Oligomeric Structural Transition of HspB1 from Chinese Hamster
Abstract
:1. Introduction
2. Results
2.1. Conformational Change of CgHspB1 Wild Type and S15D Mutant Analyzed by SV-AUC
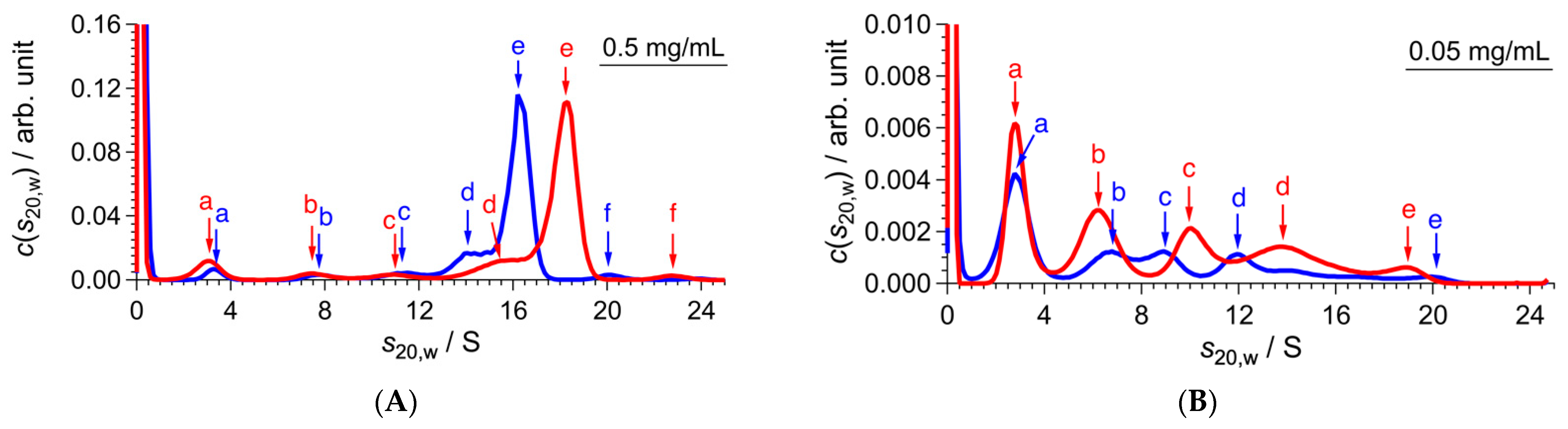
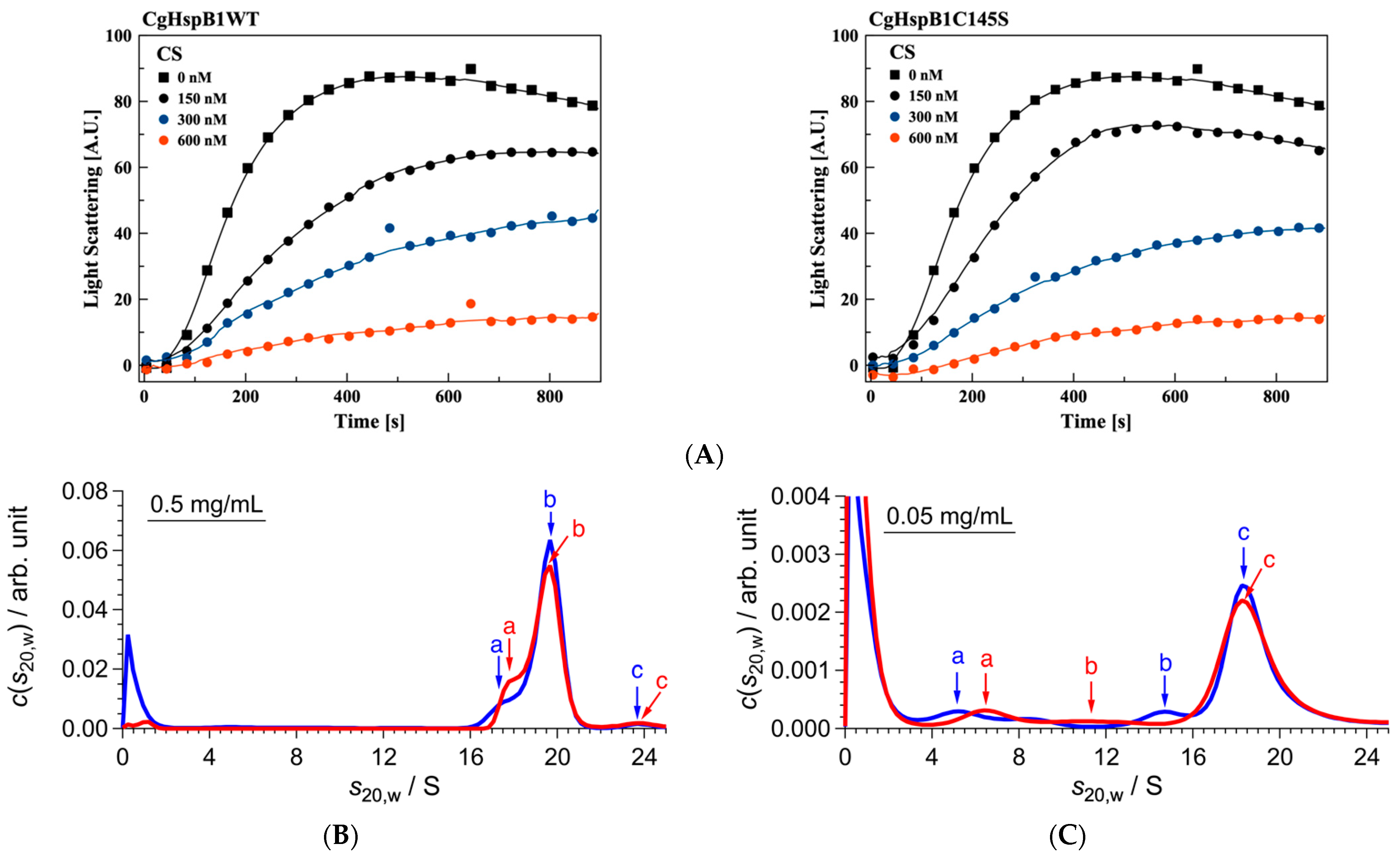
2.2. Role of the Disulfide Bond
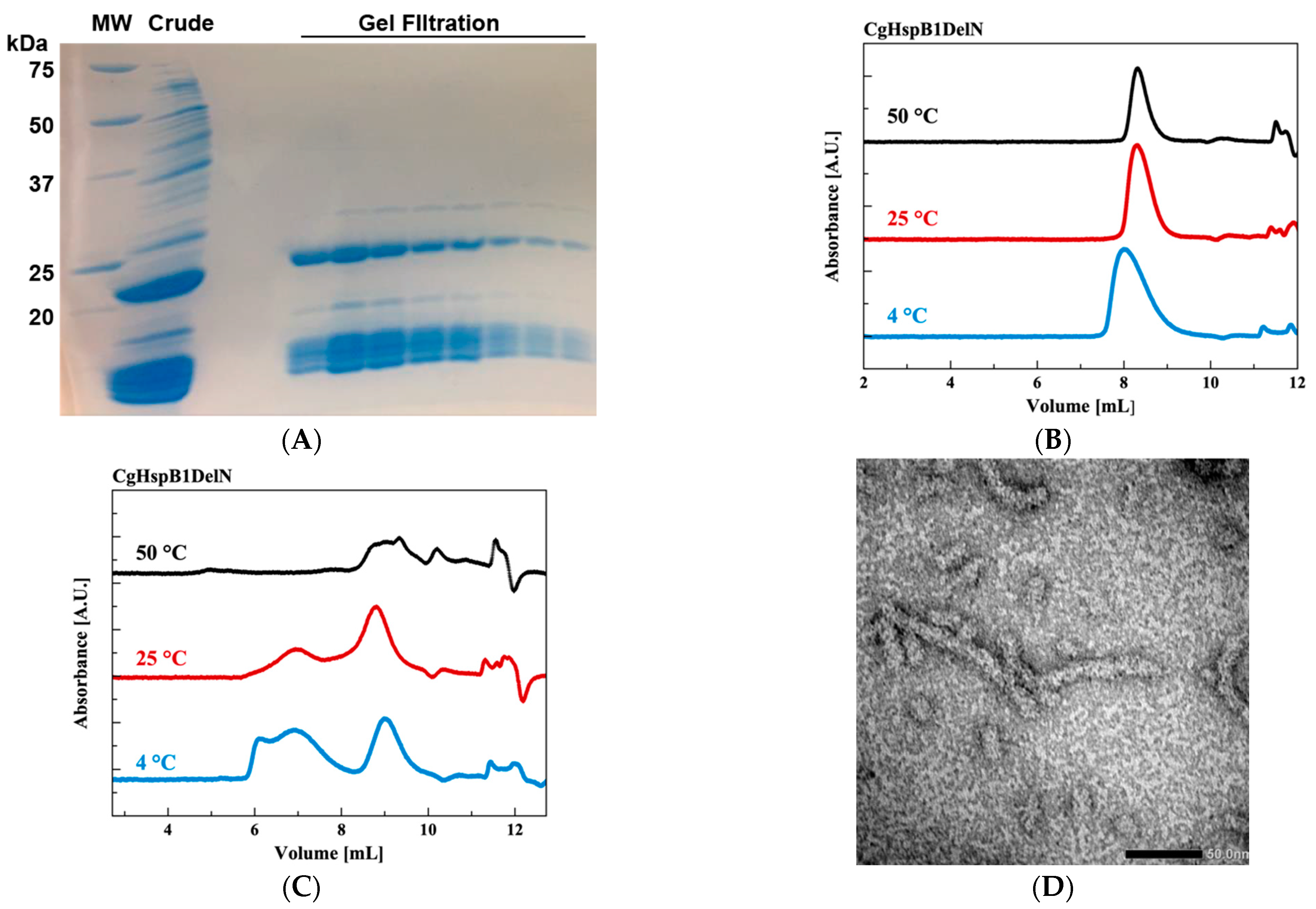
2.3. Effects of N- and C-Terminus Truncations
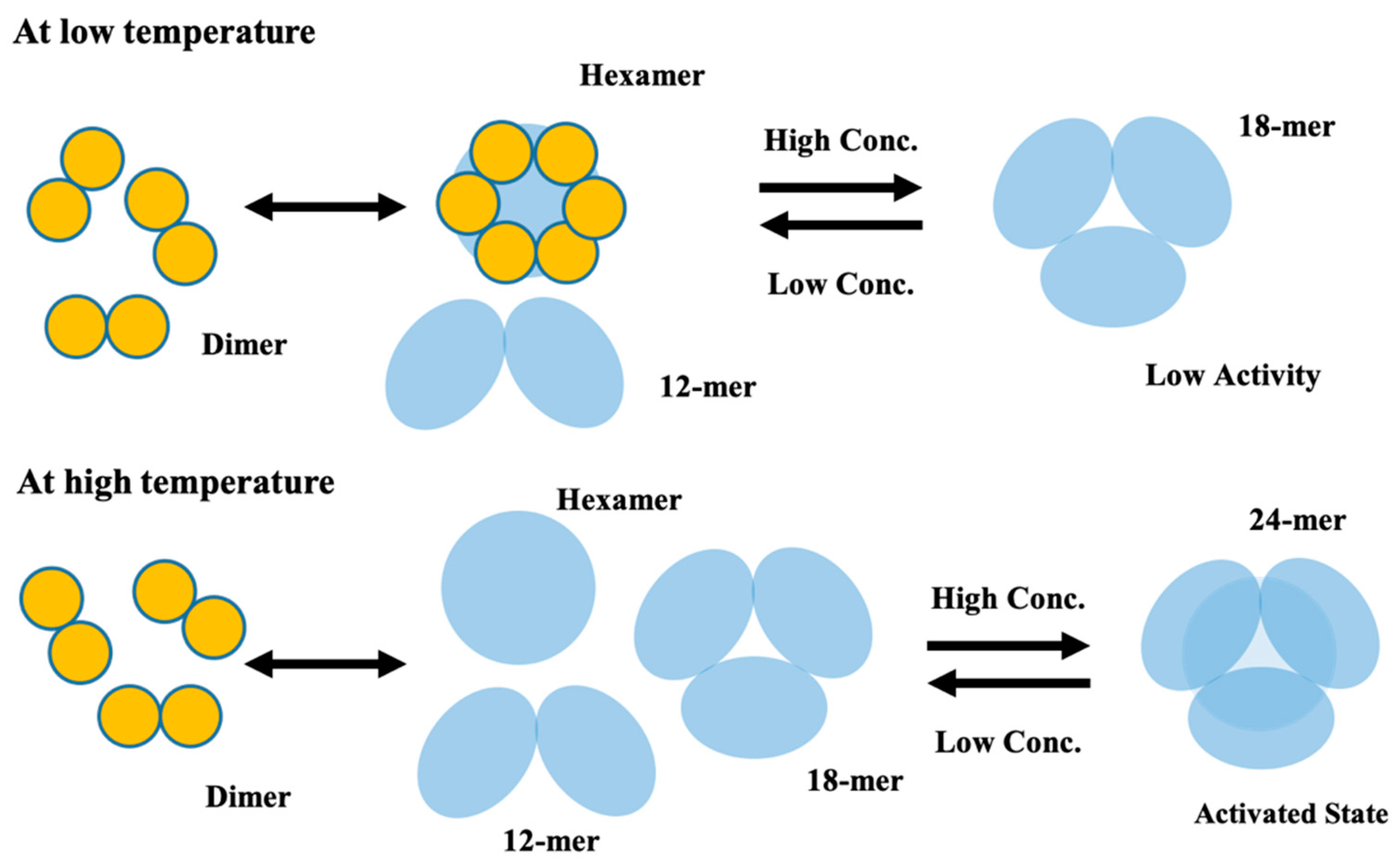
3. Discussion
4. Materials and Methods
4.1. Cloning, Expression, and Purification
4.2. Protein Aggregation Measurements
4.3. HPLC-SEC
4.4. SV-AUC Measurements
4.5. Electron Micrograph
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACD | α-crystallin domain |
| NTD | N-terminal domain |
| CTE | C-terminal extension |
| SEC | Size-exclusion chromatography |
| SEC-MALS | Size-exclusion chromatography with multiangle light scattering |
| SAXS | Small-angle X-ray scattering |
| SV-AUC | Sedimentation velocity analytical ultracentrifugation |
| CgHspB1 | HspB1 from Chinese hamster |
| CgHspB1WT | CgHspB1 wild type |
| CgHspB1C145S | CgHspB1 C145S mutant |
| CgHspB1DelN | CgHspB1 NTD deletion mutant |
| CgHspB1DelC | CgHspB1 CTE deletion mutant |
| DTT | Dithiothreitol |
| CS | Citrate synthase from porcine heart |
References
- Garrido, C.; Paul, C.; Seigneuric, R.; Kampinga, H.H. The small heat shock proteins family: The long forgotten chaperones. Int. J. Biochem. Cell Biol. 2012, 44, 1588–1592. [Google Scholar] [CrossRef]
- Bakthisaran, R.; Tangirala, R.; Rao, C.M. Small heat shock proteins: Role in cellular functions and pathology. Biochim. Biophys. Acta Prot. Proteom. 2015, 1854, 291–319. [Google Scholar] [CrossRef] [Green Version]
