Nanobody-Based Theranostic Agents for HER2-Positive Breast Cancer: Radiolabeling Strategies
Abstract
:1. Introduction
2. Nuclear Imaging and Targeted Radionuclide Therapy
3. Antibody Fragments for Nuclear Imaging and Radionuclide-Based Therapy
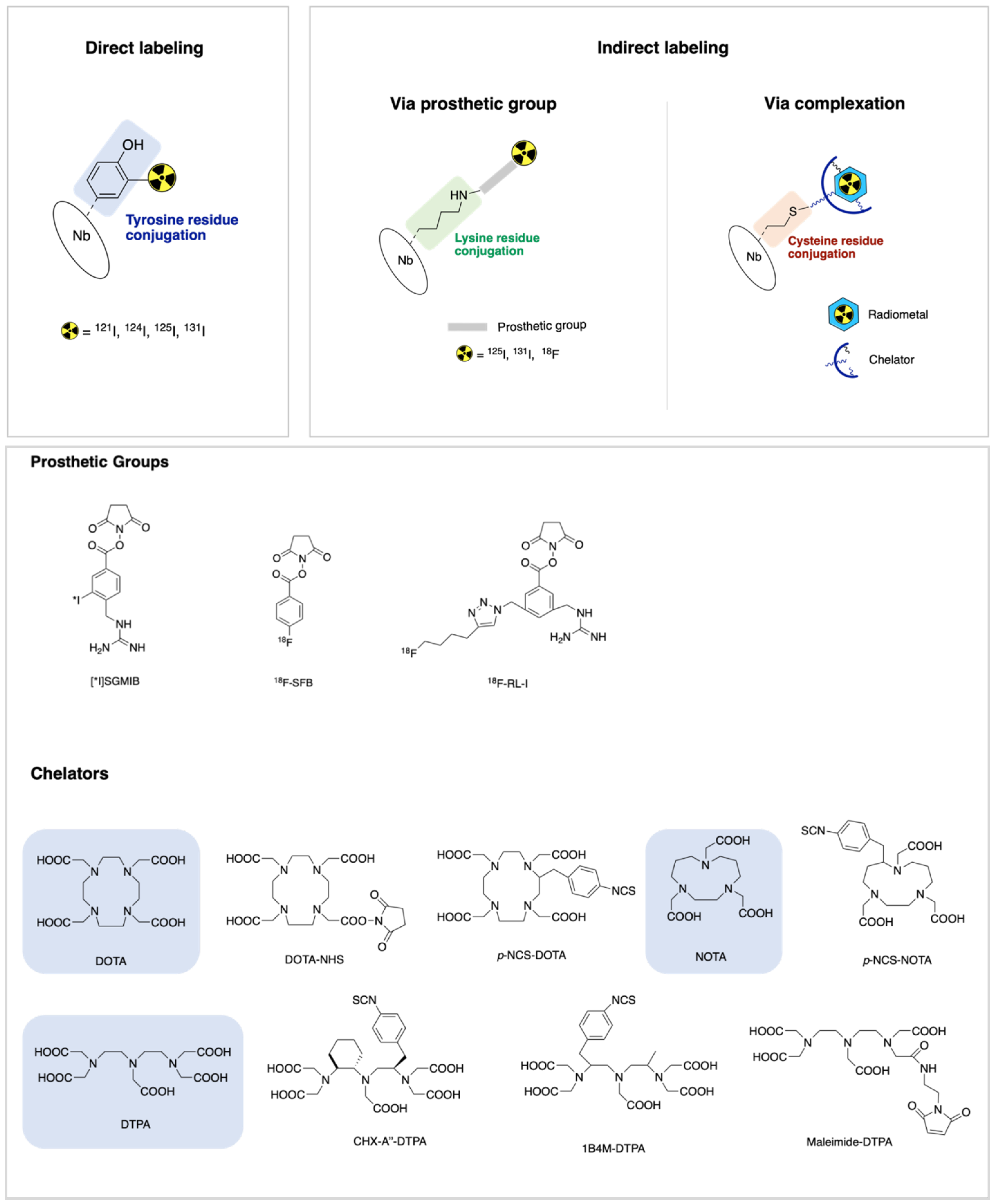
4. Radiolabeling Methods of Nanobodies
4.1. Direct Labeling
4.2. Indirect Labeling via Prosthetic Group
4.3. Indirect Labeling via Complexation
| Entry | Radiometal | Production Mode | Half-Life | Mode of Decay (%) | Maximum Energy (KeV) | Chelator Agent | HER2 Nanobody-Tracer | Phase | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68Ga | 68Ge/68Ga generator | 1.13 h | β+ (90) | 1899 | p-SCN-Bn-NOTA | 68Ga-NOTA-2Rs15d | Phase II ongoing (NCT03331601) | PET | [68,82] |
| 2 | 111In | 111Cd(p,n)111In | 2.83 d | EC (100) | 245 | maleimide-DTPA | 111In-DTPA-2Rs15d | Preclinical | SPECT | [88] |
| 3 | CHX-A″-DTPA | 111In-DTPA-2Rs15d | [86] | |||||||
| 4 | p-SCN-Bn-CHX-A″-DTPA | 111In-DTPA-2Rs15d | [87] | |||||||
| 5 | p-SCN-Bn-DOTA | 111In-DOTA-2Rs15d | [69] | |||||||
| 6 | 117Lu | 176Lu(n,g)177Lu | 6.71 d | β− (100) | 500 | CHX-A″–DTPA | 177Lu-DTPA-2Rs15d | Preclinical | TRNT | [85] |
| 7 | 1B4M–DTPA | 177Lu-DTPA-2Rs15d | ||||||||
| 8 | p-NCS–Bn–DOTA | 177Lu-DOTA-2Rs15d | ||||||||
| 9 | DOTA–NHS–ester | 177Lu-DOTA-2Rs15d | ||||||||
| 10 | 1B4M-DTPA | 177Lu-DTPA-2Rs15d | Preclinical | [86] | ||||||
| 11 | 225Ac | 226Ra(p,2n)225Ac | 10 d | α (100) | 8000 | p-SCN-Bn-DOTA | 225Ac-DOTA-2Rs15d | Preclinical | TRNT | [87] |
| 12 | p-SCN-Bn-CHX-A″-DTPA | 225Ac-DTPA-2Rs15d | Preclinical | [69] |
5. Preclinical to Clinical Studies: Radiolabeled Nbs as Potential Theranostic Agents
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Theriault, R.L.; Carlson, R.W.; Allred, C.; Anderson, B.O.; Burstein, H.J.; Edge, S.B.; Farrar, W.B.; Forero, A.; Giordano, S.H.; Goldstein, L.J. Breast cancer, version 3.2013. J. Natl. Compr. Cancer Netw. 2013, 11, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Buono, G.; Cardalesi, C.; Desideri, I.; De Placido, S.; Del Mastro, L. Hormone receptor/human epidermal growth factor receptor 2-positive breast cancer: Where we are now and where we are going. Cancer Treat. Rev. 2016, 46, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santa-Maria, C.A.; Nye, L.; Mutonga, M.B.; Jain, S.; Gradishar, W.J. Management of metastatic HER2-positive breast cancer: Where are we and where do we go from here? Oncology 2016, 30, 148–155. [Google Scholar]
