Multiscale Regulation of the Intervertebral Disc: Achievements in Experimental, In Silico, and Regenerative Research
Abstract
1. Introduction
2. IVD Extracellular Matrix in Health and Disease
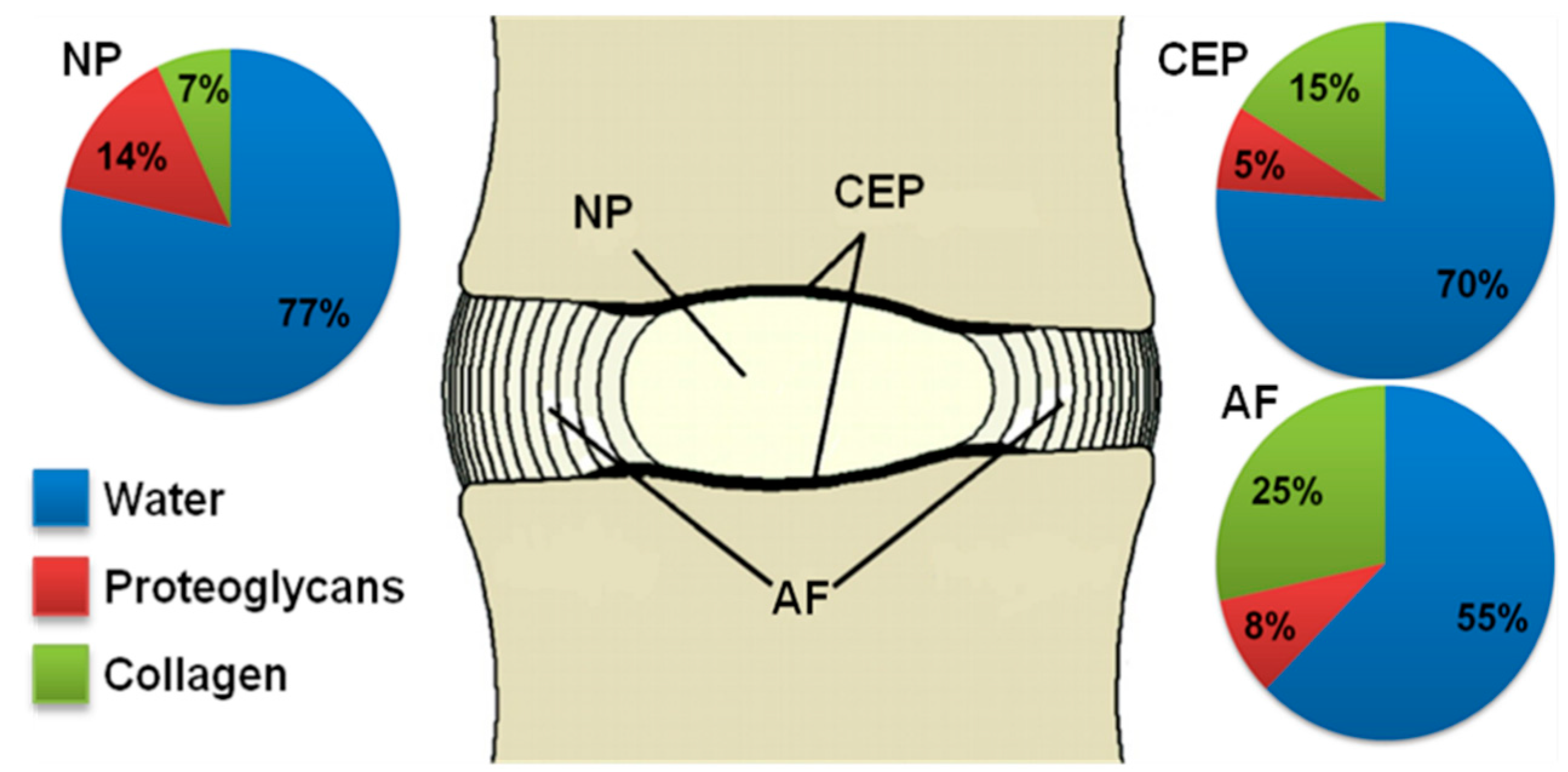
2.1. Proteoglycans
2.2. Collagen
2.3. Water
3. IVD Cell Activity and Molecular Biology in Health and Disease
3.1. Multifactorial Regulation of Cell Activity in Health
3.2. Multifactorial Regulation of Cell Activity in Disease
4. IVD Regeneration Strategies: Biological Targets and Biomaterials
4.1. Signaling Pathways and Biological Targets
- ERK activation typically occurs via mitogens and GF (e.g., platelet-derived growth factor (PDGF), transforming growth factors β1 and β3 (TGF-β1, TGF-β3), fibroblast growth factor (FGF), and insulin-like growth factor (IGF) I [242,243,244]), thereby controlling growth, differentiation, cell cycle progression, and development. In addition, ERK activation in the IVD supports cell survival following hypoxia and osmotic stress, the latter with cross talk to TonEBP [118,119,120,121,245,246,247]. Interestingly, NP-derived mesenchymal stromal cells (MSC) also respond to osmotic stimuli, whereby hyperosmotic stress was associated with ERK activation, leading to a reduction in proliferation and chondrogenic differentiation [248]. Interestingly, excessive cyclic stretch was shown to induce AF apoptosis via inhibition of ERK phosphorylation, whereby β1 integrin could inhibit the apoptotic processes [249]. Pro-inflammatory cytokines, such as TNF-α and IL-1β, as well as stimuli known to induce inflammation, such as ECM fragments, activate the ERK pathway in IVD cells, possibly mediating loss of tissue ECM proteins associated with DD [31,55,250,251,252,253], inflammatory and catabolic responses [250,254,255], apoptosis [256], and senescence [257]. Interestingly, ERK was suppressed by stimulation with the anti-inflammatory cytokine IL-10 [254]. Overall, these findings indicate that modulating ERK activity for therapeutic means is possible yet challenging due to the multifactorial role of this signaling pathway.
- The p38 signaling pathway is generally activated by stressors and is known to regulate inflammation, autophagy, apoptosis, and differentiation [241]. Numerous studies have investigated p38 in the IVD, thereby identifying hypoxia [245], hyperosmolarity [120], hyperphysiological mechanical loads [133], ER stress [258], acidity [257], high glucose levels [256], and IL-1 [253] as potent activators. Interestingly, p38 is connected to TPRV4 [133], which has previously been described to transduce mechanical, inflammatory, and pain signals in cartilage [259]. Different research fields have shown extensive cross talk between p38 and other signaling pathways, e.g., ERK [258], TGF-β/Smad, [260] or Akt [261], which should be investigated in IVD cells. Overall, inhibition of p38 is being discussed for therapeutic approaches, potentially reducing inflammation, pain, and disc matrix catabolism [253,262], although ultimate outcomes may be difficult to predict due to the extensive cross talk with other pathways.
- JNK, similar to p38, is activated by stressors, GF, and pro-inflammatory cytokines [241,253]. Stressors entail high glucose levels [256], hyperosmolarity [120,263], TNF-α and IL-1β exposure [250,251,255], syndecan-4 overexpression [264], and Propionibacterium acnes (P. acnes) infection [265]. Following activation, JNK regulates apoptosis [120,256,265], enhanced expression of MMP [250], DNA damage [263], and DD [264]. The pro-apoptotic mechanisms of JNK seem to be associated with p53 induction [266] and with toll-like receptor 2 activation [265]. Although not yet investigated in the IVD, the interaction of JNK with miRNAs (e.g., miR-138, miR-133a-3p, miR-133b-3p, miR-4268) is likely relevant [267,268,269]. Therefore, a better understanding of JNK signaling will be needed before its modulation can be effectively used as a therapeutic means.
4.2. Growth Factor-Based Strategies
4.3. Cell Therapy-Based Strategies
4.4. Biomaterials and Nanotechnologies
5. Systems’ Modeling for the Exploration of IVD Degenerative and Regenerative Mechanisms
5.1. Organ- and Tissue-Scale Simulations of the IVD Biophysical Regulation
5.2. IVD Cell Models and Integration of Experimental Cell Stimulation Data
5.3. Cell Signalling Pathway Models and Integration of Multi-Omics Data
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| AF | Annulus fibrosus |
| BMP | Bone morphogenetic protein |
| CEP | Cartilage edplate |
| DD | IVD degeneration |
| ECM | Extracellular matrix |
| ER | Endoplasmatic recticulum |
| ERK | Extracellular signal-regulated kinase |
| FA | Focal adhesion |
| FGF | Fibroblast growth factor |
| GAG | Glycosaminoglycan |
| GDF | Growth differentiation factor |
| GF | Growth factor |
| HIF | Hypoxia inducible factor |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| IVD | Intervertebral disc |
| JNK | c-Jun NH2terminal kinase |
| LBP | Low back pain |
| MAPK | Mitogen-activated protein kinase |
| MMP | Metalloproteinase |
| MP | Microparticle |
| MSC | Mesenchymal stromal cell |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor kappa B |
| NGF | Nerve growth factor |
| NP | Nucleus pulposus |
| p38 | p38 MAPK |
| PCL | Poly-ε-caprolactone |
| PDGF | Platelet-derived growth factor |
| PDLLA | Poly D,L-lactide |
| PEG | Polyethylene glycol |
| PG | Proteoglycan |
| PGA | Polyglycolic acid |
| PKN | Prior-knowledge-network |
| PLGA | Polylactic-co-glycolic acid |
| PNIPAM | Poly N-isopropylacrylamide |
| PU | Polyurethane |
| TE | Tissue engineering |
| TGF | Transforming growth factor |
| TNF-α | Tumor necrosis factor alpha |
| TonEBP | Tonicity-responsive enhancer binding protein |
| TonEBP/NFAT5 | Tonicity-responsive enhancer binding protein/nuclear factor of activated T-cells 5 |
| TRP | Transient receptor potential |
References
- Shapiro, I.M.; Risbud, M.V. The Intervertebral Disc-Molecular and Structural Studies of the Disc in Health and Disease; Springer: Vienna, Austria, 2016; ISBN 978-3-7091-1534-3. [Google Scholar]
- Urban, J.P.G.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef][Green Version]
- Le Maitre, C.L.; Pockert, A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 2007, 35, 652–655. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J. Pathol. 2004, 204, 47–54. [Google Scholar] [CrossRef]
- Roberts, S.; Caterson, B.; Menage, J.; Evans, E.H.; Jaffray, D.C.; Eisenstein, S.M. Matrix metalloproteinases and aggrecanase: Their role in disorders of the human intervertebral disc. Spine 2000, 25, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Sztrolovics, R.; Alini, M.; Roughley, P.J.; Mort, J.S. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem. J. 1997, 326, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Weiler, C.; Nerlich, A.G.; Zipperer, J.; Bachmeier, B.E.; Boos, N. SSE Award Competition in Basic Science: Expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur. Spine J. 2002, 11, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, J.; Steffen, T.; Nelson, F.; Winterbottom, N.; Hollander, A.P.; Poole, R.A.; Aebi, M.; Alini, M. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Invest. 1996, 98, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Benneker, L.M.; Heini, P.F.; Anderson, S.E. Correlation of radiographic and MRI parameters to morphological and biochemical assessment of intervertebral disc degeneration. Eur. Spine J. 2005, 14, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pfirrmann, C.W.A.; Metzdorf, A.; Zanetti, M.; Hodler, J.; Boos, N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001, 26, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Galbusera, F.; van Rijsbergen, M.; Ito, K.; Huyghe, J.M.; Brayda-Bruno, M.; Wilke, H.-J. Ageing and degenerative changes of the intervertebral disc and their impact on spinal flexibility. Eur. Spine J. 2014, 23, 324–332. [Google Scholar] [CrossRef]
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Wognum, S.; Huyghe, J.M.; Baaijens, F.P.T. Influence of osmotic pressure changes on the opening of existing cracks in 2 intervertebral disc models. Spine 2006, 31, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Osti, O.L.; Vernon-Roberts, B.; Moore, R.; Fraser, R.D. Annular tears and disc degeneration in the lumbar spine—A post-mortem study of 135 discs. J. Bone Jt. Surg. Br. 1992, 74, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Lama, P.; Le Maitre, C.L.; Dolan, P.; Tarlton, J.F.; Harding, I.J.; Adams, M.A. Do intervertebral discs degenerate before they herniate, or after? Bone Jt. J. 2013, 95, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pilgram, T.; Wippold, F.J. Association between annular tears and disk degeneration: A longitudinal study. Am. J. Neuroradiol. 2009, 30, 500–506. [Google Scholar] [CrossRef]
- Adams, M.A.; Dolan, P. Intervertebral disc degeneration: Evidence for two distinct phenotypes. J. Anat. 2012, 221, 497–506. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Urquhart, D.M.; Wang, Y.; Wluka, A.E.; O’Sullivan, R.; Jones, G.; Cicuttini, F.M. Lumbar disc degeneration is associated with modic change and high paraspinal fat content—A 3.0T magnetic resonance imaging study. BMC Musculoskelet. Disord. 2016, 17, 439. [Google Scholar] [CrossRef]
- Kerttula, L.; Luoma, K.; Vehmas, T.; Grönblad, M.; Kääpa, E. Modic type i change may predict rapid progressive, deforming disc degeneration: A prospective 1-year follow-up study. Eur. Spine J. 2012, 21, 1135–1142. [Google Scholar] [CrossRef]
- Munir, S.; Freidin, M.B.; Rade, M.; Määttä, J.; Livshits, G.; Williams, F.M.K. Endplate defect is heritable, associated with low back pain and triggers intervertebral disc degeneration: A longitudinal study from Twinsuk. Spine 2018, 43, 1496–1501. [Google Scholar] [CrossRef]
- Määttä, J.H.; Rade, M.; Freidin, M.B.; Airaksinen, O.; Karppinen, J.; Williams, F.M.K. Strong association between vertebral endplate defect and Modic change in the general population. Sci. Rep. 2018, 8, 16630. [Google Scholar] [CrossRef]
- Fardon, D.F.; Williams, A.L.; Dohring, E.J.; Murtagh, F.R.; Rothman, S.L.G.; Sze, G.K. Lumbar Disc Nomenclature: Version 2.0: Recommendations of the Combined Task Forces of the North American Spine Society, the American Society of Spine Radiology, and the American Society of Neuroradiology. Spine 2014, 39, E1448–E1465. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, A.J.; Andrew, T.; Sambrook, P.N.; Spector, T.D. Structural, psychological, and genetic influences on low back and neck pain: A study of adult female twins. Arthritis Care Res. 2004, 51, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Popham, M.; Malkin, I.; Sambrook, P.N.; MacGregor, A.J.; Spector, T.; Williams, F.M.K. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: The UK Twin Spine Study. Ann. Rheum. Dis. 2011, 70, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, K.-U.; Niederer, P.F.; Cronin, D.S.; Morrison, B., III; Muser, M.H.; Walz, F. Trauma Biomechanics; Springer: Berlin, Germany, 2019; ISBN 9783030116583. [Google Scholar]
- Battié, M.C.; Videman, T.; Kaprio, J.; Gibbons, L.E.; Gill, K.; Manninen, H.; Saarela, J.; Peltonen, L. The Twin Spine Study: Contributions to a changing view of disc degeneration†. Spine J. 2009, 9, 47–59. [Google Scholar] [CrossRef]
- Sambrook, P.N.; MacGregor, A.J.; Spector, T.D. Genetic influences on cervical and lumbar disc degeneration: A magnetic resonance imaging study in twins. Arthritis Rheum. 1999, 42, 366–372. [Google Scholar] [CrossRef]
- Hughes, S.P.F.; Freemont, A.J.; Hukins, D.W.L.; McGregor, A.H.; Roberts, S. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J. Bone Jt. Surg. J. Bone Jt. Surg. Br. 2012, 94, 1298–1304. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 2005, 7, R732–R745. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of Cytokines in Intervertebral Disc Degeneration: Pain and Disc-content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Séguin, C.A.; Pilliar, R.M.; Madri, J.A.; Kandel, R.A. TNF-α Induces MMP2 Gelatinase Activity and MT1-MMP Expression in an In Vitro Model of Nucleus Pulposus Tissue Degeneration. Spine 2008, 33, 356–365. [Google Scholar] [CrossRef]
- Wang, J.; Markova, D.; Anderson, D.G.; Zheng, Z.; Shapiro, I.M.; Risbud, M.V. TNF-a and IL-1b promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J. Biol. Chem. 2011, 286, 39738–39749. [Google Scholar] [CrossRef]
- Dudli, S.; Liebenberg, E.; Magnitsky, S.; Lu, B.; Lauricella, M.; Lotz, J.C. Modic type 1 change is an autoimmune response that requires a proinflammatory milieu provided by the “Modic disc”. Spine J. 2018, 18, 831–844. [Google Scholar] [CrossRef]
- Huang, Y.C.; Urban, J.P.G.; Luk, K.D.K. Intervertebral disc regeneration: Do nutrients lead the way? Nat. Rev. Rheumatol. 2014, 10, 561–566. [Google Scholar] [CrossRef]
- Baumgartner, L.; Reagh, J.J.; González Ballester, M.A.; Noailly, J. Simulating intervertebral disc cell behaviour within 3D multifactorial environments. Bioinformatics 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Zhu, Q.; Gao, X.; Brown, M.D. Simulation of the Progression of Interverteral Disc Degeneration due to Decreased Nutrition Supply. Spine 2014, 39, E1411–E1417. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Wills, C.; Foata, B.; González Ballester, M.Á.; Karppinen, J.; Noailly, J. Theoretical Explorations Generate New Hypotheses About the Role of the Cartilage Endplate in Early Intervertebral Disk Degeneration. Front. Physiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.P. Intervertebral disc: Anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008, 8, 18–44. [Google Scholar] [CrossRef]
- Setton, L.A.; Chen, J. Cell mechanics and mechanobiology in the intervertebral disc. Spine 2004, 29, 2710–2723. [Google Scholar] [CrossRef]
- Schroeder, Y.; Huyghe, J.M.; Van Donkelaar, C.C.; Ito, K. A biochemical/biophysical 3D FE intervertebral disc model. Biomech. Model. Mechanobiol. 2010, 9, 641–650. [Google Scholar] [CrossRef]
- DeLucca, J.F.; Cortes, D.H.; Jacobs, N.T.; Vresilovic, E.J.; Duncan, R.L.; Elliott, D.M. Human cartilage endplate permeability varies with degeneration and intervertebral disc site. J. Biomech. 2016, 49, 550–557. [Google Scholar] [CrossRef]
- Brickley-Parsons, D.; Glimcher, M.J. Is the chemistry of Collagen in Intervertebral Discs an Expression of Wolff’s Law? A Study of the Human Lumbar Spine. Spine 1984, 9, 148–163. [Google Scholar] [CrossRef]
- Tavakoli, J.; Elliott, D.M.; Costi, J.J. Structure and mechanical function of the inter-lamellar matrix of the annulus fibrosus in the disc. J. Orthop. Res. 2016, 34, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.S.; Tsitron, E.; Wachtel, E.; Roughley, P.J.; Sakkee, N.; Van Der Ham, F.; DeGroot, J.; Roberts, S.; Maroudas, A. Aggrecan turnover in human intervertebral disc as determined by the racemization of aspartic acid. J. Biol. Chem. 2006, 281, 13009–13014. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.S.; Wachtel, E.; Tsitron, E.; Sakkee, N.; Van Der Ham, F.; DeGroot, J.; Roberts, S.; Maroudas, A. Collagen turnover in normal and degenerate human intervertebral discs as determined by the racemization of aspartic acid. J. Biol. Chem. 2008, 283, 8796–8801. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Wills, C. A Computational Study of Intervertebral Disc Degeneration in Relation to Changes in Regional Tissue Composition and Disc Nutrition. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2015. [Google Scholar]
- Smith, L.J.; Nerurkar, N.L.; Choi, K.-S.; Harfe, B.D.; Elliott, D.M. Degeneration and regeneration of the intervertebral disc: Lessons from development. Dis. Model. Mech. 2011, 4, 31–41. [Google Scholar] [CrossRef]
- Roberts, S.; Urban, J.P.G. Intervertebral discs. In Encyclopaedia of Occupational Health and Safety; International Labour Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Singh, K.; Masuda, K.; Thonar, E.J.-M.; An, H.S.; Cs-Szabo, G. Age-related changes in the Extracellular Matrix of Nucleus Pulposus and Anulus Fibrosus of Human Intervertebral Disc. Spine 2009, 34, 10–16. [Google Scholar] [CrossRef]
- Fields, A.J.; Rodriguez, D.; Gary, K.N.; Liebenberg, E.C.; Lotz, J.C. Influence of biochemical composition on endplate cartilage tensile properties in the human lumbar spine. J. Orthop. Res. 2014, 32, 245–252. [Google Scholar] [CrossRef]
- Roughley, P.J.; Melching, L.I.; Heathfield, T.F.; Pearce, R.H.; Mort, J.S. The structure and degradation of aggrecan in human intervertebral disc. Eur. Spine J. 2006, 15, S326–S332. [Google Scholar] [CrossRef]
- van Rijsbergen, M.M.; Barthelemy, V.M.P.; Vrancken, A.C.T.; Crijns, S.P.M.; Wilke, H.J.; Wilson, W.; van Rietbergen, B.; Ito, K. Moderately degenerated lumbar motion segments: Are they truly unstable? Biomech. Model. Mechanobiol. 2017, 16, 537–547. [Google Scholar] [CrossRef]
- Joyce, K.; Isa, I.L.M.; Fahey, R.; Creemers, L.; Devitt, A.; Pandit, A. The Glycomic Profile of the Intervertebral Disc in Health and Degeneration for Biomaterial Functionalization. Orthop. Proc. 2018, 100, 117. [Google Scholar]
- Pandit, A.; Mohd Isa, L. United States, Patent Application Publication 2020.