- Delbecq, S.P.; Jehle, S.; Klevit, R. Binding determinants of the small heat shock protein, αb-crystallin: Recognition of the ′IxI′ motif. EMBO J. 2012, 31, 4587–4594. [Google Scholar] [CrossRef] [Green Version]
- Santhanagopalan, I.; Degiacomi, M.T.; Shepherd, D.A.; Hochberg, G.K.A.; Benesch, J.L.P.; Vierling, E. It takes a dimer to tango: Oligomeric small heat shock proteins dissociate to capture substrate. J. Biol. Chem. 2018, 293, 19511–19521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.K.; Kim, R.; Kim, S. Crystal structure of a small heat-shock protein. Nature 1998, 394, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Hanazono, Y.; Takeda, K.; Yohda, M.; Miki, K. Structural studies on the oligomeric transition of a small heat shock protein, StHsp14.0. J. Mol. Biol. 2012, 422, 100–108. [Google Scholar] [CrossRef] [PubMed]
- van Montfort, R.L.; Basha, E.; Friedrich, K.L.; Slingsby, C.; Vierling, E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Struct. Biol. 2001, 8, 1025–1030. [Google Scholar] [CrossRef]
- Hanazono, Y.; Takeda, K.; Oka, T.; Abe, T.; Tomonari, T.; Akiyama, N.; Aikawa, Y.; Yohda, M.; Miki, K. Nonequivalence observed for the 16-meric structure of a small heat shock protein, SpHsp16.0, from Schizosaccharomyces pombe. Structure 2013, 21, 220–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleckenstein, T.; Kastenmüller, A.; Stein, M.L.; Peters, C.; Daake, M.; Krause, M.; Weinfurtner, D.; Haslbeck, M.; Weinkauf, S.; Groll, M.; et al. The Chaperone Activity of the Developmental Small Heat Shock Protein Sip1 Is Regulated by pH-Dependent Conformational Changes. Mol. Cell 2015, 58, 1067–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappé, G.; Franck, E.; Verschuure, P.; Boelens, W.C.; Leunissen, J.A.M.; De Jong, W.W. The human genome encodes 10 α-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperon. 2003, 8, 53–61. [Google Scholar] [CrossRef]
