PKC Regulates YAP Expression through Alternative Splicing of YAP 3′UTR Pre-mRNA by hnRNP F
Abstract
1. Introduction
2. Results
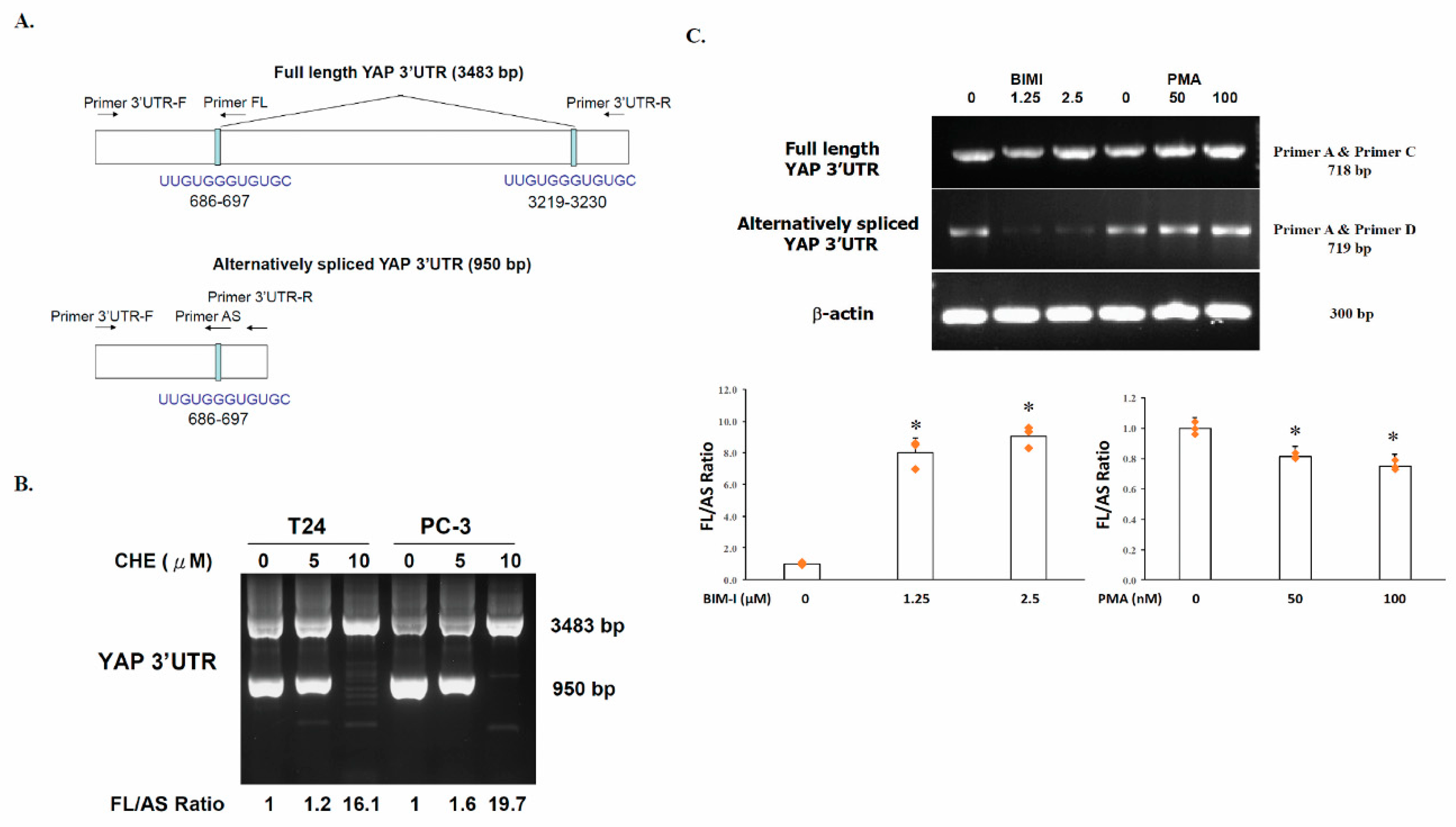
2.1. Generation of an Alternatively Spliced YAP 3′UTR in Human Cancer Cells
2.2. The Switch of YAP 3′UTR between Full Length 3′UTR and Alternatively Spliced 3′UTR Is Regulated by PKC
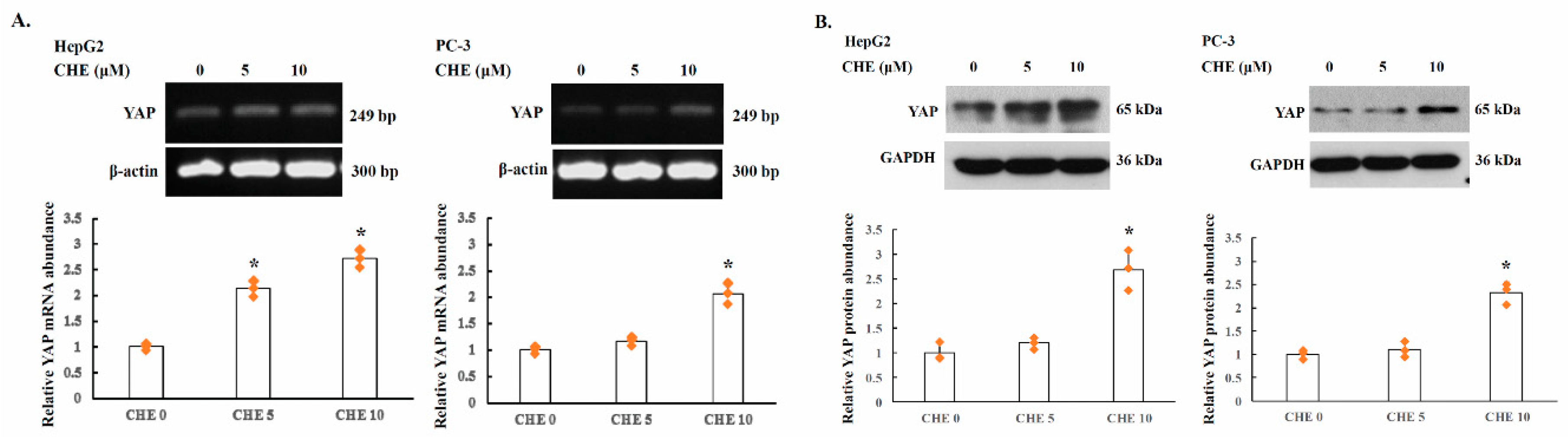
2.3. YAP Is Upregulated by PKC Inhibitors in Human Cancer Cells
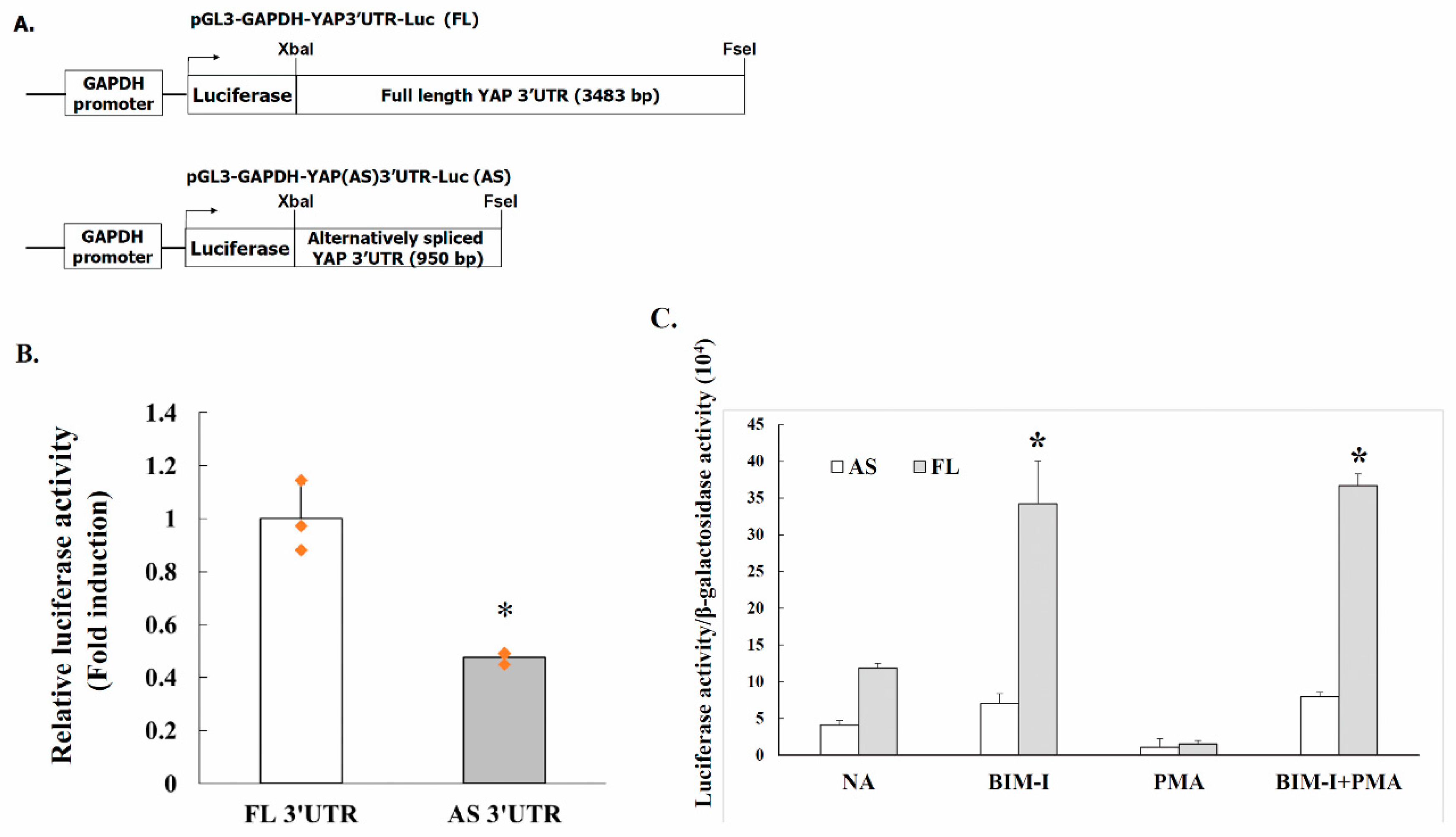
2.4. Alternative Splicing of YAP 3′UTR Contributes to Its Stability
2.5. PKC Activity Decreases YAP 3′UTR–Mediated mRNA Stability
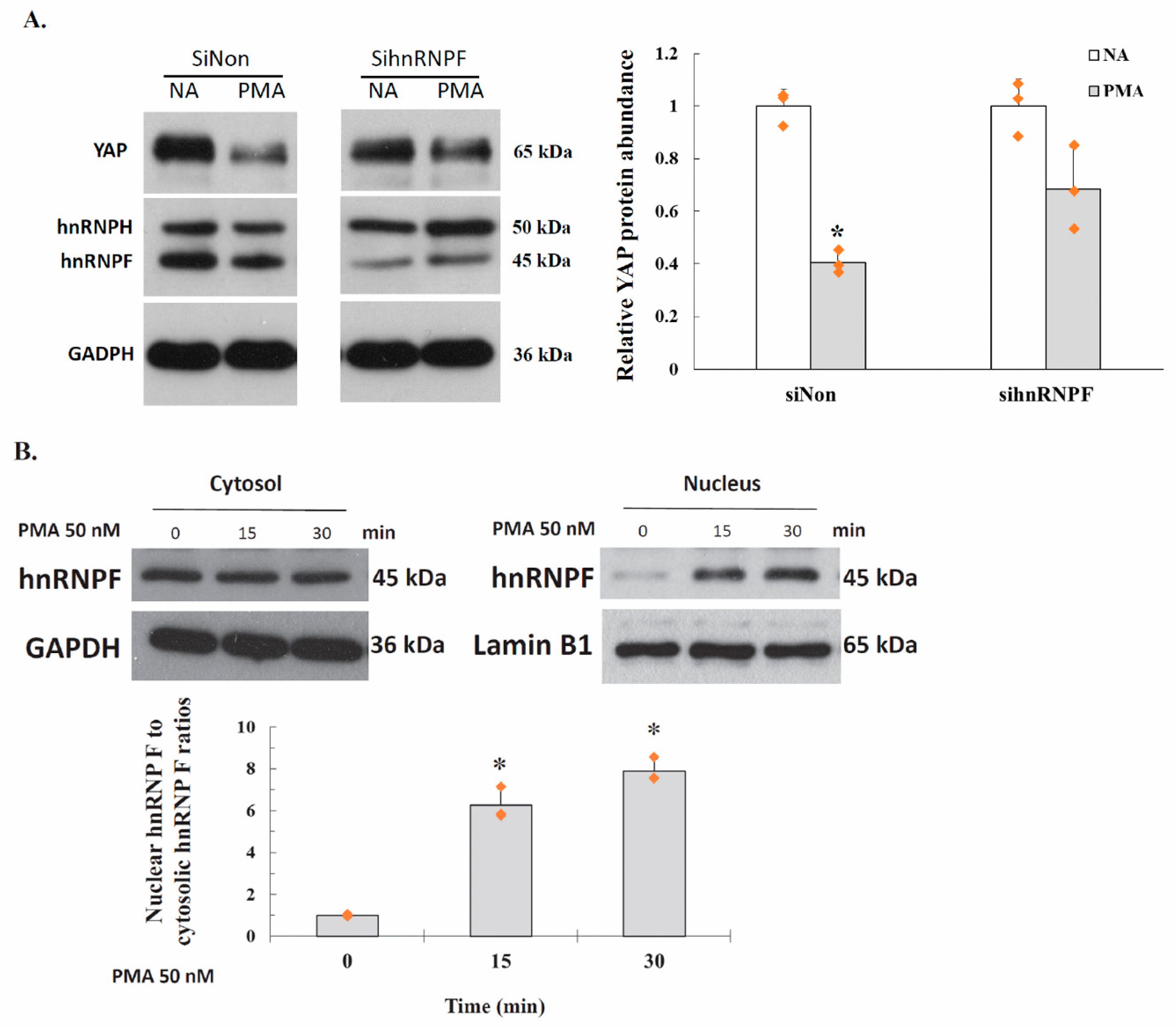
2.6. PKC Represses YAP 3′UTR–Mediated mRNA Stability Is Dependent on hnRNP F
2.7. Activation of PKC Induces Nuclear Translocation of Cytosolic hnRNP F
2.8. HnRNP F Enhances YAP3′UTR Splicing
2.9. The Regulation of YAP 3′UTR-Mediated mRNA Stability by hnRNPF Is Alternative Splicing-Dependent
3. Discussion
4. Materials and Methods
4.1. Cell Line and Cell Culture
4.2. Reverse Transcription-PCR (RT-PCR) and RT-qPCR
4.3. Construction of YAP 3′UTR Containing Luciferase Constructs
4.4. Site-Directed Mutagenesis of the YAP 3′UTR-Luciferase Reporter Vectors
4.5. Transient Transfection and Western Blot Analysis
4.6. Luciferase Reporter Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zender, L.; Spector, M.S.; Xue, W.; Flemming, P.; Cordon-Cardo, C.; Silke, J.; Fan, S.-T.; Luk, J.M.; Wigler, M.; Hannon, G.J.; et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 2006, 125, 1253–1267. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, A.A.; Gayyed, M.F.; Klein, A.P.; Dong, J.; Maitra, A.; Pan, D.; Montgomery, E.A.; Anders, R.A. Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 2008, 39, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Overholtzer, M.; Zhang, J.; Smolen, G.A.; Muir, B.; Li, W.; Sgroi, D.C.; Deng, C.-X.; Brugge, J.S.; Haber, D.A. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. USA 2006, 103, 12405–12410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Kim, J.; Ye, X.; Lai, Z.-C.; Guan, K.-L. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of Yes-Associated protein. Cancer Res. 2009, 69, 1089–1098. [Google Scholar] [CrossRef]
- Zhu, L.; Ma, G.; Liu, J.; Deng, Y.; Wu, Q.; Chen, W.; Zhou, Q. Prognostic significance of nuclear Yes-associated protein 1 in patients with nonsmall cell lung cancer. Medicine 2019, 98, e15069. [Google Scholar] [CrossRef]
- Wu, Y.; Hou, Y.; Xu, P.; Deng, Y.; Liu, K.; Wang, M.; Tian, T.; Dai, C.; Li, N.; Hao, Q.; et al. The prognostic value of YAP1 on clinical outcomes in human cancers. Aging 2019, 11, 8681–8700. [Google Scholar] [CrossRef]
- Wojciechowska, J.; Krajewski, W.; Bolanowski, M.; Kręcicki, T.; Zatoński, T. Diabetes and cancer: A review of current knowledge. Exp. Clin. Endocrinol. Diabetes 2016, 124, 263–275. [Google Scholar] [CrossRef]
- Ryu, T.Y.; Park, J.; Scherer, P.E. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. J. 2014, 38, 330–336. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, Y.; Wu, Q.; Chen, Y.; Zou, S.; Liu, X.; Zhu, G.; Zhao, Y.; Chen, Y.; Yu, Y.; et al. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat. Commun. 2017, 8, 15280. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jeong, K.; Jiang, H.; Guo, W.; Gu, C.; Lu, Y.; Liang, J. YAP/TAZ regulates the insulin signaling via IRS1/2 in endometrial cancer. Am. J. Cancer Res. 2016, 6, 996–1010. [Google Scholar] [PubMed]