- Quero, L.; Klawitter, M.; Schmaus, A.; Rothley, M.; Sleeman, J.; Tiaden, A.N.; Klasen, J.; Boos, N.; Hottiger, M.O.; Wuertz, K.; et al. Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signalling pathways. Arthritis Res. Ther. 2013, 15, R94. [Google Scholar] [CrossRef]
- Veres, S.P.; Robertson, P.; Broom, N.D. ISSLS Prize Winner: Microstructure and Mechanical Disruption of the Lumbar Disc Annulus—Part II: How the Annulus Fails Under Hydrostatic Pressure. Spine 2008, 33, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Kanna, R.M.; Senthil, N.; Raveendran, M.; Cheung, K.M.C.; Chan, D.; Subramaniam, S.; Shetty, A.P. Phenotype variations affect genetic association studies of degenerative disc disease: Conclusions of analysis of genetic association of 58 single nucleotide polymorphisms with highly specific phenotypes for disc degeneration in 332 subjects. Spine J. 2013, 13, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Urban, J.P.; Evans, H.; Eisenstein, S.M. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine 1996, 21, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.G.; Slichter, C.K.; Acosta, F.L.; Rodriguez-Soto, A.E.; Burghardt, A.J.; Majumdar, S.; Lotz, J.C. Human disc nucleus properties and vertebral endplate permeability. Spine 2011, 36, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Horvath, I.; Foran, D.J. Mechanical implications of the domain structure of fiber-forming collagens: Comparison of the molecular and fibrillar flexibilities of the α1-chains found in types I-III collagen. J. Theor. Biol. 2002, 216, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.J.; Schmid, T.M.; Eyre, D.R. Assembly of collagen types II, IX and XI into nascent hetero-fibrils by a rat chondrocyte cell line. Eur. J. Biochem. 2003, 270, 3243–3250. [Google Scholar] [CrossRef]
- Vázquez-Portalatín, N.; Kilmer, C.E.; Panitch, A.; Liu, J.C. Characterization of Collagen Type I and II Blended Hydrogels for Articular Cartilage Tissue Engineering. Biomacromolecules 2016, 17, 3145–3152. [Google Scholar] [CrossRef]
- Bruehlmann, S.B.; Rattner, J.B.; Matyas, J.R.; Duncan, N.A. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J. Anat. 2002, 201, 159–171. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Babu, J.N.; Arun, R.; Armstrong, B.R.W.; Shetty, A.P.; Murugan, S. ISSLS prize winner: A study of diffusion in human lumbar discs: A serial magnetic resonance imaging study documenting the influence of the endplate on diffusion in normal and degenerate discs. Spine 2004, 29, 2654–2667. [Google Scholar] [CrossRef]
- Accadbled, F.; Laffosse, J.M.; Ambard, D.; Gomez-Brouchet, A.; De Gauzy, J.S.; Swider, P. Influence of Location, Fluid Flow Direction, and Tissue Maturity on the Macroscopic Permeability of Vertebral End Plates. Spine 2008, 33, 612–619. [Google Scholar] [CrossRef]
- Gu, W.Y.; Mao, X.G.; Foster, R.J.; Weidenbaum, M.; Mow, V.C.; Rawlins, B.A. The anisotropic hydraulic permeability of human lumbar anulus fibrosus: Influence of age, degeneration, direction, and water content. Spine 1999, 24, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Poillot, P.; O’Donnell, J.; O’Connor, D.T.; Ul Haq, E.; Silien, C.; Tofail, S.A.M.; Huyghe, J.M. Piezoelectricity in the Intervertebral disc. J. Biomech. 2020, 102, 109622. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Wills, C.; Malandrino, A.; Van Rijsbergen, M.; Lacroix, D.; Ito, K.; Noailly, J. Simulating the sensitivity of cell nutritive environment to composition changes within the intervertebral disc. J. Mech. Phys. Solids 2016, 90, 108–123. [Google Scholar] [CrossRef]
- Iatridis, J.C.; MacLean, J.J.; O’Brien, M.; Stokes, I.A.F. Measurements of Proteoglycan and Water Content Distribution in Human Lumbar Intervertebral Discs. Spine 2007, 32, 1493–1497. [Google Scholar] [CrossRef]
- Duance, V.C.; Crean, J.K.G.; Sims, T.J.; Avery, N.; Smith, S.; Menage, J.; Eisenstein, S.M.; Roberts, S. Changes in Collagen Cross-Linking in Degenerative Disc Disease and Scoliosis. Spine 1998, 23, 2545–2551. [Google Scholar] [CrossRef]
- Pokharna, H.K.; Phillips, F.M. Collagen Crosslinks in Human Lumbar Intervertebral Disc Aging. Spine 1998, 23, 1645–1648. [Google Scholar] [CrossRef]
- Gruber, H.E.; Hanley, E.N. Observations on morphologic changes in the aging and degenerating human disc: Secondary collagen alterations. BMC Musculoskelet. Disord. 2002, 3, 9. [Google Scholar] [CrossRef][Green Version]
- Nerlich, A.G.; Boos, N.; Wiest, I.; Aebi, M. Immunolocalization of major interstitial collagen types in human lumbar intervertebral discs of various ages. Virchows Arch. 1998, 432, 67–76. [Google Scholar] [CrossRef]
- Hayes, A.; Shu, C.; Lord, M.; Little, C.; Whitelock, J.; Melrose, J. Pericellular colocalisation and interactive properties of type VI collagen and perlecan in the intervertebral disc. Eur. Cells Mater. 2016, 32, 40–57. [Google Scholar] [CrossRef]
- Hodson, N.W.; Patel, S.; Richardson, S.M.; Hoyland, J.A.; Gilbert, H.T.J. Degenerate intervertebral disc-like pH induces a catabolic mechanoresponse in human nucleus pulposus cells. JOR Spine 2018, 1, e1004. [Google Scholar] [CrossRef]
- Duncan, N.A. Cell Deformation and Micromechanical Environment in the Intervertebral Disc. J. Bone Jt. Surg. 2006, 88, 47–51. [Google Scholar]
- Wilusz, R.E.; Sanchez-Adams, J.; Guilak, F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014, 39, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Roos, R.W.; Petterson, R.; Huyghe, J.M. Confined compression and torsion experiments on a pHEMA gel in various bath concentrations. Biomech. Model. Mechanobiol. 2013, 12, 617–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huyghe, J.M.; Houben, G.B.; Drost, M.R.; van Donkelaar, C.C. An ionised/non-ionised dual porosity model of intervertebral disc tissue. Biomech. Model. Mechanobiol. 2003, 2, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, Y.; Sivan, S.; Wilson, W.; Merkher, Y.; Huyghe, J.M.; Maroudas, A.; Baaijens, F.P.T. Are Disc Pressure, Stress, and Osmolarity Affected by Intra- and Extrafibrillar Fluid Exchange? J. Orthop. Res. 2007, 25, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Encyclopaedia of Occupational Health and Safety, 4th ed.; International Labour Organization: Geneva, Switzerland, 1998.
- Maroudas, A.; Stockwell, R.A.; Nachemson, A.; Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J. Anat. 1975, 120, 113–130. [Google Scholar] [PubMed]
- Liebscher, T.; Haefeli, M.; Wuertz, K.; Nerlich, A.G.; Boos, N. Age-Related Variation in Cell Density of Human Lumbar Intervertebral Disc. Spine 2011, 36, 153–159. [Google Scholar] [CrossRef]
- Tomaszewski, K.A.; Walocha, J.; Mizia, E.; Gładysz, T.; Głowacki, R.; Tomaszewska, R. Age- and degeneration-related variations in cell density and glycosaminoglycan content in the human cervical intervertebral disc and its endplates. Polish J. Pathol. 2015, 66, 296–309. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Spine Update Aging and Degeneration of the Human Intervertebral Disc. Spine 1995, 11, 1307–1314. [Google Scholar] [CrossRef]
- Mwale, F.; Roughley, P.; Antoniou, J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: A requisite for tissue engineering of intervertebral disc. Eur. Cells Mater. 2004, 8, 58–64. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Binch, A.L.A.; Creemers, L.B.; Sammon, C.; Le Maitre, C.L. Nucleus pulposus phenotypic markers to determine stem cell differentiation: Fact or fiction? Oncotarget 2016, 7, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Schoepflin, Z.R.; Mwale, F.; Kandel, R.A.; Grad, S.; Iatridis, J.C.; Sakai, D.; Hoyland, J.A. Defining the phenotype of young healthy nucleus pulposus cells: Recommendations of the Spine Research Interest Group at the 2014 annual ORS meeting. J. Orthop. Res. 2015, 33, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Notochordal Cells in the Adult Intervertebral Disc: New Perspective on an Old Question. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 29–41. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.R.; Tamplin, O.J.; Rossant, J.; Seguin, C.A. Tracing notochord-derived cells using a Noto-cre mouse: Implications for intervertebral disc development. Dis. Model. Mech. 2012, 5, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W.; Au, T.Y.K.; Tam, V.; Cheah, K.S.E.; Chan, D. Coming together is a beginning: The making of an intervertebral disc. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Sélard, É.; Shirazi-Adl, A.; Urban, J.P.G. Finite Element Study of Nutrient Diffusion in the Human Intervertebral Disc. Spine 2003, 28, 1945–1953. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Bach, F.C.; Tryfonidou, M.A.; Le Maitre, C.L.; Mwale, F.; Diwan, A.D.; Ito, K. Leaping the hurdles in developing regenerative treatments for the intervertebral disc from preclinical to clinical. JOR Spine 2018, 1, e1027. [Google Scholar] [CrossRef]
- Chen, J.-W.; Li, B.; Yang, Y.-H.; Jiang, S.-D.; Jiang, L.-S. Significance of Hypoxia in the Physiological Function of Intervertebral Disc Cells. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 193–204. [Google Scholar] [CrossRef]
- Soukane, D.M.; Shirazi-Adl, A.; Urban, J.P. Analysis of Nonlinear Coupled Diffusion of Oxygen and Lactic Acid in Intervertebral Discs. J. Biomech. Eng. 2005, 127, 1121–1126. [Google Scholar] [CrossRef]
- Mokhbi Soukane, D.; Shirazi-Adl, A.; Urban, J.P.G. Investigation of solute concentrations in a 3D model of intervertebral disc. Eur. Spine J. 2009, 18, 254–262. [Google Scholar] [CrossRef]
- Kraemer, J.; Kolditz, D.; Gowin, R. Water and Electrolyte Content of Human Intervertebral Discs Under Variable Load. Spine 1985, 10, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Bibby, S.R.S.; Jones, D.; Ripley, R.M.; Urban, J.P.G. Metabolism of the intervertebral disc: Effects of low levels of oxygen, glucose, and pH on rates of energy metabolism of bovine nucleus pulposus cells. Spine 2005, 30, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Silagi, E.S.; Novais, E.J.; Bisetto, S.; Telonis, A.G.; Snuggs, J.; Le Maitre, C.L.; Qiu, Y.; Kurland, I.J.; Shapiro, I.M.; Philp, N.J.; et al. Lactate Efflux From Intervertebral Disc Cells Is Required for Maintenance of Spine Health. J. Bone Miner. Res. 2020, 35, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Silagi, E.S.; Schoepflin, Z.R.; Seifert, E.L.; Merceron, C.; Schipani, E.; Shapiro, I.M.; Risbud, M. V Bicarbonate Recycling by HIF-1-Dependent Carbonic Anhydrase Isoforms 9 and 12 Is Critical in Maintaining Intracellular pH and Viability of Nucleus Pulposus Cells. J. Bone Miner. Res. 2018, 33, 338–355. [Google Scholar] [CrossRef]
- Carroll, V.A.; Ashcroft, M. Targeting the molecular basis for tumour hypoxia. Expert Rev. Mol. Med. 2005, 7, 1–16. [Google Scholar] [CrossRef]
- Li, H.; Liang, C.Z.; Chen, Q.X. Regulatory Role of Hypoxia Inducible Factor in the Biological Behavior of Nucleus Pulposus Cells. Yonsei Med. J. 2013, 54, 807–812. [Google Scholar] [CrossRef]
- Chen, J.-W.; Ni, B.-B.; Zheng, X.-F.; Li, B.; Jiang, S.-D.; Jiang, L.-S. Hypoxia facilitates the survival of nucleus pulposus cells in serum deprivation by down-regulating excessive autophagy through restricting ROS generation. Int. J. Biochem. Cell Biol. 2015, 59, 1–10. [Google Scholar] [CrossRef]
- Richardson, S.M.; Knowles, R.; Tyler, J.; Mobasheri, A.; Hoyland, J.A. Expression of glucose transporters GLUT-1, GLUT-3, GLUT-9 and HIF-1α in normal and degenerate human intervertebral disc. Histochem. Cell Biol. 2008, 129, 503–511. [Google Scholar] [CrossRef]
- Meng, X.; Zhuang, L.; Wang, J.; Liu, Z.; Wang, Y.; Xiao, D.; Zhang, X. Hypoxia-inducible factor (HIF)-1alpha knockout accelerates intervertebral disc degeneration in mice. Int. J. Clin. Exp. Pathol. 2018, 11, 548–557. [Google Scholar]
- Han, S.; Xu, W.; Wang, Z.; Qi, X.; Wang, Y.; Ni, Y.; Shen, H.; Hu, Q.; Han, W. Crosstalk between the HIF-1 and Toll-like receptor/nuclear factor-κB pathways in the oral squamous cell carcinoma microenvironment. Oncotarget 2016, 7, 37773–37789. [Google Scholar] [CrossRef]
- Risbud, M.V.; Schipani, E.; Shapiro, I.M. Hypoxic regulation of nucleus pulposus cell survival: From niche to notch. Am. J. Pathol. 2010, 176, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, W.; Jiang, S.; Ye, W.; Yang, H.; Shapiro, I.M.; Risbud, M.V. Prolyl-4-hydroxylase Domain Protein 2 Controls NF-κB/p65 Transactivation and Enhances the Catabolic Effects of Inflammatory Cytokines on Cells of the Nucleus Pulposus. J. Biol. Chem. 2015, 290, 7195–7207. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.; Hogg, D.; Whitehouse, G.H.; Dangerfield, P. Quantitative analysis of diurnal variation in volume and water content of lumbar intervertebral discs. Clin. Anat. 1998, 11, 1–8. [Google Scholar] [CrossRef]
- Urban, J.P.G. The Chondrocyte: A Cell under Pressure. Rheumatology 1994, 33, 901–908. [Google Scholar] [CrossRef]
- Ishihara, H.; Warensjo, K.; Roberts, S.; Urban, J.P. Proteoglycan synthesis in the intervertebral disk nucleus: The role of extracellular osmolality. Am. J. Physiol. Physiol. 1997, 272, C1499–C1506. [Google Scholar] [CrossRef]
- van Dijk, B.; Potier, E.; Ito, K. Culturing Bovine Nucleus Pulposus Explants by Balancing Medium Osmolarity. Tissue Eng. Part C Methods 2011, 17, 1089–1096. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Shapiro, I.M.; Risbud, M.V. Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: Evolving role of TonEBP. Matrix Biol. 2014, 40, 10–16. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Danielson, K.G.; Guttapalli, A.; Oguz, E.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. TonEBP/OREBP Is a Regulator of Nucleus Pulposus Cell Function and Survival in the Intervertebral Disc. J. Biol. Chem. 2006, 281, 25416–25424. [Google Scholar] [CrossRef]
- Halterman, J.A.; Kwon, H.M.; Wamhoff, B.R. Tonicity-independent regulation of the osmosensitive transcription factor TonEBP (NFAT5). Am. J. Physiol. Physiol. 2012, 302, C1–C8. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Doolittle, A.C.; Snuggs, J.W.; Shapiro, I.M.; Le Maitre, C.L.; Risbud, M.V. TNF-α promotes nuclear enrichment of the transcription factor TonEBP/NFAT5 to selectively control inflammatory but not osmoregulatory responses in nucleus pulposus cells. J. Biol. Chem. 2017, 292, 17561–17575. [Google Scholar] [CrossRef]
- Hiyama, A.; Gajghate, S.; Sakai, D.; Mochida, J.; Shapiro, I.M.; Risbud, M.V. Activation of TonEBP by Calcium Controls β1,3-Glucuronosyltransferase-I Expression, a Key Regulator of Glycosaminoglycan Synthesis in Cells of the Intervertebral Disc. J. Biol. Chem. 2009, 284, 9824–9834. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.; Kameda, T.; Krupkova, O. Wuertz-Kozak Osmosensing, osmosignalling and inflammation: How intervertebral disc cells respond to altered osmolarity. Eur. Cells Mater. 2018, 36, 231–250. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-T.; Guttapalli, A.; Agrawal, A.; Albert, T.J.; Shapiro, I.M.; Risbud, M. V MEK/ERK Signaling Controls Osmoregulation of Nucleus Pulposus Cells of the Intervertebral Disc by Transactivation of TonEBP/OREBP. J. Bone Miner. Res. 2007, 22, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-H.; Wang, D.; Liu, T.-T.; Li, F.; Liu, R.; Wei, J.; Zhou, C. The Roles of MAPKs in Rabbit Nucleus Pulposus Cell Apoptosis Induced by High Osmolality. Glob. Spine J. 2014, 18, 2835–2845. [Google Scholar]
- Li, P.; Gan, Y.; Wang, H.; Xu, Y.; Li, S.; Song, L.; Zhang, C.; Ou, Y.; Wang, L.; Zhou, Q. Role of the ERK1/2 pathway in osmolarity effects on nucleus pulposus cell apoptosis in a disc perfusion culture. J. Orthop. Res. 2017, 35, 86–92. [Google Scholar] [CrossRef]