- Taylor, R.P.; Benjamin, I.J. Small heat shock proteins: A new classification scheme in mammals. J. Mol. Cell. Cardiol. 2005, 38, 433–444. [Google Scholar] [CrossRef]
- Acunzo, J.; Katsogiannou, M.; Rocchi, P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int. J. Biochem. Cell Biol. 2012, 44, 1622–1631. [Google Scholar] [CrossRef]
- Wettstein, G.; Bellaye, P.S.; Micheau, O.; Bonniaud, P. Small heat shock proteins and the cytoskeleton: An essential interplay for cell integrity? Int. J. Biochem. Cell Biol. 2012, 44, 1680–1686. [Google Scholar] [CrossRef]
- Concannon, C.G.; Gorman, A.M.; Samali, A. On the role of Hsp27 in regulating apoptosis. Apoptosis 2003, 8, 61–70. [Google Scholar] [CrossRef]
- Arrigo, A.-P. Hsp27: Novel Regulator of Intracellular Redox State. IUBMB Life 2001, 52, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, S.; Moens, U. Heat shock protein 27 phosphorylation: Kinases, phosphatases, functions and pathology. Cell. Mol. Life Sci. 2009, 66, 3289–3307. [Google Scholar] [CrossRef] [PubMed]
- Sha, E.; Nakamura, M.; Ankai, K.; Yamamoto, Y.; Oka, T.; Yohda, M. Functional and structural characterization of HspB1/Hsp27 from Chinese hamster ovary cells. FEBS Open Bio 2019, 9, 1826–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behlke, J.; Lutsch, G.; Gaestel, M.; Bielka, H. Supramolecular structure of the recombinant murine small heat shock protein hsp25. FEBS Lett. 1991, 288, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Ogall, T.; Ehrnsperger, N.; Preville, X.; Kotlyarov, A.; Lutsch, G.; Ducasse, C.; Paul, C.; Wieske, M.; Arrigo, A.