- Chen, J.; Harris, R.C. Interaction of the EGF receptor and the Hippo pathway in the diabetic kidney. J. Am. Soc. Nephrol. JASN 2015, 27, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, H.-I.; Yang, W.; Liu, C.; Chen, P.; You, S.; Wang, L.; Sun, C.-A.; Lu, S.-N.; Chen, D.; et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: A follow-up study in Taiwan. Gastroenterology 2008, 135, 111–121. [Google Scholar] [CrossRef]
- Ma, R.; Ren, J.-M.; Li, P.; Zhou, Y.-J.; Zhou, M.-K.; Hu, Z.; Xiao, X.-Y. Activated YAP causes renal damage of type 2 diabetic nephropathy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 755–763. [Google Scholar]
- Guo, X.; Sun, Y.; Azad, T.; van Rensburg, H.J.J.; Luo, J.; Yang, S.; Liu, P.; Lv, Z.; Zhan, M.; Lu, L.; et al. Rox8 promotes microRNA-dependent yki messenger RNA decay. Proc. Natl. Acad. Sci. USA 2020, 117, 30520–30530. [Google Scholar] [CrossRef]
- Birney, E.; Kumar, S.; Krainer, A.R. Analysis of the RNA-recognition motif and RS and RGG domains: Conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993, 21, 5803–5816. [Google Scholar] [CrossRef]
- Göhring, F.; Schwab, B.L.; Nicotera, P.; Leist, M.; Fackelmayer, F.O. The novel SAR-binding domain of scaffold attachment factor A (SAF-A) is a target in apoptotic nuclear breakdown. EMBO J. 1997, 16, 7361–7371. [Google Scholar] [CrossRef]