- Roth, I.; Leroy, V.; Kwon, H.M.; Martin, P.-Y.; Féraille, E.; Hasler, U. Osmoprotective Transcription Factor NFAT5/TonEBP Modulates Nuclear Factor-κB Activity. Mol. Biol. Cell 2010, 21, 3459–3474. [Google Scholar] [CrossRef]
- López-Rodríguez, C.; Aramburu, J.; Jin, L.; Rakeman, A.S.; Michino, M.; Rao, A. Bridging the NFAT and NF-κB Families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity 2001, 15, 47–58. [Google Scholar] [CrossRef]
- Lee, S.D.; Choi, S.Y.; Lim, S.W.; Lamitina, S.T.; Ho, S.N.; Go, W.Y.; Kwon, H.M. TonEBP stimulates multiple cellular pathways for adaptation to hypertonic stress: Organic osmolyte-dependent and -independent pathways. Am. J. Physiol. Physiol. 2011, 300, F707–F715. [Google Scholar] [CrossRef]
- Gajghate, S.; Hiyama, A.; Shah, M.; Sakai, D.; Anderson, D.G.; Shapiro, I.M.; Risbud, M. V Osmolarity and Intracellular Calcium Regulate Aquaporin2 Expression Through TonEBP in Nucleus Pulposus Cells of the Intervertebral Disc. J. Bone Miner. Res. 2009, 24, 992–1001. [Google Scholar] [CrossRef]
- Snuggs, J.W.; Day, R.E.; Bach, F.C.; Conner, M.T.; Bunning, R.A.D.; Tryfonidou, M.A.; Le Maitre, C.L. Aquaporin expression in the human and canine intervertebral disc during maturation and degeneration. JOR Spine 2019, 2, e1049. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, H.; Cheng, W.; Lin, S.; Shao, R.; Pan, H. Effects of hypoxia and ASIC3 on nucleus pulposus cells: From cell behavior to molecular mechanism. Biomed. Pharmacother. 2019, 117, 109061. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.I.; Gogate, S.S.; Day, R.; Binch, A.; Markova, D.Z.; Chiverton, N.; Cole, A.; Conner, M.; Shapiro, I.M.; Le Maitre, C.L.; et al. Aquaporin 1 and 5 expression decreases during human intervertebral disc degeneration: Novel HIF-1-mediated regulation of aquaporins in NP cells. Oncotarget 2015, 6, 11945. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Knowles, R.; Marples, D.; Hoyland, J.A.; Mobasheri, A. Aquaporin expression in the human intervertebral disc. J. Mol. Histol. 2008, 39, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Neidlinger-Wilke, C.; Galbusera, F.; Pratsinis, H.; Mavrogonatou, E.; Mietsch, A.; Kletsas, D.; Wilke, H.J. Mechanical loading of the intervertebral disc: From the macroscopic to the cellular level. Eur. Spine J. 2014, 23. [Google Scholar] [CrossRef]
- Tsai, T.T.; Cheng, C.M.; Chen, C.F.; Lai, P.L. Mechanotransduction in intervertebral discs. J. Cell. Mol. Med. 2014, 18, 2351–2360. [Google Scholar] [CrossRef]
- Iatridis, J.C.; MacLean, J.J.; Roughley, P.J.; Alini, M. Effects of Mechanical Loading on intervertebral Disc Metabolism In Vivo. J. Bone Jt. Surg. 2006, 88, 41–46. [Google Scholar]
- Cambria, E.; Arlt, M.J.E.; Wandel, S.; Krupkova, O.; Hitzl, W.; Passini, F.S.; Hausmann, O.N.; Snedeker, J.G.; Ferguson, S.J.; Wuertz-kozak, K. TRPV4 Inhibition and CRISPR-Cas9 Knockout Reduce Inflammation Induced by Hyperphysiological Stretching in Human Annulus Fibrosus Cells. Cells 2020, 9, 1736. [Google Scholar] [CrossRef]
- Krupkova, O.; Zvick, J.; Wuertz-Kozak, K. The role of transient receptor potential channels in joint diseases. Eur. Cells Mater. 2017, 34, 180–201. [Google Scholar] [CrossRef]
- Neidlinger-Wilke, C.; Würtz, K.; Liedert, A.; Schmidt, C.; Börm, W.; Ignatius, A.; Wilke, H.-J.; Claes, L. A three-dimensional collagen matrix as a suitable culture system for the comparison of cyclic strain and hydrostatic pressure effects on intervertebral disc cells. J. Neurosurg. Spine 2005, 2, 457–465. [Google Scholar] [CrossRef]
- Chan, S.C.W.; Walser, J.; Käppeli, P.; Shamsollahi, M.J.; Ferguson, S.J.; Gantenbein-Ritter, B. Region Specific Response of Intervertebral Disc Cells to Complex Dynamic Loading: An Organ Culture Study Using a Dynamic Torsion-Compression Bioreactor. PLoS ONE 2013, 8, e72489. [Google Scholar] [CrossRef]
- Setton, L.A.; Chen, J. Mechanobiology of the Intervertebral Disc and Relevance to Disc Degeneration. J. Bone Jt. Surg. 2006, 88, 52–57. [Google Scholar]
- Latridis, J.C.; Godburn, K.; Wuertz, K.; Alini, M.; Roughley, P.J. Region-dependent aggrecan degradation patterns in the rat intervertebral disc are affected by mechanical loading in vivo. Spine 2011, 36, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The Biology of YAP/TAZ: Hippo Signaling and Beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Pocaterra, A.; Romani, P.; Dupont, S. YAP/TAZ functions and their regulation at a glance. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef]
- Boopathy, G.T.K.; Hong, W. Role of Hippo Pathway-YAP/TAZ Signaling in Angiogenesis. Front. Cell Dev. Biol. 2019, 7, 49. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Fearing, B.V.; Jing, L.; Barcellona, M.N.; Witte, S.E.; Buchowski, J.M.; Zebala, L.P.; Kelly, M.P.; Luhmann, S.; Gupta, M.C.; Pathak, A.; et al. Mechanosensitive transcriptional coactivators MRTF-A and YAP/TAZ regulate nucleus pulposus cell phenotype through cell shape. FASEB J. 2019, 33, 14022–14035. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Gullbrand, S.E.; Ashinsky, B.G.; Tsinman, T.K.; Elliott, D.M.; Chao, P.G.; Smith, H.E.; Mauck, R.L. Aberrant mechanosensing in injured intervertebral discs as a result of boundary-constraint disruption and residual-strain loss. Nat. Biomed. Eng. 2019, 3, 998–1008. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Xie, Z.; Chen, L.; Sinkemani, A.; Yu, H.; Wang, K.; Mao, L.; Wu, X. Dysregulation of YAP by the Hippo pathway is involved in intervertebral disc degeneration, cell contact inhibition, and cell senescence. Oncotarget 2018, 9, 2175–2192. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Xie, Z.; Chen, L.; Sinkemani, A.; Yu, H.; Wu, X. AMOT130 linking F-actin to YAP is involved in intervertebral disc degeneration. Cell Prolif. 2018, 51, e12492. [Google Scholar] [CrossRef]
- Vo, N.V.; Hartman, R.A.; Patil, P.R.; Risbud, M.V.; Kletsas, D.; Iatridis, J.C.; Hoyland, J.A.; Le Maitre, C.L.; Sowa, G.A.; Kang, J.D. Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res. 2016, 34, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, P.P.A.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.W.; Welting, T.J.; van Royen, B.J.; van Dieën, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Lama, P.; Claireaux, H.; Flower, L.; Harding, I.J.; Dolan, T.; Le Maitre, C.L.; Adams, M.A. Physical disruption of intervertebral disc promotes cell clustering and a degenerative phenotype. Cell Death Discov. 2019, 5, 154. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernández, C.; Francisco, V.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Gualillo, O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? Int. J. Mol. Sci. 2019, 20, E2030. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory biomarkers of low back pain and disc degeneration: A review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Schoepflin, Z.R.; Choi, H.; Shapiro, I.M.; Risbud, M.V. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur. Cells Mater. 2015, 30, 104–117. [Google Scholar] [CrossRef]
- Wuertz, K.; Haglund, L. Inflammatory mediators in intervertebral disk degeneration and discogenic pain. Glob. Spine J. 2013, 3, 175–184. [Google Scholar] [CrossRef]
- Phillips, K.L.E.; Cullen, K.; Chiverton, N.; Michael, A.L.R.; Cole, A.A.; Breakwell, L.M.; Haddock, G.; Bunning, R.A.D.; Cross, A.K.; Le Maitre, C.L. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: Interleukin-1 is a master regulator of catabolic processes. Osteoarthr. Cartil. 2015, 23, 1165–1177. [Google Scholar] [CrossRef]
- Hoyland, J.A.; Le maitre, C.; Freemont, A.J. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology 2008, 47, 809–814. [Google Scholar] [CrossRef]
- Binch, A.L.A.; Shapiro, I.M.; Risbud, M.V. Syndecan-4 in intervertebral disc and cartilage: Saint or synner? Matrix Biol. 2016, 52–54, 355–362. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Han, X.; Mao, C.; Zhang, K.; Zhao, T.; Zhao, J. The imbalance between TIMP3 and matrix-degrading enzymes plays an important role in intervertebral disc degeneration. Biochem. Biophys. Res. Commun. 2016, 469, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.S.; Hayes, A.J.; Wachtel, E.; Caterson, B.; Merkher, Y.; Maroudas, A.; Brown, S.; Roberts, S. Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc. Eur. Spine J. 2014, 23, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Neidlinger-Wilke, C.; Mietsch, A.; Rinkler, C.; Wilke, H.J.; Ignatius, A.; Urban, J. Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells. J. Orthop. Res. 2012, 30, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Crean, J.K.G.; Roberts, S.; Jaffray, D.C.; Eisenstein, S.M.; Duance, V.C. Matrix Metalloproteinases in the Human Intervertebral Disc: Role in Disc Degeneration and Scoliosis. Spine 1997, 22, 2877–2884. [Google Scholar] [CrossRef]
- Vo, N.V.; Hartman, R.A.; Yurube, T.; Jacobs, L.J.; Sowa, G.A.; Kang, J.D. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013, 13, 331–341. [Google Scholar] [CrossRef]
- Pockert, A.J.; Richardson, S.M.; Le Maitre, C.L.; Lyon, M.; Deakin, J.A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009, 60, 482–491. [Google Scholar] [CrossRef]
- Ohtori, S.; Miyagi, M.; Inoue, G. Sensory nerve ingrowth, cytokines, and instability of discogenic low back pain: A review. Spine Surg. Relat. Res. 2018, 2, 11–17. [Google Scholar] [CrossRef]
- Krock, E.; Currie, J.B.; Weber, M.H.; Ouellet, J.A.; Stone, L.S.; Rosenzweig, D.H.; Haglund, L. Nerve growth factor is regulated by toll-like receptor 2 in human intervertebral discs. J. Biol. Chem. 2016, 291, 3541–3551. [Google Scholar] [CrossRef]
- LA Binch, A.; Cole, A.A.; Breakwell, L.M.; Michael, A.L.; Chiverton, N.; Cross, A.K.; Le Maitre, C.L. Expression and regulation of neurotrophic and angiogenic factors during human intervertebral disc degeneration. Arthritis Res. Ther. 2014, 16, 416. [Google Scholar] [CrossRef]
- Kao, T.-H.; Peng, Y.-J.; Tsou, H.-K.; Salter, D.M.; Lee, H.-S. Nerve growth factor promotes expression of novel genes in intervertebral disc cells that regulate tissue degradation. J. Neurosurg. Spine 2014, 21, 653–661. [Google Scholar] [CrossRef]
- Krock, E.; Rosenzweig, D.H.; Chabot-Doré, A.-J.; Jarzem, P.; Weber, M.H.; Ouellet, J.A.; Stone, L.S.; Haglund, L. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J. Cell. Mol. Med. 2014, 18, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Sakai, D.; Mochida, J. Cell Signaling Pathways Related to Pain Receptors in the Degenerated Disk. Glob. Spine J. 2013, 3, 165–174. [Google Scholar] [CrossRef]
- Richardson, S.M.; Purmessur, D.; Baird, P.; Probyn, B.; Freemont, A.J.; Hoyland, J.A. Degenerate Human Nucleus Pulposus Cells Promote Neurite Outgrowth in Neural Cells. PLoS ONE 2012, 7, e47735. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.; Hoelscher, G.; Bethea, S.; Hanley, E. Interleukin 1-beta upregulates brain-derived neurotrophic factor, neurotrophin 3 and neuropilin 2 gene expression and NGF production in annulus cells. Biotech. Histochem. 2012, 87, 506–511. [Google Scholar] [CrossRef]
- Navone, S.E.; Marfia, G.; Canzi, L.; Ciusani, E.; Canazza, A.; Visintini, S.; Campanella, R.; Parati, E.A. Expression of neural and neurotrophic markers in nucleus pulposus cells isolated from degenerated intervertebral disc. J. Orthop. Res. 2012, 30, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Binch, A.L.A.; Cole, A.A.; Breakwell, L.M.; Michael, A.L.R.; Chiverton, N.; Creemers, L.B.; Cross, A.K.; Le Maitre, C.L. Class 3 semaphorins expression and association with innervation and angiogenesis within the degenerate human intervertebral disc. Oncotarget 2015, 6, 18338. [Google Scholar] [CrossRef]
- Jung, W.-W.; Kim, H.-S.; Shon, J.-R.; Lee, M.; Lee, S.-H.; Sul, D.; Na, H.S.; Kim, J.H.; Kim, B.-J. Intervertebral Disc Degeneration-induced Expression of Pain-related Molecules: Glial Cell-derived Neurotropic Factor as a Key Factor. J. Neurosurg. Anesthesiol. 2011, 23, 329–334. [Google Scholar] [CrossRef]
- Lee, J.M.; Song, J.Y.; Baek, M.; Jung, H.-Y.; Kang, H.; Han, I.B.; Kwon, Y.D.; Shin, D.E. Interleukin-1β induces angiogenesis and innervation in human intervertebral disc degeneration. J. Orthop. Res. 2011, 29, 265–269. [Google Scholar] [CrossRef]
- García-Cosamalón, J.; Del Valle, M.E.; Calavia, M.G.; García-Suárez, O.; López-Muñiz, A.; Otero, J.; Vega, J.A. Intervertebral disc, sensory nerves and neurotrophins: Who is who in discogenic pain? J. Anat. 2010, 217, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Purmessur, D.; Freemont, A.J.; Hoyland, J.A. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res. Ther. 2008, 10, R99. [Google Scholar] [CrossRef]
- Johnson, W.E.B.; Sivan, S.; Wright, K.T.; Eisenstein, S.M.; Maroudas, A.; Roberts, S. Human Intervertebral Disc Cells Promote Nerve Growth Over Substrata of Human Intervertebral Disc Aggrecan. Spine 2006, 31, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, L.; Peng, B. Ingrowth of Nociceptive Receptors into Diseased Cervical Intervertebral Disc Is Associated with Discogenic Neck Pain. Pain Med. 2019, 20, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Lama, P.; Le Maitre, C.L.; Harding, I.J.; Dolan, P.; Adams, M.A. Nerves and blood vessels in degenerated intervertebral discs are confined to physically disrupted tissue. J. Anat. 2018, 233, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Jones, B.; Marrero, E.; Hanley, E.N. Proinflammatory Cytokines IL-1β and TNF-α Influence Human Annulus Cell Signaling Cues for Neurite Growth: In Vitro Coculture Studies. Spine 2017, 42, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Binch, A.L.A.; Cole, A.A.; Breakwell, L.M.; Michael, A.L.R.; Chiverton, N.; Creemers, L.B.; Cross, A.K.; Le Maitre, C.L. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res. Ther. 2015, 17, 370. [Google Scholar] [CrossRef]
- Stefanakis, M.; Al-Abbasi, M.; Harding, I.; Pollintine, P.; Dolan, P.; Tarlton, J.; Adams, M.A. Annulus Fissures Are Mechanically and Chemically Conducive to the Ingrowth of Nerves and Blood Vessels. Spine 2012, 37, 1883–1891. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.; Tao, Y.; Shen, C.; Li, F.; Shi, Z.; Han, B.; Chen, Q. New hypothesis of chronic back pain: Low pH promotes nerve ingrowth into damaged intervertebral disks. Acta Anaesthesiol. Scand. 2013, 57, 271–277. [Google Scholar] [CrossRef]
- Tolofari, S.K.; Richardson, S.M.; Freemont, A.J.; Hoyland, J.A. Expression of semaphorin 3A and its receptors in the human intervertebral disc: Potential role in regulating neural ingrowth in the degenerate intervertebral disc. Arthritis Res. Ther. 2010, 12, R1. [Google Scholar] [CrossRef]
- Freemont, A.J.; Watkins, A.; Le Maitre, C.; Baird, P.; Jeziorska, M.; Knight, M.T.N.; Ross, E.R.S.; O’Brien, J.P.; Hoyland, J.A. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol. 2002, 197, 286–292. [Google Scholar] [CrossRef]
- Freemont, A.; Peacock, T.; Goupille, P.; Hoyland, J.; O’Brien, J.; Jayson, M. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, X.; Shen, H.; Zhang, C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int. J. Mol. Med. 2016, 37, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.; Yang, K.; Guo, S.; Wang, J.; Feng, C.; Chen, H. Hyper-osmolarity environment-induced oxidative stress injury promotes nucleus pulposus cell senescence in vitro. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Falabella, M.; Saeed, A.; Lee, D.; Kaufman, B.; Shiva, S.; Croix, C.S.; Van Houten, B.; Niedernhofer, L.J.; Robbins, P.D.; et al. Oxidative stress-induced senescence markedly increases disc cell bioenergetics. Mech. Ageing Dev. 2019, 180, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Niedernhofer, L.J.; Robbins, P.D.; Lee, J.; Sowa, G.; Vo, N. Cellular Senescence in Intervertebral Disc Aging and Degeneration. Curr. Mol. Biol. Reports 2018, 4, 180–190. [Google Scholar] [CrossRef]