- Nitta, H.; Kelly, B.D.; Allred, C.; Jewell, S.; Banks, P.; Dennis, E.; Grogan, T.M. The assessment of HER2 status in breast cancer: The past, the present, and the future. Pathol. Int. 2016, 66, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Dendukuri, N.; Khetani, K.; McIsaac, M.; Brophy, J. Testing for HER2-positive breast cancer: A systematic review and cost-effectiveness analysis. Cmaj 2007, 176, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- Sapino, A.; Goia, M.; Recupero, D.; Marchiò, C. Current challenges for HER2 testing in diagnostic pathology: State of the art and controversial issues. Front. Oncol. 2013, 3, 129. [Google Scholar] [CrossRef] [Green Version]
- Niikura, N.; Liu, J.; Hayashi, N.; Mittendorf, E.A.; Gong, Y.; Palla, S.L.; Tokuda, Y.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Ueno, N.T. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J. Clin. Oncol. 2012, 30, 593. [Google Scholar] [CrossRef] [Green Version]
- Fabi, A.; Di Benedetto, A.; Metro, G.; Perracchio, L.; Nisticò, C.; Di Filippo, F.; Ercolani, C.; Ferretti, G.; Melucci, E.; Buglioni, S. HER2 protein and gene variation between primary and metastatic breast cancer: Significance and impact on patient care. Clin. Cancer Res. 2011, 17, 2055–2064. [Google Scholar] [CrossRef] [Green Version]
- Santinelli, A.; Pisa, E.; Stramazzotti, D.; Fabris, G. HER-2 status discrepancy between primary breast cancer and metastatic sites. Impact on target therapy. Int. J. Cancer 2008, 122, 999–1004. [Google Scholar] [CrossRef]
- Rack, B.; Zombirt, E.; Trapp, E.; Jückstock, J.; Andergassen, U.; Neugebauer, J.; Kost, B.; Weissenbacher, T.; Jeschke, U.; Schindlbeck, C. Comparison of HER2 expression in primary tumor and disseminated tumor cells in the bone marrow of breast cancer patients. Oncology 2016, 90, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Massicano, A.V.; Marquez-Nostra, B.V.; Lapi, S.E. Targeting HER2 in nuclear medicine for imaging and therapy. Mol. Imaging 2018, 17, 1536012117745386. [Google Scholar] [CrossRef]
- Henry, K.E.; Ulaner, G.A.; Lewis, J.S. Human epidermal growth factor receptor 2-targeted PET/single-photon emission computed tomography imaging of breast cancer: Noninvasive measurement of a biomarker integral to tumor treatment and prognosis. PET Clin. 2017, 12, 269–288. [Google Scholar] [CrossRef] [Green Version]
- Capala, J.; Bouchelouche, K. Molecular imaging of HER2-positive breast cancer-a step toward an individualized “Image and Treat” strategy. Curr. Opin. Oncol. 2010, 22, 559. [Google Scholar] [CrossRef] [Green Version]
- Mendes, D.; Alves, C.; Afonso, N.; Cardoso, F.; Passos-Coelho, J.L.; Costa, L.; Andrade, S.; Batel-Marques, F. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer–a systematic review. Breast Cancer Res. 2015, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Akbari, V.; Chou, C.P.; Abedi, D. New insights into affinity proteins for HER2-targeted therapy: Beyond trastuzumab. Biochim. Biophys. Acta BBA Rev. Cancer 2020, 188448. [Google Scholar] [CrossRef]