-P.; Buchner, J.; et al. Regulation of Hsp27 Oligomerization, Chaperone Function, and Protective Activity against Oxidative Stress/Tumor Necrosis Factor by Phosphorylation. J. Biol. Chem. 1999, 274, 18947–18956. [Google Scholar]
- Lelj-Garolla, B.; Mauk, A.G. Self-association of a small heat shock protein. J. Mol. Biol. 2005, 345, 631–642. [Google Scholar] [CrossRef]
- Lelj-Garolla, B.; Mauk, A.G. Self-association and chaperone activity of Hsp27 are thermally activated. J. Biol. Chem. 2006, 281, 8169–8174. [Google Scholar] [CrossRef] [Green Version]
- Jovcevski, B.; Kelly, M.A.; Rote, A.P.; Berg, T.; Gastall, H.Y.; Benesch, J.L.P.; Aquilina, J.A.; Ecroyd, H. Phosphomimics destabilize Hsp27 oligomeric assemblies and enhance chaperone activity. Chem. Biol. 2015, 22, 186–195. [Google Scholar] [CrossRef]
- Nappi, L.; Aguda, A.H.; Nakouzi, N.A.; Lelj-Garolla, B.; Beraldi, E.; Lallous, N.; Thi, M.; Moore, S.; Fazli, L.; Battsogt, D.; et al. Ivermectin inhibits HSP27 and potentiates efficacy of oncogene targeting in tumor models. J. Clin. Investig. 2020, 130, 699–714. [Google Scholar] [CrossRef]
- Baranova, E.V.; Weeks, S.D.; Beelen, S.; Bukach, O.V.; Gusev, N.B.; Strelkov, S.V. Three-dimensional structure of α-crystallin domain dimers of human small heat shock proteins HSPB1 and HSPB6. J. Mol. Biol. 2011, 411, 110–122. [Google Scholar] [CrossRef]
- Hochberga, G.K.A.; Ecroydb, H.; Liud, C.; Coxb, D.; Casciod, D.; Sawayad, M.R.; Colliera, M.P.; Stroudd, J.; Carverg, J.A.; Baldwina, A.J.; et al. The structured core domain of αB-crystallin can prevent amyloid fibrillation and associated toxicity. Proc. Natl. Acad. Sci. USA 2014, 111, E1562–E1570. [Google Scholar] [CrossRef] [Green Version]
