus nephritis, cryoglobulinemia, bacterial pneumonia | IV | 90 µg/kg × 3 doses | CS, RTX, plasmapheresis | NR | none | 1 |
| Pathak et al. [102] | 2015 | 47 ± 19 years | ANCA vasculitis (n = 9) | IV | 75 µg/kg × 4 doses | CS, CPM or RTX, IVIG Plasmapheresis | CR (9/9) | none | 1/9 |
| Good pasture’s syndrome (n = 3) | CR (2/3), NR (1/3) | none | 1/3 | ||||||
| SLE (n = 2) | CR (2/2) | none | 0 | ||||||
| ITP (n = 1) | CR (1/1) | none | 0 | ||||||
| Cryoglobulinemia (n = 1) | CR (1/1) | none | 0 | ||||||
| Baker et al. [75] | 2016 | F/23 | SLE | IP | 50 µg/kg × 1dose | CS, EACA, IVIG, CPM, RTX, plasmapheresis | PR | none | 1 |
| Diaz et al. [103] | 2019 | M/67 | Wegener’s granulomatosis, bacterial pneumonia | IP | 30 µg/kg × 1 dose | CS, desmopressin | CR | none | 0 |
| F/61 | SLE, pulmonary HTN, pneumonia | IP | 50 µg/kg × 1dose | CS, plasmapheresis | CR | none | 1 | ||
| F/22 | Pulmonary sarcoidosis, pulmonary embolism, pneumonia | IP | 50 µg/kg × 1dose | ECMO | CR | none | 1 | ||
| Non-immune related | |||||||||
| Meijer et al. [104] | 2000 | M/49 | ALL, fungal pneumonia | IV | 90 µg/kg × 1 dose | TXA, antifungal agents | CR | none | 1 |
| White et al. [105] | 2001 | M/ns | MDS, AML, Aspergillus pneumonia | IV | 90 µg/kg × 4 doses | Antifungal agents | CR | none | 0 |
| Hicks et al. [106] | 2002 | F/35 | AML, HCT, GVHD, fungal pneumonia | IV | 90 µg/kg × 4 doses | CS, EACA, desmopressin | CR | none | 0 |
| Pastores et al. [4] | 2003 | M/48 | NHL, HCT, GVHD | IV | 90 µg/kg × 2 doses | CS | CR | none | 0 |
| Henke et al. [6] | 2004 | M/28 | Acute leukemia, HCT | IV | 120 µg/kg→180 µg/kg | CS | CR | none | 1 |
| Yildirim et al. [107] | 2006 | M/23 | Pulmonary renal syndrome | IV | 90 µg/kg × 3 doses | none | CR | none | 0 |
| Macdonald et al. [108] | 2006 | M/52 | CAP | IV | 90 µg/kg × 1 dose | none | CR | none | 0 |
| Heslet et al. [5] | 2006 | M/46 | CLL, HCT, GVHD, CMV pneumonia | IV /IP | 50 µg/kg IV × 3 doses/50 µg/kg IP × 2 doses | TXA, aprotinin | PR | none | 1 |
| M/44 | AML, pneumonia | IP | 50 µg/kg × 1 dose | TXA, aprotinin | CR | none | 0 | ||
| F/44 | AIDS, C. difficile colitis, Pseudomonas pneumonia, CMV infection | IP | 50 µg/kg × 1 doses | TXA, aprotinin | PR | none | 1 | ||
| M/63 | AML, HCT, GVHD, pneumonia | IP | 50 µg/kg × 1 dose | TXA, aprotinin | CR | none | 0 | ||
| Shenoy et al. [109] | 2007 | F/NA | AML, HCT, pneumonia | IV | 90 µg/kg × 2 doses | CS | CR | none | 0 |
| Gupta et al. [110] | 2007 | n = 24 | HCT | NA | NA | CS (n = 24), desmopressin (n = 4), EACA (n = 3) | NA | none | NA |
| Estella et al. [98] | 2008 | F/39 | AML, renal failure | IP | 50 µg/kg × 1dose | None | CR | none | 0 |
| M/46 | IV drug abuse, hepatitis B and C, HIV, myocarditis, aspirin & clopidogrel | IP | 50 µg/kg × 1dose | None | CR | none | 0 | ||
| Lau et al. [111] | 2009 | M/33 | Cystic fibrosis | IV | 90 µg/kg × 1 dose | BAE | CR | none | 0 |
| M/22 | Cystic fibrosis, liver failure, liver TPL, pneumonia | IV | 90 µg/kg × 1 dose | BAE | CR | none | 1 | ||
| F/23 | Cystic fibrosis, lung infection, ARDS | IV | 120 µg/kg × 2 doses | BAE | CR | none | 1 | ||
| M/27 | Cystic fibrosis, suppurative lung disease | IV | 90 µgkg × 2 doses | BAE | PR/CR | none | 0 | ||