- Dominguez, C.; Fisette, J.-F.; Chabot, B.; Allain, F.H.-T. Structural basis of G-tract recognition and encaging by hnRNP F quasi-RRMs. Nat. Struct. Mol. Biol. 2010, 17, 853–861. [Google Scholar] [CrossRef]
- Garneau, D.; Revil, T.; Fisette, J.-F.; Chabot, B. Heterogeneous nuclear ribonucleoprotein F/H proteins modulate the alternative splicing of the apoptotic mediator Bcl-x. J. Biol. Chem. 2005, 280, 22641–22650. [Google Scholar] [CrossRef]
- Xu, C.; Xie, N.; Su, Y.; Sun, Z.; Liang, Y.; Zhang, N.; Liu, D.; Jia, S.; Xing, X.; Han, L.; et al. HnRNP F/H associate with hTERC and telomerase holoenzyme to modulate telomerase function and promote cell proliferation. Cell Death Differ. 2019, 27, 1998–2013. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-S.; Shi, Y.; Chang, S.-Y.; Abdo, S.; Chenier, I.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.-L.; Chan, J.S. Overexpression of heterogeneous nuclear ribonucleoprotein F stimulates renal Ace-2 gene expression and prevents TGF-β1-induced kidney injury in a mouse model of diabetes. Diabetologia 2015, 58, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-S.; Miyata, K.N.; Zhao, S.; Ghosh, A.; Chang, S.-Y.; Chenier, I.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.-L.; Chan, J.S. Tubular deficiency of heterogeneous nuclear ribonucleoprotein F elevates systolic blood pressure and induces glycosuria in mice. Sci. Rep. 2019, 9, 15765. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-S.; Shi, Y.; Chenier, I.; Ghosh, A.; Wu, C.-H.; Cailhier, J.-F.; Ethier, J.; Lattouf, J.-B.; Filep, J.G.; Ingelfinger, J.R.; et al. Heterogeneous nuclear ribonucleoprotein F stimulates sirtuin-1 gene expression and attenuates nephropathy progression in diabetic mice. Diabetes 2017, 66, 1964–1978. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-K.; Hung, L.-M.; Hou, C.-W.; Chen, J.-K. Heterogeneous ribonucleoprotein F regulates YAP expression via a G-tract in 3′UTR. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Conne, B.; Stutz, A.; Vassalli, J.-D. The 3′ untranslated region of messenger RNA: A molecular ‘hotspot’ for pathology? Nat. Med. 2000, 6, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Sayed-Ahmed, N.; Besbas, N.; Mundy, J.; Muchaneta-Kubara, E.; Cope, G.; Pearson, C.; El Nahas, M. Upregulation of epidermal growth factor and its receptor in the kidneys of rats with streptozotocin-induced diabetes. Exp. Nephrol. 1996, 4, 330–339. [Google Scholar]
- Saad, S.; Stevens, V.A.; Wassef, L.; Poronnik, P.; Kelly, D.J.; Gilbert, R.E.; Pollock, C.A. High glucose transactivates the EGF receptor and up-regulates serum glucocorticoid kinase in the proximal tubule. Kidney Int. 2005, 68, 985–997. [Google Scholar] [CrossRef]
- Szeto, S.G.; Narimatsu, M.; Lu, M.; He, X.; Sidiqi, A.M.; Tolosa, M.F.; Chan, L.; De Freitas, K.; Bialik, J.F.; Majumder, S.; et al. YAP/TAZ Are mechanoregulators of TGF-β-smad signaling and renal fibrogenesis. J. Am. Soc. Nephrol. JASN 2016, 27, 3117–3128. [Google Scholar] [CrossRef]
- Liang, M.; Yu, M.; Xia, R.; Song, K.; Wang, J.; Luo, J.; Chen, G.; Cheng, J. Yap/Taz deletion in Gli+ cell-derived myofibroblasts attenuates fibrosis. J. Am. Soc. Nephrol. JASN 2017, 28, 3278–3290. [Google Scholar] [CrossRef]
