Computational Identification of Master Regulators Influencing Trypanotolerance in Cattle
Abstract
1. Introduction
Master Regulators as Drug Targets
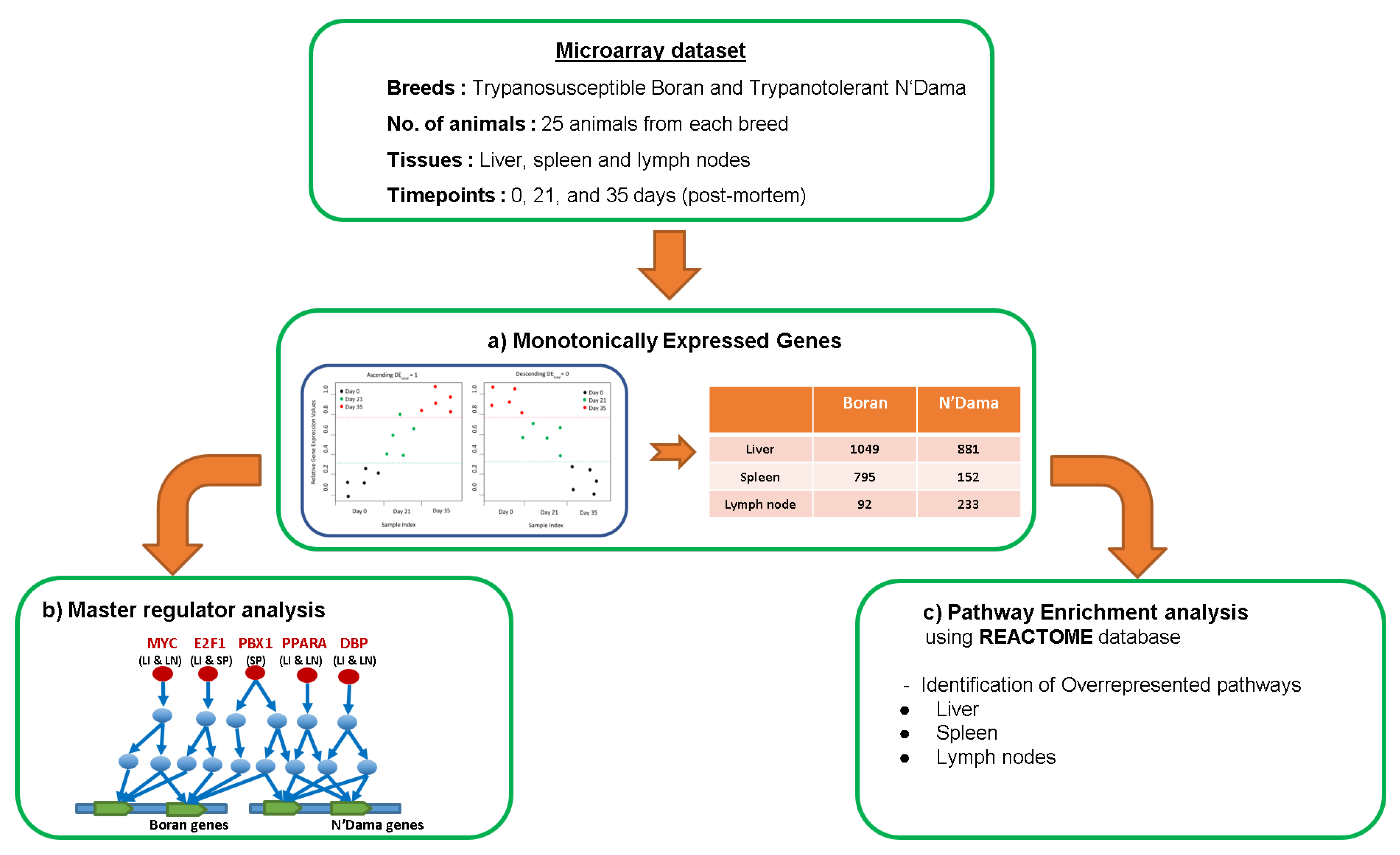
2. Materials and Methods
2.1. Gene Sets
2.2. Microarray Data Set
2.3. Monotonically Expressed Genes
2.4. Finding Master Regulators and Over-Represented Pathways
3. Results
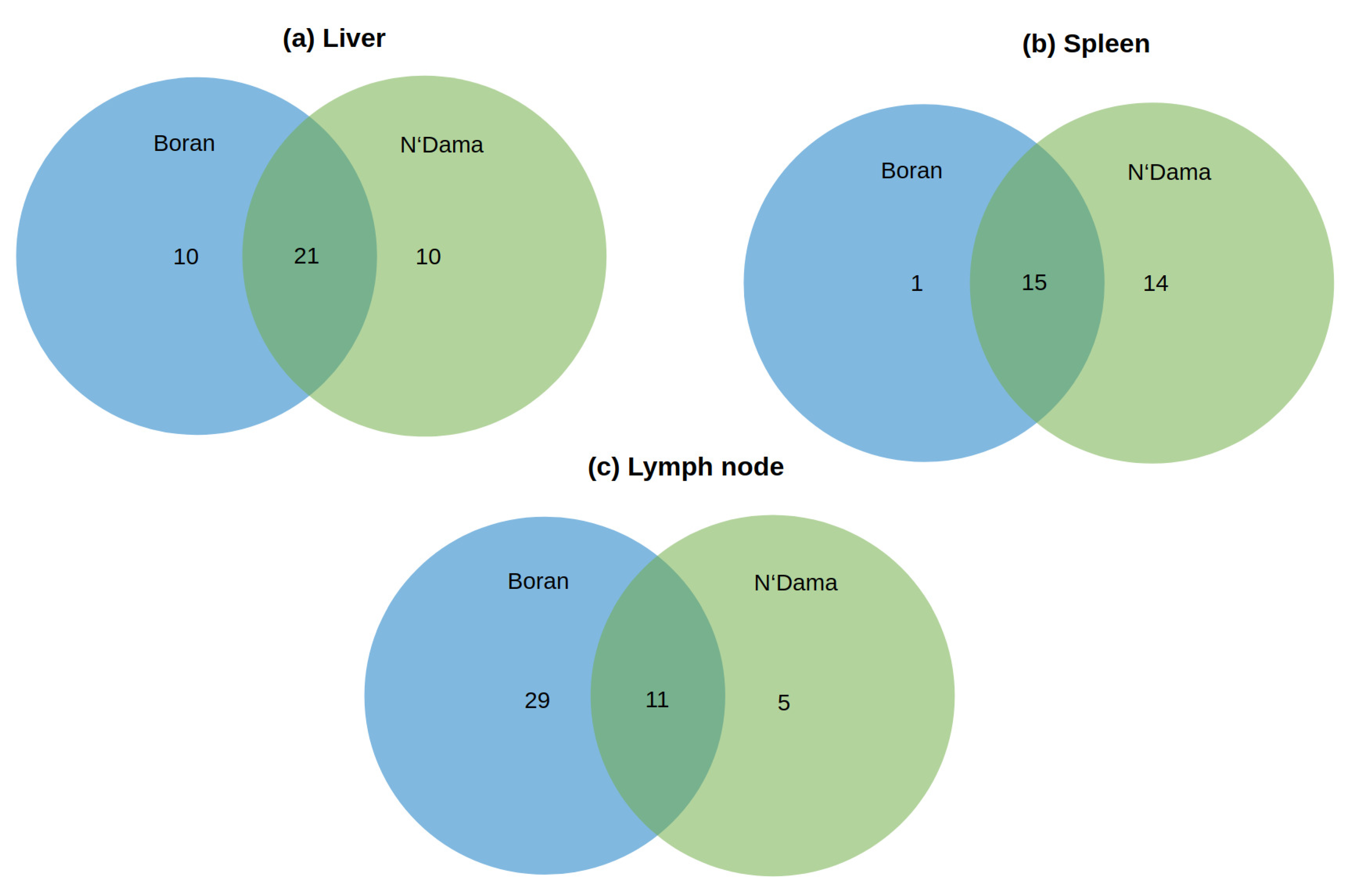
3.1. Master Regulator Analysis
3.1.1. Master Regulators in Liver
3.1.2. Master Regulators in Spleen
3.1.3. Master Regulators in Lymph Node
3.2. Pathway Analyses
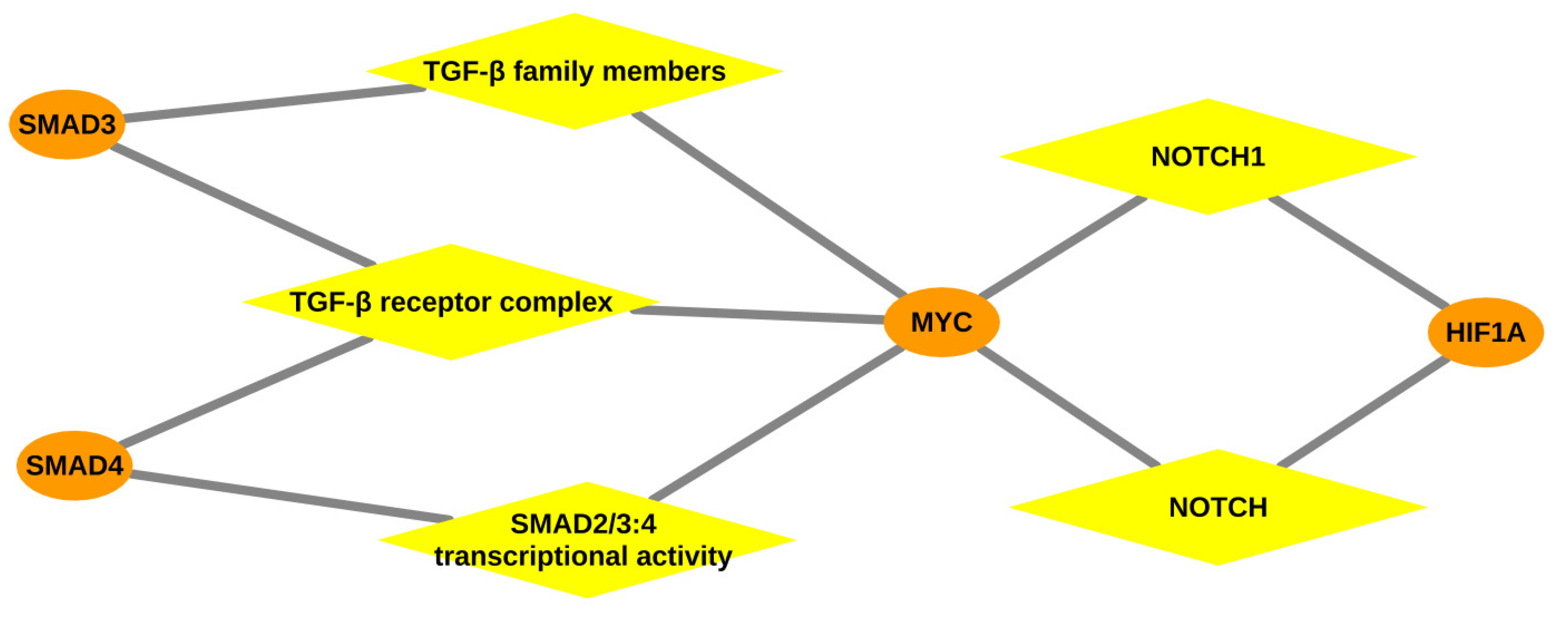
3.2.1. Over-Represented Pathways Found for Liver Tissue
3.2.2. Over-Represented Pathways Found for Spleen Tissue
3.2.3. Over-Represented Pathways Found for Lymph Node Tissue
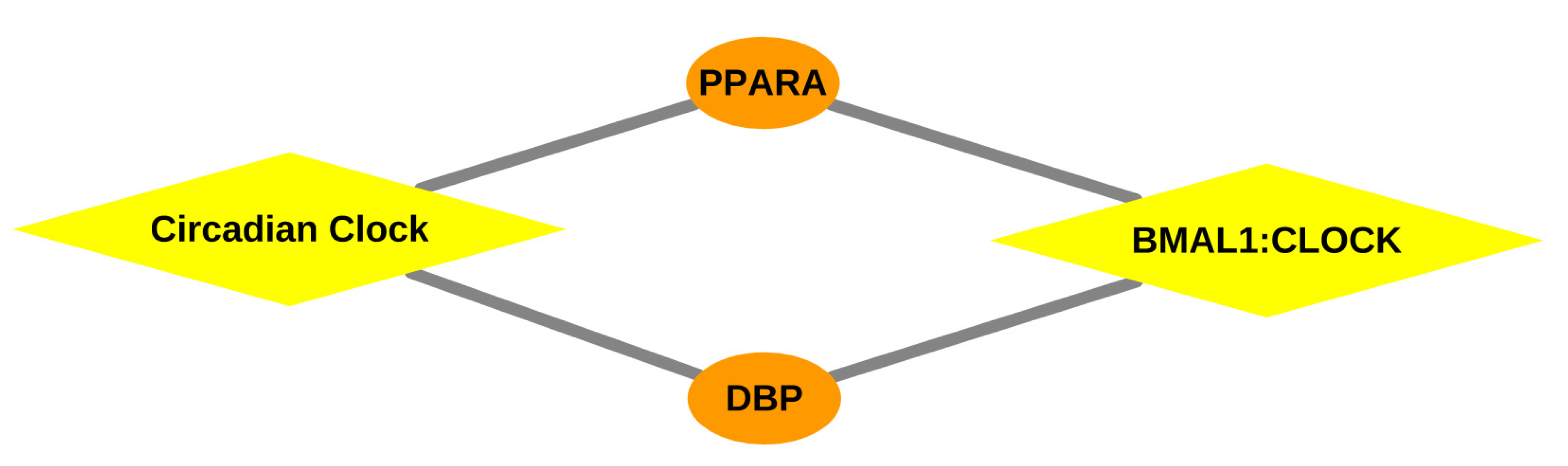
3.3. Analysis of Gene Expression Profiles of DBP and MYC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steverding, D. Sleeping Sickness and Nagana Disease Caused by Trypanosoma. In Arthropod Borne Diseases; Springer: Berlin/Heidelberg, Germany, 2016; p. 277. [Google Scholar]
- Steverding, D. The history of African trypanosomiasis. Parasites Vectors 2008. [Google Scholar] [CrossRef] [PubMed]
- Hursey, B.; Slingenbergh, J. The tsetse fly and its effects on agriculture in sub-Saharan Africa. World Anim. Rev. 1995, 3–4, 67–73. [Google Scholar]
- Losos, G.J.; Ikede, B. Review of pathology of diseases in domestic and laboratory animals caused by Trypanosoma congolense, T. vivax, T. brucei, T. rhodesiense and T. gambiense. Vet. Pathol. 1972, 9, 1–79. [Google Scholar] [CrossRef]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef] [PubMed]
- Angara, T.; Ismail, A.; Ibrahim, A. Research Paper Veterinary An Overview on the Economic Impacts of Animal Trypanosomiasis; Global Journal for Research Analysis: Ahmedabad, India, 2014. [Google Scholar]
- Morrison, L.J.; Vezza, L.; Rowan, T.; Hope, J.C. Animal African trypanosomiasis: Time to increase focus on clinically relevant parasite and host species. Trends Parasitol. 2016, 32, 599–607. [Google Scholar] [CrossRef]
- Naessens, J. Bovine trypanotolerance: A natural ability to prevent severe anaemia and haemophagocytic syndrome? Int. J. Parasitol. 2006. [Google Scholar] [CrossRef]
- Murray, M.; Trail, J.C.; Davis, C.E.; Black, S.J. Genetic resistance to African trypanosomiasis. J. Infect. Dis. 1984. [Google Scholar] [CrossRef]
- Murray, M.; Morrison, W.I.; Whitelaw, D. Host susceptibility to African trypanosomiasis: Trypanotolerance. Adv. Parasitol. 1982, 21, 1–68. [Google Scholar]
- Courtin, D.; Berthier, D.; Thevenon, S.; Dayo, G.K.; Garcia, A.; Bucheton, B. Host genetics in African trypanosomiasis. Infect. Genet. Evol. 2008. [Google Scholar] [CrossRef]
- Starkey, P. N’Dama Cattle—A Productive Trypanotolerant Breed. FAO World Anim. Rev. 1984, 50, 2–15. [Google Scholar]
- Dargie, J.; Murray, P.; Murray, M.; Grimshaw, W.; McIntyre, W. Bovine trypanosomiasis: The red cell kinetics of Ndama and Zebu cattle infected with Trypanosoma congolense. Parasitology 1979, 78, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A. Climate and cattle in North Africa: A first approximation. In The Origins and Development of African Livestock: Archaeology, Genetics, Linguistics and Ethnography; University College London Press, Taylor & Francis Group: Oxford, UK, 2006; p. 61. [Google Scholar]
- Mattioli, R.; Wilson, R. Trypanosomes, tsetse and trypanotolerance: Coevolution in tropical Africa. Parassitologia 1996, 38, 531–535. [Google Scholar] [PubMed]
- Paling, R.; Moloo, S.; Scott, J.; Gettinby, G.; McOdimba, F.; Murray, M. Susceptibility of N’Dama and Boran cattle to sequential challenges with tsetse-transmitted clones of Trypanosoma congolense. Parasite Immunol. 1991, 13, 427–445. [Google Scholar] [CrossRef]
- Murray, M.; Trail, J.; d’Ieteren, G. Trypanotolerance in cattle and prospects for the control of trypanosomiasis by selective breeding. Rev. Sci. Tech. 1990, 9, 369–386. [Google Scholar] [CrossRef]
- Roelants, G.E. Natural resistance to African trypanosomiasis. Parasite Immunol. 1986. [Google Scholar] [CrossRef] [PubMed]
- Epstein, H. The Origin of the Domestic Animals of Africa; Africana Publishing Corporation: New York, NY, USA, 1971. [Google Scholar]
- Mekonnen, Y.A.; Gültas, M.; Effa, K.; Hanotte, O.; Schmitt, A.O. Identification of Candidate Signature Genes and Key Regulators Associated With Trypanotolerance in the Sheko Breed. Front. Genet. 2019, 10, 1095. [Google Scholar] [CrossRef]
- Kim, S.J.; Ka, S.; Ha, J.W.; Kim, J.; Yoo, D.; Kim, K.; Lee, H.K.; Lim, D.; Cho, S.; Hanotte, O.; et al. Cattle genome-wide analysis reveals genetic signatures in trypanotolerant N’Dama. BMC Genom. 2017, 18, 371. [Google Scholar] [CrossRef]
- O’Gorman, G.M.; Park, S.D.; Hill, E.W.; Meade, K.G.; Coussens, P.M.; Agaba, M.; Naessens, J.; Kemp, S.J.; MacHugh, D.E. Transcriptional profiling of cattle infected with Trypanosoma congolense highlights gene expression signatures underlying trypanotolerance and trypanosusceptibility. BMC Genom. 2009, 10, 207. [Google Scholar] [CrossRef]