- Dam, J.; Schuck, P. Calculating sedimentation coefficient distributions by direct modeling of sedimentation velocity concentration profiles. Methods Enzymol. 2004, 384, 185–212. [Google Scholar]
- Chalova, A.S.; Sudnitsyna, M.V.; Semenyuk, P.I.; Orlov, V.N.; Gusev, N.B. Effect of disulfide crosslinking on thermal transitions and chaperone-like activity of human small heat shock protein HspB1. Cell Stress Chaperon. 2014, 19, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Zavialov, A.; Benndorf, R.; Ehrnsperger, M.; Zav’yalov, V.; Dudich, I.; Buchner, J.; Gaestel, M. The effect of the intersubunit disulfide bond on the structural and functional properties of the small heat shock protein Hsp25. Int. J. Biol. Macromol. 1998, 22, 163–173. [Google Scholar] [CrossRef]
- Franzmann, T.M.; Wühr, M.; Richter, K.; Walter, S.; Buchner, J. The activation mechanism of Hsp26 does not require dissociation of the oligomer. J. Mol. Biol. 2005, 350, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Hatipoglu, O.F.; Ishii, N.; Yohda, M. Role of the N-terminal region of the crenarchaeal sHsp, StHsp14.0, in thermal-induced disassembly of the complex and molecular chaperone activity. Biochem. Biophys. Res. Commun. 2004, 315, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Iizuka, R.; Yoshida, T.; Abe, T.; Kidokoro, S.; Ishii, N.; Yohda, M. Role of the IXI/V motif in oligomer assembly and function of StHsp14.0, a small heat shock protein from the acidothermophilic archaeon, Sulfolobus tokodaii strain 7. Proteins Struct. Funct. Genet. 