Effects of Mephedrone and Amphetamine Exposure during Adolescence on Spatial Memory in Adulthood: Behavioral and Neurochemical Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Procedures
2.3.1. Barnes Maze Task
2.3.2. Habituation
2.3.3. Acquisition phase
2.3.4. Probe Trial
2.3.5. Reversal Learning
2.4. Locomotor Activity Test
2.5. Biochemical Experiments
2.5.1. Western Blot
2.5.2. ELISA Assay
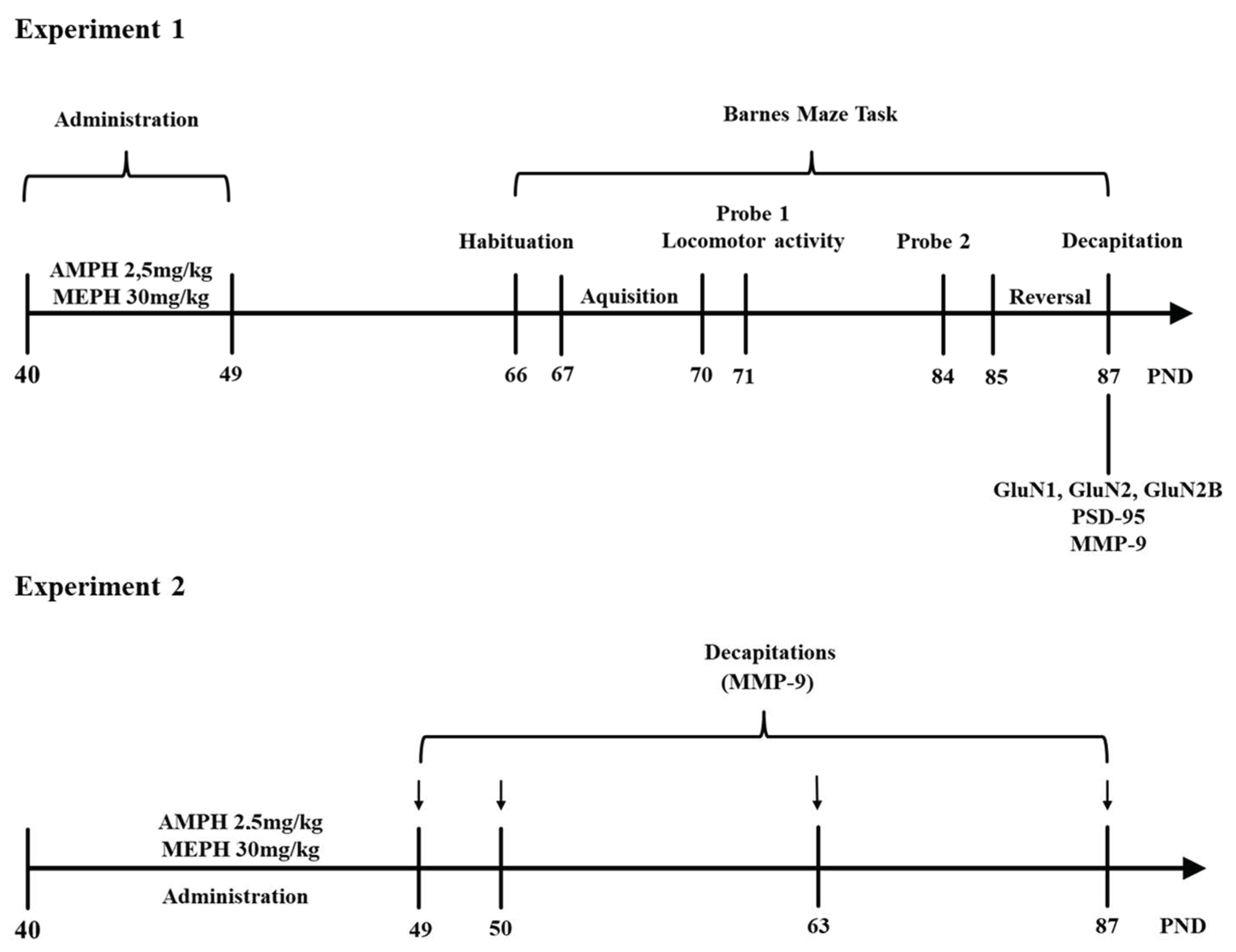
2.5.3. Experimental Design
Experiment 1
Experiment 2
2.6. Statistical Analysis
3. Results
3.1. Experiment 1
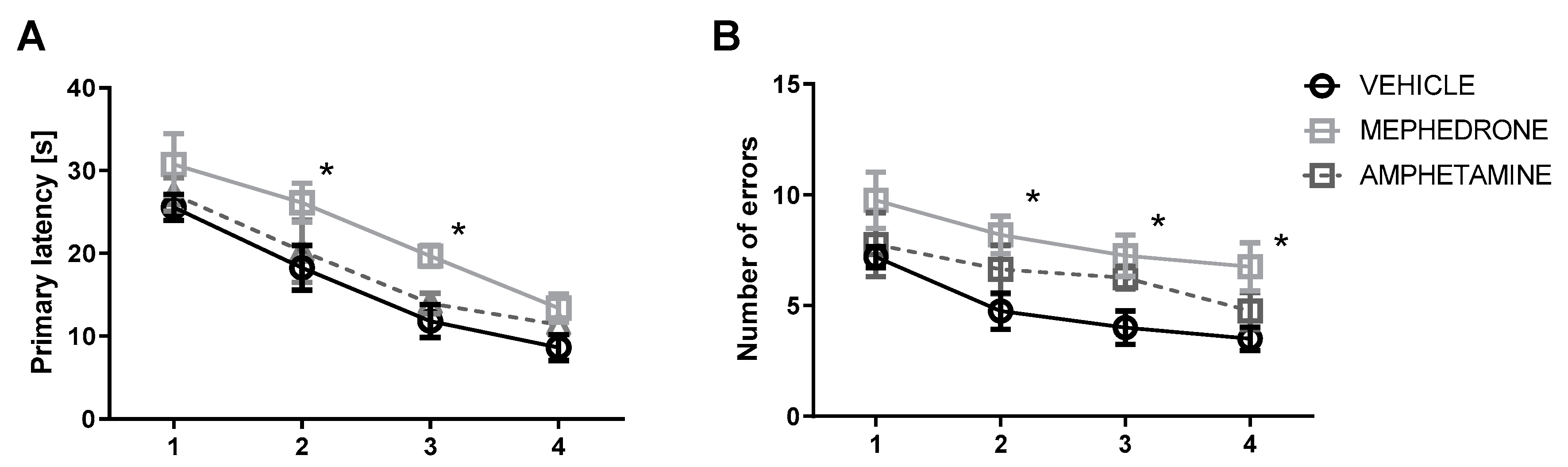
3.1.1. The Influence of Repeated Mephedrone and Amphetamine Administration during Late Adolescence on Acquisition Memory of the Barnes Maze Task in Adult Rats
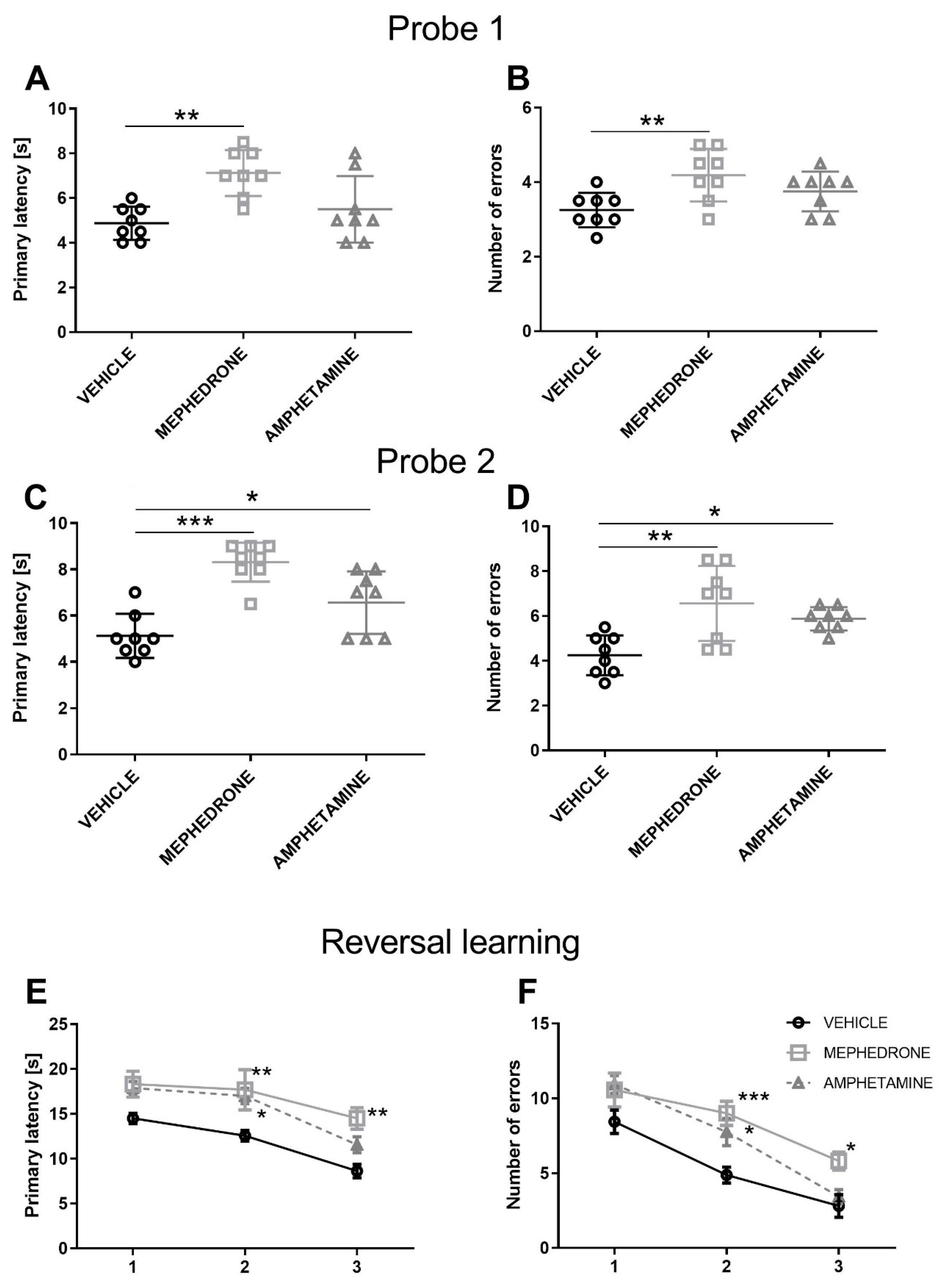
3.1.2. The Influence of Repeated Mephedrone and Amphetamine Administration during Late Adolescence on Spatial Memory Retrieval in Probe Trial 1 and Probe Trial 2 of the Barnes Maze Task in Adult Rats
3.1.3. The Influence of Repeated Mephedrone and Amphetamine Administration during Late Adolescence on Reversal Learning of the Barnes Maze Task in Adult Rats
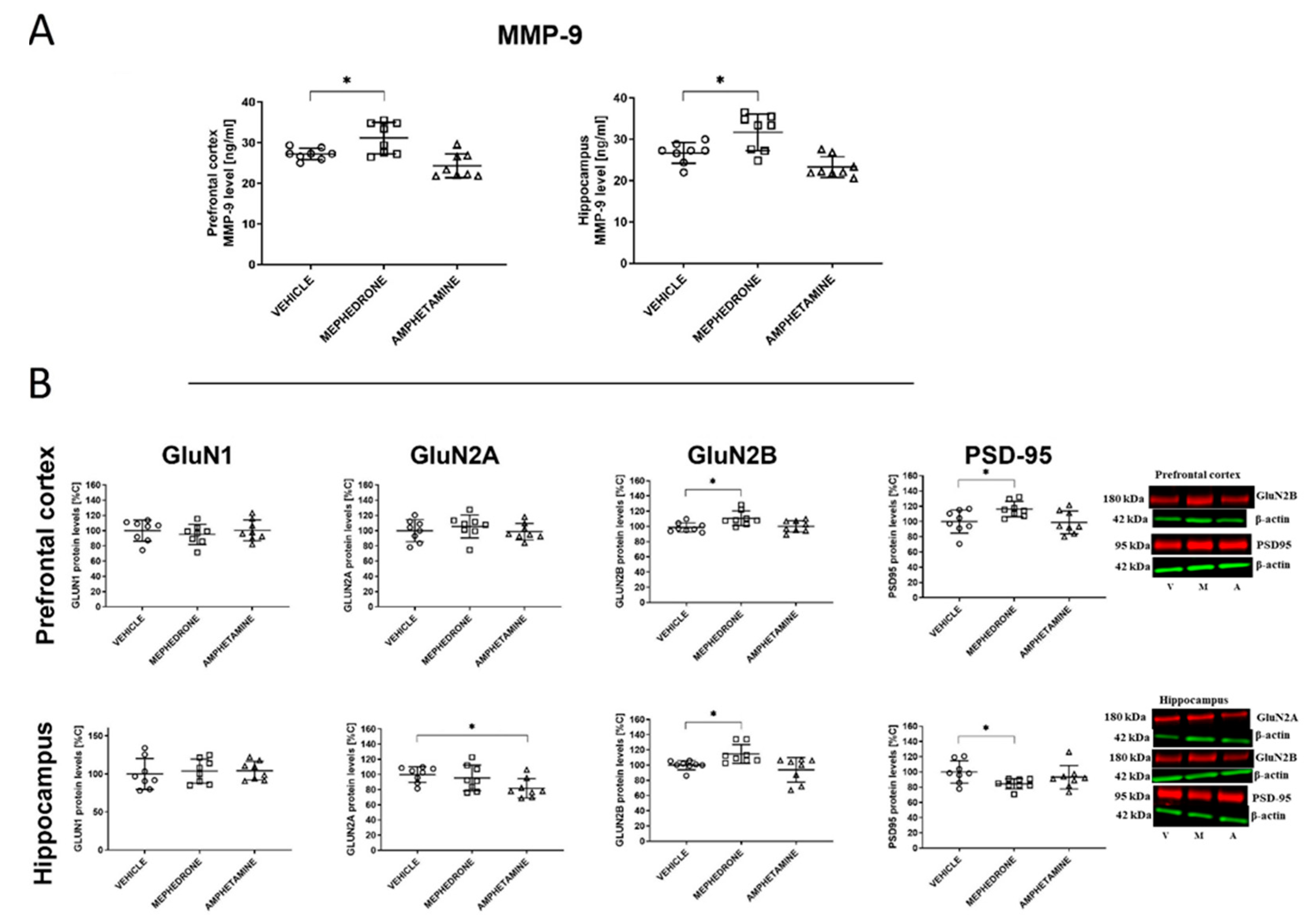
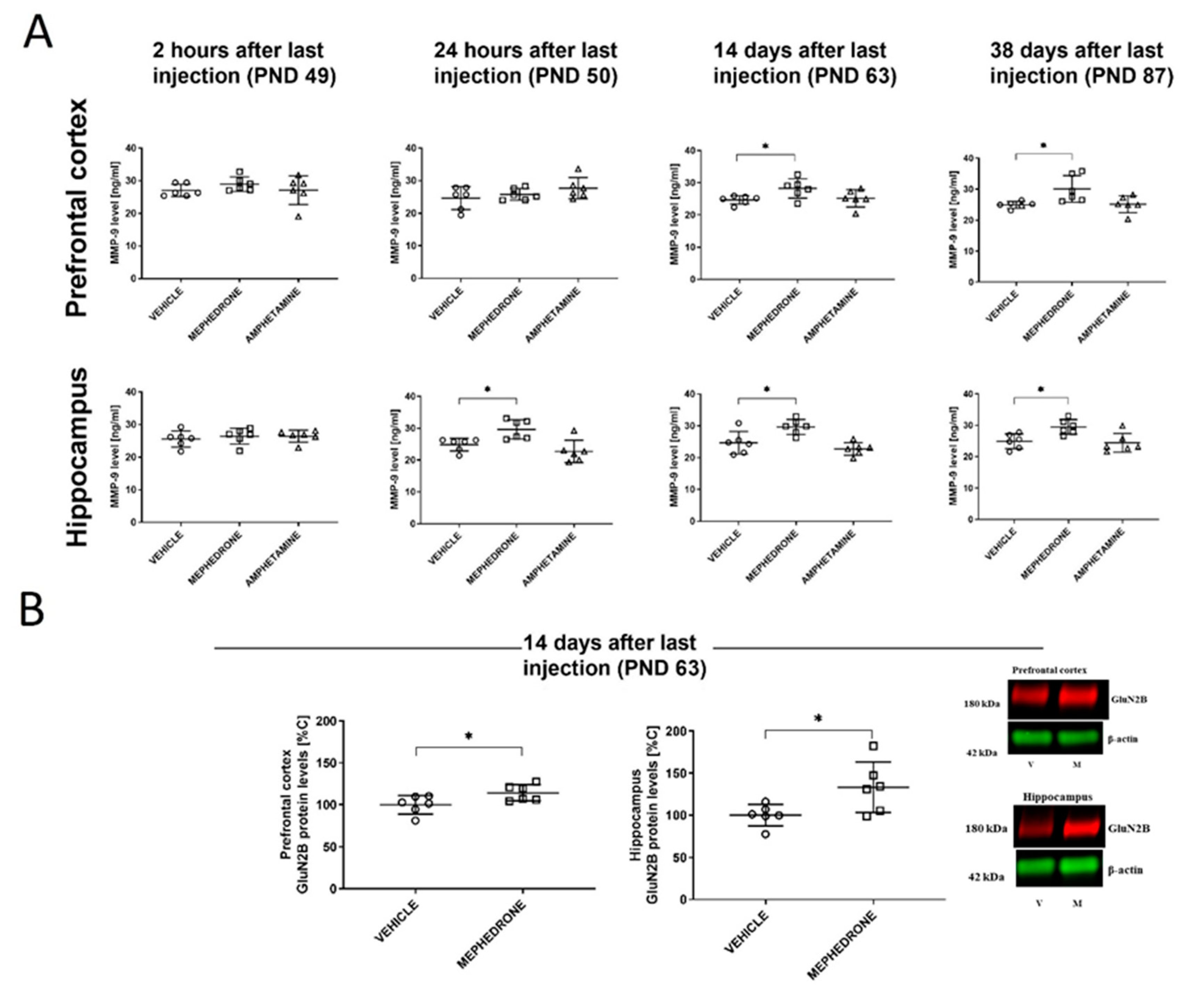
3.1.4. The Influence of Repeated Mephedrone and Amphetamine Administration during Late Adolescence on the Expression of NMDA Receptor Subunits (GluN1, GluN2A, and GluN2B), PSD-95, and MMP-9 Proteins in the PC and HC of Adult Rats That Underwent the Barnes-Maze Task
3.1.5. The Influence of Repeated Mephedrone and Amphetamine Exposure during Late Adolescence on Adult Rat Locomotor Activity
3.2. Experiment 2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bava, S.; Tapert, S.F. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychol. Rev. 2010, 20, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004, 1021, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Steinberg, L. Cognitive and affective development in adolescence. Trends Cogn. Sci. 2005, 9, 69–74. [Google Scholar] [CrossRef]
- Izenwasser, S. Differential effects of psychoactive drugs in adolescents and adults. Crit. Rev. Neurobiol. 2005, 17, 51–67. [Google Scholar] [CrossRef]
- Coleman, L.G., Jr.; He, J.; Lee, J.; Styner, M.; Crews, F.T. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol. Clin. Exp. Res. 2011, 35, 671–688. [Google Scholar] [CrossRef]
- Gleason, K.A.; Birnbaum, S.G.; Shukla, A.; Ghose, S. Susceptibility of the adolescent brain to cannabinoids: Long-term hippocampal effects and relevance to schizophrenia. Transl. Psychiatry 2012, 2, e199. [Google Scholar] [CrossRef]
- Portugal, G.S.; Wilkinson, D.S.; Turner, J.R.; Blendy, J.A.; Gould, T.J. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol. Learn. Mem. 2012, 97, 482–494. [Google Scholar] [CrossRef]
- Mooney-Leber, S.M.; Gould, T.J. The long-term cognitive consequences of adolescent exposure to recreational drugs of abuse. Learn. Mem. 2018, 25, 481–491. [Google Scholar] [CrossRef]
