16S rRNA of Mucosal Colon Microbiome and CCL2 Circulating Levels Are Potential Biomarkers in Colorectal Cancer
Abstract
:1. Introduction
2. Results
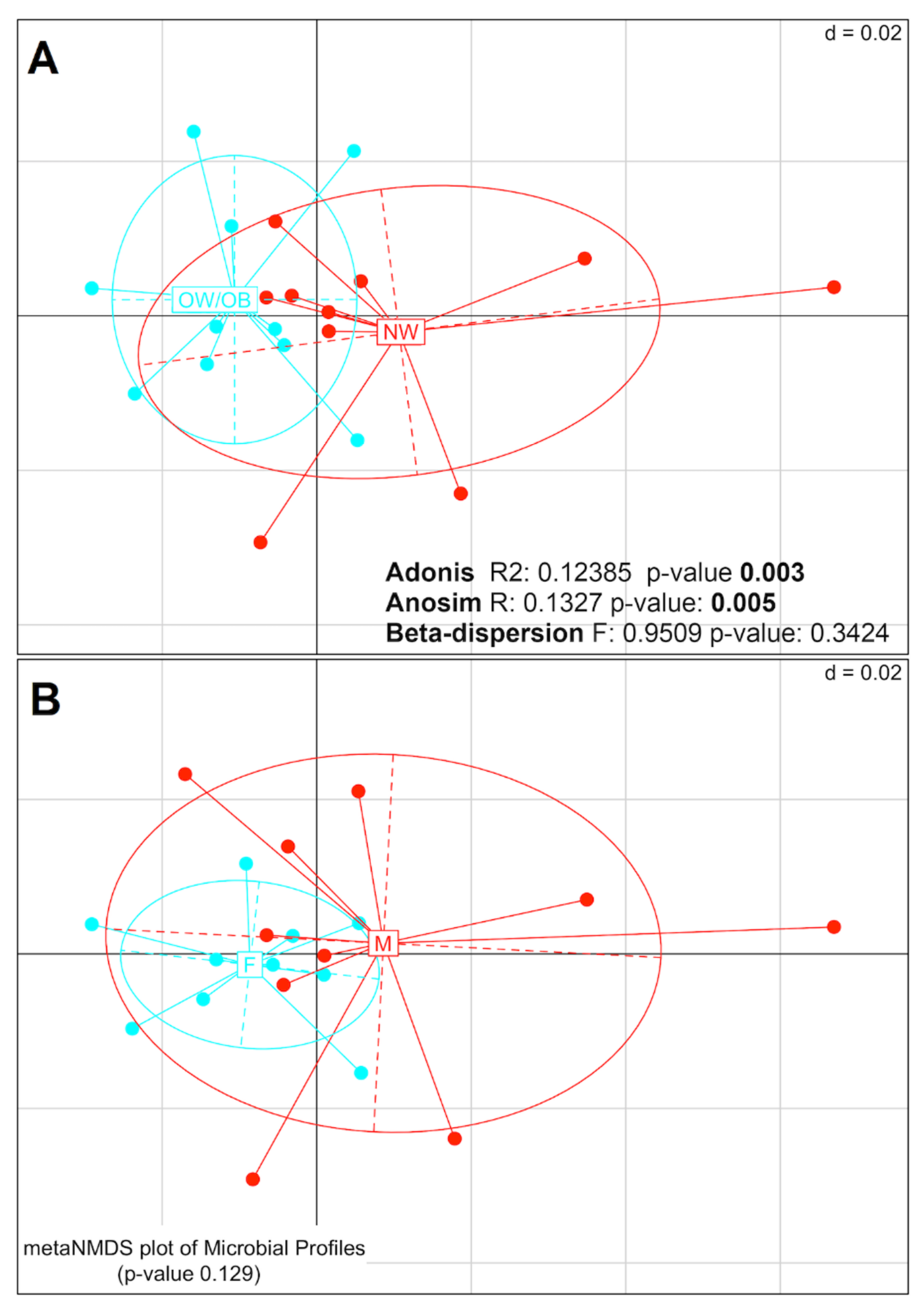
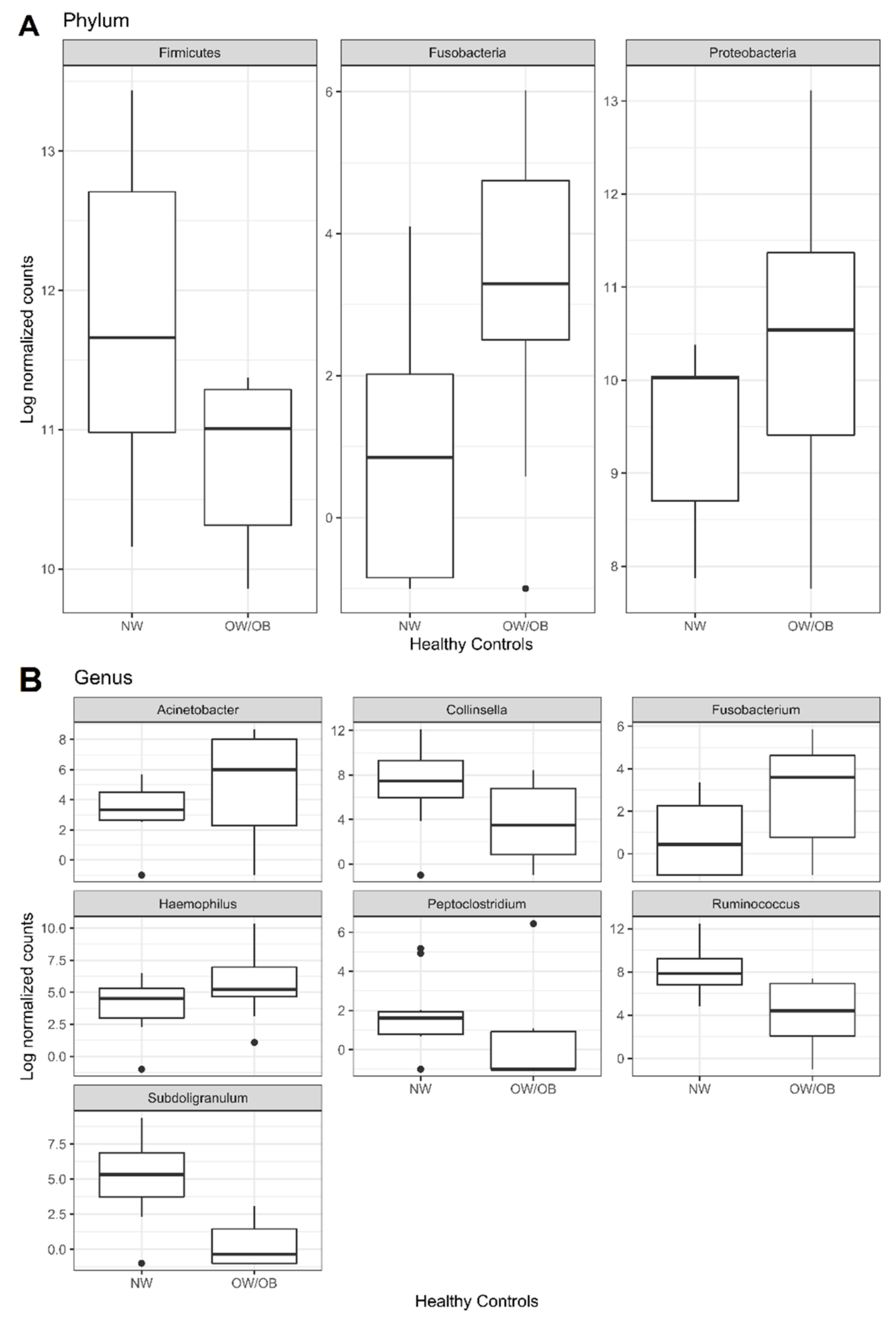
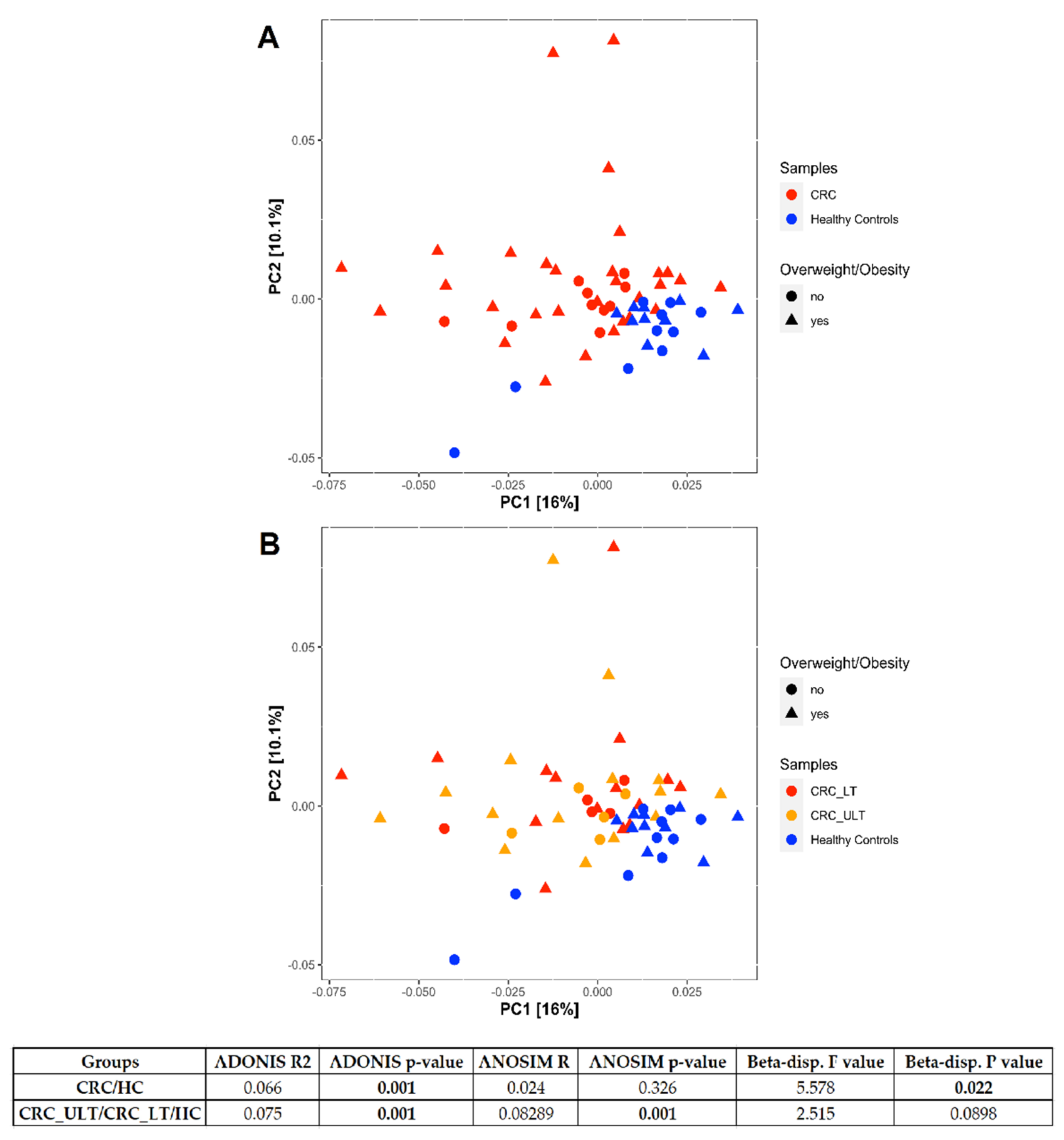
2.1. Mucosal Colon 16S rRNA Analysis
2.2. Impact of Aging on Microbiome Composition
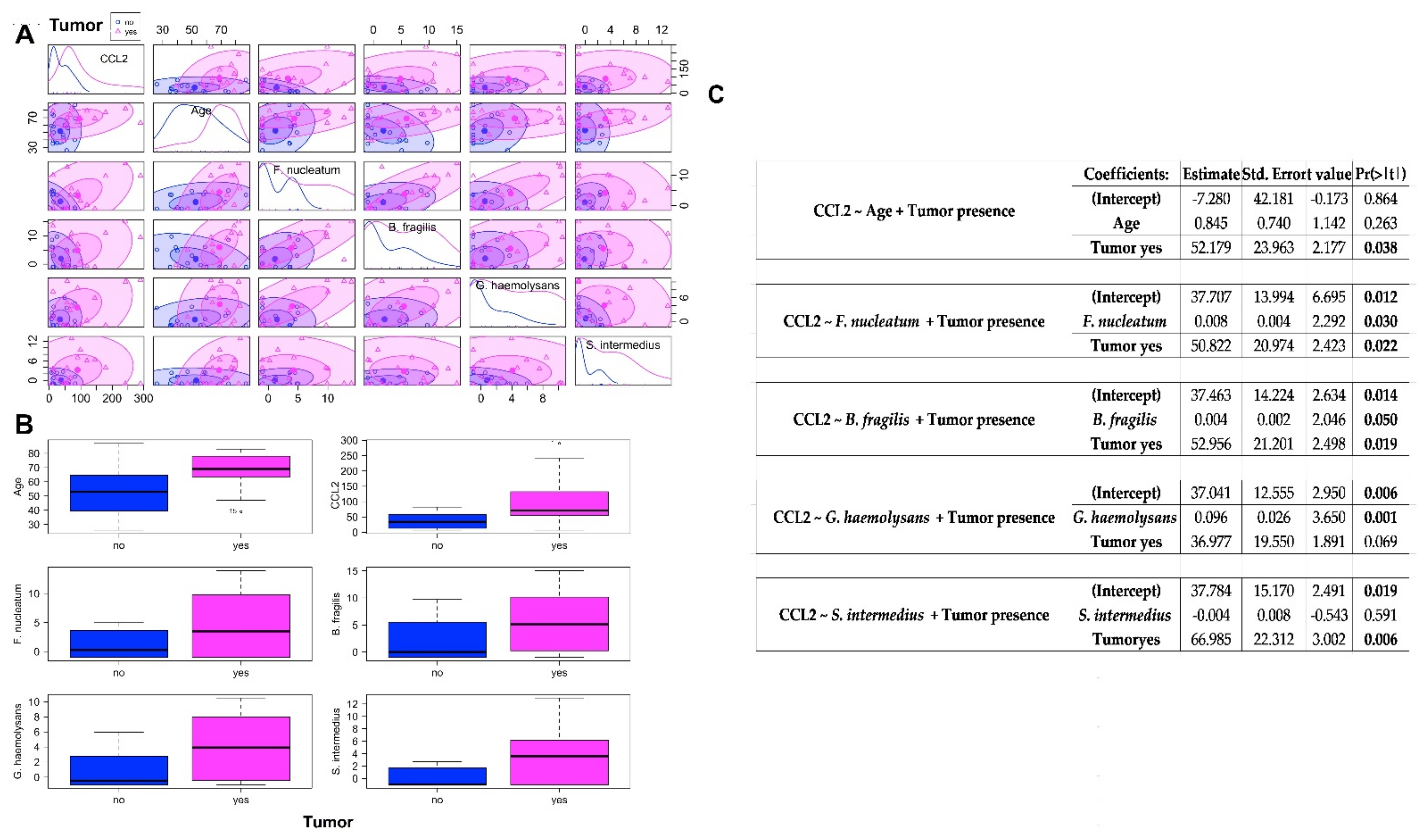
2.3. Association between CCL2 Blood Levels in CRC Patients and Healthy Controls and the Abundances of Bacteria Significantly CRC-Associated
3. Discussion
3.1. Mucosal Colon Microbiome Was Different between NW and OW/OB Control Subjects
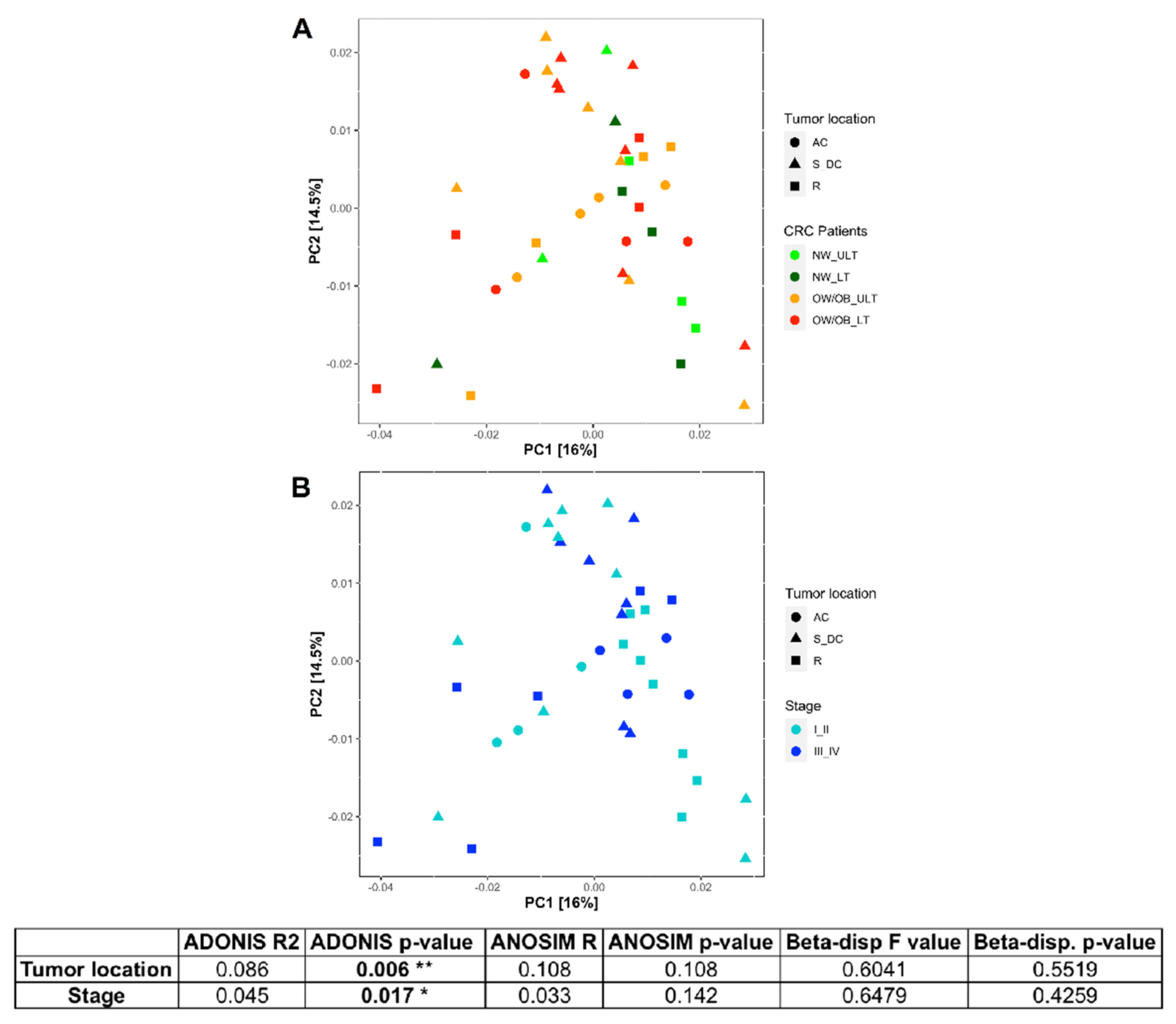
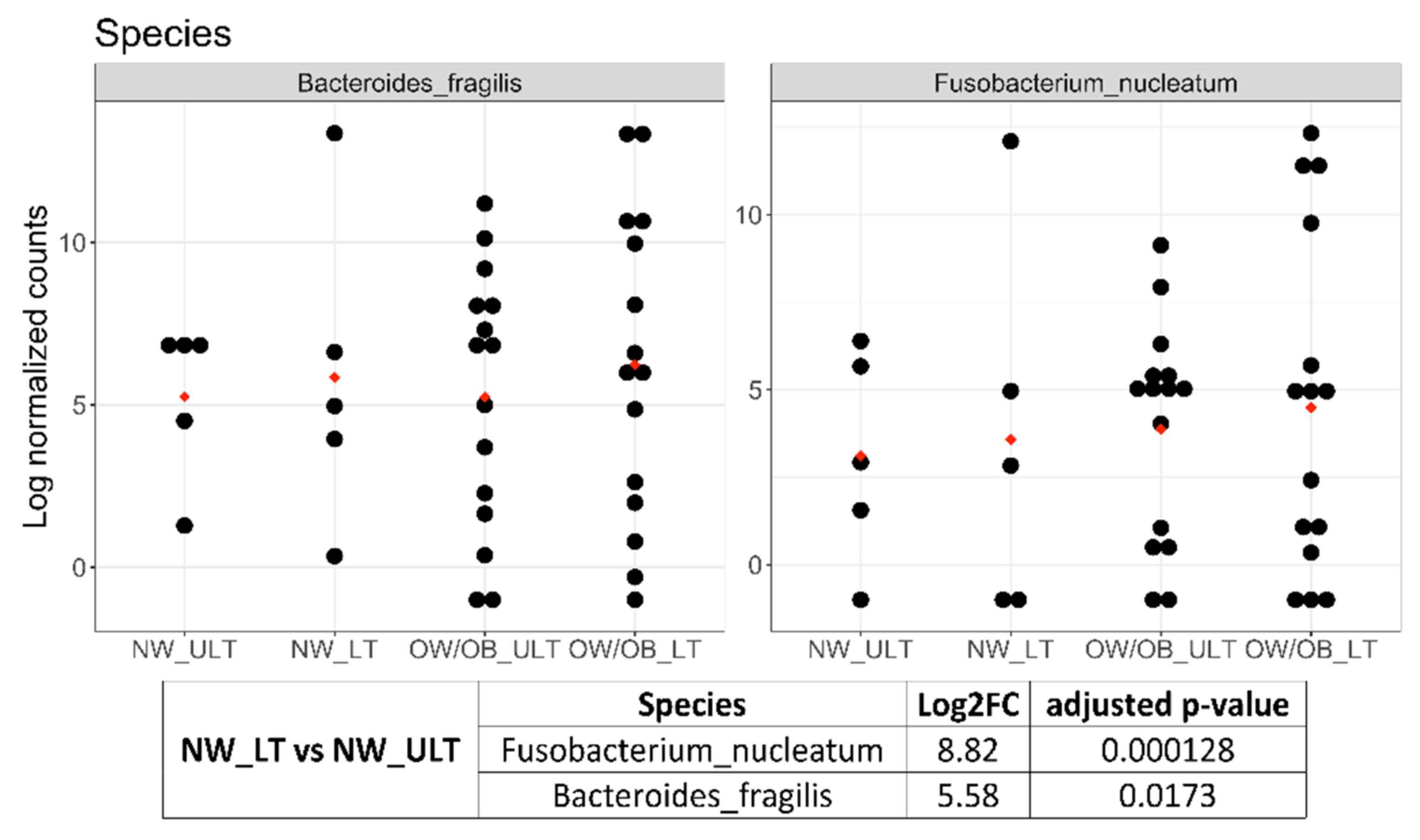
3.2. Differences in Mucosal Colon Microbiome between Tumor Lesioned and Unlesioned Tissues of CRC Patients
3.3. Mucosal Colon CRC Microbiome Differs Significantly from That of Healthy Controls
3.4. Circulating Levels of CCL2 in CRC and Healthy Controls
4. Materials and Methods
4.1. Patients and Controls
4.2. Sample Collection and Storage
4.3. CCL2 Assay
4.4. DNA Isolation and 16S rRNA Sequencing
4.5. Reads Processing
4.6. Bioinformatics and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Day, J.C. Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050; U.S. Bureau of the Census, Current Population Reports: Washington, DC, USA, 1996.
- AIRTUM Working Group. I tumori in Italia Rapporto 2016. Sopravvivenza. Epidemiol. Prev. 2018, 41 (Suppl. 1). [Google Scholar]
- Lee, M.S.; Menter, D.G.; Kopetz, S. Right versus left colon cancer biology: Integrating the consensus molecular subtypes. J. Natl. Compr. Cancer Netw. 2017, 15, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Mana, M.D.; Hussey, A.M.; Tzouanas, C.N.; Imada, S.; Millan, Y.B.; Bahceci, D.; Saiz, D.R.; Webb, A.T.; Lewis, C.A.; Carmeliet, P.; et al. High-fat diet-activated fatty acid oxidation mediates intestinal stemness and tumorigenicity. Cell Rep. 2021, 35, 109212. [Google Scholar] [CrossRef]