- Feng, C.; Liu, H.; Yang, M.; Zhang, Y.; Huang, B.; Zhou, Y. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways. Cell Cycle 2016, 15, 1674–1684. [Google Scholar] [CrossRef]
- Heathfield, S.; Le Maitre, C.; Hoyland, J. Caveolin-1 expression and stress-induced premature senescence in human intervertebral disc degeneration. Arthritis Res. Ther. 2008, 10, R87. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. Accelerated cellular senescence in degenerate intervertebral discs: A possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res. Ther. 2007, 9, R45. [Google Scholar] [CrossRef]
- Gruber, H.E.; Ingram, J.A.; Norton, H.J.; Hanley, E.N. Senescence in Cells of the Aging and Degenerating Intervertebral Disc: Immunolocalization of Senescence-Associated Beta-Galactosidase in Human and Sand Rat Discs. Spine 2007, 32, 321–327. [Google Scholar] [CrossRef]
- Roberts, S.; Evans, E.H.; Kletsas, D.; Jaffray, D.C.; Eisenstein, S.M. Senescence in human intervertebral discs. Eur. Spine J. 2006, 15, 312–316. [Google Scholar] [CrossRef]
- Gorth, D.J.; Shapiro, I.M.; Risbud, M. V Disc-overy of the Drivers of Inflammation Induced Chronic Low Back Pain: From Bacteria to Diabetes. Discov Med. 2015, 20, 177–184. [Google Scholar]
- Martirosyan, N.L.; Patel, A.A.; Carotenuto, A.; Kalani, M.Y.S.; Belykh, E.; Walker, C.T.; Preul, M.C.; Theodore, N. Genetic Alterations in Intervertebral Disc Disease. Front. Surg. 2016, 3, 59. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, X.-H.; Wang, C.; He, W.-S.; Zhang, S.-J.; Yan, Y.-G.; Zhang, J.; Xiang, Y.-X.; Wang, W.-J. Interleukin-1β in intervertebral disk degeneration. Clin. Chim. Acta 2015, 450, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.T.J.; Nagra, N.S.; Freemont, A.J.; Millward-Sadler, S.J.; Hoyland, J.A. Integrin–Dependent Mechanotransduction in Mechanically Stimulated Human Annulus Fibrosus Cells: Evidence for an Alternative Mechanotransduction Pathway Operating with Degeneration. PLoS ONE 2013, 8, e72994. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.T.J.; Hoyland, J.A.; Millward-Sadler, S.J. The response of human anulus fibrosus cells to cyclic tensile strain is frequency-dependent and altered with disc degeneration. Arthritis Rheum. 2010, 62, 3385–3394. [Google Scholar] [CrossRef] [PubMed]
- Le Maitre, C.L.; Frain, J.; Fotheringham, A.P.; Freemont, A.J.; Hoyland, J.A. Human cells derived from degenerate intervertebral discs respond differently to those derived from non-degenerate intervertebral discs following application of dynamic hydrostatic pressure. Biorheology 2008, 45, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.L.E.; Chiverton, N.; Michael, A.L.; Cole, A.A.; Breakwell, L.M.; Haddock, G.; Bunning, R.A.; Cross, A.K.; Le Maitre, C.L. The cytokine and chemokine expression profile of nucleus pulposus cells: Implications for degeneration and regeneration of the intervertebral disc. Arthritis Res. Ther. 2013, 15, R213. [Google Scholar] [CrossRef]
- Tessier, S.; Tran, V.A.; Ottone, O.K.; Novais, E.J.; Doolittle, A.; DiMuzio, M.J.; Shapiro, I.M.; Risbud, M.V. TonEBP-deficiency accelerates intervertebral disc degeneration underscored by matrix remodeling, cytoskeletal rearrangements, and changes in proinflammatory gene expression. Matrix Biol. 2020, 87, 94–111. [Google Scholar] [CrossRef]
- Guo, Y.; Meng, Y.; Liu, H.; Wang, B.; Ding, C.; Rong, X.; Yang, Y.; Hong, Y. Acid-sensing ion channels mediate the degeneration of intervertebral disc via various pathways—A systematic review. Channels 2019, 13, 367–373. [Google Scholar] [CrossRef]
- Bibby, S.R.S.; Urban, J.P.G. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur. Spine J. 2004, 13, 695–701. [Google Scholar] [CrossRef]
- Rinkler, C.; Heuer, F.; Pedro, M.T.; Mauer, U.M.; Ignatius, A.; Neidlinger-Wilke, C. Influence of low glucose supply on the regulation of gene expression by nucleus pulposus cells and their responsiveness to mechanical loading. J. Neurosurg. Spine J Neurosurg Spine 2010, 13, 535–542. [Google Scholar] [CrossRef]
- Horner, H.A.; Urban, J.P.G. 2001 Volvo Award Winner in Basic Science Studies: Effect of Nutrient Supply on the Viability of Cells From the Nucleus Pulposus of the Intervertebral Disc. Spine 2001, 26, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, H.T.J.; Hodson, N.; Baird, P.; Richardson, S.M.; Hoyland, J.A. Acidic pH promotes intervertebral disc degeneration: Acid-sensing ion channel -3 as a potential therapeutic target. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Ciobanu, I.; Giannitsios, D.; Roughley, P.; Steffen, T.; Antoniou, J. Effect of oxygen levels on proteoglycan synthesis by intervertebral disc cells. Spine 2011, 36, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Sakai, D.; Risbud, M.V.; Tanaka, M.; Arai, F.; Abe, K.; Mochida, J. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010, 62, 3036–3047. [Google Scholar] [CrossRef]
- Ding, F.; Shao, Z.W.; Xiong, L.M. Cell death in intervertebral disc degeneration. Apoptosis 2013, 18, 777–785. [Google Scholar] [CrossRef]
- Zhao, C.-Q.; Jiang, L.-S.; Dai, L.-Y. Programmed cell death in intervertebral disc degeneration. Apoptosis 2006, 11, 2079–2088. [Google Scholar] [CrossRef]
- Gruber, H.E.; Hanley, E.N. Analysis of aging and degeneration of the human intervertebral disc: Comparison of surgical specimens with normal controls. Spine 1998, 23, 751–757. [Google Scholar] [CrossRef]
- Kong, C.G.; Park, J.B.; Kim, M.S.; Park, E.Y. High glucose accelerates autophagy in adult rat intervertebral disc cells. Asian Spine J. 2014, 8, 543–548. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, X.; Zheng, X.; Ru, A.; Ni, X.; Wu, Y.; Tian, N.; Huang, Y.; Xue, E.; Wang, X.; et al. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: Implications for diabetic intervertebral disc degeneration. J. Orthop. Res. 2013, 31, 692–702. [Google Scholar] [CrossRef]
- Ye, W.; Zhu, W.; Xu, K.; Liang, A.; Peng, Y.; Huang, D.; Li, C. Increased macroautophagy in the pathological process of intervertebral disc degeneration in rats. Connect. Tissue Res. 2013, 54, 22–28. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Hao, J.; Shen, J.; Fang, J.; Dong, W.; Wang, D.; Zhang, X.; Shui, W.; Luo, Y.; et al. SIRT1 protects against apoptosis by promoting autophagy in degenerative human disc nucleus pulposus cells. Sci. Rep. 2014, 4, 7456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-J.; Yang, W.; Wang, C.; He, W.-S.; Deng, H.-Y.; Yan, Y.-G.; Zhang, J.; Xiang, Y.-X.; Wang, W.-J. Autophagy: A double-edged sword in intervertebral disk degeneration. Clin. Chim. Acta. 2016, 457, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yan, J.; Jiang, L.-S.; Dai, L.-Y. Autophagy in rat annulus fibrosus cells: Evidence and possible implications. Arthritis Res. Ther. 2011, 13, R132. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-Q.; Liu, D.; Li, H.; Jiang, L.-S.; Dai, L.-Y. Interleukin-1beta enhances the effect of serum deprivation on rat annular cell apoptosis. Apoptosis 2007, 12, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Lopiccolo, J.; Blumenthal, G.; Bernstein, W.; Dennis, P. Targeting the PI3K/Akt/mTOR pathway: Effective combinations and clinical considerations. Drug Resist. Updat. 2008, 11, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, D.M. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc. Natl. Acad. Sci. USA 2017, 114, 11818–11825. [Google Scholar] [CrossRef] [PubMed]
- Hebert, D.N.; Molinari, M. In and Out of the ER: Protein Folding, Quality Control, Degradation, and Related Human Diseases. Physiol. Rev. 2007, 87, 1377–1408. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Yurube, T.; Ito, M.; Kakiuchi, Y.; Kuroda, R.; Kakutani, K. Autophagy and mTOR signaling during intervertebral disc aging and degeneration. JOR Spine 2020, 3, e1082. [Google Scholar] [CrossRef]
- Ma, K.-G.; Shao, Z.-W.; Yang, S.-H.; Wang, J.; Wang, B.-C.; Xiong, L.-M.; Wu, Q.; Chen, S.-F. Autophagy is activated in compression-induced cell degeneration and is mediated by reactive oxygen species in nucleus pulposus cells exposed to compression. Osteoarthr. Cartil. 2013, 21, 2030–2038. [Google Scholar] [CrossRef]
- Chen, J.-W.; Ni, B.-B.; Li, B.; Yang, Y.-H.; Jiang, S.-D.; Jiang, L.-S. The Responses of Autophagy and Apoptosis to Oxidative Stress in Nucleus Pulposus Cells: Implications for Disc Degeneration. Cell. Physiol. Biochem. 2014, 34, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yurube, T.; Kakutani, K.; Maeno, K.; Takada, T.; Terashima, Y.; Kakiuchi, Y.; Takeoka, Y.; Miyazaki, S.; Kuroda, R.; et al. Selective interference of mTORC1/RAPTOR protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism with Akt and autophagy induction. Osteoarthr. Cartil. 2017, 25, 2134–2146. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, Y.; Yurube, T.; Kakutani, K.; Takada, T.; Ito, M.; Takeoka, Y.; Kanda, Y.; Miyazaki, S.; Kuroda, R.; Nishida, K. Pharmacological inhibition of mTORC1 but not mTORC2 protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism through Akt and autophagy induction. Osteoarthr. Cartil. 2019, 27, 965–976. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Liu, Z.; Xiao, X.; Hu, W.; Sun, Z. Osteogenic protein-1 attenuates nucleus pulposus cell apoptosis through activating the PI3K/Akt/mTOR pathway in a hyperosmotic culture. Biosci. Rep. 2018, 38, BSR20181708. [Google Scholar] [CrossRef]
- Ngo, K.; Yurube, T.; Pohl, P.; Qing, D.; Miller, R.; Roughley, P.; Sowa, G.A.; Kang, J.D. Effects Of The Anti-aging Agent Rapamycin On Disc Matrix Homeostasis. In Proceedings of the ORS 2014 Annual Meeting, New Orleans, LO, USA, 15–18 March 2014; p. 1647. [Google Scholar]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef]
- Bray, S.J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 2016, 17, 722–735. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Y.; Wang, J.; Phillips, K.L.E.; Binch, A.L.A; Dunn, S.; Cross, A.; Chiverton, N.; Zheng, Z.; Shapiro, I.M.; et al. Inflammatory cytokines induce NOTCH signaling in nucleus pulposus cells: Implications in intervertebral disc degeneration. J. Biol. Chem. 2013, 288, 16761–16774. [Google Scholar] [CrossRef]
- Long, J.; Wang, X.; Du, X.; Pan, H.; Wang, J.; Li, Z.; Liu, H.; Li, X.; Zheng, Z. JAG2/Notch2 inhibits intervertebral disc degeneration by modulating cell proliferation, apoptosis, and extracellular matrix. Arthritis Res. Ther. 2019, 21, 213. [Google Scholar] [CrossRef]
- Hiyama, A.; Skubutyte, R.; Markova, D.; Anderson, D.G.; Yadla, S.; Sakai, D.; Mochida, J.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: Implications in degenerative disc disease. Arthritis Rheum. 2011, 63, 1355–1364. [Google Scholar] [CrossRef]
- Millward-Sadler, S.J.; Costello, P.W.; Freemont, A.J.; Hoyland, J.A. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: Implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res. Ther. 2009, 11, R65. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J.; et al. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Cambria, E.; Besse, L.; Besse, A.; Bowles, R.; Wuertz-Kozak, K. The potential of CRISPR/Cas9 genome editing for the study and treatment of intervertebral disc pathologies. JOR Spine 2018, 1, e1003. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, K.; Vo, N.; Kletsas, D.; Boos, N. Inflammatory and Catabolic Signalling in Intervertebral Discs: The Roles of NF-ΚB and MAP Kinases. JOR Spine 2012, 23, 103–120. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Guttapalli, A.; Oguz, E.; Chen, L.-H.; Vaccaro, A.R.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. Fibroblast Growth Factor-2 Maintains the Differentiation Potential of Nucleus Pulposus Cells In Vitro: Implications for cell-based transplantation therapy. Spine 2007, 32, 495–502. [Google Scholar] [CrossRef]
- Pratsinis, H.; Kletsas, D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur. Spine J. 2007, 16, 1858–1866. [Google Scholar] [CrossRef]
- Risbud, M.V.; Di Martino, A.; Guttapalli, A.; Seghatoleslami, R.; Denaro, V.; Vaccaro, A.R.; Albert, T.J.; Shapiro, I.M. Toward an Optimum System for Intervertebral Disc Organ Culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine 2006, 31, 884–890. [Google Scholar] [CrossRef]
- Risbud, M.V.; Guttapalli, A.; Albert, T.J.; Shapiro, I.M. Hypoxia Activates MAPK Activity in Rat Nucleus Pulposus Cells: Regulation of Integrin Expression and Cell Survival. Spine 2005, 30, 2503–2509. [Google Scholar] [CrossRef]
- Risbud, M.V.; Fertala, J.; Vresilovic, E.J.; Albert, T.J.; Shapiro, I.M. Nucleus Pulposus Cells Upregulate PI3K/Akt and MEK/ERK Signaling Pathways Under Hypoxic Conditions and Resist Apoptosis Induced by Serum Withdrawal. Spine 2005, 30, 882–889. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Kletsas, D. Effect of varying osmotic conditions on the response of bovine nucleus pulposus cells to growth factors and the activation of the ERK and Akt pathways. J. Orthop. Res. 2010, 28, 1276–1282. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, F.; Chen, G.; Chen, Q. The Influence of Hyperosmolarity in the Intervertebral Disc on the Proliferation and Chondrogenic Differentiation of Nucleus Pulposus-Derived Mesenchymal Stem Cells. Cells Tissues Organs 2018, 205, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ding, W.; Sun, W.; Sun, X.; Xie, Y.; Zhao, C.; Zhao, J. Beta1 integrin inhibits apoptosis induced by cyclic stretch in annulus fibrosus cells via ERK1/2 MAPK pathway. Apoptosis 2016, 21, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Séguin, C.A.; Bojarski, M.; Pilliar, R.M.; Roughley, P.J.; Kandel, R.A. Differential regulation of matrix degrading enzymes in a TNFα-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 2006, 25, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Wuertz, K.; Quero, L.; Sekiguchi, M.; Klawitter, M.; Nerlich, A.; Konno, S.-I.; Kikuchi, S.-I.; Boos, N. The Red Wine Polyphenol Resveratrol Shows Promising Potential for the Treatment of Nucleus Pulposus–Mediated Pain In Vitro and In Vivo. Spine 2011, 36, E1373–E1384. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Zhu, Y. Fibronectin fragment activation of ERK increasing integrin α 5 and β 1 subunit expression to degenerate nucleus pulposus cells. J. Orthop. Res. 2011, 29, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.; Binch, A.A.L.; Le Maitre, C.L. Inhibiting IL-1 signaling pathways to inhibit catabolic processes in disc degeneration. J. Orthop. Res. 2017, 35, 74–85. [Google Scholar] [CrossRef]
- Ge, J.; Yan, Q.; Wang, Y.; Cheng, X.; Song, D.; Wu, C.; Yu, H.; Yang, H.; Zou, J. IL-10 delays the degeneration of intervertebral discs by suppressing the p38 MAPK signaling pathway. Free Radic. Biol. Med. 2020, 147, 262–270. [Google Scholar] [CrossRef]
- Krupkova, O.; Sekiguchi, M.; Klasen, J.; Hausmann, O.; Konno, S.; Ferguson, S.J.; Wuertz-Kozak, K. Epigallocatechin 3-gallate suppresses interleukin-1β-induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur. Cell. Mater. 2014, 28, 372–386. [Google Scholar] [CrossRef]
- Shan, L.; Yang, D.; Zhu, D.; Feng, F.; Li, X. High glucose promotes annulus fibrosus cell apoptosis through activating the JNK and p38 MAPK pathways. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Fu, J.; Yu, W.; Jiang, D. Acidic pH promotes nucleus pulposus cell senescence through activating the p38 MAPK pathway. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Krupkova, O.; Sadowska, A.; Kameda, T.; Hitzl, W.; Hausmann, O.N.; Klasen, J.; Wuertz-Kozak, K. p38 MAPK Facilitates Crosstalk Between Endoplasmic Reticulum Stress and IL-6 Release in the Intervertebral Disc. Front. Immunol. 2018, 9, 1706. [Google Scholar] [CrossRef] [PubMed]