- Dias, R.; Aggleton, J.P. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: Differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioural flexibility. Eur. J. Neurosci. 2000, 12, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.; Baunez, C.; Robbins, T.W. Functional disconnection of the medial prefrontal cortex and subthalamic nucleus in attentional performance: Evidence for corticosubthalamic interaction. J. Neurosci. 2003, 23, 5477–5485. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.; Robbins, T.W. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J. Neurosci. 2003, 23, 8771–8780. [Google Scholar] [CrossRef] [PubMed]
- Boehme, R.; Lorenz, R.C.; Gleich, T.; Romund, L.; Pelz, P.; Golde, S.; Flemming, E.; Wold, A.; Deserno, L.; Behr, J.; et al. Reversal learning strategy in adolescence is associated with prefrontal cortex activation. Eur. J. Neurosci. 2017, 45, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Stubley-Weatherly, L.; Harding, J.W.; Wright, J.W. Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res. 1996, 716, 29–38. [Google Scholar] [CrossRef]
- Logue, S.F.; Paylor, R.; Wehner, J.M. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav. Neurosci. 1997, 111, 104–113. [Google Scholar] [CrossRef]
- Gould, T.J.; Lommock, J.A. Nicotine enhances contextual fear conditioning and ameliorates ethanol-induced deficits in contextual fear conditioning. Behav. Neurosci. 2003, 117, 1276–1282. [Google Scholar] [CrossRef]
- Arguello, P.A.; Jentsch, J.D. Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology 2004, 177, 141–150. [Google Scholar] [CrossRef]
- Pamplona, F.A.; Takahashi, R.N. WIN 55212-2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci. Lett. 2006, 397, 88–92. [Google Scholar] [CrossRef]
- Semenova, S.; Contet, C.; Roberts, A.J.; Markou, A. Mice lacking the β4 subunit of the nicotinic acetylcholine receptor show memory deficits, altered anxiety- and depression-like behavior, and diminished nicotine-induced analgesia. Nicotine Tob. Res. 2012, 14, 1346–1355. [Google Scholar] [CrossRef]
- Portugal, G.S.; Wilkinson, D.S.; Kenney, J.W.; Sullivan, C.; Gould, T.J. Strain-dependent effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Behav. Genet. 2012, 42, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Schifano, F.; Albanese, A.; Fergus, S.; Stair, J.L.; Deluca, P.; Corazza, O.; Davey, Z.; Corkery, J.; Siemann, H.; Scherbaum, N.; et al. Mephedrone (4-methylmethcathinone; ‘meow meow’): Chemical, pharmacological and clinical issues. Psychopharmacology 2011, 214, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Pantano, F.; Tittarelli, R.; Mannocchi, G.; Pacifici, R.; di Luca, A.; Busardò, F.P.; Marinelli, E. Neurotoxicity Induced by Mephedrone: An up-to-date Review. Curr. Neuropharmacol. 2017, 15, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Ayestas, M.A., Jr.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 2012, 7, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R. Psychoactive “bath salts”: Not so soothing. Eur. J. Pharmacol. 2013, 698, 1–5. [Google Scholar] [CrossRef]
- Rickli, A.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: Para-halogenated amphetamines and pyrovalerone cathinones. Eur. Neuropsychopharmacol. 2015, 25, 365–376. [Google Scholar] [CrossRef]