- Biragyn, A.; Ferrucci, L. Gut dysbiosis: A potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 2018, 19, e295–e304. [Google Scholar] [CrossRef]
- Saus, E.; Iraola-Guzmán, S.; Willis, J.R.; Brunet-Vega, A.; Gabaldón, T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol. Asp. Med. 2019, 69, 93–106. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The role of the gut microbiome in colorectal cancer development and therapy response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef]
- Kho, Z.Y.; Lal, S.K. The human gut microbiome—A potential controller of wellness and disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nat. Cell Biol. 2016, 535, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: A foe or ally? Cell. Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Q.; Vadgama, J.V.; Wang, P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun. Signal. 2020, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Diaz, T.; Verduzco, A.D.R.; Magyar, C.E.; Zhong, J.; Elshimali, Y.; Rettig, M.B.; Henning, S.M.; Vadgama, J.V.; Wang, P. Arctigenin inhibits prostate tumor growth in high-fat diet fed mice through dual actions on adipose tissue and tumor. Sci. Rep. 2020, 10, 1403–1413. [Google Scholar] [CrossRef]
- Fujita, K.; Hayashi, T.; Matsushita, M.; Uemura, M.; Nonomura, N. Obesity, inflammation, and prostate cancer. J. Clin. Med. 2019, 8, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Chen, Q.; Corey, E.; Xie, W.; Fan, J.; Mizokami, A.; Zhang, J. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clin. Exp. Metastasis 2008, 26, 161–169. [Google Scholar] [CrossRef]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 671–682. [Google Scholar] [CrossRef]
- Brennan, C.A.; Garrett, W.S. Fusobacterium nucleatum—Symbiont, opportunist and oncobacterium. Nat. Rev. Genet. 