2008, 71, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Oka, T.; Nakagome, A.; Tsukada, Y.; Yasunaga, T.; Yohda, M. StHsp14.0, a small heat shock protein of Sulfolobus tokodaii strain 7, protects denatured proteins from aggregation in the partially dissociated conformation. J. Biochem. 2011, 150, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
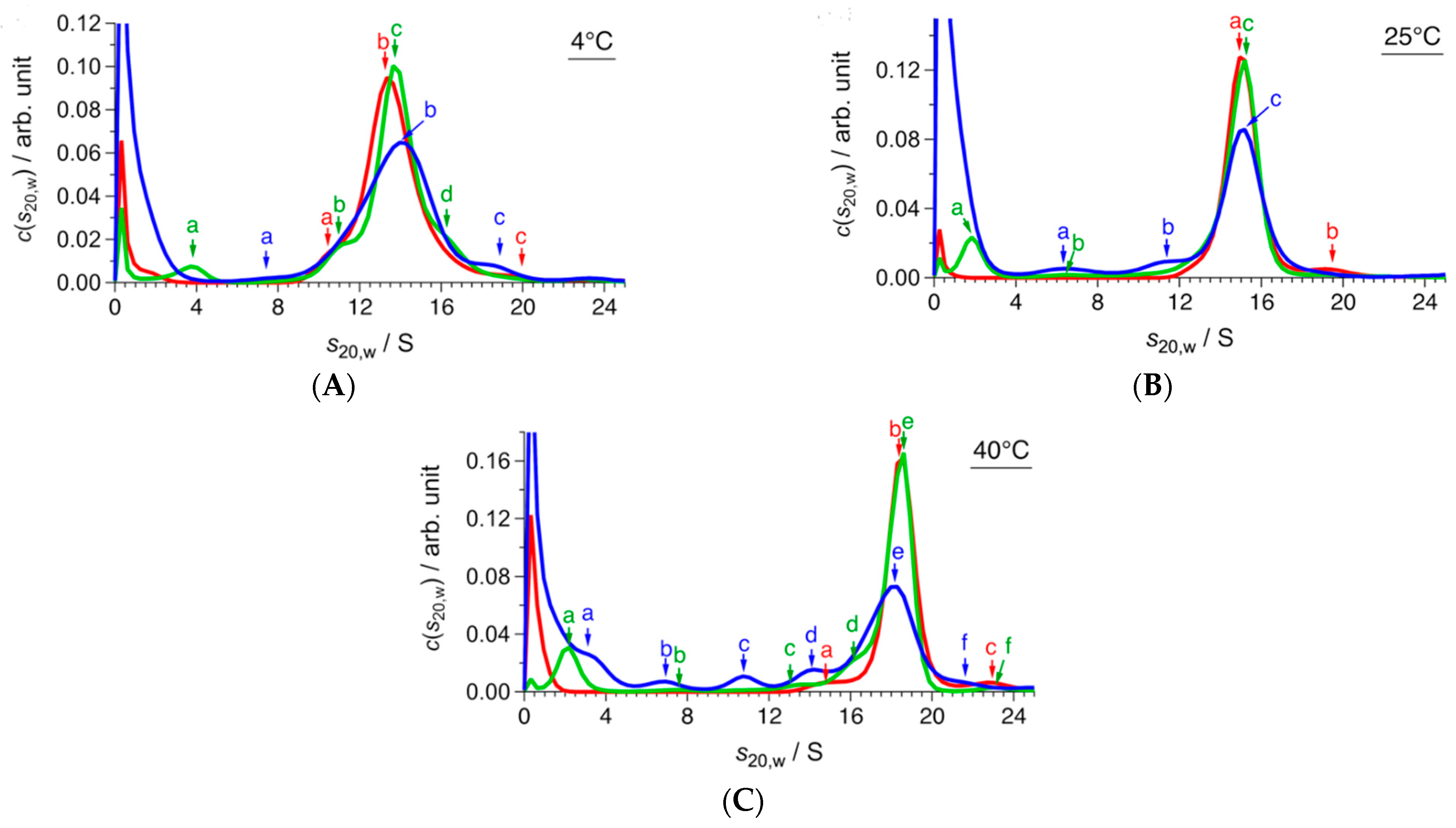


| (A) 4 °C | |||||
| c/mg mL−1 | f/f0 | Peak | s20,w/S | M/kDa | Association Number |
| 1.5 | 1.40 | a | 10.5 | 266 | 11 |
| b | 13.5 | 395 | 17 | ||
| c | 19.8 | 688 | 30 | ||
| 0.5 | 1.46 | a | 3.7 | 55 | 2 |
| b | 11.1 | 310 | 13 | ||
| c | 13.6 | 447 | 19 | ||
| d | 19.8 | 552 | 24 | ||
| 0.1 | 1.44 | a | 7.8 | 176 | 7 |
| b | 13.9 | 447 | 19 | ||
| c | 18.5 | 653 | 28 | ||
| (B) 25 °C | |||||
| c/mg mL−1 | f/f0 | Peak | s20,w/S | M/kDa | Association Number |
| 1.5 | 1.43 | a | 15.0 | 460 | 20 |
| b | 19.6 | 694 | 30 | ||
| 0.5 | 1.39 | a | 1.8 | 20 | 1 |
| b | 6.3 | 131 | 6 | ||
| c | 15.1 | 432 | 18 | ||
| 0.1 | 1.41 | a | 6.3 | 133 | 6 |
| b | 11.5 | 308 | 13 | ||
| c | 15.0 | 447 | 19 | ||
| (C) 40 °C | |||||
| c/mg mL−1 | f/f0 | Peak | s20,w/S | M/kDa | Association Number |
| 1.5 | 1.42 | a | 15.1 | 468 | 20 |
| b | 18.4 | 623 | 27 | ||
| c | 22.6 | 885 | 38 | ||
| 0.5 | 1.36 | a | 2.2 | 24 | 1 |
| b | 7.6 | 151 | 6 | ||
| c | 13.6 | 334 | 14 | ||
| d | 16.4 | 491 | 21 | ||
| e | 18.5 | 567 | 24 | ||
| f | 23.0 | 832 | 36 | ||
| 0.1 | 1.40 | a | 3.2 | 43 | 2 |
| b | 6.9 | 143 | 6 | ||
| c | 10.7 | 227 | 12 | ||
| d | 14.2 | 405 | 17 | ||
| e | 18.2 | 598 | 26 | ||
| f | 21.9 | 790 | 34 | ||
| (A) 4 °C | |||||
| Sample | c/mg mL−1 | f/f0 | s20,w/S | M/kDa | Association Number |