- Fisher, P.; Hedeler, C.; Wolstencroft, K.; Hulme, H.; Noyes, H.; Kemp, S.; Stevens, R.; Brass, A. A systematic strategy for large-scale analysis of genotype–phenotype correlations: Identification of candidate genes involved in African trypanosomiasis. Nucleic Acids Res. 2007, 35, 5625–5633. [Google Scholar] [CrossRef]
- Hill, E.W.; O’Gorman, G.M.; Agaba, M.; Gibson, J.P.; Hanotte, O.; Kemp, S.J.; Naessens, J.; Coussens, P.M.; MacHugh, D.E. Understanding bovine trypanosomiasis and trypanotolerance: The promise of functional genomics. Vet. Immunol. Immunopathol. 2005, 105, 247–258. [Google Scholar] [CrossRef]
- Hanotte, O.; Ronin, Y.; Agaba, M.; Nilsson, P.; Gelhaus, A.; Horstmann, R.; Sugimoto, Y.; Kemp, S.; Gibson, J.; Korol, A.; et al. Mapping of quantitative trait loci controlling trypanotolerance in a cross of tolerant West African N’Dama and susceptible East African Boran cattle. Proc. Natl. Acad. Sci. USA 2003, 100, 7443–7448. [Google Scholar] [CrossRef] [PubMed]
- Noyes, H.; Brass, A.; Obara, I.; Anderson, S.; Archibald, A.L.; Bradley, D.G.; Fisher, P.; Freeman, A.; Gibson, J.; Gicheru, M.; et al. Genetic and expression analysis of cattle identifies candidate genes in pathways responding to Trypanosoma congolense infection. Proc. Natl. Acad. Sci. USA 2011, 108, 9304–9309. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, A.; Heinrich, F.; Schmitt, A.O.; Gültas, M. Identifying Cattle Breed-Specific Partner Choice of Transcription Factors during the African Trypanosomiasis Disease Progression Using Bioinformatics Analysis. Vaccines 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Molina, L.; Conquet, F.; Dubois-Dauphin, M.; Schibler, U. The DBP gene is expressed according to a circadian rhythm in the suprachiasmatic nucleus and influences circadian behavior. EMBO J. 1997, 16, 6762–6771. [Google Scholar] [CrossRef] [PubMed]
- Fonjallaz, P.; Ossipow, V.; Wanner, G.; Schibler, U. The two PAR leucine zipper proteins, TEF and DBP, display similar circadian and tissue-specific expression, but have different target promoter preferences. EMBO J. 1996, 15, 351–362. [Google Scholar] [CrossRef]
- Wuarin, J.; Schibler, U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell 1990, 63, 1257–1266. [Google Scholar] [CrossRef]
- Orozco-Solis, R.; Aguilar-Arnal, L. Circadian Regulation of Immunity Through Epigenetic Mechanisms. Front. Cell. Infect. Microbiol. 2020, 10, 96. [Google Scholar] [CrossRef]
- Barik, S. Molecular Interactions between Pathogens and the Circadian Clock. Int. J. Mol. Sci. 2019, 20, 5824. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Hawking, F. Circadian rhythms of Trypanosoma congolense in laboratory rodents. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 592–595. [Google Scholar] [CrossRef]
- Taylor, K.A. Immune responses of cattle to African trypanosomes: Protective or pathogenic? Int. J. Parasitol. 1998, 28, 219–240. [Google Scholar] [CrossRef]
- Andrianarivo, A.G.; Muiya, P.; Opollo, M.; Loganhenfrey, L.L. Trypanosoma congolense: Comparative Effects of a Primary Infection on Bone Marrow Progenitor Cells from N’Dama and Boran Cattle. Exp. Parasitol. 1995, 80, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, F.; Klees, S.; Schmitt, A.O.; Cavero, D.; Gültas, M. Identification of Age-Specific and Common Key Regulatory Mechanisms Governing Eggshell Strength in Chicken Using Random Forests. Genes 2020, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Wlochowitz, D.; Haubrock, M.; Arackal, J.; Bleckmann, A.; Wolff, A.; Beißbarth, T.; Wingender, E.; Gültas, M. Computational identification of key regulators in two different colorectal cancer cell lines. Front. Genet. 2016, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Hosen, M.R.; Goody, P.R.; Zietzer, A.; Nickenig, G.; Jansen, F. MicroRNAs as master regulators of atherosclerosis: From pathogenesis to novel therapeutic options. Antioxidants Redox Signal. 2020. [Google Scholar] [CrossRef]
- Cai, W.; Zhou, W.; Han, Z.; Lei, J.; Zhuang, J.; Zhu, P.; Wu, X.; Yuan, W. Master regulator genes and their impact on major diseases. PeerJ 2020, 8, e9952. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, Y.; Laws, M.J.; Guillen, V.S.; Kim, S.H.; Dey, P.; Smith, B.P.; Gong, P.; Bindman, N.; Zhao, Y.; Carlson, K.; et al. Suppression of FOXM1 activities and breast cancer growth in vitro and in vivo by a new class of compounds. NPJ Breast Cancer 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Picaud, S.; Leonards, K.; Lambert, J.P.; Dovey, O.; Wells, C.; Fedorov, O.; Monteiro, O.; Fujisawa, T.; Wang, C.Y.; Lingard, H.; et al. Promiscuous targeting of bromodomains by bromosporine identifies BET proteins as master regulators of primary transcription response in leukemia. Sci. Adv. 2016, 2, e1600760. [Google Scholar] [CrossRef]
- Barillot, E.; Calzone, L.; Hupe, P.; Vert, J.P.; Zinovyev, A. Computational Systems Biology of Cancer; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Mellman, I. Dendritic cells: Master regulators of the immune response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef]
- Vargas, D.M.d.; De Bastiani, M.A.; Zimmer, E.R.; Klamt, F. Alzheimer’s disease master regulators analysis: Search for potential molecular targets and drug repositioning candidates. Alzheimer Res. Ther. 2018, 10, 59. [Google Scholar] [CrossRef]