2018, 17, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, C.; Granata, I.; D’Argenio, V.; Tramontano, S.; Compare, D.; Guarracino, M.R.; Nardone, G.; Pilone, V.; Sacchetti, L. Characterization of the duodenal mucosal microbiome in obese adult subjects by 16S rRNA sequencing. Microorganisms 2020, 8, 485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, I.; Nardelli, C.; D’Argenio, V.; Tramontano, S.; Compare, D.; Guarracino, M.R.; Nardone, G.; Pilone, V.; Sacchetti, L. Duodenal metatranscriptomics to define human and microbial functional alterations associated with severe obesity: A pilot study. Microorganisms 2020, 8, 1811. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Gerner, R.R.; Moschen, A.R. The intestinal microbiota in colorectal cancer. Cancer Cell 2018, 33, 954–964. [Google Scholar] [CrossRef] [Green Version]
- Dejea, C.M.; Wick, E.C.; Hechenbleikner, E.M.; White, J.R.; Welch, J.L.M.; Rossetti, B.J.; Peterson, S.N.; Snesrud, E.C.; Borisy, G.G.; Lazarev, M.; et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 18321–18326. [Google Scholar] [CrossRef] [Green Version]
- Burns, M.B.; Lynch, J.; Starr, T.K.; Knights, D.; Blekhman, R. Virulence genes are a signature of the microbiome in the colorectal tumor microenvironment. Genome Med. 2015, 7, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Flemer, B.; Warren, R.D.; Barrett, M.P.; Cisek, K.; Das, A.; Jeffery, I.; Hurley, E.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2017, 67, 1454–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Chan, A.T.; Sun, J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical activity, obesity and sedentary behavior in cancer etiology: Epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body fatness and cancer—Viewpoint of the IARC working group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [Green Version]
- Whisner, C.; Aktipis, C.A. The role of the microbiome in cancer initiation and progression: How microbes and cancer cells utilize excess energy and promote one another’s growth. Curr. Nutr. Rep. 2019, 8, 42–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andoh, A.; Nishida, A.; Takahashi, K.; Inatomi, O.; Imaeda, H.; Bamba, S.; Kito, K.; Sugimoto, M.; Kobayashi, T. Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 2016, 59, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; De Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [Green Version]