- McNulty, A.L.; Leddy, H.A.; Liedtke, W.; Guilak, F. TRPV4 as a therapeutic target for joint diseases. Naunyn. Schmiedebergs. Arch. Pharmacol. 2015, 388, 437–450. [Google Scholar] [CrossRef] [PubMed]
- TAN, Y.; XU, Q.; LI, Y.; MAO, X.; ZHANG, K. Crosstalk between the p38 and TGF-β signaling pathways through TβRI, TβRII and Smad3 expression in plancental choriocarcinoma JEG-3 cells. Oncol. Lett. 2014, 8, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Kucuksayan, H.; Akca, H. The crosstalk between p38 and Akt signaling pathways orchestrates EMT by regulating SATB2 expression in NSCLC cells. Tumor Biol. 2017, 1–9. [Google Scholar] [CrossRef]
- Studer, R.K.; Aboka, A.M.; Gilbertson, L.G.; Georgescu, H.; Sowa, G.; Vo, N.; Kang, J.D. p38 MAPK Inhibition in Nucleus Pulposus Cells: A potential target for treating intervertebral disc degeneration. Spine 2007, 32, 2827–2833. [Google Scholar] [CrossRef]
- Mavrogonatou, E.; Kletsas, D. Differential response of nucleus pulposus intervertebral disc cells to high salt, sorbitol, and urea. J. Cell. Physiol. 2012, 227, 1179–1187. [Google Scholar] [CrossRef]
- Ge, J.; Cheng, X.; Yuan, C.; Qian, J.; Wu, C.; Cao, C.; Yang, H.; Zhou, F.; Zou, J. Syndecan-4 is a Novel Therapeutic Target for Intervertebral Disc Degeneration via Suppressing JNK/p53 Pathway. Int. J. Biol. Sci. 2020, 16, 766–776. [Google Scholar] [CrossRef]
- Lin, Y.; Jiao, Y.; Yuan, Y.; Zhou, Z.; Zheng, Y.; Xiao, J.; Li, C.; Chen, Z.; Cao, P. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell apoptosis via the TLR2/JNK/mitochondrial-mediated pathway. Emerg. Microbes Infect. 2018, 7, 1–8. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK signaling in apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Zhao, L.; Xue, M.; Zhang, L.; Guo, B.; Qin, Y.; Jiang, Q.; Sun, R.; Yang, J.; Wang, L.; Liu, L.; et al. MicroRNA-4268 inhibits cell proliferation via AKT/JNK signalling pathways by targeting Rab6B in human gastric cancer. Cancer Gene Ther. 2020, 27, 461–472. [Google Scholar] [CrossRef]
- Xie, S.-J.; Li, J.-H.; Chen, H.-F.; Tan, Y.-Y.; Liu, S.-R.; Zhang, Y.; Xu, H.; Yang, J.-H.; Liu, S.; Zheng, L.-L.; et al. Inhibition of the JNK/MAPK signaling pathway by myogenesis-associated miRNAs is required for skeletal muscle development. Cell Death Differ. 2018, 25, 1581–1597. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Chen, S.D.; Fan, J.; Zhang, G.; Li, J.B. miRNA-138 regulates MLK3/JNK/MAPK pathway to protect BV-2 cells from H2O2-induced apoptosis. Bratislava Med. J. 2018, 119, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.H.C.N.; Fraga, C.A.M. NF-κB-IKKβ Pathway as a Target for Drug Development: Realities, Challenges and Perspectives. Curr. Drug Targets 2018, 19, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- LIU, Z.; MA, C.; SHEN, J.; WANG, D.; HAO, J.; HU, Z. SDF-1/CXCR4 axis induces apoptosis of human degenerative nucleus pulposus cells via the NF-κB pathway. Mol. Med. Rep. 2016, 14, 783–789. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Henkel, T. Function and Activation of NF-kappaB in the Immune System. Annu. Rev. Immunol. 1994, 12, 141–179. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, Z.; Liu, C.; Tian, J. The Changes in the Expression of NF-KB in a Degenerative Human Intervertebral Disc model. Cell Biochem. Biophys. 2015, 72, 115–122. [Google Scholar] [CrossRef]
- Zhongyi, S.; Sai, Z.; Chao, L.; Jiwei, T. Effects of nuclear factor kappa B signaling pathway in human intervertebral disc degeneration. Spine 2015, 40, 224–232. [Google Scholar] [CrossRef]
- Tisherman, R.; Coelho, P.; Phillibert, D.; Wang, D.; Dong, Q.; Vo, N.; Kang, J.; Sowa, G. NF-κB Signaling Pathway in Controlling Intervertebral Disk Cell Response to Inflammatory and Mechanical Stressors. Phys. Ther. 2016, 96, 704–711. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Sun, Z.; Yan, P.; Liang, H.; Huang, K.; Li, C.; Tian, J. IL-1β increases asporin expression via the NF-κB p65 pathway in nucleus pulposus cells during intervertebral disc degeneration. Sci. Rep. 2017, 7, 4112. [Google Scholar] [CrossRef]
- Wako, M.; Ohba, T.; Ando, T.; Arai, Y.; Koyama, K.; Hamada, Y.; Nakao, A.; Haro, H. Mechanism of Signal Transduction in Tumor Necrosis Factor-Like Weak Inducer of Apoptosis-Induced Matrix Degradation by MMP-3 Upregulation in Disc Tissues. Spine 2008, 33, 2489–2494. [Google Scholar] [CrossRef]
- Pichika, R.; Akeda, K.; Gemba, T.; Miyamoto, K.; An, H.; Masuda, K. Transcription Factor Decoy for NFKB Inhibits the Appearance of Active MMPS and ADAMTS4 in the Medium of Human Intervertebral Disc Cells Cultured in Alginate. In Proceedings of the 51 st Annual Meeting of the Orthopaedic Research Society, Washington, DC, USA, 20–23 February 2005; p. 1298. [Google Scholar]
- Wang, Z.; Hutton, W.; Yoon, S. BMP-7 suppresses TNF-mediated induction of the aggrecanases ADAMTS4/5 through antagonized activity of the transcription factor NF-қB in the intervertebral discs cells. Trans Orthop Res Soc 2012, 37, 65. [Google Scholar]
- Glaeser, J.D.; Salehi, K.; Kanim, L.E.A.; NaPier, Z.; Kropf, M.A.; Cuéllar, J.M.; Perry, T.G.; Bae, H.W.; Sheyn, D. NF-κB inhibitor, NEMO-binding domain peptide attenuates intervertebral disc degeneration. Spine J. 2020. [Google Scholar]
- Ma, T.; Guo, C.-J.; Zhao, X.; Wu, L.; Sun, S.-X.; Jin, Q.-H. The effect of Curcumin on NF-κB expression in rat with lumbar intervertebral disc degeneration. Euroean Rev. Med. Pharmacol. Sci. 2015, 19, 1305–1314. [Google Scholar]
- Jiang, Y.; Dong, G.; Song, Y. Nucleus pulposus cell senescence is alleviated by resveratrol through regulating the ROS/NF-κB pathway under high-magnitude compression. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.-S.; Li, X.-J.; Huang, C.-R.; Yu, H.-J.; Yang, X.-X.; Wang, B.-X. Overexpression of miR-150 Inhibits the NF-κB Signal Pathway in Intervertebral Disc Degeneration through Targeting P2X7. Cells Tissues Organs 2019, 207, 165–176. [Google Scholar] [CrossRef]
- Dong, W.; Liu, J.; Lv, Y.; Wang, F.; Liu, T.; Sun, S.; Liao, B.; Shu, Z.; Qian, J. miR-640 aggravates intervertebral disc degeneration via NF-κB and WNT signalling pathway. Cell Prolif. 2019, 52, e12664. [Google Scholar] [CrossRef]
- Cazzanelli, P.; Wuertz-kozak, K. MicroRNAs in Intervertebral Disc Degeneration, Apoptosis, Inflammation, and Mechanobiology. Int. J. Mol. Sci. 2020, 21, 3601. [Google Scholar] [CrossRef]
- Komiya, Y.; Habas, R. Wnt signal transduction pathways. Organogenesis 2008, 4, 68–75. [Google Scholar] [CrossRef]
- Nusse, R. The Wnt homepage. Available online: http://web.stanford.edu/group/nusselab/cgi-bin/wnt/ (accessed on 4 January 2021).
- Kondo, N.; Yuasa, T.; Shimono, K.; Tung, W.; Okabe, T.; Yasuhara, R.; Pacifici, M.; Zhang, Y.; Iwamoto, M.; Enomoto-Iwamoto, M. Intervertebral Disc Development Is Regulated by Wnt/β-catenin Signaling. Spine 2011, 36, E513–E518. [Google Scholar] [CrossRef]
- Winkler, T.; Mahoney, E.J.; Sinner, D.; Wylie, C.C.; Dahia, C.L. Wnt Signaling Activates Shh Signaling in Early Postnatal Intervertebral Discs, and Re-Activates Shh Signaling in Old Discs in the Mouse. PLoS ONE 2014, 9, e98444. [Google Scholar] [CrossRef]
- Holguin, N.; Silva, M.J. In-Vivo Nucleus Pulposus-Specific Regulation of Adult Murine Intervertebral Disc Degeneration via Wnt/Beta-Catenin Signaling. Sci. Rep. 2018, 8, 11191. [Google Scholar] [CrossRef] [PubMed]
- Smolders, L.A.; Meij, B.P.; Onis, D.; Riemers, F.M.; Bergknut, N.; Wubbolts, R.; Grinwis, G.C.; Houweling, M.; Groot Koerkamp, M.J.; van Leenen, D.; et al. Gene expression profiling of early intervertebral disc degeneration reveals a down-regulation of canonical Wnt signaling and caveolin-1 expression: Implications for development of regenerative strategies. Arthritis Res. Ther. 2013, 15, R23. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Jian, Y.; Fu, H.; Li, B. MiR-532 downregulation of the Wnt/β-catenin signaling via targeting Bcl-9 and induced human intervertebral disc nucleus pulposus cells apoptosis. J. Pharmacol. Sci. 2018, 138, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Pizzute, T.; He, F.; Zhang, X.-B.; Pei, M. Impact of Wnt signals on human intervertebral disc cell regeneration. J. Orthop. Res. 2018, 36, 3196–3207. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Yokoyama, K.; Nukaga, T.; Sakai, D.; Mochida, J. A complex interaction between Wnt signaling and TNF-α in nucleus pulposus cells. Arthritis Res. Ther. 2013, 15, R189. [Google Scholar] [CrossRef]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef]
- Dan, H.C.; Cooper, M.J.; Cogswell, P.C.; Duncan, J.A.; Ting, J.P.-Y.; Baldwin, A.S. Akt-dependent regulation of NF- B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008, 22, 1490–1500. [Google Scholar] [CrossRef]
- Vasudevan, K.M.; Gurumurthy, S.; Rangnekar, V.M. Suppression of PTEN Expression by NF-κB Prevents Apoptosis. Mol. Cell. Biol. 2004, 24, 1007–1021. [Google Scholar] [CrossRef]
- Pratsinis, H.; Constantinou, V.; Pavlakis, K.; Sapkas, G.; Kletsas, D. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J. Orthop. Res. 2012, 30, 958–964. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, K.; Fu, W.; Zhang, H. Insulin-Like Growth Factor 1 Activates PI3k/Akt Signaling to Antagonize Lumbar Disc Degeneration. Cell. Physiol. Biochem. 2015, 37, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, B.A.; Restuccia, D.F. PI3K-PKB/Akt Pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011189. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.-B.; Li, B.; Yang, Y.-H.; Chen, J.-W.; Chen, K.; Jiang, S.-D.; Jiang, L.-S. The effect of transforming growth factor β1 on the crosstalk between autophagy and apoptosis in the annulus fibrosus cells under serum deprivation. Cytokine 2014, 70, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Ellman, M.B.; An, H.S.; van Wijnen, A.J.; Borgia, J.A.; Im, H.-J. Insulin-like growth factor 1 synergizes with bone morphogenetic protein 7-mediated anabolism in bovine intervertebral disc cells. Arthritis Rheum. 2010, 62, 3706–3715. [Google Scholar] [CrossRef]
- Yang, S.-D.; Ma, L.; Yang, D.-L.; Ding, W.-Y. Combined effect of 17β-estradiol and resveratrol against apoptosis induced by interleukin-1β in rat nucleus pulposus cells via PI3K/Akt/caspase-3 pathway. PeerJ 2016, 4, e1640. [Google Scholar] [CrossRef]
- Krupkova, O.; Handa, J.; Hlavna, M.; Klasen, J.; Ospelt, C.; Ferguson, S.J.; Wuertz-Kozak, K. The Natural Polyphenol Epigallocatechin Gallate Protects Intervertebral Disc Cells from Oxidative Stress. Oxid. Med. Cell. Longev. 2016, 2016, 7031397. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Liu, X.; Xiao, S.; Zhang, Y.; Xiang, T.; Shen, X.; Wang, G.; Sheng, B. miR-21 Promotes Human Nucleus Pulposus Cell Proliferation through PTEN/AKT Signaling. Int. J. Mol. Sci. 2014, 15, 4007–4018. [Google Scholar] [CrossRef]
- Liu, G.; Cao, P.; Chen, H.; Yuan, W.; Wang, J.; Tang, X. MiR-27a Regulates Apoptosis in Nucleus Pulposus Cells by Targeting PI3K. PLoS ONE 2013, 8, e75251. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Fu, W.-Q.; Zhao, S.; Zhao, X. Regulation of insulin-like growth factor 1 receptor signaling by microRNA-4458 in the development of lumbar disc degeneration. Am. J. Transl. Res. 2016, 8, 2309–2316. [Google Scholar]
- Sadowska, A.; Hitzl, W.; Karol, A.; Jaszczuk, P.; Cherif, H.; Haglund, L.; Hausmann, O.N.; Wuertz-Kozak, K. Differential regulation of TRP channel gene and protein expression by intervertebral disc degeneration and back pain. Sci. Rep. 2019, 9, 18889. [Google Scholar] [CrossRef]
- Klawitter, M.; Hakozaki, M.; Kobayashi, H.; Krupkova, O.; Quero, L.; Ospelt, C.; Gay, S.; Hausmann, O.; Liebscher, T.; Meier, U.; et al. Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur. Spine J. 2014, 23, 1878–1891. [Google Scholar] [CrossRef] [PubMed]
- Krock, E.; Rosenzweig, D.H.; Currie, J.B.; Bisson, D.G.; Ouellet, J.A.; Haglund, L. Toll-like Receptor Activation Induces Degeneration of Human Intervertebral Discs. Sci. Rep. 2017, 7, 17184. [Google Scholar] [CrossRef] [PubMed]
- Pelinkovic, D.; Hao, H.; Pazomiño, P.; Markel, D.C.; Wooley, P.H. TLR-4 expression in intervertebral disc tissue: A possible link to degenerative disc disease. In Proceedings of the 52nd Annual Meeting of the Orthopaedic Research Society, Chicago, IL, USA, 19–22 March 2006. Paper No. 1163. [Google Scholar]
- Seidel, M.F.; Wise, B.L.; Lane, N.E. Nerve growth factor: An update on the science and therapy. Osteoarthr. Cartil. 2013, 21, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Mwale, F.; Demers, C.N.; Petit, A.; Roughley, P.; Poole, A.R.; Steffen, T.; Aebi, M.; Antoniou, J. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J. Cell. Biochem. 2003, 88, 1202–1213. [Google Scholar] [CrossRef]
- Kennon, J.C.; Awad, M.E.; Chutkan, N.; DeVine, J.; Fulzele, S. Current insights on use of growth factors as therapy for Intervertebral Disc Degeneration. Biomol. Concepts 2018, 9, 43–52. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Shen, B.; Diwan, A.; Hoyland, J.A.; Richardson, S.M. Therapeutic potential of growth differentiation factors in the treatment of degenerative disc diseases. JOR SPINE 2019, 2, e1045. [Google Scholar] [CrossRef]
- Peeters, M.; Detiger, S.E.L.; Karfeld-Sulzer, L.S.; Smit, T.H.; Yayon, A.; Weber, F.E.; Helder, M.N. BMP-2 and BMP-2/7 Heterodimers Conjugated to a Fibrin/Hyaluronic Acid Hydrogel in a Large Animal Model of Mild Intervertebral Disc Degeneration. Biores. Open Access 2015, 4, 398–406. [Google Scholar] [CrossRef]
- Willems, N.; Bach, F.C.; Plomp, S.G.M.; van Rijen, M.H.; Wolfswinkel, J.; Grinwis, G.C.; Bos, C.; Strijkers, G.J.; Dhert, W.J.; Meij, B.P.; et al. Intradiscal application of rhBMP-7 does not induce regeneration in a canine model of spontaneous intervertebral disc degeneration. Arthritis Res. Ther. 2015, 17, 137. [Google Scholar] [CrossRef][Green Version]
- Gulati, T.; Chung, S.A.; Wei, A.; Diwan, A.D. Localization of bone morphogenetic protein 13 in human intervertebral disc and its molecular and functional effects in vitro in 3D culture. J. Orthop. Res. 2015, 33, 1769–1775. [Google Scholar] [CrossRef]
- Wei, A.; Williams, L.A.; Bhargav, D.; Shen, B.; Kishen, T.; Duffy, N.; Diwan, A.D. BMP13 Prevents the Effects of Annular Injury in an Ovine Model. Int. J. Biol. Sci. 2009, 5, 388–396. [Google Scholar] [CrossRef]
- Miyazaki, S.; Diwan, A.D.; Kato, K.; Cheng, K.; Bae, W.C.; Sun, Y.; Yamada, J.; Muehleman, C.; Lenz, M.E.; Inoue, N.; et al. ISSLS PRIZE IN BASIC SCIENCE 2018: Growth differentiation factor-6 attenuated pro-inflammatory molecular changes in the rabbit anular-puncture model and degenerated disc-induced pain generation in the rat xenograft radiculopathy model. Eur. Spine J. 2018, 27, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; An, H.S.; Akeda, K.; Miyamoto, K.; Muehleman, C.; Attawia, M.; Andersson, G.; Masuda, K. Effects of Growth Differentiation Factor-5 on the Intervertebral Disc−In Vitro Bovine Study and In Vivo Rabbit Disc Degeneration Model Study. Spine 2006, 31, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- DePuy Spine A Multicenter, Randomized, Double-blind, Placebo Controlled, Clinical Trial to Evaluate the Safety, Tolerability and Preliminary Effectiveness of 2 Doses of Intradiscal rhGDF-5 (Single Administration) for the Treatment of Early Stage Lumbar Disc Degenerat. Available online: Clinicaltrials.gov/ct2/show/study/NCT01124006 (accessed on 4 January 2021).