- Pan, Z.; Li, L.; Fang, Q.; Qian, Y.; Zhang, Y.; Zhu, J.; Ge, M.; Huang, P. Integrated bioinformatics analysis of master regulators in anaplastic thyroid carcinoma. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Wang, H.W.; Sun, H.J.; Chang, T.Y.; Lo, H.H.; Cheng, W.C.; Tseng, G.C.; Lin, C.T.; Chang, S.J.; Pal, N.R.; Chung, I.F. Discovering monotonic stemness marker genes from time-series stem cell microarray data. B Genom. Biomed. Cent. 2015, 16, S2. [Google Scholar] [CrossRef]
- Wingender, E.; Kel, A.E. geneXplain — eine integrierte Bioinformatik-Plattform. BIOspektrum 2012, 18, 554–556. [Google Scholar] [CrossRef]
- Koschmann, J.; Bhar, A.; Stegmaier, P.; Kel, A.E.; Wingender, E. “Upstream analysis”: An integrated promoter-pathway analysis approach to causal interpretation of microarray data. Microarrays 2015, 4, 270–286. [Google Scholar] [CrossRef] [PubMed]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2019, 48, D498–D503. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.K.; Kyba, M. What is a master regulator? J. Stem Cell Res. Ther. 2013, 3, 114. [Google Scholar]
- Benetatos, L.; Benetatou, A.; Vartholomatos, G. Enhancers and MYC interplay in hematopoiesis. J. Mol. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016. [Google Scholar] [CrossRef]
- Conacci-Sorrell, M.; McFerrin, L.; Eisenman, R.N. An overview of MYC and its interactome. Cold Spring Harb. Perspect. Med. 2014. [Google Scholar] [CrossRef]
- Delgado, M.D.; León, J. Myc roles in hematopoiesis and leukemia. Genes Cancer 2010, 1, 605–616. [Google Scholar] [CrossRef]
- Guo, Y.; Niu, C.; Breslin, P.; Tang, M.; Zhang, S.; Wei, W.; Kini, A.R.; Paner, G.P.; Alkan, S.; Morris, S.W.; et al. c-Myc–mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Blood, J. Am. Soc. Hematol. 2009, 114, 2097–2106. [Google Scholar] [CrossRef]
- Ohanian, M.; Rozovski, U.; Kanagal-Shamanna, R.; Abruzzo, L.V.; Loghavi, S.; Kadia, T.; Futreal, A.; Bhalla, K.; Zuo, Z.; Huh, Y.O.; et al. MYC protein expression is an important prognostic factor in acute myeloid leukemia. Leuk. Lymphoma 2019, 60, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Matsuura, S.; Mowery, C.T.; Stoner, S.A.; Lam, K.; Ran, D.; Davis, A.G.; Lo, M.C.; Zhang, D.E. Restoration of MYC-repressed targets mediates the negative effects of GM-CSF on RUNX1-ETO leukemogenicity. Leukemia 2017, 31, 159–169. [Google Scholar] [CrossRef]
- Matsushita, H.; Nakajima, H.; Nakamura, Y.; Tsukamoto, H.; Tanaka, Y.; Jin, G.; Yabe, M.; Asai, S.; Ono, R.; Nosaka, T.; et al. C/EBPα and C/EBPϵ induce the monocytic differentiation of myelomonocytic cells with the MLL-chimeric fusion gene. Oncogene 2008, 27, 6749–6760. [Google Scholar] [CrossRef]
- Shroff, E.H.; Eberlin, L.S.; Dang, V.M.; Gouw, A.M.; Gabay, M.; Adam, S.J.; Bellovin, D.I.; Trand, P.T.; Philbrick, W.M.; Garcia-Ocana, A.; et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proc. Natl. Acad. Sci. USA 2015. [Google Scholar] [CrossRef]
- Ventura, A.; Kirsch, D.G.; McLaughlin, M.E.; Tuveson, D.A.; Grimm, J.; Lintault, L.; Newman, J.; Reczek, E.E.; Weissleder, R.; Jacks, T. Restoration of p53 function leads to tumour regression in vivo. Nature 2007. [Google Scholar] [CrossRef]
- Hoffman, B.; Amanullah, A.; Shafarenko, M.; Liebermann, D.A. The proto-oncogene c-myc in hematopoietic development and leukemogenesis. Oncogene 2002. [Google Scholar] [CrossRef]
- Franco, M.; Shastri, A.J.; Boothroyd, J.C. Infection by Toxoplasma gondii specifically induces host c-Myc and the genes this pivotal transcription factor regulates. Eukaryot. Cell 2014, 13, 483–493. [Google Scholar] [CrossRef]
- Dessauge, F.; Hilaly, S.; Baumgartner, M.; Blumen, B.; Werling, D.; Langsley, G. c-Myc activation by Theileria parasites promotes survival of infected B-lymphocytes. Oncogene 2005, 24, 1075–1083. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, N.; Xu, J.; Kong, B.; Copple, B.; Guo, G.L.; Wang, L. E2F1 is a novel fibrogenic gene that regulates cholestatic liver fibrosis through the Egr-1/SHP/EID1 network. Hepatology 2014, 60, 919–930. [Google Scholar] [CrossRef]
- Lai, Q.; Giralt, A.; Le May, C.; Zhang, L.; Cariou, B.; Denechaud, P.D.; Fajas, L. E2F1 inhibits circulating cholesterol clearance by regulating Pcsk9 expression in the liver. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Biryomumaisho, S.; Katunguka-Rwakishaya, E.; Rubaire-Akiiki, C. Serum biochemical changes in experimental Trypanosoma congolense and Trypanosoma brucei infection in Small East Africa goats. Vet. Arh. 2003, 73, 167–180. [Google Scholar]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236. [Google Scholar] [CrossRef]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Hasenfuss, S.C.; Bakiri, L.; Thomsen, M.K.; Williams, E.G.; Auwerx, J.; Wagner, E.F. Regulation of steatohepatitis and PPARγ signaling by distinct AP-1 dimers. Cell Metab. 2014, 19, 84–95. [Google Scholar] [CrossRef]
- Yamazaki, T.; Shiraishi, S.; Kishimoto, K.; Miura, S.; Ezaki, O. An increase in liver PPARγ2 is an initial event to induce fatty liver in response to a diet high in butter: PPARγ2 knockdown improves fatty liver induced by high-saturated fat. J. Nutr. Biochem. 2011, 22, 543–553. [Google Scholar] [CrossRef]
- Katunguka-Rwakishaya, E.; Murray, M.; Holmes, P. Pathophysiology of Trypanosoma congolense infection in two breeds of sheep, Scottish blackface and Finn Dorset. Vet. Parasitol. 1997, 68, 215–225. [Google Scholar] [CrossRef]