- Ley, R.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.; Turnbaugh, P.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Turnbaugh, P.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, S.; Lobley, G.E.; Holtrop, G.; Ince, J.; Johnstone, A.; Louis, P.; Flint, H.J. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. 2008, 32, 1720–1724. [Google Scholar] [CrossRef] [Green Version]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef] [Green Version]
- Sze, M.A.; Schloss, P.D. Looking for a signal in the noise: Revisiting obesity and the microbiome. mBio 2016, 7, e01018-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greathouse, K.L.; White, J.R.; Padgett, R.N.; Perrotta, B.G.; Jenkins, G.D.; Chia, N.; Chen, J. Gut microbiome meta-analysis reveals dysbiosis is independent of body mass index in predicting risk of obesity-associated CRC. BMJ Open Gastroenterol. 2019, 6, e000247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Dai, Z.; Coker, O.O.; Nakatsu, G.; Wu, W.K.K.; Zhao, L.; Chen, Z.; Chan, F.K.L.; Kristiansen, K.; Sung, J.J.Y.; Wong, S.H.; et al. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome 2018, 6, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Flemer, B.; Lynch, D.B.; Brown, J.M.R.; Jeffery, I.; Ryan, F.; Claesson, M.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef]
- Tahara, T.; Hirata, I.; Nakano, N.; Tahara, S.; Horiguchi, N.; Kawamura, T.; Okubo, M.; Ishizuka, T.; Yamada, H.; Yoshida, D.; et al. Potential link between Fusobacterium enrichment and DNA methylation accumulation in the inflammatory colonic mucosa in ulcerative colitis. Oncotarget 2017, 8, 61917–61926. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Kanno, S.; Nosho, K.; Sukawa, Y.; Mitsuhashi, K.; Kurihara, H.; Igarashi, H.; Takahashi, T.; Tachibana, M.; Takahashi, H.; et al. Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway. Int. J. Cancer 2015, 137, 1258–1268. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatumin colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [Green Version]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-Cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Goodman, B.; Gardner, H. The microbiome and cancer. J. Pathol. 2018, 244, 667–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, A.C.; Shields, C.E.D.; Wu, S.; Huso, D.L.; Wu, X.; Murray, T.S.; Hacker-Prietz, A.; Rabizadeh, S.; Woster, P.; Sears, C.L.; et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15354–15359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C.; et al. The bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef]
- Vizcaino, M.I.; Crawford, J.M. The colibactin warhead crosslinks DNA. Nat. Chem. 2015, 7, 411–417. [Google Scholar] [CrossRef]
- Bonnet, M.; Buc, E.; Sauvanet, P.; Darcha, C.; Dubois, D.; Pereira, B.; Déchelotte, P.; Bonnet, R.; Pezet, D.; Darfeuille-Michaud, A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin. Cancer Res. 2014, 20, 859–867. [Google Scholar] [CrossRef] [Green Version]
- Nakatsu, G.; Li, X.; Zhou, H.; Sheng, J.; Wong, S.H.; Wu, W.K.K.; Ng, S.C.; Tsoi, H.; Dong, Y.; Zhang, N.; et al. Gut mucosal microbiome across stages of colorectal carcinogenesis. Nat. Commun. 2015, 6, 8727. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef]
- Allali, I.; Delgado, S.; Marron, P.I.; Astudillo, A.; Yeh, J.J.; Ghazal, H.; Amzazi, S.; Keku, T.; Azcarate-Peril, M.A. Gut microbiome compositional and functional differences between tumor and non-tumor adjacent tissues from cohorts from the US and Spain. Gut Microbes 2015, 6, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Tsoi, H.; Chu, E.S.; Zhang, X.; Sheng, J.; Nakatsu, G.; Ng, S.C.; Chan, A.; Chan, F.K.; Sung, J.J.Y.; Yu, J. Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology 2017, 152, 1419–1433.e5. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.Y.; Yuzhalin, A.; Gordon-Weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conley, M.N.; Wong, C.P.; Duyck, K.M.; Hord, N.; Ho, E.; Sharpton, T.J. Aging and serum MCP-1 are associated with gut microbiome composition in a murine model. PeerJ 2016, 4, e1854. [Google Scholar] [CrossRef] [Green Version]