- Brandt, S.D.; Sumnall, H.R.; Measham, F.; Cole, J. Analyses of second-generation ‘legal highs’ in the UK: Initial findings. Drug Test. Anal. 2010, 2, 377–382. [Google Scholar] [CrossRef]
- Hockenhull, J.; Murphy, K.G.; Paterson, S. Mephedrone use is increasing in London. Lancet 2016, 387, 1719–1720. [Google Scholar] [CrossRef][Green Version]
- Papaseit, E.; Pérez-Mañá, C.; Mateus, J.A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; de la Torre, R.; Farré, M. Human pharmacology of mephedrone in comparison with MDMA. Neuropsychopharmacology 2016, 41, 2704–2713. [Google Scholar] [CrossRef]
- Mayo, L.M.; de Wit, H. Acquisition of responses to a methamphetamine-associated cue in healthy humans: Self-report, behavioral, and psychophysiological measures. Neuropsychopharmacology 2015, 40, 1734–1741. [Google Scholar] [CrossRef]
- Dolder, P.C.; Strajhar, P.; Vizeli, P.; Hammann, F.; Odermatt, A.; Liechti, M.E. Pharmacokinetics and pharmacodynamics of lisdexamfetamine compared with D-amphetamine in healthy subjects. Front. Pharmacol. 2017, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Dolder, P.C.; Müller, F.; Schmid, Y.; Borgwardt, S.J.; Liechti, M.E. Direct comparison of the acute subjective, emotional, autonomic, and endocrine effects of MDMA, methylphenidate, and modafinil in healthy subjects. Psychopharmacology 2018, 235, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Winstock, A.; Mitcheson, L.; Ramsey, J.; Davies, S.; Puchnarewicz, M.; Marsden, J. Mephedrone: Use, subjective effects and health risks. Addiction 2011, 106, 1991–1996. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.P.; Morgan, C.J.; Vaughn-Jones, J.; Hussain, N.; Karimi, K.; Curran, H.V. Cognitive and subjective effects of mephedrone and factors influencing use of a ‘new legal high’. Addiction 2012, 107, 792–800. [Google Scholar] [CrossRef]
- De Sousa Fernandes Perna, E.B.; Papaseit, E.; Pérez-Mañá, C.; Mateus, J.; Theunissen, E.L.; Kuypers, K.; de la Torre, R.; Farré, M.; Ramaekers, J.G. Neurocognitive performance following acute mephedrone administration, with and without alcohol. J. Psychopharmacol. 2016, 30, 1305–1312. [Google Scholar] [CrossRef]
- Jones, L.; Reed, P.; Parrott, A. Mephedrone and 3,4-methylenedioxy-methamphetamine: Comparative psychobiological effects as reported by recreational polydrug users. J. Psychopharmacol. 2016, 30, 1313–1320. [Google Scholar] [CrossRef]
- den Hollander, B.; Rozov, S.; Linden, A.M.; Uusi-Oukari, M.; Ojanperä, I.; Korpi, E.R. Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol. Biochem. Behav. 2013, 103, 501–519. [Google Scholar] [CrossRef]
- Motbey, C.P.; Clemens, K.J.; Apetz, N.; Winstock, A.R.; Ramsey, J.; Li, K.M.; Wyatt, N.; Callaghan, P.D.; Bowen, M.T.; Cornish, J.L.; et al. High levels of intravenous mephedrone (4-methylmethcathinone) self-administration in rats: Neural consequences and comparison with methamphetamine. J. Psychopharmacol. 2013, 27, 823–836. [Google Scholar] [CrossRef]