| Tatopoulous et al. [112] | 2010 | M/53 | Leptospirosis, ARF, ARDS | IV | 105 µg/kg × 1 dose | CS | CR | none | 0 |
| Dabar et al. [97] | 2011 | NA | Leukemia | NA | 90 µg/kg × 1 dose | none | CR | none | 0 |
| Elinoff et al. [96] | 2014 | n = 23 (M:F =13:10), Age 36 (9–66) years | Diagnosis: ALL (n = 1), AML (n = 3), CLL (n = 2), HD (n = 1), AA (n = 6), MDS (n = 1), other (n = 5). Preceding conditions: HCT (n = 23), CMV (n = 14), aGVHD (n = 1), cGVHD (n = 8), DLI (n = 6), stem cell boost (n = 5), ARDS | IV | 41 µg/kg × 3 doses, total dose 16mg, (4.8–37.6 mg) | CS (n = 23), Desmopressin (n = 8), EACA (n = 2), Estrogen (n = 1) | NA | 44/43 episodes: blood clot obstruction an e-tube (n = 1), basilic vein thrombosis (n = 1). DIC (n = 2) | 15/23 |
| Pathak et al. [102] | 2015 | years | HCT (n = 7) | IV | 75 µg/kg × 4 doses | CS, CPM or RTX, IVIG Plasmapheresis | CR (7/7) | none | 6/7 |
| Baker et al. [75] | 2016 | F/49 | MDS, HCT | IP | 30 µg/kg × 2 dose | CS, EACA | CR | none | 0 |
| M/64 | End-stage liver disease, clopidogrel treatment | IP | 30 µg/kg × 2 dose | CS | CR | none | 0 | ||
| F/68 | Metastatic anal cell carcinoma, ARDS | IP | 30 µg/kg × 2 dose | CS | CR | none | 0 | ||
| F/23 | HCT | IP | 30 µg/kg × 1 dose | CS | CR | none | 0 | ||
| F/84 | Burn, inhaled injury | IP | 60 µg/kg × 1 dose | CS, EACA | CR | none | 0 | ||
| Diaz et al. [103] | 2019 | F/46 | Septic shock | IP | 50 µg/kg × 1dose | none | CR | none | 0 |
| Shimizu et al. [113] | 2020 | M/68 | Acute ischemic stroke, t-PA treatment | IV | 75 µg/kg × 1dose | CS | CR | none | 0 |
| M/54 | Acute ischemic stroke, t-PA treatment | IP | 75 µg/kg × 1dose | CS | CR | none | 0 | ||
| Reference | Year | Patients (Sex/Age) | Previous History | Route | Dose of FVIIa | Additional Therapies | Outcome | TE Complication | Case of Death |
|---|---|---|---|---|---|---|---|---|---|
| Immune-related | |||||||||
| Bafaquih et al. [121] | 2015 | 3/8 patients, (M:F = 4:4), 2 (0.5–9) years | Connective tissue disorder (n = 3), respiratory infection (n = 3), MOF (n = 1) | IP | 35–50 µg/kg × 3–6 dose | TXA | 3CR | none | 0 |
| Congestive heart failure associated | |||||||||
| Veldman et al. [122] | 2002 | M/preterm | VLBW, RDS, PDA, IVH, PDA ligation | IV | 200 µg/kg × 2 doses | none | CR | none | 0 |
| Olomu et al. [123] | 2002 | M/preterm | VLBW, RDS, PDA, sepsis, DIC, PIE | IV | 50 µg/kg × 6 doses | none | CR | none | 0 |
| F/preterm | VLBW, RDS, PDA, Sepsis, DIC | IV | 50 µg/kg × 16 doses | none | CR | none | 0 | ||
| Leibovitch et al. [124] | 2003 | F/2 months | Down syndrome, CHD, cardiac surgery | IV | 100 µg/kg × 4 doses | TXA | CR | none | 0 |
| Bafaquih et al. [121] | 2015 | 1/8 patients | Cardiovascular disease, ARDS, infection | IP | 35–50 µg/kg × 3–6 dose | TXA | 1CR | none | 0 |
| Miscellaneous | |||||||||
| Blatt et al. [125] | 2001 | F/8 years | AML, HCT, HC | IV | 270 µg/kg × 1 dose → 90 µg/kg × 28 doses | CS | PR | none | 1 |
| Cetin et al. [3] | 2006 | M/preterm | LBW, RDS, sepsis, DIC | IV | 120 µg/kg × 3 doses | none | PR | none | 0 |
| Brady et al. [94] | 2006 | F/2 days | MMA, DIC, HD for hyperammonemia | IV | 90 µg/kg × 2 doses | CR | none | 1 | |