- Heintz, C.; Mair, W. You are what you host: Microbiome modulation of the aging process. Cell 2014, 156, 408–411. [Google Scholar] [CrossRef] [Green Version]
- Garborg, K.; Bretthauer, M.; De Lange, T. Quality indicators in colonoscopy. Curr. Treat. Options Gastroenterol. 2017, 15, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Chen, C.-Y.; Chen-Deng, A.; Chen, Y.-L.; Wang, J.-Y.; Hou, Y.-I.; Lin, M.-C. Joining Illumina paired-end reads for classifying phylogenetic marker sequences. BMC Bioinform. 2020, 21, 105–117. [Google Scholar] [CrossRef]
- Agnihotry, S.; Sarangi, A.N.; Aggarwal, R. Construction & assessment of a unified curated reference database for improving the taxonomic classification of bacteria using 16S rRNA sequence data. Indian J. Med. Res. 2020, 151, 93–103. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- McMurdie, P.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahti, L.; Shetty, S. Bioconductor, 2017-Tools for Microbiome Analysis in R. Microbiome Package Version. Available online: http://microbiome.github.io/microbiome (accessed on 5 August 2021).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5–7. Available online: https://CRAN.R-project.org/package=vegan (accessed on 5 August 2021).
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
| Level | Taxa | Log2FC | Adjusted p-Value |
|---|---|---|---|
| Phylum | Firmicutes | −1.25 | 0.0072 |
| Fusobacteria | 2.3 | 0.0282 | |
| Proteobacteria | 1.57 | 0.0134 | |
| Class | Clostridia (Firmicutes) | −2.15 | 0.0009 |
| Coriobacteriia (Actinobacteria) | −2.52 | 0.0250 | |
| Fusobacteriia (Fusobacteria) | 2.11 | 0.0444 | |
| Gammaproteobacteria (Proteobacteria) | 1.84 | 0.0143 | |
| Order | Bifidobacteriales (Actinobacteria) | −3.8 | 0.0002 |
| Clostridiales (Firmicutes) | −3.29 | 0.0001 | |
| Coriobacteriales (Actinobacteria) | −4.06 | 0.0002 | |
| Family | Bifidobacteriaceae (Actinobacteria) | −3.53 | 0.0030 |