- Ciudad-Roberts, A.; Duart-Castells, L.; Camarasa, J.; Pubill, D.; Escubedo, E. The combination of ethanol with mephedrone increases the signs of neurotoxicity and impairs neurogenesis and learning in adolescent CD-1 mice. Toxicol. Appl. Pharmacol. 2016, 293, 10–20. [Google Scholar] [CrossRef]
- López-Arnau, R.; Martínez-Clemente, J.; Rodrigo, T.; Pubill, D.; Camarasa, J.; Escubedo, E. Neuronal changes and oxidative stress in adolescent rats after repeated exposure to mephedrone. Toxicol. Appl. Pharmacol. 2015, 286, 27–35. [Google Scholar] [CrossRef]
- Shortall, S.E.; Macerola, A.E.; Swaby, R.T.; Jayson, R.; Korsah, C.; Pillidge, K.E.; Wigmore, P.M.; Ebling, F.J.; Richard Green, A.; Fone, K.C.; et al. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur. Neuropsychopharmacol. 2013, 23, 1085–1095. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Kurzepa, J.; Biała, G.; Kaszubska, K.; Grot, K.; Tarkowski, P.; Kowalczyk, J.; Silvestro, S.; Faggio, C.; Budzyńska, B. Mephedrone impact on matrix metalloproteinases activity—Do they influence the memory processes? Curr. Mol. Pharmacol. 2019, 12, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Anneken, J.H.; Kuhn, D.M. Neurotoxicology of synthetic cathinone analogs. Curr. Top. Behav. Neurosci. 2017, 32, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Dzwonek, J.; Rylski, M.; Kaczmarek, L. Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett. 2004, 567, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, A.; Lapinska, J.; Rylski, M.; McKay, R.D.; Kaczmarek, L. Matrix metalloproteinase-9 undergoes expression and activation during dendritic remodeling in adult hippocampus. J. Neurosci. 2002, 22, 920–930. [Google Scholar] [CrossRef]
- Vaillant, C.; Didier-Bazès, M.; Hutter, A.; Belin, M.F.; Thomasset, N. Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum. J. Neurosci. 1999, 19, 4994–5004. [Google Scholar] [CrossRef]
- Bednarek, N.; Clément, Y.; Lelièvre, V.; Olivier, P.; Loron, G.; Garnotel, R.; Gressens, P. Ontogeny of MMPs and TIMPs in the murine neocortex. Pediatr. Res. 2009, 65, 296–300. [Google Scholar] [CrossRef]
- Sbai, O.; Ould-Yahoui, A.; Ferhat, L.; Gueye, Y.; Bernard, A.; Charrat, E.; Mehanna, A.; Risso, J.J.; Chauvin, J.P.; Fenouillet, E.; et al. Differential vesicular distribution and trafficking of MMP-2, MMP-9, and their inhibitors in astrocytes. Glia 2010, 58, 344–366. [Google Scholar] [CrossRef]
- Huntley, G.W. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci. 2012, 13, 743–757. [Google Scholar] [CrossRef]
- Dziembowska, M.; Wlodarczyk, J. MMP9: A novel function in synaptic plasticity. Int. J. Biochem. Cell Biol. 2012, 44, 709–713. [Google Scholar] [CrossRef]
- Nagy, V.; Bozdagi, O.; Matynia, A.; Balcerzyk, M.; Okulski, P.; Dzwonek, J.; Costa, R.M.; Silva, A.J.; Kaczmarek, L.; Huntley, G.W. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J. Neurosci. 2006, 26, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Gorkiewicz, T.; Balcerzyk, M.; Kaczmarek, L.; Knapska, E. Matrix metalloproteinase 9 (MMP-9) is indispensable for long term potentiation in the central and basal but not in the lateral nucleus of the amygdala. Front. Cell. Neurosci. 2015, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Bozdagi, O.; Nagy, V.; Kwei, K.T.; Huntley, G.W. In vivo roles for matrix metalloproteinase-9 in mature hippocampal synaptic physiology and plasticity. J. Neurophysiol. 2007, 98, 334–344. [Google Scholar] [CrossRef]