| CgHspB1 WT | 1.5 | 1.40 | 13.5 | 395 | 17 |
| CgHspB1 S15D | 1.45 | 13.9 | 427 | 18 | |
| CgHspB1 WT | 0.5 | 1.46 | 13.6 | 447 | 19 |
| CgHspB1 S15D | 1.44 | 13.9 | 449 | 19 | |
| CgHspB1 WT | 0.1 | 1.44 | 13.9 | 447 | 19 |
| CgHspB1 S15D | 1.43 | 14.0 | 435 | 19 | |
| (B) 25 °C | |||||
| Sample | c/mg mL−1 | f/f0 | s20,w/S | M/kDa | Association Number |
| CgHspB1 WT | 1.5 | 1.43 | 15.0 | 460 | 20 |
| CgHspB1 S15D | 1.35 | 15.4 | 443 | 19 | |
| CgHspB1 WT | 0.5 | 1.39 | 15.1 | 432 | 18 |
| CgHspB1 S15D | 1.39 | 15.4 | 435 | 19 | |
| CgHspB1 WT | 0.1 | 1.41 | 15.0 | 447 | 19 |
| CgHspB1 S15D | 1.34 | 15.1 | 413 | 18 | |
| (C) 40 °C | |||||
| Sample | c/mg mL−1 | f/f0 | s20,w/S | M/kDa | Association Number |
| CgHspB1 WT | 1.5 | 1.42 | 18.4 | 623 | 27 |
| CgHspB1 S15D | 1.40 | 18.9 | 628 | 27 | |
| CgHspB1 WT | 0.5 | 1.36 | 18.5 | 567 | 24 |
| CgHspB1 S15D | 1.39 | 18.9 | 609 | 26 | |
| CgHspB1 WT | 0.1 | 1.40 | 18.2 | 598 | 26 |
| CgHspB1 S15D | 1.35 | 18.9 | 597 | 26 | |
| 0.5 mg/mL | |||||
| DTT | f/f0 | Peak | s20,w/S | M/kDa | Association Number |
| + | 1.42 | a | 3.3 | 47 | 2 |
| b | 7.7 | 169 | 7 | ||
| c | 11.2 | 299 | 13 | ||
| d | 14.0 | 420 | 18 | ||
| e | 16.2 | 518 | 22 | ||
| f | 20.0 | 713 | 30 | ||
| − | 1.40 | a | 3.1 | 43 | 2 |
| b | 7.5 | 161 | 7 | ||
| c | 10.9 | 282 | 12 | ||
| d | 15.5 | 475 | 20 | ||
| e | 18.3 | 612 | 26 | ||
| f | 22.7 | 845 | 36 | ||
| 0.50 mg/mL | |||||
| DTT | f/f0 | Peak | s20,w/S | M/kDa | Association Number |
| + | 1.45 | a | 2.8 | 38 | 2 |
| b | 6.6 | 139 | 6 | ||
| c | 8.9 | 217 | 9 | ||
| d | 11.9 | 336 | 14 | ||
| e | 20.1 | 738 | 32 | ||
| − | 1.47 | a | 2.8 | 39 | 2 |
| b | 6.3 | 132 | 6 | ||
| c | 10.0 | 264 | 11 | ||
| d | 13.8 | 428 | 18 | ||