- Bozek, K.; Relógio, A.; Kielbasa, S.M.; Heine, M.; Dame, C.; Kramer, A.; Herzel, H. Regulation of clock-controlled genes in mammals. PLoS ONE 2009, 4, e4882. [Google Scholar] [CrossRef]
- Takahashi, J.S. Molecular components of the circadian clock in mammals. Diabetes, Obes. Metab. 2015, 17, 6–11. [Google Scholar] [CrossRef]
- Schrem, H.; Klempnauer, J.; Borlak, J. Liver-enriched transcription factors in liver function and development. Part II: The C/EBPs and D site-binding protein in cell cycle control, carcinogenesis, circadian gene regulation, liver regeneration, apoptosis, and liver-specific gene regulation. Pharmacol. Rev. 2004, 56, 291–330. [Google Scholar] [CrossRef]
- Lavery, D.J.; Lopez-Molina, L.; Margueron, R.; Fleury-Olela, F.; Conquet, F.; Schibler, U.; Bonfils, C. Circadian expression of the steroid 15 α-hydroxylase (Cyp2a4) and coumarin 7-hydroxylase (Cyp2a5) genes in mouse liver is regulated by the PAR leucine zipper transcription factor DBP. Mol. Cell. Biol. 1999, 19, 6488–6499. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Alberta, J.A.; Gonzalez, F.J.; Waxman, D.J. Multiple, functional DBP sites on the promoter of the cholesterol 7 alpha-hydroxylase P450 gene, CYP7. Proposed role in diurnal regulation of liver gene expression. J. Biol. Chem. 1994, 269, 14681–14689. [Google Scholar] [PubMed]
- Lavery, D.J.; Schibler, U. Circadian transcription of the cholesterol 7 alpha hydroxylase gene may involve the liver-enriched bZIP protein DBP. Genes Dev. 1993, 7, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Kierstein, S.; Noyes, H.; Naessens, J.; Nakamura, Y.; Pritchard, C.; Gibson, J.; Kemp, S.; Brass, A. Gene expression profiling in a mouse model for African trypanosomiasis. Genes Immun. 2006, 7, 667–679. [Google Scholar] [CrossRef]
- Kersten, S.; Rakhshandehroo, M.; Knoch, B.; Müller, M. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010. [Google Scholar] [CrossRef]
- Mandard, S.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor α target genes. Cell. Mol. Life Sci. CMLS 2004, 61, 393–416. [Google Scholar] [CrossRef]
- Gervois, P.; Mansouri, R.M. PPARα as a therapeutic target in inflammation-associated diseases. Expert Opin. Ther. Targets 2012, 16, 1113–1125. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2011. [Google Scholar] [CrossRef]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα). J. Biol. Chem. 1998. [Google Scholar] [CrossRef]
- Abdelmegeed, M.A.; Moon, K.H.; Hardwick, J.P.; Gonzalez, F.J.; Song, B.J. Role of peroxisome proliferator-activated receptor-α in fasting-mediated oxidative stress. Free. Radic. Biol. Med. 2009. [Google Scholar] [CrossRef]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [PubMed]
- Dimartino, J.F.; Selleri, L.; Traver, D.; Firpo, M.T.; Rhee, J.; Warnke, R.; O’Gorman, S.; Weissman, I.L.; Cleary, M.L. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood 2001. [Google Scholar] [CrossRef] [PubMed]
- Fournier, M.; Lebert-Ghali, C.E.; Krosl, G.; Bijl, J.J. HOXA4 induces expansion of hematopoietic stem cells in vitro and confers enhancement of pro-B-cells in vivo. Stem Cells Dev. 2012, 21, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zewdu, R.; Risolino, M.; Barbulescu, A.; Ramalingam, P.; Butler, J.M.; Selleri, L. Spleen hypoplasia leads to abnormal stress hematopoiesis in mice with loss of Pbx homeoproteins in splenic mesenchyme. J. Anat. 2016, 229, 153–169. [Google Scholar] [CrossRef]
- Fang, F.; Wang, Y.; Li, R.; Zhao, Y.; Guo, Y.; Jiang, M.; Sun, J.; Ma, Y.; Ren, Z.; Tian, Z.; et al. Transcription factor E2F1 suppresses dendritic cell maturation. J. Immunol. 2010, 184, 6084–6091. [Google Scholar] [CrossRef]
- Magez, S.; Radwanska, M.; Drennan, M.; Fick, L.; Baral, T.N.; Brombacher, F.; Baetselier, P.D. Interferon-γ and nitric oxide in combination with antibodies are key protective host immune factors during Trypanosoma congolense Tc13 infections. J. Infect. Dis. 2006, 193, 1575–1583. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, M.; Wang, J.; Wang, Q.; Xia, D.; Sun, W.; Zhang, L.; Yu, H.; Liu, Y.; Cao, X. Interferon-γ is an autocrine mediator for dendritic cell maturation. Immunol. Lett. 2004, 94, 141–151. [Google Scholar] [CrossRef]
- Wu, H.; Liu, G.; Shi, M. Interferon gamma in African trypanosome infections: Friends or foes? Front. Immunol. 2017, 8, 1105. [Google Scholar] [CrossRef]
- Cox, C.J.; Espinoza, H.M.; McWilliams, B.; Chappell, K.; Morton, L.; Hjalt, T.A.; Semina, E.V.; Amendt, B.A. Differential regulation of gene expression by PITX2 isoforms. J. Biol. Chem. 2002, 277, 25001–25010. [Google Scholar] [CrossRef]
- Casacuberta-Serra, S.; Soucek, L. Myc and Ras, the Bonnie and Clyde of immune evasion. Transl. Cancer Res. 2018, 7, S457. [Google Scholar] [CrossRef]
- Kortlever, R.M.; Sodir, N.M.; Wilson, C.H.; Burkhart, D.L.; Pellegrinet, L.; Swigart, L.B.; Littlewood, T.D.; Evan, G.I. Myc cooperates with Ras by programming inflammation and immune suppression. Cell 2017, 171, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Gallo, E.; Abbas, A.K. STAT1-activating cytokines limit Th17 responses through both T-bet–dependent and–independent mechanisms. J. Immunol. 2010, 185, 6461–6471. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.J.; Sullivan, B.M.; Peng, S.L.; Glimcher, L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003, 21, 713–758. [Google Scholar] [CrossRef] [PubMed]