- Ganguly, K.; Rejmak, E.; Mikosz, M.; Nikolaev, E.; Knapska, E.; Kaczmarek, L. Matrix metalloproteinase (MMP) 9 transcription in mouse brain induced by fear learning. J. Biol. Chem. 2013, 288, 20978–20991. [Google Scholar] [CrossRef] [PubMed]
- Nagy, V.; Bozdagi, O.; Huntley, G.W. The extracellular protease matrix metalloproteinase-9 is activated by inhibitory avoidance learning and required for long-term memory. Learn. Mem. 2007, 14, 655–664. [Google Scholar] [CrossRef][Green Version]
- Vafadari, B.; Salamian, A.; Kaczmarek, L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016, 139, 91–114. [Google Scholar] [CrossRef]
- Reinhard, S.M.; Razak, K.; Ethell, I.M. A delicate balance: Role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front. Cell. Neurosci. 2015, 9, 280. [Google Scholar] [CrossRef]
- Beroun, A.; Mitra, S.; Michaluk, P.; Pijet, B.; Stefaniuk, M.; Kaczmarek, L. MMPs in learning and memory and neuropsychiatric disorders. Cell. Mol. Life Sci. 2019, 76, 3207–3228. [Google Scholar] [CrossRef]
- Wiera, G.; Wozniak, G.; Bajor, M.; Kaczmarek, L.; Mozrzymas, J.W. Maintenance of long-term potentiation in hippocampal mossy fiber-CA3 pathway requires fine-tuned MMP-9 proteolytic activity. Hippocampus 2013, 23, 529–543. [Google Scholar] [CrossRef]
- Smith, A.C.; Scofield, M.D.; Kalivas, P.W. The tetrapartite synapse: Extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res. 2015, 1628 Pt A, 29–39. [Google Scholar] [CrossRef]
- Gawel, K.; Gibula, E.; Marszalek-Grabska, M.; Filarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents-methodological consideration. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pitts, M.W. Barnes Maze Procedure for Spatial Learning and Memory in Mice. Bio Protoc. 2018, 8, e2744. [Google Scholar] [CrossRef] [PubMed]
- Bingham, B.; McFadden, K.; Zhang, X.; Bhatnagar, S.; Beck, S.; Valentino, R. Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology 2011, 36, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Laviola, G.; Macrì, S.; Morley-Fletcher, S.; Adriani, W. Risk-taking behavior in adolescent mice: Psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev. 2003, 27, 19–31. [Google Scholar] [CrossRef]
- Kotlinska, J.H.; Gibula-Bruzda, E.; Koltunowska, D.; Raoof, H.; Suder, P.; Silberring, J. Modulation of neuropeptide FF (NPFF) receptors influences the expression of amphetamine-induced conditioned place preference and amphetamine withdrawal anxiety-like behavior in rats. Peptides 2012, 33, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Lisek, R.; Xu, W.; Yuvasheva, E.; Chiu, Y.T.; Reitz, A.B.; Liu-Chen, L.Y.; Rawls, S.M. Mephedrone (‘bath salt’) elicits conditioned place preference and dopamine-sensitive motor activation. Drug Alcohol Depend. 2012, 126, 257–262. [Google Scholar] [CrossRef]