- Masuda, K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur. Spine J. 2008, 17, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.R.; Silva-Correia, J.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Salgado, A.J.; Oliveira, J.M.; Mano, J.F.; Sousa, N.; Reis, R.L. Development of Gellan Gum-Based Microparticles/Hydrogel Matrices for Application in the Intervertebral Disc Regeneration. Tissue Eng. Part C Methods 2011, 17, 961–972. [Google Scholar] [CrossRef]
- Tsaryk, R.; Gloria, A.; Russo, T.; Anspach, L.; De Santis, R.; Ghanaati, S.; Unger, R.E.; Ambrosio, L.; Kirkpatrick, C.J. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015, 20, 10–21. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Stening, J.Z.; White, L.J.; Shakesheff, K.M.; Hoyland, J.A.; Richardson, S.M. Microparticles for controlled growth differentiation factor 6 delivery to direct adipose stem cell-based nucleus pulposus regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 1406–1417. [Google Scholar] [CrossRef]
- Urits, I.; Capuco, A.; Sharma, M.; Kaye, A.D.; Viswanath, O.; Cornett, E.M.; Orhurhu, V. Stem Cell Therapies for Treatment of Discogenic Low Back Pain: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 65. [Google Scholar] [CrossRef]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef]
- Melrose, J. Strategies in regenerative medicine for intervertebral disc repair using mesenchymal stem cells and bioscaffolds. Regen. Med. 2016, 11, 705–724. [Google Scholar] [CrossRef]
- Chan, S.; Gantenbein-Ritter, B. Intervertebral disc regeneration or repair with biomaterials and stem cell therapy—Feasible or fiction? Swiss Med. Wkly. 2012, 142, w13598. [Google Scholar] [CrossRef]
- Gay, M.; Mehrkens, A.; Rittmann, M.; Haug, M.; Barbero, A.; Martin, I.; Schaeren, S. Nose to back: Compatibility of nasal chondrocytes with environmental conditions mimicking a degenerated intervertebral disc. Eur. Cells Mater. 2019, 37, 214–232. [Google Scholar] [CrossRef] [PubMed]
- Vedicherla, S.; Buckley, C.T. In vitro extracellular matrix accumulation of nasal and articular chondrocytes for intervertebral disc repair. Tissue Cell 2017, 49, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Acosta, F.L.; Metz, L.; Adkisson, H.D.; Liu, J.; Carruthers-Liebenberg, E.; Milliman, C.; Maloney, M.; Lotz, J.C. Porcine Intervertebral Disc Repair Using Allogeneic Juvenile Articular Chondrocytes or Mesenchymal Stem Cells. Tissue Eng. Part A 2011, 17, 3045–3055. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; He, R.; Ma, K.; Wang, Z.; Cui, M.; Hu, H.; Rai, S.; Wang, B.; Shao, Z. Intervertebral Disc-Derived Stem/Progenitor Cells as a Promising Cell Source for Intervertebral Disc Regeneration. Stem Cells Int. 2018, 2018, 7412304. [Google Scholar] [CrossRef]
- Lyu, F.-J.; Cheung, K.M.; Zheng, Z.; Wang, H.; Sakai, D.; Leung, V.Y. IVD progenitor cells: A new horizon for understanding disc homeostasis and repair. Nat. Rev. Rheumatol. 2019, 15, 102–112. [Google Scholar] [CrossRef]
- Rosenzweig, D.H.; Fairag, R.; Mathieu, A.P.; Li, L.; Eglin, D.; D’Este, M.; Steffen, T.; Weber, M.H.; Ouellet, J.A.; Haglund, L. Thermoreversible hyaluronan-hydrogel and autologous nucleus pulposus cell delivery regenerates human intervertebral discs in an ex vivo, physiological organ culture model. Eur. Cell. Mater. 2018, 36, 200–217. [Google Scholar] [CrossRef]
- Arkesteijn, I.T.M.; Potier, E.; Ito, K. The Regenerative Potential of Notochordal Cells in a Nucleus Pulposus Explant. Glob. Spine J. 2017, 7, 14–20. [Google Scholar] [CrossRef]
- Erwin, W.M.; Islam, D.; Inman, R.D.; Fehlings, M.G.; Tsui, F.W.L. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: Implications for the mechanisms of intervertebral disc degeneration. Arthritis Res. Ther. 2011, 13, R215. [Google Scholar] [CrossRef]
- Vadalà, G.; Russo, F.; Ambrosio, L.; Loppini, M.; Denaro, V. Stem cells sources for intervertebral disc regeneration. World J. Stem Cells 2016, 8, 185–201. [Google Scholar] [CrossRef]
- Xia, K.; Gong, Z.; Zhu, J.; Yu, W.; Wang, Y.; Wang, J.; Xu, A.; Zhou, X.; Tao, H.; Li, F.; et al. Differentiation of Pluripotent Stem Cells into Nucleus Pulposus Progenitor Cells for Intervertebral Disc Regeneration. Curr. Stem Cell Res. Ther. 2019, 14, 57–64. [Google Scholar] [CrossRef]
- Colombier, P.; Halgand, B.; Chédeville, C.; Chariau, C.; François-Campion, V.; Kilens, S.; Vedrenne, N.; Clouet, J.; David, L.; Guicheux, J.; et al. NOTO Transcription Factor Directs Human Induced Pluripotent Stem Cell-Derived Mesendoderm Progenitors to a Notochordal Fate. Cells 2020, 9, E509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Chen, P.; Ma, C.Y.; Li, C.; Au, T.Y.K.; Tam, V.; Peng, Y.; Wu, R.; Cheung, K.M.C.; et al. Directed Differentiation of Notochord-like and Nucleus Pulposus-like Cells Using Human Pluripotent Stem Cells. Cell Rep. 2020, 30, 2791–2806.e5. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Xing, R.; Jiang, L.; Li, Z.; Liu, P.; Wang, H.; Li, X.; Dong, J. Thermosensitive hydrogels loaded with human-induced pluripotent stem cells overexpressing growth differentiation factor-5 ameliorate intervertebral disc degeneration in rats. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Sheyn, D.; Ben-David, S.; Tawackoli, W.; Zhou, Z.; Salehi, K.; Bez, M.; De Mel, S.; Chan, V.; Roth, J.; Avalos, P.; et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics 2019, 9, 7506–7524. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Zhu, J.; Hua, J.; Gong, Z.; Yu, C.; Zhou, X.; Wang, J.; Huang, X.; Yu, W.; Li, L.; et al. Intradiscal Injection of Induced Pluripotent Stem Cell-Derived Nucleus Pulposus-Like Cell-Seeded Polymeric Microspheres Promotes Rat Disc Regeneration. Stem Cells Int. 2019, 2019, 6806540. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Jing, L.; Willard, V.P.; Wu, C.; Guilak, F.; Chen, J.; Setton, L.A. Differentiation of human induced pluripotent stem cells into nucleus pulposus-like cells. Stem Cell Res. Ther. 2018, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Leung, V.Y.; Cheung, K.M. Clinical trials of intervertebral disc regeneration: Current status and future developments. Int. Orthop. 2019, 43, 1003–1010. [Google Scholar] [CrossRef]
- Clouet, J.; Fusellier, M.; Camus, A.; Le Visage, C.; Guicheux, J. Intervertebral disc regeneration: From cell therapy to the development of novel bioinspired endogenous repair strategies. Adv. Drug Deliv. Rev. 2019, 146, 306–324. [Google Scholar] [CrossRef]
- Henry, N.; Clouet, J.; Le Bideau, J.; Le Visage, C.; Guicheux, J. Innovative strategies for intervertebral disc regenerative medicine: From cell therapies to multiscale delivery systems. Biotechnol. Adv. 2018, 36, 281–294. [Google Scholar] [CrossRef]
- Pennicooke, B.; Moriguchi, Y.; Hussain, I.; Bonssar, L.; Härtl, R. Biological Treatment Approaches for Degenerative Disc Disease: A Review of Clinical Trials and Future Directions. Cureus 2016, 8, e892. [Google Scholar] [CrossRef]
- Smith, L.J.; Silverman, L.; Sakai, D.; Le Maitre, C.L.; Mauck, R.L.; Malhotra, N.R.; Lotz, J.C.; Buckley, C.T. Advancing cell therapies for intervertebral disc regeneration from the lab to the clinic: Recommendations of the ORS spine section. JOR Spine 2018, 1, e1036. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, G.J.; Yee, A.J.M.; Erwin, W.M. Bedside to bench and back to bedside: Translational implications of targeted intervertebral disc therapeutics. J. Orthop. Transl. 2017, 10, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, M.; Bunger, C.; Colombier, P.; Le Visage, C.; Roberts, S.; Sakai, D.; Urban, J.P.G. Biological challenges for regeneration of the degenerated disc using cellular therapies. Acta Orthop. 2016, 87, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shi, R.; Cai, F.; Wang, Y.-T.; Wu, X.-T. Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev. 2015, 24, 2479–2495. [Google Scholar] [CrossRef]
- Vickers, L.; Thorpe, A.A.; Snuggs, J.; Sammon, C.; Le Maitre, C.L. Mesenchymal stem cell therapies for intervertebral disc degeneration: Consideration of the degenerate niche. JOR Spine 2019, 2, e1055. [Google Scholar] [CrossRef]
- Loibl, M.; Wuertz-Kozak, K.; Vadala, G.; Lang, S.; Fairbank, J.; Urban, J.P. Controversies in regenerative medicine: Should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine 2019, 2, e1043. [Google Scholar] [CrossRef]
- Choi, Y.; Park, M.H.; Lee, K. Tissue Engineering Strategies for Intervertebral Disc Treatment Using Functional Polymers. Polymers 2019, 11, 872. [Google Scholar] [CrossRef]
- Frauchiger, D.A.; Tekari, A.; Wöltje, M.; Fortunato, G.; Benneker, L.M.; Gantenbein, B. A review of the application of reinforced hydrogels and silk as biomaterials for intervertebral disc repair. Eur. Cell. Mater. 2017, 34, 271–290. [Google Scholar] [CrossRef]
- Stergar, J.; Gradisnik, L.; Velnar, T.; Maver, U. Intervertebral disc tissue engineering: A brief review. Bosn. J. Basic Med. Sci. 2019, 19, 130–137. [Google Scholar] [CrossRef]
- van Uden, S.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Current strategies for treatment of intervertebral disc degeneration: Substitution and regeneration possibilities. Biomater. Res. 2017, 21, 22. [Google Scholar] [CrossRef]
- Shamsah, A.H.; Cartmell, S.H.; Richardson, S.M.; Bosworth, L.A. Tissue Engineering the Annulus Fibrosus Using 3D Rings of Electrospun PCL:PLLA Angle-Ply Nanofiber Sheets. Front. Bioeng. Biotechnol. 2020, 7, 437. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Shi, C.; Wang, H.; Zhang, W.; Yang, H.; Li, B. Strategies for Annulus Fibrosus Regeneration: From Biological Therapies to Tissue Engineering. Front. Bioeng. Biotechnol. 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J. Tissue Engineering of the Intervertebral Disc’s Annulus Fibrosus: A Scaffold-Based Review Study. Tissue Eng. Regen. Med. 2017, 14, 81–91. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Mojica-Santiago, J.; Grunert, P.; Pennicooke, B.; Berlin, C.; Khair, T.; Navarro-Ramirez, R.; Ricart Arbona, R.J.; Nguyen, J.; Härtl, R.; et al. Total disc replacement using tissue-engineered intervertebral discs in the canine cervical spine. PLoS ONE 2017, 12, e0185716. [Google Scholar] [CrossRef] [PubMed]
- Schutgens, E.M.; Tryfonidou, M.A.; Smit, T.H.; Öner, F.C.; Krouwels, A.; Ito, K.; Creemers, L.B. Biomaterials for intervertebral disc regeneration: Past performance and possible future strategies. Eur. Cell. Mater. 2015, 30, 210–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, D.; Zhao, Q.; Chen, Z.; Liu, K.; Chen, S.; Sun, X.; Yue, Q.; Zhang, R.; Feng, G. Fabrication of a novel whole tissue-engineered intervertebral disc for intervertebral disc regeneration in the porcine lumbar spine. RSC Adv. 2018, 8, 39013–39021. [Google Scholar] [CrossRef]
- Daly, C.; Ghosh, P.; Jenkin, G.; Oehme, D.; Goldschlager, T. A Review of Animal Models of Intervertebral Disc Degeneration: Pathophysiology, Regeneration, and Translation to the Clinic. Biomed Res. Int. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Moriguchi, Y.; Alimi, M.; Khair, T.; Manolarakis, G.; Berlin, C.; Bonassar, L.J.; Härtl, R. Biological Treatment Approaches for Degenerative Disk Disease: A Literature Review of In Vivo Animal and Clinical Data. Glob. Spine J. 2016, 6, 497–518. [Google Scholar] [CrossRef]
- Baer, A.E.; Wang, J.Y.; Kraus, V.B.; Setton, L.A. Collagen gene expression and mechanical properties of intervertebral disc cell-alginate cultures. J. Orthop. Res. 2001, 19, 2–10. [Google Scholar] [CrossRef]
- Growney Kalaf, E.A.; Pendyala, M.; Bledsoe, J.G.; Sell, S.A. Characterization and restoration of degenerated IVD function with an injectable, in situ gelling alginate hydrogel: An in vitro and ex vivo study. J. Mech. Behav. Biomed. Mater. 2017, 72, 229–240. [Google Scholar] [CrossRef]
- Chou, A.I.; Nicoll, S.B. Characterization of photocrosslinked alginate hydrogels for nucleus pulposus cell encapsulation. J. Biomed. Mater. Res. Part A 2009, 91, 187–194. [Google Scholar] [CrossRef]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Frauchiger, D.A.; May, R.D.; Bakirci, E.; Tekari, A.; Chan, S.C.W.; Wöltje, M.; Benneker, L.M.; Gantenbein, B. Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model. J. Funct. Biomater. 2018, 9, E40. [Google Scholar] [CrossRef] [PubMed]
- Likhitpanichkul, M.; Dreischarf, M.; Illien-Junger, S.; Walter, B.A.; Nukaga, T.; Long, R.G.; Sakai, D.; Hecht, A.C.; Iatridis, J.C. Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: Performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests. Eur. Cell. Mater. 2014, 28, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Coric, D. A Study Comparing the Safety and Effectiveness of Cartilage Cell Injected Into the Lumbar Disc as Compared to a Placebo. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT01771471 (accessed on 4 January 2021).
- Bron, J.L.; Koenderink, G.H.; Everts, V.; Smit, T.H. Rheological characterization of the nucleus pulposus and dense collagen scaffolds intended for functional replacement. J. Orthop. Res. 2009, 27, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.-J.; Heuer, F.; Neidlinger-Wilke, C.; Claes, L. Is a collagen scaffold for a tissue engineered nucleus replacement capable of restoring disc height and stability in an animal model? Eur. Spine J. 2006, 15 (Suppl. 3), S433–S438. [Google Scholar] [CrossRef]
- Priyadarshani, P.; Li, Y.; Yang, S.; Yao, L. Injectable hydrogel provides growth-permissive environment for human nucleus pulposus cells. J. Biomed. Mater. Res. A 2016, 104, 419–426. [Google Scholar] [CrossRef]
- Omlor, G.W.; Nerlich, A.G.; Lorenz, H.; Bruckner, T.; Richter, W.; Pfeiffer, M.; Gühring, T. Injection of a polymerized hyaluronic acid/collagen hydrogel matrix in an in vivo porcine disc degeneration model. Eur. Spine J. 2012, 21, 1700–1708. [Google Scholar] [CrossRef]
- Sakai, D.; Mochida, J.; Iwashina, T.; Hiyama, A.; Omi, H.; Imai, M.; Nakai, T.; Ando, K.; Hotta, T. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials 2006, 27, 335–345. [Google Scholar] [CrossRef]
- Sakai, D.; Mochida, J.; Yamamoto, Y.; Nomura, T.; Okuma, M.; Nishimura, K.; Nakai, T.; Ando, K.; Hotta, T. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: A potential therapeutic model for disc degeneration. Biomaterials 2003, 24, 3531–3541. [Google Scholar] [CrossRef]
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Hughes, N.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. Human mesenchymal stem cell differentiation to NP-like cells in chitosan-glycerophosphate hydrogels. Biomaterials 2008, 29, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Silva-Correia, J.; Oliveira, J.M.; Caridade, S.G.; Oliveira, J.T.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J. Tissue Eng. Regen. Med. 2011, 5, e97–e107. [Google Scholar] [CrossRef] [PubMed]
- Bacelar, A.H.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Recent progress in gellan gum hydrogels provided by functionalization strategies. J. Mater. Chem. B 2016, 4, 6164–6174. [Google Scholar] [CrossRef]
- Tsaryk, R.; Silva-Correia, J.; Oliveira, J.M.; Unger, R.E.; Landes, C.; Brochhausen, C.; Ghanaati, S.; Reis, R.L.; Kirkpatrick, C.J. Biological performance of cell-encapsulated methacrylated gellan gum-based hydrogels for nucleus pulposus regeneration. J. Tissue Eng. Regen. Med. 2017, 11, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.R.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Pandit, A.; Biggs, M.J. Nanocellulose reinforced gellan-gum hydrogels as potential biological substitutes for annulus fibrosus tissue regeneration. Nanomedicine 2018, 14, 897–908. [Google Scholar] [CrossRef]
- Crevensten, G.; Walsh, A.J.L.; Ananthakrishnan, D.; Page, P.; Wahba, G.M.; Lotz, J.C.; Berven, S. Intervertebral disc cell therapy for regeneration: Mesenchymal stem cell implantation in rat intervertebral discs. Ann. Biomed. Eng. 2004, 32, 430–434. [Google Scholar] [CrossRef]
- Mohd Isa, I.L.; Abbah, S.A.; Kilcoyne, M.; Sakai, D.; Dockery, P.; Finn, D.P.; Pandit, A. Implantation of hyaluronic acid hydrogel prevents the pain phenotype in a rat model of intervertebral disc injury. Sci. Adv. 2018, 4, eaaq0597. [Google Scholar] [CrossRef]
- Brown, R. Safety and Preliminary Efficacy Study of Mesenchymal Precursor Cells (MPCs) in Subjects with Lumbar Back Pain 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT01290367?term=mesenchymal+precursor+cells+in+subjects+with+chronic+discogenic+lumbar+back+pain&rank=1 (accessed on 4 January 2021).