- Okwor, I.; Muleme, H.; Jia, P.; Uzonna, J.E. Altered proinflammatory cytokine production and enhanced resistance to Trypanosoma congolense infection in lymphotoxin β-deficient mice. J. Infect. Dis. 2009, 200, 361–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, M.; Pan, W.; Tabel, H. Experimental African trypanosomiasis: IFN-γ mediates early mortality. Eur. J. Immunol. 2003, 33, 108–118. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, B.; Xu, X.; Tong, X.; Sun, R.; Tian, Z.; Wei, H. Requirement for PBX1 in developmental programming of natural killer cells. J. Immunol. 2017, 198, 202–215. [Google Scholar]
- Cnops, J.; De Trez, C.; Stijlemans, B.; Keirsse, J.; Kauffmann, F.; Barkhuizen, M.; Keeton, R.; Boon, L.; Brombacher, F.; Magez, S. NK-, NKT-and CD8-Derived IFNγ drives myeloid cell activation and erythrophagocytosis, resulting in trypanosomosis-associated acute anemia. PLoS Pathog. 2015, 11, e1004964. [Google Scholar] [CrossRef]
- Ueda, H.R.; Hayashi, S.; Chen, W.; Sano, M.; Machida, M.; Shigeyoshi, Y.; Iino, M.; Hashimoto, S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 2005, 37, 187–192. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Mitsui, S.; Yan, L.; Yagita, K.; Miyake, S.; Okamura, H. Role of DBP in the circadian oscillatory mechanism. Mol. Cell. Biol. 2000, 20, 4773–4781. [Google Scholar] [CrossRef]
- Silver, A.C.; Arjona, A.; Hughes, M.E.; Nitabach, M.N.; Fikrig, E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav. Immun. 2012, 26, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.N.; Viola, A.U.; Kyriakopoulou, V.; von Schantz, M.; Dijk, D.J. Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep 2008, 31, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Shimba, S.; Tezuka, M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol. Pharm. Bull. 2007, 30, 621–626. [Google Scholar] [CrossRef]
- Arjona, A.; Sarkar, D.K. The circadian gene mPer2 regulates the daily rhythm of IFN-γ. J. Interferon Cytokine Res. 2006, 26, 645–649. [Google Scholar] [CrossRef]
- Arjona, A.; Sarkar, D.K. Evidence supporting a circadian control of natural killer cell function. Brain, Behav. Immun. 2006, 20, 469–476. [Google Scholar] [CrossRef]
- Arjona, A.; Sarkar, D.K. Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. J. Immunol. 2005, 174, 7618–7624. [Google Scholar] [CrossRef]
- Boivin, D.B.; James, F.O.; Wu, A.; Cho-Park, P.F.; Xiong, H.; Sun, Z.S. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood 2003, 102, 4143–4145. [Google Scholar] [CrossRef]
- Sha, Z.; Compans, R.W. Induction of CD4+ T-cell-independent immunoglobulin responses by inactivated influenza virus. J. Virol. 2000, 74, 4999–5005. [Google Scholar] [CrossRef]
- Oxenius, A.; Zinkernagel, R.M.; Hengartner, H. CD4+ T-cell induction and effector functions: A comparison of immunity against soluble antigens and viral infections. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 70, pp. 313–367. [Google Scholar]
- Parker, D.C. T cell-dependent B cell activation. Annu. Rev. Immunol. 1993, 11, 331–360. [Google Scholar] [CrossRef]
- Taylor, K.A.; Lutje, V.; Kennedy, D.; Authié, E.; Boulangé, A.; Logan-Henfrey, L.; Gichuki, B.; Gettinby, G. Trypanosoma congolense: B-lymphocyte responses differ between trypanotolerant and trypanosusceptible cattle. Exp. Parasitol. 1996, 83, 106–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bollinger, T.; Leutz, A.; Leliavski, A.; Skrum, L.; Kovac, J.; Bonacina, L.; Benedict, C.; Lange, T.; Westermann, J.; Oster, H.; et al. Circadian clocks in mouse and human CD4+ T cells. PLoS ONE 2011, 6, e29801. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.C.; Ding, X.; Daynes, R.A. Nuclear Receptor Peroxisome Proliferator-activated Receptor α (PPARα) Is Expressed in Resting Murine Lymphocytes The Pparα In T and B Lymphocytes Is Both Transactivation and Transrepression Competent. J. Biol. Chem. 2002, 277, 6838–6845. [Google Scholar] [CrossRef] [PubMed]
- Cunard, R.; Ricote, M.; DiCampli, D.; Archer, D.C.; Kahn, D.A.; Glass, C.K.; Kelly, C.J. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J. Immunol. 2002, 168, 2795–2802. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gocke, A.R.; Lovett-Racke, A.; Drew, P.D.; Racke, M.K. PPAR alpha regulation of the immune response and autoimmune encephalomyelitis. PPAR Res. 2008, 2008. [Google Scholar] [CrossRef]
- Cunard, R.; DiCampli, D.; Archer, D.C.; Stevenson, J.L.; Ricote, M.; Glass, C.K.; Kelly, C.J. WY14, 643, a PPARα ligand, has profound effects on immune responses in vivo. J. Immunol. 2002, 169, 6806–6812. [Google Scholar] [CrossRef]
- Delerive, P.; De Bosscher, K.; Besnard, S.; Berghe, W.V.; Peters, J.M.; Gonzalez, F.J.; Fruchart, J.C.; Tedgui, A.; Haegeman, G.; Staels, B. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J. Biol. Chem. 1999, 274, 32048–32054. [Google Scholar] [CrossRef]
- Jantsch, J.; Schödel, J. Hypoxia and hypoxia-inducible factors in myeloid cell-driven host defense and tissue homeostasis. Immunobiology 2015, 220, 305–314. [Google Scholar] [CrossRef]