| e | 18.9 | 687 | 29 | ||
| 0.5 mg/mL | |||||
| DTT | f/f0 | Peak | s20,w/S | M/kDa | Association Number |
| + | 1.25 | a | 17.5 | 484 | 21 |
| b | 19.7 | 575 | 25 | ||
| c | 23.7 | 760 | 32 | ||
| − | 1.26 | a | 17.8 | 495 | 21 |
| b | 19.7 | 576 | 25 | ||
| c | 23.7 | 762 | 33 | ||
| 0.50 mg/mL | |||||
| DTT | f/f0 | Peak | s20,w/S | M/kDa | Association Number |
| + | 1.26 | a | 5.2 | 79 | 3 |
| b | 14.6 | 375 | 16 | ||
| c | 18.2 | 523 | 22 | ||
| − | 1.27 | a | 6.4 | 109 | 5 |
| b | 18.2 | 523 | 22 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurokawa, N.; Midorikawa, R.; Nakamura, M.; Noguchi, K.; Morishima, K.; Inoue, R.; Sugiyama, M.; Yohda, M. Oligomeric Structural Transition of HspB1 from Chinese Hamster. Int. J. Mol. Sci. 2021, 22, 10797. https://doi.org/10.3390/ijms221910797
Kurokawa N, Midorikawa R, Nakamura M, Noguchi K, Morishima K, Inoue R, Sugiyama M, Yohda M. Oligomeric Structural Transition of HspB1 from Chinese Hamster. International Journal of Molecular Sciences. 2021; 22(19):10797. https://doi.org/10.3390/ijms221910797
Chicago/Turabian StyleKurokawa, Nina, Rio Midorikawa, Manami Nakamura, Keiichi Noguchi, Ken Morishima, Rintaro Inoue, Masaaki Sugiyama, and Masafumi Yohda. 2021. "Oligomeric Structural Transition of HspB1 from Chinese Hamster" International Journal of Molecular Sciences 22, no. 19: 10797. https://doi.org/10.3390/ijms221910797
APA StyleKurokawa, N., Midorikawa, R., Nakamura, M., Noguchi, K., Morishima, K., Inoue, R., Sugiyama, M., & Yohda, M. (2021). Oligomeric Structural Transition of HspB1 from Chinese Hamster. International Journal of Molecular Sciences, 22(19), 10797. https://doi.org/10.3390/ijms221910797