- Li, Z.; Kaplan, K.M.; Wertzel, A.; Peroglio, M.; Amit, B.; Alini, M.; Grad, S.; Yayon, A. Biomimetic fibrin-hyaluronan hydrogels for nucleus pulposus regeneration. Regen. Med. 2014, 9, 309–326. [Google Scholar] [CrossRef]
- Malonzo, C.; Chan, S.C.W.; Kabiri, A.; Eglin, D.; Grad, S.; Bonél, H.M.; Benneker, L.M.; Gantenbein-Ritter, B. A papain-induced disc degeneration model for the assessment of thermo-reversible hydrogel-cells therapeutic approach. J. Tissue Eng. Regen. Med. 2015, 9, E167–E176. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Boyes, V.L.; Sammon, C.; Le Maitre, C.L. Thermally triggered injectable hydrogel, which induces mesenchymal stem cell differentiation to nucleus pulposus cells: Potential for regeneration of the intervertebral disc. Acta Biomater. 2016, 36, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, A.A.; Freeman, C.; Farthing, P.; Callaghan, J.; Hatton, P.V.; Brook, I.M.; Sammon, C.; Le Maitre, C.L. In vivo safety and efficacy testing of a thermally triggered injectable hydrogel scaffold for bone regeneration and augmentation in a rat model. Oncotarget 2018, 9, 18277–18295. [Google Scholar] [CrossRef] [PubMed]
- Peroglio, M.; Eglin, D.; Benneker, L.M.; Alini, M.; Grad, S. Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells. Spine J. 2013, 13, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Peroglio, M.; Grad, S.; Mortisen, D.; Sprecher, C.M.; Illien-Jünger, S.; Alini, M.; Eglin, D. Injectable thermoreversible hyaluronan-based hydrogels for nucleus pulposus cell encapsulation. Eur. Spine J. 2012, 21, 839–849. [Google Scholar] [CrossRef]
- Jeong, C.G.; Francisco, A.T.; Niu, Z.; Mancino, R.L.; Craig, S.L.; Setton, L.A. Screening of hyaluronic acid-poly(ethylene glycol) composite hydrogels to support intervertebral disc cell biosynthesis using artificial neural network analysis. Acta Biomater. 2014, 10, 3421–3430. [Google Scholar] [CrossRef]
- Francisco, A.T.; Hwang, P.Y.; Jeong, C.G.; Jing, L.; Chen, J.; Setton, L.A. Photocrosslinkable laminin-functionalized polyethylene glycol hydrogel for intervertebral disc regeneration. Acta Biomater. 2014, 10, 1102–1111. [Google Scholar] [CrossRef]
- Benz, K.; Stippich, C.; Osswald, C.; Gaissmaier, C.; Lembert, N.; Badke, A.; Steck, E.; Aicher, W.K.; Mollenhauer, J.A. Rheological and biological properties of a hydrogel support for cells intended for intervertebral disc repair. BMC Musculoskelet. Disord. 2012, 13, 54. [Google Scholar] [CrossRef]
- Schmocker, A.; Khoushabi, A.; Frauchiger, D.A.; Gantenbein, B.; Schizas, C.; Moser, C.; Bourban, P.-E.; Pioletti, D.P. A photopolymerized composite hydrogel and surgical implanting tool for a nucleus pulposus replacement. Biomaterials 2016, 88, 110–119. [Google Scholar] [CrossRef]
- Isa, I.L.M.; Srivastava, A.; Tiernan, D.; Owens, P.; Rooney, P.; Dockery, P.; Pandit, A. Hyaluronic Acid Based Hydrogels Attenuate Inflammatory Receptors and Neurotrophins in Interleukin-1β Induced Inflammation Model of Nucleus Pulposus Cells. Biomacromolecules 2015, 16, 1714–1725. [Google Scholar] [CrossRef]
- Joshi, A.; Fussell, G.; Thomas, J.; Hsuan, A.; Lowman, A.; Karduna, A.; Vresilovic, E.; Marcolongo, M. Functional compressive mechanics of a PVA/PVP nucleus pulposus replacement. Biomaterials 2006, 27, 176–184. [Google Scholar] [CrossRef]
- Wang, B.H.; Campbell, G. Formulations of polyvinyl alcohol cryogel that mimic the biomechanical properties of soft tissues in the natural lumbar intervertebral disc. Spine 2009, 34, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Millon, L.E.; Oates, C.J.; Wan, W. Compression properties of polyvinyl alcohol--bacterial cellulose nanocomposite. J. Biomed. Mater. Res. B. Appl. Biomater. 2009, 90, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Wong, E. Poly (Vinyl Alcohol) Nanocomposite Hydrogels for Intervertebral Disc Prostheses. Ph.D. Thesis, The University of Western Ontario, Richmond St, ON, Canada, 2012. [Google Scholar]
- Neo, P.Y.; Shi, P.; Goh, J.C.-H.; Toh, S.L. Characterization and mechanical performance study of silk/PVA cryogels: Towards nucleus pulposus tissue engineering. Biomed. Mater. 2014, 9, 65002. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef]
- Miller, A.F.; Saiani, A. Engineering peptide based biomaterials Structure, properties and application. Chem. Today 2010, 28, 34–38. [Google Scholar]
- Liu, X.; Wang, X.; Wang, X.; Ren, H.; He, J.; Qiao, L.; Cui, F.-Z. Functionalized self-assembling peptide nanofiber hydrogels mimic stem cell niche to control human adipose stem cell behavior in vitro. Acta Biomater. 2013, 9, 6798–6805. [Google Scholar] [CrossRef]
- Zhou, M.; Lozano, N.; Wychowaniec, J.K.; Hodgkinson, T.; Richardson, S.M.; Kostarelos, K.; Hoyland, J.A. Graphene oxide: A growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019, 96, 271–280. [Google Scholar] [CrossRef]
- Lee, W.C.; Loh, K.P.; Lim, C.T. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials 2018, 155, 236–250. [Google Scholar]
- Wan, S.; Borland, S.; Richardson, S.M.; Merry, C.L.R.; Saiani, A.; Gough, J.E. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016, 46, 29–40. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, Q.; Wu, Y.; Liu, Y.; Guo, X.; Wu, W. Culture of nucleus pulposus cells from intervertebral disc on self-assembling KLD-12 peptide hydrogel scaffold. Mater. Sci. Eng. C 2010, 30, 975–980. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Y.; Shao, Z.; Yang, S.; Che, B.; Sun, C.; Ma, Z.; Zhang, Y. Functionalized self-assembling peptide nanofiber hydrogel as a scaffold for rabbit nucleus pulposus cells. J. Biomed. Mater. Res. A 2012, 100, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Zhang, Y.; Wang, C.-F.; Zhang, C.; Wang, X.-M.; Wang, D.-L.; Bai, X.-D.; Wen, T.-Y.; Xin, H.-K.; Wu, J.-H.; et al. Biological evaluation of human degenerated nucleus pulposus cells in functionalized self-assembling peptide nanofiber hydrogel scaffold. Tissue Eng. Part. A 2014, 20, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Ligorio, C.; Zhou, M.; Wychowaniec, J.K.; Zhu, X.; Bartlam, C.; Miller, A.F.; Vijayaraghavan, A.; Hoyland, J.A.; Saiani, A. Graphene oxide containing self-assembling peptide hybrid hydrogels as a potential 3D injectable cell delivery platform for intervertebral disc repair applications. Acta Biomater. 2019, 92, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Kim, H.-J.; Kaplan, D.; Vunjak-Novakovic, G.; Kandel, R.A. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur. Spine J. 2007, 16, 1848–1857. [Google Scholar] [CrossRef]
- Park, S.-H.; Gil, E.S.; Cho, H.; Mandal, B.B.; Tien, L.W.; Min, B.-H.; Kaplan, D.L. Intervertebral disk tissue engineering using biphasic silk composite scaffolds. Tissue Eng. Part. A 2012, 18, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, B.K.; Kaplan, D.L.; Mandal, B.B. Silk-based multilayered angle-ply annulus fibrosus construct to recapitulate form and function of the intervertebral disc. Proc. Natl. Acad. Sci. USA 2018, 115, 477–482. [Google Scholar] [CrossRef]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Shao, X.; Hunter, C.J. Developing an alginate/chitosan hybrid fiber scaffold for annulus fibrosus cells. J. Biomed. Mater. Res. A 2007, 82, 701–710. [Google Scholar] [CrossRef]
- Renani, H.B.; Ghorbani, M.; Beni, B.H.; Karimi, Z.; Mirhosseini, M.; Zarkesh, H.; Kabiri, A. Determination and comparison of specifics of nucleus pulposus cells of human intervertebral disc in alginate and chitosan-gelatin scaffolds. Adv. Biomed. Res. 2012, 1, 81. [Google Scholar]
- Lee, K.-I.; Moon, S.-H.; Kim, H.; Kwon, U.-H.; Kim, H.-J.; Park, S.-N.; Suh, H.; Lee, H.-M.; Kim, H.-S.; Chun, H.-J.; et al. Tissue engineering of the intervertebral disc with cultured nucleus pulposus cells using atelocollagen scaffold and growth factors. Spine 2012, 37, 452–458. [Google Scholar] [CrossRef]
- Sato, M.; Asazuma, T.; Ishihara, M.; Ishihara, M.; Kikuchi, T.; Kikuchi, M.; Fujikawa, K. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine 2003, 28, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Levene, H.B.; Gu, W.; Huang, C.-Y.C. Enhancement of Energy Production of the Intervertebral Disc by the Implantation of Polyurethane Mass Transfer Devices. Ann. Biomed. Eng. 2017, 45, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lang, G.; Chen, X.; Sacks, H.; Mantzur, C.; Tropp, U.; Mader, K.T.; Smallwood, T.C.; Sammon, C.; Richards, R.G.; et al. Polyurethane scaffold with in situ swelling capacity for nucleus pulposus replacement. Biomaterials 2016, 84, 196–209. [Google Scholar] [CrossRef]
- Xin, L.; Xu, W.; Yu, L.; Fan, S.; Wang, W.; Yu, F.; Wang, Z. Effects of annulus defects and implantation of poly(lactic-co-glycolic acid) (PLGA)/fibrin gel scaffolds on nerves ingrowth in a rabbit model of annular injury disc degeneration. J. Orthop. Surg. Res. 2017, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Roy, A.K.; Vacanti, C.A.; Kojima, K.; Ueda, M.; Bonassar, L.J. Tissue-engineered composites of anulus fibrosus and nucleus pulposus for intervertebral disc replacement. Spine 2004, 29, 1290-7-8. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.J.; Li, W.-J.; Shanti, R.M.; Jiang, Y.J.; Jackson, W.; Freedman, B.A.; Kuklo, T.R.; Giuliani, J.R.; Tuan, R.S. Intervertebral disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold (HANFS) amalgam. Tissue Eng. Part. A 2008, 14, 1527–1537. [Google Scholar] [CrossRef]
- Casagrande, S.; Tiribuzi, R.; Cassetti, E.; Selmin, F.; Gervasi, G.L.; Barberini, L.; Freddolini, M.; Ricci, M.; Schoubben, A.; Cerulli, G.G.; et al. Biodegradable composite porous poly(dl-lactide-co-glycolide) scaffold supports mesenchymal stem cell differentiation and calcium phosphate deposition. Artif. Cells Nanomed. Biotechnol. 2018, 46, 219–229. [Google Scholar] [CrossRef]
- Helen, W.; Gough, J.E. Cell viability, proliferation and extracellular matrix production of human annulus fibrosus cells cultured within PDLLA/Bioglass composite foam scaffolds in vitro. Acta Biomater. 2008, 4, 230–243. [Google Scholar] [CrossRef]
- López, A.; Persson, C.; Hilborn, J.; Engqvist, H. Synthesis and characterization of injectable composites of poly[D,L-lactide-co-(ε-caprolactone)] reinforced with β-TCP and CaCO3 for intervertebral disk augmentation. J. Biomed. Mater. Res. B. Appl. Biomater. 2010, 95, 75–83. [Google Scholar] [CrossRef]
- Martin, J.T.; Milby, A.H.; Chiaro, J.A.; Kim, D.H.; Hebela, N.M.; Smith, L.J.; Elliott, D.M.; Mauck, R.L. Translation of an engineered nanofibrous disc-like angle-ply structure for intervertebral disc replacement in a small animal model. Acta Biomater. 2014, 10, 2473–2481. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Baker, B.M.; Sen, S.; Wible, E.E.; Elliott, D.M.; Mauck, R.L. Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus. Nat. Mater. 2009, 8, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Du, L.; Zhang, J.; Zhu, M.; Ji, S.; Zhang, Y.; Kong, D.; Ma, X.; Yang, Q.; Wang, L. Circumferentially oriented microfiber scaffold prepared by wet-spinning for tissue engineering of annulus fibrosus. RSC Adv. 2015, 5, 42705–42713. [Google Scholar] [CrossRef]
- Kohane, D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Tellegen, A.R.; Rudnik-Jansen, I.; Beukers, M.; Miranda-Bedate, A.; Bach, F.C.; de Jong, W.; Woike, N.; Mihov, G.; Thies, J.C.; Meij, B.P.; et al. Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J. Control. Release 2018, 286, 439–450. [Google Scholar] [CrossRef]
- Viswanathan, V.K.; Rajaram Manoharan, S.R.; Subramanian, S.; Moon, A. Nanotechnology in Spine Surgery: A Current Update and Critical Review of the Literature. World Neurosurg. 2019, 123, 142–155. [Google Scholar] [CrossRef]
- Antunes, J.C.; Pereira, C.L.; Teixeira, G.Q.; Silva, R.V.; Caldeira, J.; Grad, S.; Gonçalves, R.M.; Barbosa, M.A. Poly(γ-glutamic acid) and poly(γ-glutamic acid)-based nanocomplexes enhance type II collagen production in intervertebral disc. J. Mater. Sci. Mater. Med. 2017, 28, 6. [Google Scholar] [CrossRef]
- Liang, C.; Li, H.; Li, C.; Yang, Z.; Zhou, X.; Tao, Y.; Xiao, Y.; Li, F.; Chen, Q. Fabrication of a Layered Microstructured Polymeric Microspheres as a Cell Carrier for Nucleus Pulposus Regeneration. J. Biomater. Sci. Polym. Ed. 2012, 23, 2287–2302. [Google Scholar] [CrossRef]
- Mukherjee, S.; Nazemi, M.; Jonkers, I.; Geris, L. Use of Computational Modeling to Study Joint Degeneration: A Review. Front. Bioeng. Biotechnol. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Malandrino, A.; Jackson, A.R.; Huyghe, J.M.; Noailly, J. Poroelastic modeling of the intervertebral disc: A path toward integrated studies of tissue biophysics and organ degeneration. MRS Bull. 2015, 40, 324–332. [Google Scholar] [CrossRef]
- Biot, M.A. General theory of three-dimensional consolidation. J. Appl. Phys. 1941, 12, 155–164. [Google Scholar] [CrossRef]
- Mow, V.C.; Kuei, S.C.; Lai, W.M.; Armstrong, C.G. Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. J. Biomech. Eng. 1980, 102, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, A.; Noailly, J.; Lacroix, D. The effect of sustained compression on oxygen metabolic transport in the intervertebral disc decreases with degenerative changes. PLoS Comput. Biol. 2011, 7, e1002112. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.; Van Donkelaar, C.C.; Van Rietbergen, B.; Huiskes, R. A fibril-reinforced poroviscoelastic swelling model for articular cartilage. J. Biomech. 2005, 38, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, Y.; Wilson, W.; Huyghe, J.M.; Baaijens, F.P.T. Osmoviscoelastic finite element model of the intervertebral disc. Eur. Spine J. 2006, 15, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Van Rijsbergen, M.; Van Rietbergen, B.; Barthelemy, V.; Eltes, P.; Lazáry, Á.; Lacroix, D.; Noailly, J.; Tho, M.C.H.B.; Wilson, W.; Ito, K. Comparison of patient-specific computational models vs. clinical follow-up, for adjacent segment disc degeneration and bone remodelling after spinal fusion. PLoS ONE 2018, 13, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Gao, X.; Brown, M.D.; Eismont, F.; Gu, W. Effects of diurnal loading on the transport of charged antibiotics into intervertebral discs. J. Biomech. 2019, 87, 177–182. [Google Scholar] [CrossRef]
- Jackson, A.R.; Eismont, A.; Yu, L.; Li, N.; Gu, W.; Eismont, F.; Brown, M.D. Diffusion of antibiotics in intervertebral disc. J. Biomech. 2018, 76, 259–262. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Gu, W.Y. Effects of Mechanical Compression on Metabolism and Distribution of Oxygen and Lactate in Intervertebral Disc. J. Biomech 2008, 41, 1184–1196. [Google Scholar] [CrossRef]
- Malandrino, A.; Pozo, J.M.; Castro-Mateos, I.; Frangi, A.F.; van Rijsbergen, M.M.; Ito, K.; Wilke, H.-J.; Dao, T.T.; Ho Ba Tho, M.-C.; Noailly, J. On the Relative Relevance of Subject-Specific Geometries and Degeneration-Specific Mechanical Properties for the Study of Cell Death in Human Intervertebral Disk Models. Front. Bioeng. Biotechnol. 2015, 3, 1–15. [Google Scholar] [CrossRef]
- Urban, J.P.G.; Smith, S.Dp.; Fairbank, J.C.T. Nutrition of the Intervertebral Disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef]
- Benneker, L.M.; Heini, P.F.; Alini, M.; Anderson, S.E.; Ito, K. 2004 Young Investigator Award Winner: Vertebral Endplate Marrow Contact Channel Occlusions and Intervertebral Disc Degeneration. Spine 2005, 30, 167–173. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, M.; Lezuo, P.; Maissen, O.; van Donkelaar, C.C.; Ito, K. Inhibition of vertebral endplate perfusion results in decreased intervertebral disc intranuclear diffusive transport. J. Anat. 2007, 211, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Malandrino, A.; Lacroix, D.; Hellmich, C.; Ito, K.; Ferguson, S.J.; Noailly, J. The role of endplate poromechanical properties on the nutrient availability in the intervertebral disc. Osteoarthr. Cartil. 2014, 22, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Dudli, S.; Fields, A.J.; Samartzis, D.; Karppinen, J.; Lotz, J.C. Pathobiology of Modic changes. Eur. Spine J. 2016, 25, 3723–3734. [Google Scholar] [CrossRef] [PubMed]
- Määttä, J.H.; Kraatari, M.; Wolber, L.; Niinimäki, J.; Wadge, S.; Karppinen, J.; Williams, F.M.K. Vertebral endplate change as a feature of intervertebral disc degeneration: A heritability study. Eur. Spine J. 2014, 23, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, L.; Xenarios, I. A method for the generation of standardized qualitative dynamical systems of regulatory networks. Theor. Biol. Med. Model. 2006, 3, 13. [Google Scholar] [CrossRef]
- Baumgartner, L.; González Ballester, M.Á.; Noailly, J. Simulation of the Multifactorial Cellular Environment within the Intervertebral disc to better understand Microtrauma Emergence. In Proceedings of the IRC-19-67; 2019; pp. 484–485. [Google Scholar]
- Machado, D.; Costa, R.S.; Rocha, M.; Ferreira, E.C.; Tidor, B.; Rocha, I. Modeling formalisms in systems biology. AMB Express 2011, 1, 45. [Google Scholar] [CrossRef]
- Munir, S.; Rade, M.; Määttä, J.H.; Freidin, M.B.; Williams, F.M.K. Intervertebral Disc Biology: Genetic Basis of Disc Degeneration. Curr. Mol. Biol. Reports 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Bradshaw, R.A.; Dennis, E.A. Handbook of Cell Signaling, Three-Volume Set; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 9780121245467. [Google Scholar]
- Klamt, S.; Saez-Rodriguez, J.; Lindquist, J.A.; Simeoni, L.; Gilles, E.D. A methodology for the structural and functional analysis of signaling and regulatory networks. BMC Bioinform. 2006, 7, 56. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Garapati, P.; Gillespie, M.; Hausmann, K.; Haw, R.; Jassal, B.; Jupe, S.; Korninger, F.; McKay, S.; et al. The Reactome pathway Knowledgebase. Nucleic Acids Res. 2016, 44, D481–D487. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Slenter, D.N.; Kutmon, M.; Hanspers, K.; Riutta, A.; Windsor, J.; Nunes, N.; Mélius, J.; Cirillo, E.; Coort, S.L.; Digles, D.; et al. WikiPathways: A multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018, 46, D661–D667. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.G.; Gross, B.E.; Demir, E.; Rodchenkov, I.; Babur, O.; Anwar, N.; Schultz, N.; Bader, G.D.; Sander, C. Pathway Commons, a web resource for biological pathway data. Nucleic Acids Res. 2011, 39, D685–D690. [Google Scholar] [CrossRef] [PubMed]
- Türei, D.; Korcsmáros, T.; Saez-Rodriguez, J. OmniPath: Guidelines and gateway for literature-curated signaling pathway resources. Nat. Methods 2016, 13, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Clerc, O.; Deniaud, M.; Vallet, S.D.; Naba, A.; Rivet, A.; Perez, S.; Thierry-Mieg, N.; Ricard-Blum, S. MatrixDB: Integration of new data with a focus on glycosaminoglycan interactions. Nucleic Acids Res. 2019, 47, D376–D381. [Google Scholar] [CrossRef] [PubMed]
- Mitsos, A.; Melas, I.N.; Morris, M.K.; Saez-Rodriguez, J.; Lauffenburger, D.A.; Alexopoulos, L.G. Non Linear Programming (NLP) Formulation for Quantitative Modeling of Protein Signal Transduction Pathways. PLoS ONE 2012, 7, e50085. [Google Scholar] [CrossRef][Green Version]
- Morris, M.K.; Saez-Rodriguez, J.; Sorger, P.K.; Lauffenburger, D.A. Logic-Based Models for the Analysis of Cell Signaling Networks. Biochemistry 2010, 49, 3216–3224. [Google Scholar] [CrossRef]
- Aldridge, B.B.; Saez-Rodriguez, J.; Muhlich, J.L.; Sorger, P.K.; Lauffenburger, D.A. Fuzzy Logic Analysis of Kinase Pathway Crosstalk in TNF/EGF/Insulin-Induced Signaling. PLoS Comput. Biol. 2009, 5, e1000340. [Google Scholar] [CrossRef]
- Yue, H.; Brown, M.; Knowles, J.; Wang, H.; Broomhead, D.S.; Kell, D.B. Insights into the behaviour of systems biology models from dynamic sensitivity and identifiability analysis: A case study of an NF-κB signalling pathway. Mol. BioSyst. 2006, 2, 640–649. [Google Scholar] [CrossRef]
- Orton, R.J.; Sturm, O.E.; Vyshemirsky, V.; Calder, M.; Gilbert, D.R.; Kolch, W. Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem. J. 2005, 392, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Krumsiek, J.; Pölsterl, S.; Wittmann, D.M.; Theis, F.J. Odefy—From discrete to continuous models. BMC Bioinformatics 2010, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Disc4All—Training Network to Advance Integrated Computational Simulations in Translational Medicine, Applied to Intervertebral disc Degeneration. Available online: https://www.upf.edu/web/disc4all (accessed on 4 January 2021).