- Arena, E.T.; Tinevez, J.Y.; Nigro, G.; Sansonetti, P.J.; Marteyn, B.S. The infectious hypoxia: Occurrence and causes during Shigella infection. Microbes Infect. 2017, 19, 157–165. [Google Scholar] [CrossRef]
- Waghabi, M.C.; Keramidas, M.; Bailly, S.; Degrave, W.; Mendonça-Lima, L.; Maria de Nazaré, C.S.; Maria de Nazareth, L.M.; Paciornik, S.; Araújo-Jorge, T.C.; Feige, J.J. Uptake of host cell transforming growth factor-β by Trypanosoma cruzi amastigotes in cardiomyocytes: Potential role in parasite cycle completion. Am. J. Pathol. 2005, 167, 993–1003. [Google Scholar] [CrossRef]
- Gantt, K.R.; Schultz-Cherry, S.; Rodriguez, N.; Jeronimo, S.M.; Nascimento, E.T.; Goldman, T.L.; Recker, T.J.; Miller, M.A.; Wilson, M.E. Activation of TGF-β by Leishmania chagasi: Importance for parasite survival in macrophages. J. Immunol. 2003, 170, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Boutard, V.; Havouis, R.; Fouqueray, B.; Philippe, C.; Moulinoux, J.P.; Baud, L. Transforming growth factor-beta stimulates arginase activity in macrophages. Implications for the regulation of macrophage cytotoxicity. J. Immunol. 1995, 155, 2077–2084. [Google Scholar] [PubMed]
- Bogdan, C.; Nathan, C. Modulation of Macrophage Function by Transforming Growth Factor β, Interleukin-4, and Interleukin-10 a. Ann. N. Y. Acad. Sci. 1993, 685, 713–739. [Google Scholar] [CrossRef]
- Johnston, C.J.; Smyth, D.J.; Dresser, D.W.; Maizels, R.M. TGF-β in tolerance, development and regulation of immunity. Cell. Immunol. 2016, 299, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.; McIntire, J.J.; Yeung, V.P.; Berry, G.; Thorbecke, G.J.; Chen, L.; DeKruyff, R.H.; Umetsu, D.T. CD4+ T helper cells engineered to produce latent TGF-β1 reverse allergen-induced airway hyperreactivity and inflammation. J. Clin. Investig. 2000, 105, 61–70. [Google Scholar] [CrossRef]
- Ferrão, P.M.; Nisimura, L.M.; Moreira, O.C.; Land, M.G.; Pereira, M.C.; de Mendonça-Lima, L.; Araujo-Jorge, T.C.; Waghabi, M.C.; Garzoni, L.R. Inhibition of TGF-β pathway reverts extracellular matrix remodeling in T. cruzi-infected cardiac spheroids. Exp. Cell Res. 2018, 362, 260–267. [Google Scholar] [CrossRef]
- MacLean, L.; Chisi, J.E.; Odiit, M.; Gibson, W.C.; Ferris, V.; Picozzi, K.; Sternberg, J.M. Severity of human African trypanosomiasis in East Africa is associated with geographic location, parasite genotype, and host inflammatory cytokine response profile. Infect. Immun. 2004, 72, 7040–7044. [Google Scholar] [CrossRef][Green Version]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef]
- Wang, J.; Mauvoisin, D.; Martin, E.; Atger, F.; Galindo, A.N.; Dayon, L.; Sizzano, F.; Palini, A.; Kussmann, M.; Waridel, P.; et al. Nuclear proteomics uncovers diurnal regulatory landscapes in mouse liver. Cell Metab. 2017, 25, 102–117. [Google Scholar] [CrossRef]
- Mauvoisin, D.; Wang, J.; Jouffe, C.; Martin, E.; Atger, F.; Waridel, P.; Quadroni, M.; Gachon, F.; Naef, F. Circadian clock-dependent and-independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl. Acad. Sci. USA 2014, 111, 167–172. [Google Scholar] [CrossRef]
- Panda, S.; Antoch, M.P.; Miller, B.H.; Su, A.I.; Schook, A.B.; Straume, M.; Schultz, P.G.; Kay, S.A.; Takahashi, J.S.; Hogenesch, J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002, 109, 307–320. [Google Scholar] [CrossRef]
- Taghon, T.; Thys, K.; De Smedt, M.; Weerkamp, F.; Staal, F.; Plum, J.; Leclercq, G. Homeobox gene expression profile in human hematopoietic multipotent stem cells and T-cell progenitors: Implications for human T-cell development. Leukemia 2003, 17, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Taghon, T.; Stolz, F.; De Smedt, M.; Cnockaert, M.; Verhasselt, B.; Plum, J.; Leclercq, G. HOX-A10 regulates hematopoietic lineage commitment: Evidence for a monocyte-specific transcription factor. Blood, J. Am. Soc. Hematol. 2002, 99, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Res, P.; Spits, H. Developmental stages in the human thymus. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 11, pp. 39–46. [Google Scholar]
- Magli, M.C.; Largman, C.; Lawrence, H.J. Effects of HOX homeobox genes in blood cell differentiation. J. Cell. Physiol. 1997, 173, 168–177. [Google Scholar] [CrossRef]
- Niwa, H. How is pluripotency determined and maintained? Development 2007, 134, 635–646. [Google Scholar] [CrossRef]
- Niwa, H.; Miyazaki, J.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Niu, Z.; Peng, J.; Li, Q.; Xiong, W.; Langnas, A.N.; Ma, M.Y.; Zhao, Y. MOP3, a component of the molecular clock, regulates the development of B cells. Immunology 2006, 119, 451–460. [Google Scholar] [CrossRef]
- Kuriakose, S.M.; Singh, R.; Uzonna, J.E. Host intracellular signaling events and pro-inflammatory cytokine production in African trypanosomiasis. Front. Immunol. 2016, 7, 181. [Google Scholar] [CrossRef]
- Fatouros, M.; Bourantas, K.; Bairaktari, E.; Elisaf, M.; Tsolas, O.; Cassioumis, D. Role of the spleen in lipid metabolism. Br. J. Surg. 1995, 82, 1675–1677. [Google Scholar] [CrossRef]