- Bach, M.E.; Hawkins, R.D.; Osman, M.; Kandel, E.R.; Mayford, M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell 1995, 81, 905–915. [Google Scholar] [CrossRef]
- O’Leary, T.P.; Brown, R.E. The effects of apparatus design and test procedure on learning and memory performance of C57BL/6J mice on the Barnes maze. J. Neurosci. Methods 2012, 203, 315–324. [Google Scholar] [CrossRef]
- Salmanzadeh, H.; Ahmadi-Soleimani, S.M.; Pachenari, N.; Azadi, M.; Halliwell, R.F.; Rubino, T.; Azizi, H. Adolescent drug exposure: A review of evidence for the development of persistent changes in brain function. Brain Res. Bull. 2020, 156, 105–117. [Google Scholar] [CrossRef]
- Sherrill, L.K.; Stanis, J.J.; Gulley, J.M. Age-dependent effects of repeated amphetamine exposure on working memory in rats. Behav. Brain Res. 2013, 242, 84–94. [Google Scholar] [CrossRef]
- North, A.; Swant, J.; Salvatore, M.F.; Gamble-George, J.; Prins, P.; Butler, B.; Mittal, M.K.; Heltsley, R.; Clark, J.T.; Khoshbouei, H. Chronic methamphetamine exposure produces a delayed, long-lasting memory deficit. Synapse 2013, 67, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Pozos, H.; Phillips, T.J.; Izquierdo, A. Long-term effects of exposure to methamphetamine in adolescent rats. Drug Alcohol Depend. 2014, 138, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.; Brigman, J.L.; Radke, A.K.; Rudebeck, P.H.; Holmes, A. The neural basis of reversal learning: An updated perspective. Neuroscience 2017, 345, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.C.; Groman, S.M.; Morales, A.M.; London, E.D. An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 2013, 38, 259–274. [Google Scholar] [CrossRef]
- Potvin, S.; Stavro, K.; Rizkallah, E.; Pelletier, J. Cocaine and cognition: A systematic quantitative review. J. Addict. Med. 2014, 8, 368–376. [Google Scholar] [CrossRef]
- London, E.D.; Kohno, M.; Morales, A.M.; Ballard, M.E. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2015, 1628, 174–185. [Google Scholar] [CrossRef]
- Bernheim, A.; See, R.E.; Reichel, C.M. Chronic methamphetamine self-administration disrupts cortical control of cognition. Neurosci. Biobehav. Rev. 2016, 69, 36–48. [Google Scholar] [CrossRef]
- Meighan, S.E.; Meighan, P.C.; Choudhury, P.; Davis, C.J.; Olson, M.L.; Zornes, P.A.; Wright, J.W.; Harding, J.W. Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem. 2006, 96, 1227–1241. [Google Scholar] [CrossRef]
- Wright, J.W.; Brown, T.E.; Harding, J.W. Inhibition of hippocampal matrix metalloproteinase-3 and -9 disrupts spatial memory. Neural Plast. 2007, 2007, 73813. [Google Scholar] [CrossRef]
- Knapska, E.; Lioudyno, V.; Kiryk, A.; Mikosz, M.; Górkiewicz, T.; Michaluk, P.; Gawlak, M.; Chaturvedi, M.; Mochol, G.; Balcerzyk, M.; et al. Reward learning requires activity of matrix metalloproteinase-9 in the central amygdala. J. Neurosci. 2013, 33, 14591–14600. [Google Scholar] [CrossRef]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Brown, S.; Shaikh, J.; Fishback, J.A.; Matsumoto, R.R. Relationship between methamphetamine exposure and matrix metalloproteinase 9 expression. Neuroreport 2008, 19, 1407–1409. [Google Scholar] [CrossRef] [PubMed]