| M/2 days | Pseudomonal sepsis | IV | 90 µg/kg × 1 dose | CR | none | 0 | |||
| Grizelj et al. [126] | 2006 | NA/0 days | MAS, ventilator care | IV | 170 µg/kg × 1 dose | EACA | CR | none | 0 |
| NA/13 days | HLH, postsurgical resuscitation | 130 µg/kg × 1 dose | EACA | CR | none | 0 | |||
| NA/2 days | HLH, postsurgical resuscitation | 222 µg/kg × 1 dose | EACA | CR | none | 0 | |||
| Young et al. [119] | 2009 | 12 patients | NA | IV | 90 µg/kg × 1 dose (range, 20.3–353 µg/kg) | 4 CR, 5PR, 3NR | 1 LV thrombus | NA | |
| Bhat et al. [95] | 2011 | M/14 years | DSS, sepsis | IV | 70 µg/kg × 1 dose | Anti-D | CR | none | 0 |
| M/13 years | AML, TLS, ARF, acute pancreatitis | IV | 90 µg/kg × 1 dose | none | PR | none | 1 | ||
| F/9 years | Thalassemia, major, HCT, ARDS | IV | 90 µg/kg × 1 dose | octreotide | CR | none | 0 | ||
| F/13 years | ALL, febrile neutropenia, sepsis | IV | 70 µg/kg × 1 dose | none | NR | none | 1 | ||
| M/10 years | AML | IV | 75 µg/kg × 1 dose | none | NR | none | 1 | ||
| Colin et al. [126] | 2010 | M/17 years | AML, pancytopenia, sepsis | IP | 50 µg/kg × 1 dose | CS | CR | none | 0 |
| Larcombe et al. [99] | 2014 | M/2 years | AML, HCT, hepatic SOS, GVHD | IP | 50 µg/kg × 1 dose | None | CR | ETT thrombus | 0 |
| Park et al. [81] | 2015 | F/11 years | MDS, HCT, HC, TMA | IP | 60 µg/kg × 1 dose | CS, TXA, RTX | CR | none | 1 |
| M/15 years | AML, DIC, cytarabine syndrome | IP | 45 µg/kg × 1 dose | CS | CR | none | 0 | ||
| M/6 years | T-LL, chickenpox infection, hepatic sinusoidal obstruction syndrome | IP | 43 µg/kg × 1 dose | CS | CR | none | 0 | ||
| M/14 years | AML, DIC | IP | 52 µg/kg × 1 dose | CS | CR | none | 0 | ||
| F/10 months | HLH, HCT, CMV infection, hepatic sinusoidal obstruction syndrome | IP | 63 µg/kg × 1 dose | CS, TXA, RTX | CR | none | 1 | ||
| Bafaquih et al. [121] | 2015 | 4/8 patients, (M:F = 4:4), 2 (0.5–9) years | Connective tissue disorder (n = 3), respiratory infection (n = 7), MOF (n = 1), cardiovascular disease (n = 1), ALL (n = 1), HLH (n = 1) | IP | 35–50 µg/kg × 1 dose | TXA | 2 CR, 2 PR | none | 2/8 |
| Idiopathic | |||||||||
| Bhat et al. [95] | 2011 | F/6 years | IPH, pneumothorax, and septic shock | IV | 60 µg/kg × 1 dose | none | PR | none | 1 |
| Park et al. [81] | 2015 | F/11 years | IPH | IP | 57 µg/kg × 1 dose | CS | CR | none | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.A. Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis. Int. J. Mol. Sci. 2021, 22, 793. https://doi.org/10.3390/ijms22020793
Park JA. Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis. International Journal of Molecular Sciences. 2021; 22(2):793. https://doi.org/10.3390/ijms22020793
Chicago/Turabian StylePark, Jeong A. 2021. "Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis" International Journal of Molecular Sciences 22, no. 2: 793. https://doi.org/10.3390/ijms22020793
APA StylePark, J. A. (2021). Treatment of Diffuse Alveolar Hemorrhage: Controlling Inflammation and Obtaining Rapid and Effective Hemostasis. International Journal of Molecular Sciences, 22(2), 793. https://doi.org/10.3390/ijms22020793