- Aviram, M.; Brook, J.; Tatarsky, I.; Levy, Y.; Carter, A. Increased low-density lipoprotein levels after splenectomy: A role for the spleen in cholesterol metabolism in myeloproliferative disorders. Am. J. Med Sci. 1986, 291, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Egan, C.; Sukhumavasi, W.; Butcher, B.; Denkers, E. Functional aspects of Toll-like receptor/MyD88 signalling during protozoan infection: Focus on Toxoplasma gondii. Clin. Exp. Immunol. 2009, 156, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, S.; Onyilagha, C.; Singh, R.; Olayinka-Adefemi, F.; Jia, P.; Uzonna, J.E. TLR-2-dependent activation of MAPK and STAT proteins regulates proinflammatory cytokine response and immunity to experimental Trypanosoma congolense infection. Front. Immunol. 2019, 10, 2673. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and IL-23 induce innate IL-17 production from gamma delta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef]
- Barkhuizen, M.; Magez, S.; Atkinson, R.A.; Brombacher, F. Interleukin-12p70-dependent interferon-γ production is crucial for resistance in African trypanosomiasis. J. Infect. Dis. 2007, 196, 1253–1260. [Google Scholar] [CrossRef]
- Liu, Y.; Ragaa, E.; Li, Z.; Nuortio, L.; Mustafa, A.; Bakhiet, M. Interferon-gamma and interleukin-12 genes are preferentially expressed during early experimental African trypanosomiasis and suppressed by denervation of the spleen. Scand. J. Immunol. 1999, 50, 485–491. [Google Scholar] [CrossRef]
- Tian, S. Identification of monotonically differentially expressed genes for non-small cell lung cancer. BMC Bioinform. 2019, 20, 177. [Google Scholar] [CrossRef]
- Hergenhan, S.; Holtkamp, S.; Scheiermann, C. Molecular interactions between components of the circadian clock and the immune system. J. Mol. Biol. 2020. [Google Scholar] [CrossRef]
- Altman, B.J.; Hsieh, A.L.; Sengupta, A.; Krishnanaiah, S.Y.; Stine, Z.E.; Walton, Z.E.; Gouw, A.M.; Venkataraman, A.; Li, B.; Goraksha-Hicks, P.; et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 2015, 22, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Gery, S.; Koeffler, H. The Role of Circadian Regulation in Cancer; Cold Spring Harbor Symposia on Quantitative Biology, Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2007; Volume 72, pp. 459–464. [Google Scholar]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat. Med. 2018, 24, 1795. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.J.; Barnes, M.; Tang, H.; Pritchard, M.T.; Nagy, L.E. Kupffer cells in the liver. Compr. Physiol. 2013, 3, 785–797. [Google Scholar] [PubMed]
- Stout, R.D.; Suttles, J. Functional plasticity of macrophages: Reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004, 76, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Hörhold, F.; Eisel, D.; Oswald, M.; Kolte, A.; Röll, D.; Osen, W.; Eichmüller, S.B.; König, R. Reprogramming of macrophages employing gene regulatory and metabolic network models. PLoS Comput. Biol. 2020, 16, e1007657. [Google Scholar] [CrossRef]
- Uzonna, J.; Kaushik, R.; Gordon, J.; Tabel, H. Cytokines and antibody responses during Trypanosoma congolense infections in two inbred mouse strains that differ in resistance. Parasite Immunol. 1999, 21, 57–71. [Google Scholar]
- Kel, A.E. Search for Master Regulators in Walking Cancer Pathways. In Biological Networks and Pathway Analysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 161–191. [Google Scholar]
- Kel, A.E.; Stegmaier, P.; Valeev, T.; Koschmann, J.; Poroikov, V.; Kel-Margoulis, O.V.; Wingender, E. Multi-omics “upstream analysis” of regulatory genomic regions helps identifying targets against methotrexate resistance of colon cancer. EuPA Open Proteom. 2016, 13, 1–13. [Google Scholar] [CrossRef]
- Sikdar, S.; Datta, S. A novel statistical approach for identification of the master regulator transcription factor. BMC Bioinform. 2017, 18, 1–11. [Google Scholar] [CrossRef]

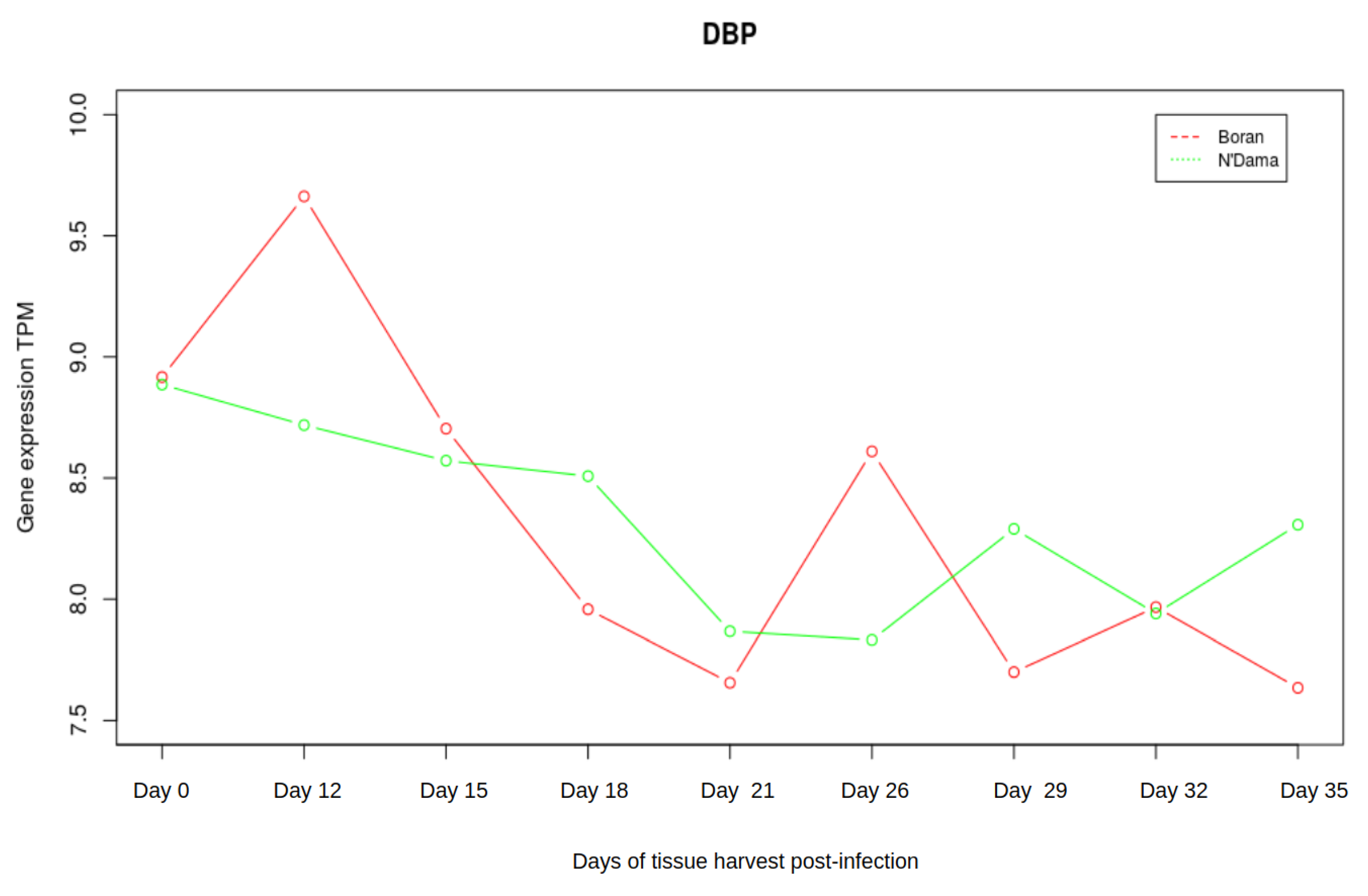
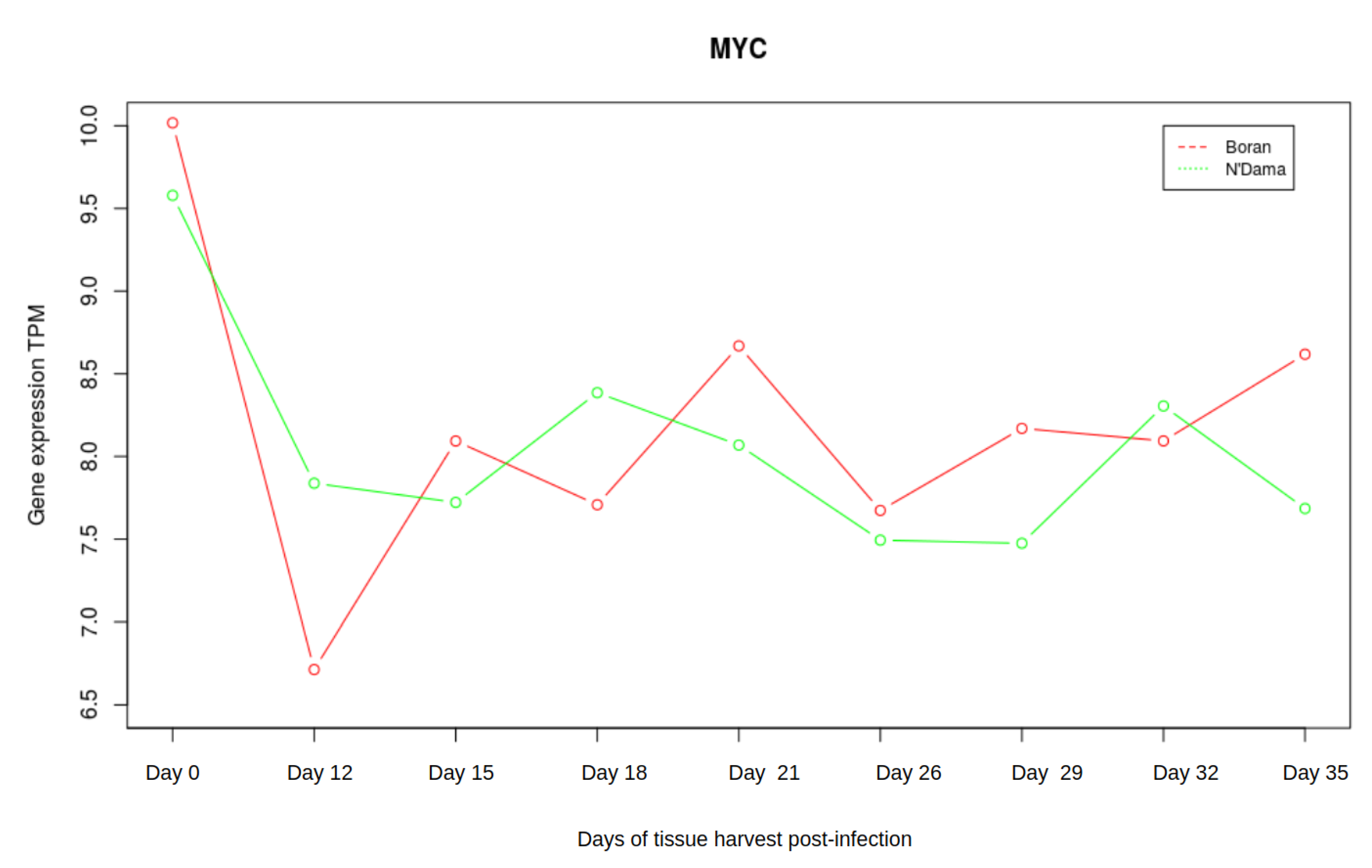
| Boran | N’Dama | |||
|---|---|---|---|---|
| Ascending | Descending | Ascending | Descending | |
| Liver | 741 | 308 | 757 | 124 |
| Spleen | 669 | 126 | 13 | 139 |
| Lymph node | 87 | 5 | 119 | 114 |
| Boran | N’Dama | |
|---|---|---|
| Liver | MYC, E2F1, PPARG | DBP, PBX1, HOXA4, PPARA |
| Spleen | PITX2, E2F1, PBX1 | PBX1 |
| Lymph node | MYC, pSTAT1, PBX1 | DBP, PPARA |
| Liver | |||
|---|---|---|---|
| Pathway Name | Hit Names | Adjusted p-Value | |
| Boran | Cellular responses to external stimuli | Arnt, Fos, Hif1a, Hsf1 | 4.64 × 10 |
| Regulation of beta-cell development | Foxo1, Hnf4g, Nkx2.2 | 0.0032 | |
| Regulation of Hypoxia-inducible Factor (HIF) by oxygen | Arnt, Hif1a | 0.0033 | |
| Cellular response to hypoxia | Arnt, Hif1a | 0.0033 | |
| Signaling by TGF-beta Receptor Complex | Myc, Smad3, Smad4 | 0.0036 | |
| Signaling by TGF-beta family members | Myc, Smad3, Smad4 | 0.0063 | |
| Signaling by NOTCH1 | Hif1a, Myc | 0.0105 | |
| Transcriptional activity of SMAD2/SMAD3:SMAD4 heterotrimer | Myc, Smad4 | 0.0147 | |
| Signaling by NOTCH | Hif1a, Myc | 0.0349 | |
| Interleukin-1 family signaling | Nfkb1, Smad3 | 0.0371 | |
| Cellular Senescence | Fos, Jun | 0.0416 | |
| N’Dama | PTEN Regulation | Atf, Jun | 0.0025 |
| Activation of HOX genes during differentiation | Hoxa4, Jun, Meis1 | 0.0036 | |
| Activation of anterior HOX genes in hindbrain development during early embryogenesis | Hoxa4, Jun, Meis1 | 0.0036 | |