- Dziembowska, M.; Milek, J.; Janusz, A.; Rejmak, E.; Romanowska, E.; Gorkiewicz, T.; Tiron, A.; Bramham, C.R.; Kaczmarek, L. Activity-dependent local translation of matrix metalloproteinase-9. J. Neurosci. 2012, 32, 14538–14547. [Google Scholar] [CrossRef]
- Gawlak, M.; Górkiewicz, T.; Gorlewicz, A.; Konopacki, F.A.; Kaczmarek, L.; Wilczynski, G.M. High resolution in situ zymography reveals matrix metalloproteinase activity at glutamatergic synapses. Neuroscience 2009, 158, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Conant, K.; Wang, Y.; Szklarczyk, A.; Dudak, A.; Mattson, M.P.; Lim, S.T. Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience 2010, 166, 508–521. [Google Scholar] [CrossRef]
- Kelly, E.A.; Tremblay, M.E.; Gahmberg, C.G.; Tian, L.; Majewska, A.K. Subcellular localization of intercellular adhesion molecule-5 (telencephalin) in the visual cortex is not developmentally regulated in the absence of matrix metalloproteinase-9. J. Comp. Neurol. 2014, 522, 676–688. [Google Scholar] [CrossRef]
- Michaluk, P.; Mikasova, L.; Groc, L.; Frischknecht, R.; Choquet, D.; Kaczmarek, L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J. Neurosci. 2009, 29, 6007–6012. [Google Scholar] [CrossRef]
- Okulski, P.; Jay, T.M.; Jaworski, J.; Duniec, K.; Dzwonek, J.; Konopacki, F.A.; Wilczynski, G.M.; Sánchez-Capelo, A.; Mallet, J.; Kaczmarek, L. TIMP-1 abolishes MMP-9-dependent long-lasting long-term potentiation in the prefrontal cortex. Biol. Psychiatry 2007, 62, 359–562. [Google Scholar] [CrossRef]
- Wiera, G.; Szczot, M.; Wojtowicz, T.; Lebida, K.; Koza, P.; Mozrzymas, J.W. Impact of matrix metalloproteinase-9 overexpression on synaptic excitatory transmission and its plasticity in rat CA3-CA1 hippocampal pathway. J. Physiol. Pharmacol. 2015, 66, 309–315. [Google Scholar]
- Michaluk, P.; Wawrzyniak, M.; Alot, P.; Szczot, M.; Wyrembek, P.; Mercik, K.; Medvedev, N.; Wilczek, E.; De Roo, M.; Zuschratter, W.; et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 2011, 124 Pt 19, 3369–3380. [Google Scholar] [CrossRef]
- Shipton, O.A.; Paulsen, O. GluN2A and GluN2B subunit-containing NMDA receptors in hippocampal plasticity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 369, 20130163. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.F.; Yamamoto, B.K. Methamphetamine neurotoxicity and striatal glutamate release: Comparison to 3,4-methylenedioxymethamphetamine. Brain Res. 1992, 581, 237–243. [Google Scholar] [CrossRef]
- Roche, K.W.; Standley, S.; McCallum, J.; Dune Ly, C.; Ehlers, M.D.; Wenthold, R.J. Molecular determinants of NMDA receptor internalization. Nat. Neurosci. 2001, 4, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Held, R.G.; Chang, S.C.; Yang, L.; Delpire, E.; Ghosh, A.; Hall, B.J. A critical role for GluN2B-containing NMDA receptors in cortical development and function. Neuron 2011, 72, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Zamzow, D.R.; Elias, V.; Shumaker, M.; Larson, C.; Magnusson, K.R. An increase in the association of GluN2B containing NMDA receptors with membrane scaffolding proteins was related to memory declines during aging. J. Neurosci. 2013, 33, 12300–12305. [Google Scholar] [CrossRef]