- Zhang, W.; Chien, J.; Yong, J.; Kuang, R. Network-based machine learning and graph theory algorithms for precision oncology. NPJ Precis. Oncol. 2017, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, L.G.; Saez-Rodriguez, J.; Espelin, C.W. High-Throughput Protein-Based Technologies and Computational Models for Drug Development, Efficacy, and Toxicity. In Drug Efficacy, Safety, and Biologics Discovery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 29–52. [Google Scholar]
- Clarke, D.C.; Morris, M.K.; Lauffenburger, D.A. Normalization and Statistical Analysis of Multiplexed Bead-based Immunoassay Data Using Mixed-effects Modeling. Mol. Cell. Proteomics 2013, 12, 245–262. [Google Scholar] [CrossRef]
- Saez-Rodriguez, J.; Alexopoulos, L.G.; Epperlein, J.; Samaga, R.; Lauffenburger, D.A.; Klamt, S.; Sorger, P.K. Discrete logic modelling as a means to link protein signalling networks with functional analysis of mammalian signal transduction. Mol. Syst. Biol. 2009, 5, 331. [Google Scholar] [CrossRef]
- Mitsos, A.; Melas, I.N.; Siminelakis, P.; Chairakaki, A.D.; Saez-Rodriguez, J.; Alexopoulos, L.G. Identifying Drug Effects via Pathway Alterations using an Integer Linear Programming Optimization Formulation on Phosphoproteomic Data. PLoS Comput. Biol. 2009, 5, e1000591. [Google Scholar] [CrossRef]
- Terfve, C.; Cokelaer, T.; Henriques, D.; MacNamara, A.; Goncalves, E.; Morris, M.K.; Iersel, M. van; Lauffenburger, D.A.; Saez-Rodriguez, J. CellNOptR: A flexible toolkit to train protein signaling networks to data using multiple logic formalisms. BMC Syst. Biol. 2012, 6, 133. [Google Scholar] [CrossRef]
- von Kamp, A.; Thiele, S.; Hädicke, O.; Klamt, S. Use of CellNetAnalyzer in biotechnology and metabolic engineering. J. Biotechnol. 2017, 261, 221–228. [Google Scholar] [CrossRef]
- Garcia-Alonso, L.; Holland, C.H.; Ibrahim, M.M.; Turei, D.; Saez-Rodriguez, J. Benchmark and integration of resources for the estimation of human transcription factor activities. Genome Res. 2019, 29, 1363–1375. [Google Scholar] [CrossRef]
- Choi, K.-S.; Harfe, B.D. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc. Natl. Acad. Sci. USA 2011, 108, 9484–9489. [Google Scholar] [CrossRef]
- Shen, L.; Xiao, Y.; Wu, Q.; Liu, L.; Zhang, C.; Pan, X. TLR4/NF-κB axis signaling pathway-dependent up-regulation of miR-625-5p contributes to human intervertebral disc degeneration by targeting COL1A1. Am. J. Transl. Res. 2019, 11, 1374–1388. [Google Scholar] [PubMed]
- Liang, H.; Yang, X.; Liu, C.; Sun, Z.; Wang, X. Effect of nf-kb signaling pathway on the expression of mif, tnf-α, il-6 in the regulation of intervertebral disc degeneration. J. Musculoskelet. Neuronal Interact. 2018, 18, 551–556. [Google Scholar] [PubMed]
- Fang, W.; Zhou, X.; Wang, J.; Xu, L.; Zhou, L.; Yu, W.; Tao, Y.; Zhu, J.; Hu, B.; Liang, C.; et al. Wogonin mitigates intervertebral disc degeneration through the Nrf2/ARE and MAPK signaling pathways. Int. Immunopharmacol. 2018, 65, 539–549. [Google Scholar] [CrossRef] [PubMed]
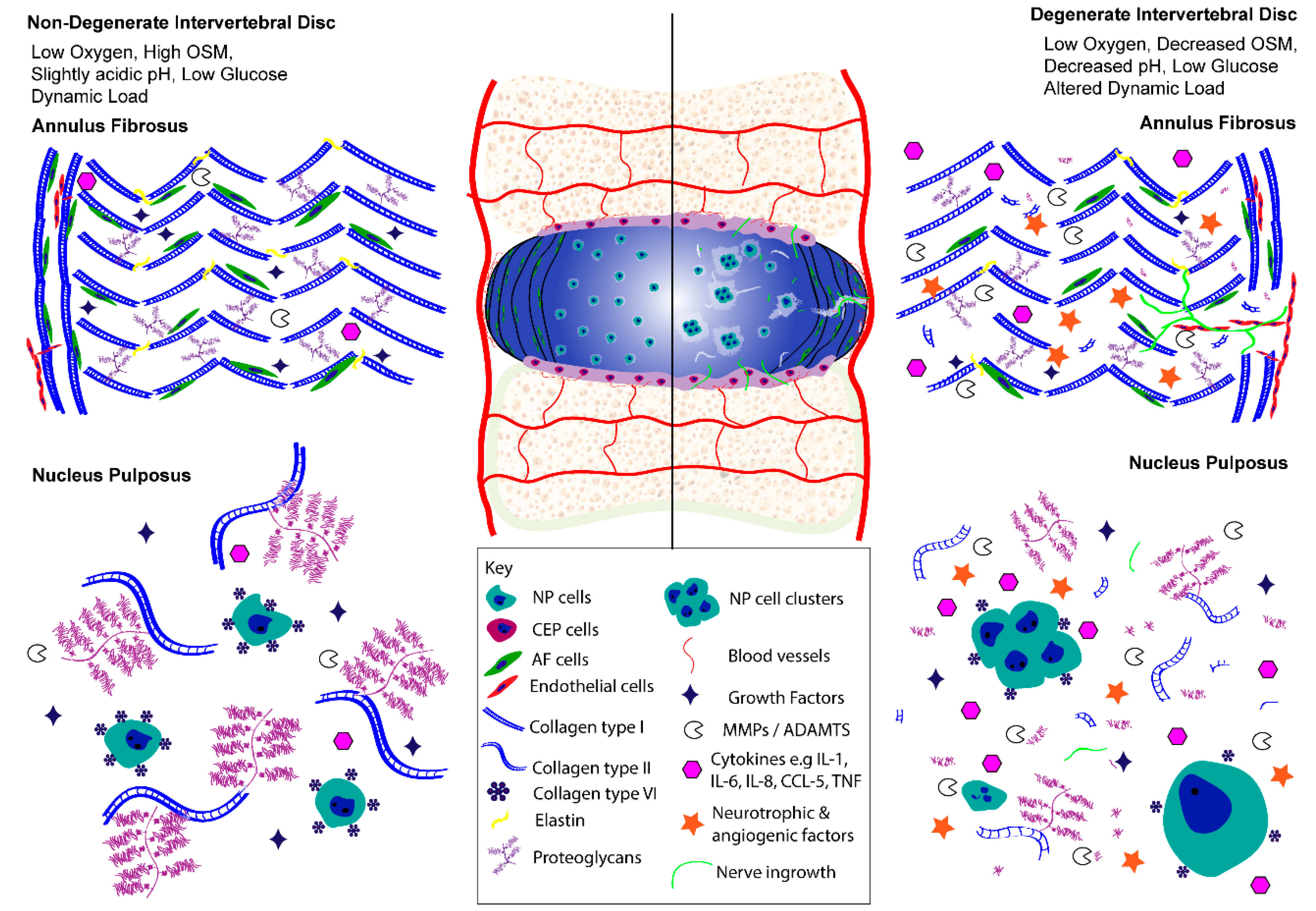
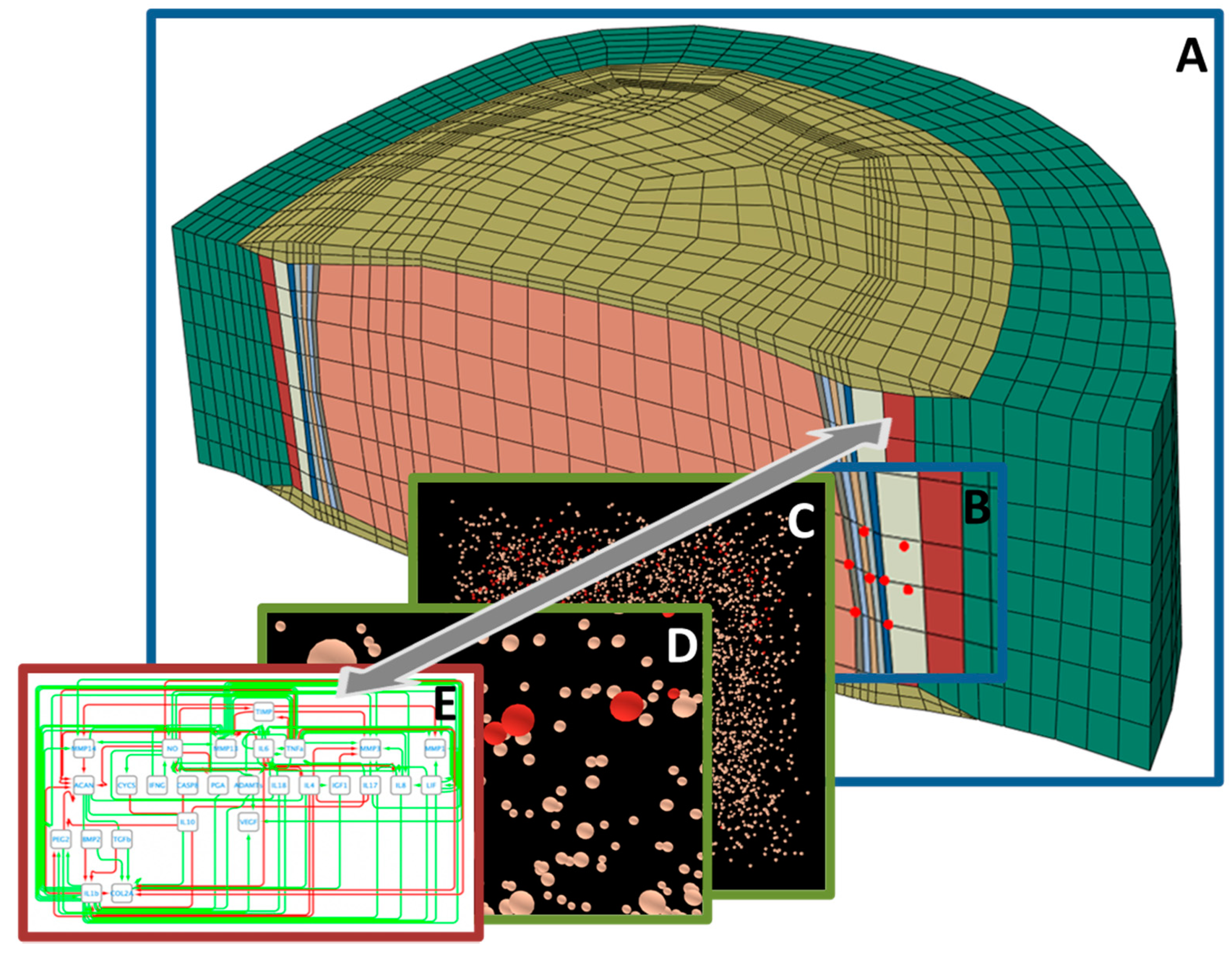
| Hydrogels for IVD TE | ||
|---|---|---|
| Natural Hydrogels | ||
| Type | Material Biomechanical Properties | IVD Studies |
| Alginate |
| |
| Fibrin |
| |
| Collagen |
|
|
| Atelo-collagen |
|
|
| Chitosan | ||
| Gellan gum |
|
|
| Hyaluronan (hyaluronic acid) |
| |
| Synthetic Hydrogels | ||
| Type | Material Biomechanical Properties | IVD Studies |
| Poly N-isopropyl-acrylamide (pNIPAM)-based hydrogels | Laponite crosslinked pNIPAM-co-DMAc: | |
|
| |
| HA-pNIPAM hydrogel: | ||
|
| |
| Polyethylene glycol (PEG)-based hydrogels | Composites:
| |
| Polyvinyl alcohol (PVA)-based hydrogels |
|
|
| Self-assembling peptide hydrogels (SAPH) |
|
|
| Scaffolds for IVD TE | ||
| Natural Polymers | ||
| Type | Material Biomechanical Properties | IVD Studies |
| Silk fibroin |
|
|
| Alginate | Composites: | |
| Atelo- collagen |
|
|
| Synthetic Polymers | ||
| Type | Material biological and mechanical properties | IVD Studies |
| Poly-urethane (PU) |
|
|
| Polylactic acid polyglycolic acid (PGA) Copolymer: polylactic-co-glycolic acid (PLGA) |
|
|
| Poly D,L-lactide (PDLLA) |
| |
| Poly-ε-caprolactone (PCL) |
|
|
| Title | Content | Size | Address |
|---|---|---|---|
| KEGG | Integrated database resource consisting of 18 databases including systems, genomic, chemical, and health information on the molecular interaction networks in biological systems | KEGG Pathway: 536 pathways | https://www.genome.jp/kegg/ [466] |
| Reactome | Pathway database with interactive web visualization tool | 2272 pathways, 10,833 proteins, 12,505 interactions | https://reactome.org/ [467] |
| STRING | Protein–protein interaction networks | 5000 organisms, 24.6 mio proteins, >2000 mio interactions | https://string-db.org/ [468] |
| WikiPathways | Pathways of different species stored in wiki format | 2785 pathways, 28 species | https://wikipathways.org/ [469] |
| Pathway Commons | Biological pathway data extracted from various databases with visualization tool | 4700 pathways, 2.3 mio interactions | https://pathwaycommons.org/ [470] |
| Omnipath | Literature-curated mammalian signaling pathways from >50 databases | 10,934 proteins, 53,542 interactions | http://omnipathdb.org/[471] |
| MatrixDB | Database focused on interactions established by extracellular matrix proteins, PG and polysaccharides | 106,453 associations from 38,921 experiments | http://matrixdb.univ-lyon1.fr/ [472] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgartner, L.; Wuertz-Kozak, K.; Le Maitre, C.L.; Wignall, F.; Richardson, S.M.; Hoyland, J.; Ruiz Wills, C.; González Ballester, M.A.; Neidlin, M.; Alexopoulos, L.G.; et al. Multiscale Regulation of the Intervertebral Disc: Achievements in Experimental, In Silico, and Regenerative Research. Int. J. Mol. Sci. 2021, 22, 703. https://doi.org/10.3390/ijms22020703
Baumgartner L, Wuertz-Kozak K, Le Maitre CL, Wignall F, Richardson SM, Hoyland J, Ruiz Wills C, González Ballester MA, Neidlin M, Alexopoulos LG, et al. Multiscale Regulation of the Intervertebral Disc: Achievements in Experimental, In Silico, and Regenerative Research. International Journal of Molecular Sciences. 2021; 22(2):703. https://doi.org/10.3390/ijms22020703
Chicago/Turabian StyleBaumgartner, Laura, Karin Wuertz-Kozak, Christine L. Le Maitre, Francis Wignall, Stephen M. Richardson, Judith Hoyland, Carlos Ruiz Wills, Miguel A. González Ballester, Michael Neidlin, Leonidas G. Alexopoulos, and et al. 2021. "Multiscale Regulation of the Intervertebral Disc: Achievements in Experimental, In Silico, and Regenerative Research" International Journal of Molecular Sciences 22, no. 2: 703. https://doi.org/10.3390/ijms22020703
APA StyleBaumgartner, L., Wuertz-Kozak, K., Le Maitre, C. L., Wignall, F., Richardson, S. M., Hoyland, J., Ruiz Wills, C., González Ballester, M. A., Neidlin, M., Alexopoulos, L. G., & Noailly, J. (2021). Multiscale Regulation of the Intervertebral Disc: Achievements in Experimental, In Silico, and Regenerative Research. International Journal of Molecular Sciences, 22(2), 703. https://doi.org/10.3390/ijms22020703