| BMAL1:CLOCK, NPAS2 activates circadian gene expression | Dbp, Ppara | 0.0161 | |
| PIP3 activates AKT signaling | Atf, Jun | 0.0229 | |
| Transcriptional regulation of pluripotent stem cells | Pbx1, Pou5f1 | 0.0229 | |
| Intracellular signaling by second messengers | Atf2, Jun | 0.0280 | |
| Transcriptional regulation by RUNX2 | Sox9, Stat1 | 0.0364 | |
| Transcriptional regulation of white adipocyte differentiation | Pparg, Rxra | 0.0424 | |
| Circadian Clock | Dbp, Ppara | 0.0488 | |
| Spleen | |||
|---|---|---|---|
| Pathway Name | Hit Names | Adjusted p-Value | |
| Boran | POU5F1 (OCT4), SOX2, NANOG activate genes related to proliferation | Pou5f1, Stat3 | 0.0034 |
| N’Dama | Oxidative Stress Induced Senescence | Fos, Jun | 0.0033 |
| BMAL1:CLOCK NPAS2 activates circadian gene expression | Dbp, Ppara | 0.0052 | |
| MAPK6/MAPK4 signaling | Foxo1, Jun | 0.0052 | |
| Signaling by NOTCH3 | Hes1, Pbx1 | 0.0052 | |
| Cellular responses to stress | Fos, Hsf1, Jun | 0.0062 | |
| Fc epsilon receptor (FCERI) signaling | Fos, Jun | 0.0067 | |
| Cellular responses to external stimuli | Fos, Hsf1, Jun | 0.0135 | |
| MAPK family signaling cascades | Foxo1, Jun | 0.0166 | |
| Circadian Clock | Dbp, Ppara | 0.0166 | |
| Signaling by NOTCH | Hes1, Pbx1 | 0.0203 | |
| Generic Transcription Pathway | E2f1, Hes1, Sox9, Stat1, Tead1 | 0.0243 | |
| Cellular Senescence | Fos, Jun | 0.0243 | |
| RNA Polymerase II Transcription | E2f1, Hes1, Sox9, Stat1, Tead1 | 0.0350 | |
| Regulation of lipid metabolism by Peroxisome proliferator-activated receptor alpha (PPARalpha) | PPara, Rxra | 0.0432 | |
| Lymph Node | |||
|---|---|---|---|
| Pathway Name | Hit Names | Adjusted p-Value | |
| Boran | MAP kinase activation in TLR cascade | Atf1, Atf2, Fos, Jun, Nfkb1 | 2.37 |
| Interleukin-17 signaling | Atf1, Atf2, Fos, Jun, Nfkb1 | 1.83 × 10 | |
| MAPK targets/ Nuclear events mediated by MAP kinases | Atf1, Atf2, Fos, Jun | 3.66 × 10 | |
| MyD88 cascade initiated on plasma membrane | Atf1, Atf2, Fos, Jun, Nfkb1 | 5.54 × 10 | |
| MyD88 dependent cascade initiated on endosome | Atf1, Atf2, Fos, Jun, Nfkb1 | 7.62 × 10 | |
| MyD88:Mal cascade initiated on plasma membrane | Atf1, Atf2, Fos, Jun, Nfkb1 | 1.03 × 10 | |
| MyD88-independent TLR4 cascade | Atf1, Atf2, Fos, Jun, Nfkb1 | 2.27 × 10 | |
| Toll Like Receptor 3 (TLR3) Cascade | Atf1, Atf2, Fos, Jun, Nfkb1 | 2.88 × 10 | |
| Toll-Like Receptors Cascades | Atf1, Atf2, Fos, Jun, Nfkb1 | 4.39 × 10 | |
| MAPK6/MAPK4 signaling | Foxo1, Jun, Myc | 4.66 × 10 | |
| Innate Immune System | Atf1, Atf2, Fos, Jun, Ltf, Nfkb1 | 9.27 × 10 | |
| Signaling by Interleukins | Atf1, Atf2, Fos, Jun, Nfkb1, Stat1, Stat3 | 0.0010 | |
| PTEN Regulation | Atf2, Jun | 0.0016 | |
| MAPK family signaling cascades | Foxo1, Jun, Myc | 0.0028 | |
| Oxidative Stress Induced Senescence | Fos, Jun | 0.0069 | |
| Cytokine Signaling in Immune system | Atf1, Atf2, Fos, Jun, Nfkb1, Stat1, Stat3 | 0.0081 | |
| Fc epsilon receptor (FCERI) signaling | Fos, Jun | 0.0138 | |
| PIP3 activates AKT signaling | Atf2, Jun | 0.0154 | |
| Transcriptional activity of SMAD2/SMAD3:SMAD4 heterotrimer | Myc, Smad4 | 0.0171 | |
| NGF signalling via TRKA from the plasma membrane | Atf1, Stat3 | 0.0171 | |
| Intracellular signaling by second messengers | Atf2, Jun | 0.0189 | |
| Immune System | Atf1, Atf2, Fos, Jun, Ltf, Nfkb1, Relb, Stat1, Stat3 | 0.0241 | |
| Transcriptional regulation by the AP-2 (TFAP2) family of transcription factors | Mybl2, Myc | 0.0311 | |
| Generic Transcription Pathway | E2f1, Mybl2, Myc, Smad4, Sox9, Stat1 | 0.0338 | |
| Mitotic G2-G2/M phases | Foxm1, Mybl2 | 0.0381 | |
| Mitotic G1-G1/S phases | E2f1, Mybl2 | 0.0381 | |
| Interleukin-1 family signaling | Nfkb1, Stat3 | 0.0430 | |
| Signaling by TGF-beta Receptor Complex | Myc, Smad4 | 0.0456 | |
| Cellular Senescence | Fos, Jun | 0.0482 | |
| N’Dama | POU5F1 (OCT4), SOX2, NANOG activate genes related to proliferation | Pou5f1, Stat3 | 0.0042 |
| BMAL1:CLOCK, NPAS2 activates circadian gene expression | Dbp, Ppara | 0.0078 | |
| Circadian Clock | Dbp, Ppara | 0.0244 | |
| Factors involved in megakaryocyte development and platelet production | Irf1, Irf2 | 0.0354 | |
| Interleukin-12 family signaling | Stat1, Stat3 | 0.0375 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajavel, A.; Schmitt, A.O.; Gültas, M. Computational Identification of Master Regulators Influencing Trypanotolerance in Cattle. Int. J. Mol. Sci. 2021, 22, 562. https://doi.org/10.3390/ijms22020562
Rajavel A, Schmitt AO, Gültas M. Computational Identification of Master Regulators Influencing Trypanotolerance in Cattle. International Journal of Molecular Sciences. 2021; 22(2):562. https://doi.org/10.3390/ijms22020562
Chicago/Turabian StyleRajavel, Abirami, Armin Otto Schmitt, and Mehmet Gültas. 2021. "Computational Identification of Master Regulators Influencing Trypanotolerance in Cattle" International Journal of Molecular Sciences 22, no. 2: 562. https://doi.org/10.3390/ijms22020562
APA StyleRajavel, A., Schmitt, A. O., & Gültas, M. (2021). Computational Identification of Master Regulators Influencing Trypanotolerance in Cattle. International Journal of Molecular Sciences, 22(2), 562. https://doi.org/10.3390/ijms22020562