| Coriobacteriaceae (Actinobacteria) | −3.22 | 0.0051 | |
| Ruminococcaceae (Firmicutes) | −3.22 | 0.0030 | |
| Genus | Acinetobacter (Proteobacteria) | 3.14 | 0.0138 |
| Collinsella (Actinobacteria) | −3.3 | 0.0210 | |
| Fusobacterium (Fusobacteria) | 2.79 | 0.0418 | |
| Haemophilus (Proteobacteria) | 2.55 | 0.0210 | |
| Peptoclostridium (Firmicutes) | −4.03 | 0.0138 | |
| Ruminococcus (Firmicutes) | −4.2 | 0.0002 | |
| Subdoligranulum (Firmicutes) | −6.31 | 0.0000 | |
| Species | Acinetobacter lwoffii (Proteobacteria) | 4.17 | 0.0062 |
| Bacteroides dorei (Bacteroidetes) | −5.88 | 0.0002 | |
| Bifidobacterium longum (Firmicutes) | −3.91 | 0.0051 | |
| Escherichia coli (Proteobacteria) | 2.62 | 0.0089 | |
| Fusobacterium nucleatum (Fusobacteria) | 3.26 | 0.0176 | |
| Haemophilus parainfluenzae (Proteobacteria) | 2.07 | 0.0487 | |
| Peptoclostridium difficile (Firmicutes) | −3.26 | 0.0454 | |
| Ruminococcus torques (Firmicutes) | −3.22 | 0.0089 | |
| Subdoligranulum variabile (Firmicutes) | −4.67 | 0.0002 |
| Species | Log2FC | Adjusted p-Value | |
|---|---|---|---|
| CRC vs. Healthy Controls | Streptococcus intermedius (Firmicutes) | 5.72 | 9 × 10−9 |
| Gemella haemolysans (Firmicutes) | 5.4 | 1.15 × 10−8 | |
| Ruminococcus torques (Firmicutes) | −3 | 9.91 × 10−5 | |
| Fusobacterium nucleatum (Fusobacteria) | 3.88 | 5.65 × 10−4 | |
| Escherichia coli (Proteobacteria) | 1.85 | 5.65 × 10−4 | |
| Bacteroides fragilis (Bacteroidetes) | 2.44 | 1.43 × 10−2 | |
| Haemophilus parainfluenzae (Proteobacteria) | 2.11 | 4.60 × 10−2 | |
| CRC_ULT vs. Healthy Controls | Gemella haemolysans | 4.84 | 3.76 × 10−5 |
| Streptococcus intermedius | 4.72 | 5.52 × 10−5 | |
| Ruminococcus torques | −3.21 | 3.24 × 10−3 | |
| Escherichia coli | 1.84 | 5.08 × 10−3 | |
| Fusobacterium nucleatum | 3.4 | 2.31 × 10−2 | |
| Ruminococcus gnavus (Firmicutes) | 2.03 | 4.85 × 10−2 | |
| CRC_LT vs. Healthy Controls | Streptococcus intermedius | 7.06 | 2.65 × 10−10 |
| Fusobacterium nucleatum | 7.33 | 5.33 × 10−8 | |
| Gemella haemolysans | 5.85 | 6.43 × 10−8 | |