- Franchini, L.; Carrano, N.; Di Luca, M.; Gardoni, F. Synaptic GluN2A-Containing NMDA Receptors: From Physiology to Pathological Synaptic Plasticity. Int. J. Mol. Sci. 2020, 21, 1538. [Google Scholar] [CrossRef]
- Laurie, D.J.; Bartke, I.; Schoepfer, R.; Naujoks, K.; Seeburg, P.H. Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Res. Mol. Brain Res. 1997, 51, 23–32. [Google Scholar] [CrossRef]
- Yashiro, K.; Philpot, B.D. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 2008, 55, 1081–1094. [Google Scholar] [CrossRef]
- Spanagel, R. Animal models of addiction. Dialogues Clin. Neurosci. 2017, 19, 247–258. [Google Scholar] [CrossRef]
| Effect of Repeated Mephedrone or Amphetamine Administration on Locomotor Activity Measured Probe Trial-1 Day (PND 71) of Barnes Maze Task | ||
|---|---|---|
| Compounds: | N | Distance traveled (m) ± SEM |
| Vehicle | 8 | 56.24 ± 1.972 (NS) |
| Mephedrone (3 × 10 mg/kg) | 8 | 66.16 ± 3.574 (NS) |
| Amphetamine (3 × 2.5 mg/kg) | 8 | 60.22 ± 3.840 (NS) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochecki, P.; Smaga, I.; Lopatynska-Mazurek, M.; Gibula-Tarlowska, E.; Kedzierska, E.; Listos, J.; Talarek, S.; Marszalek-Grabska, M.; Hubalewska-Mazgaj, M.; Korga-Plewko, A.; et al. Effects of Mephedrone and Amphetamine Exposure during Adolescence on Spatial Memory in Adulthood: Behavioral and Neurochemical Analysis. Int. J. Mol. Sci. 2021, 22, 589. https://doi.org/10.3390/ijms22020589
Grochecki P, Smaga I, Lopatynska-Mazurek M, Gibula-Tarlowska E, Kedzierska E, Listos J, Talarek S, Marszalek-Grabska M, Hubalewska-Mazgaj M, Korga-Plewko A, et al. Effects of Mephedrone and Amphetamine Exposure during Adolescence on Spatial Memory in Adulthood: Behavioral and Neurochemical Analysis. International Journal of Molecular Sciences. 2021; 22(2):589. https://doi.org/10.3390/ijms22020589
Chicago/Turabian StyleGrochecki, Pawel, Irena Smaga, Malgorzata Lopatynska-Mazurek, Ewa Gibula-Tarlowska, Ewa Kedzierska, Joanna Listos, Sylwia Talarek, Marta Marszalek-Grabska, Magdalena Hubalewska-Mazgaj, Agnieszka Korga-Plewko, and et al. 2021. "Effects of Mephedrone and Amphetamine Exposure during Adolescence on Spatial Memory in Adulthood: Behavioral and Neurochemical Analysis" International Journal of Molecular Sciences 22, no. 2: 589. https://doi.org/10.3390/ijms22020589
APA StyleGrochecki, P., Smaga, I., Lopatynska-Mazurek, M., Gibula-Tarlowska, E., Kedzierska, E., Listos, J., Talarek, S., Marszalek-Grabska, M., Hubalewska-Mazgaj, M., Korga-Plewko, A., Dudka, J., Marzec, Z., Filip, M., & Kotlinska, J. H. (2021). Effects of Mephedrone and Amphetamine Exposure during Adolescence on Spatial Memory in Adulthood: Behavioral and Neurochemical Analysis. International Journal of Molecular Sciences, 22(2), 589. https://doi.org/10.3390/ijms22020589