- Connolly, S.B.; Sadlier, D.; Kieran, N.E.; Doran, P.P.; Brady, H.R. Transcriptome profiling and the pathogenesis of diabetic complications. J. Am. Soc. Nephrol. JASN 2003, 14, 279S–283S. [Google Scholar] [CrossRef] [PubMed]
- Mauger, D.M.; Lin, C.; Garcia-Blanco, M.A. hnRNP H and hnRNP F complex with Fox2 to silence fibroblast growth factor receptor 2 exon IIIc. Mol. Cell. Biol. 2008, 28, 5403–5419. [Google Scholar] [CrossRef] [PubMed]
- Baltz, A.G.; Munschauer, M.; Schwanhäusser, B.; Vasile, A.; Murakawa, Y.; Schueler, M.; Youngs, N.; Penfold-Brown, D.; Drew, K.; Milek, M.; et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell 2012, 46, 674–690. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, W.-K.; Hung, L.-M.; Hou, C.-W.; Chen, J.-K. PKC Regulates YAP Expression through Alternative Splicing of YAP 3′UTR Pre-mRNA by hnRNP F. Int. J. Mol. Sci. 2021, 22, 694. https://doi.org/10.3390/ijms22020694
Chu W-K, Hung L-M, Hou C-W, Chen J-K. PKC Regulates YAP Expression through Alternative Splicing of YAP 3′UTR Pre-mRNA by hnRNP F. International Journal of Molecular Sciences. 2021; 22(2):694. https://doi.org/10.3390/ijms22020694
Chicago/Turabian StyleChu, Wing-Keung, Li-Man Hung, Chun-Wei Hou, and Jan-Kan Chen. 2021. "PKC Regulates YAP Expression through Alternative Splicing of YAP 3′UTR Pre-mRNA by hnRNP F" International Journal of Molecular Sciences 22, no. 2: 694. https://doi.org/10.3390/ijms22020694
APA StyleChu, W.-K., Hung, L.-M., Hou, C.-W., & Chen, J.-K. (2021). PKC Regulates YAP Expression through Alternative Splicing of YAP 3′UTR Pre-mRNA by hnRNP F. International Journal of Molecular Sciences, 22(2), 694. https://doi.org/10.3390/ijms22020694