| Bacteroides fragilis | 4.18 | 7.34 × 10−4 | |
| Haemophilus parainfluenzae | −3.44 | 2.96 × 10−3 | |
| Escherichia coli | 1.85 | 3.23 × 10−3 | |
| Bacteroides vulgatus (Bacteroidetes) | −2.03 | 4.52 × 10−2 | |
| NW_ULT_CRC vs. NW_HC | Escherichia coli | 3.36 | 4.48 × 10−3 |
| Ruminococcus torques | −5.18 | 4.48 × 10−3 | |
| Gemella haemolysans | 4.63 | 4.74 × 10−2 | |
| OW/OB_ULT_CRC vs. OW/OB_HC | Streptococcus intermedius | 7.72 | 1.78 × 10−5 |
| Gemella haemolysans | 4.45 | 5.68 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardelli, C.; Granata, I.; Nunziato, M.; Setaro, M.; Carbone, F.; Zulli, C.; Pilone, V.; Capoluongo, E.D.; De Palma, G.D.; Corcione, F.; et al. 16S rRNA of Mucosal Colon Microbiome and CCL2 Circulating Levels Are Potential Biomarkers in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 10747. https://doi.org/10.3390/ijms221910747
Nardelli C, Granata I, Nunziato M, Setaro M, Carbone F, Zulli C, Pilone V, Capoluongo ED, De Palma GD, Corcione F, et al. 16S rRNA of Mucosal Colon Microbiome and CCL2 Circulating Levels Are Potential Biomarkers in Colorectal Cancer. International Journal of Molecular Sciences. 2021; 22(19):10747. https://doi.org/10.3390/ijms221910747
Chicago/Turabian StyleNardelli, Carmela, Ilaria Granata, Marcella Nunziato, Mario Setaro, Fortunata Carbone, Claudio Zulli, Vincenzo Pilone, Ettore Domenico Capoluongo, Giovanni Domenico De Palma, Francesco Corcione, and et al. 2021. "16S rRNA of Mucosal Colon Microbiome and CCL2 Circulating Levels Are Potential Biomarkers in Colorectal Cancer" International Journal of Molecular Sciences 22, no. 19: 10747. https://doi.org/10.3390/ijms221910747
APA StyleNardelli, C., Granata, I., Nunziato, M., Setaro, M., Carbone, F., Zulli, C., Pilone, V., Capoluongo, E. D., De Palma, G. D., Corcione, F., Matarese, G., Salvatore, F., & Sacchetti, L. (2021). 16S rRNA of Mucosal Colon Microbiome and CCL2 Circulating Levels Are Potential Biomarkers in Colorectal Cancer. International Journal of Molecular Sciences, 22(19), 10747. https://doi.org/10.3390/ijms221910747