- Muyldermans, S.; Baral, T.; Retamozzo, V.C.; De Baetselier, P.; De Genst, E.; Kinne, J.; Leonhardt, H.; Magez, S.; Nguyen, V.; Revets, H. Camelid immunoglobulins and nanobody technology. Vet. Immunol. Immunopathol. 2009, 128, 178–183. [Google Scholar] [CrossRef] [Green Version]
- D’Huyvetter, M.; Xavier, C.; Caveliers, V.; Lahoutte, T.; Muyldermans, S.; Devoogdt, N. Radiolabeled nanobodies as theranostic tools in targeted radionuclide therapy of cancer. Expert Opin. Drug Deliv. 2014, 11, 1939–1954. [Google Scholar] [CrossRef]
- Gopalan, D.; Bomanji, J.B.; Costa, D.C.; Ell, P.J. Nuclear medicine in primary breast cancer imaging. Clin. Radiol 2002, 57, 565–574. [Google Scholar] [CrossRef]
- Zimmermann, R. Nuclear Medicine Radioactivity for Diagnosis and Therapy, 2nd ed.; EDP Sciences: Les Ulis, France, 2017; pp. 13–24. [Google Scholar]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.; Haberkorn, U.; Mier, W. Cancer stratification by molecular imaging. Int. J. Mol. Sci. 2015, 16, 4918–4946. [Google Scholar] [CrossRef]
- Miller, P.W.; Long, N.J.; Vilar, R.; Gee, A.D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew Chem. Int. Ed. Engl. 2008, 47, 8998–9033. [Google Scholar] [CrossRef]
- Cassidy, P.J.; Radda, G.K. Molecular imaging perspectives. J. R. Soc. Interface 2005, 2, 133–144. [Google Scholar] [CrossRef] [Green Version]
- van Dongen, G.A.; Visser, G.W.; Lub-de Hooge, M.N.; de Vries, E.G.; Perk, L.R. Immuno-PET: A navigator in monoclonal antibody development and applications. Oncologist 2007, 12, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef]
- Kaur, S.; Venktaraman, G.; Jain, M.; Senapati, S.; Garg, P.K.; Batra, S.K. Recent trends in antibody-based oncologic imaging. Cancer Lett. 2012, 315, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Gomes, C.M.; Abrunhosa, A.J.; Ramos, P.; Pauwels, E.K. Molecular imaging with SPECT as a tool for drug development. Adv. Drug Deliv. Rev. 2011, 63, 547–554. [Google Scholar] [CrossRef]
- Kalles, V.; Zografos, G.C.; Provatopoulou, X.; Koulocheri, D.; Gounaris, A. The current status of positron emission mammography in breast cancer diagnosis. Breast Cancer 2013, 20, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Velikyan, I. Prospective of 68Ga-radiopharmaceutical development. Theranostics 2013, 4, 47–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ametamey, S.M.; Honer, M.; Schubiger, P.A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Rosenkrans, Z.T.; Liu, J.; Huang, G.; Luo, Q.Y.; Cai, W. ImmunoPET: Concept, Design, and Applications. Chem. Rev. 2020, 120, 3787–3851. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Hyman, D.M.; Ross, D.S.; Corben, A.; Chandarlapaty, S.; Goldfarb, S.; McArthur, H.; Erinjeri, J.P.; Solomon, S.B.; Kolb, H.; et al. Detection of HER2-Positive Metastases in Patients with HER2-Negative Primary Breast Cancer Using 89Zr-Trastuzumab PET/CT. J. Nucl. Med. 2016, 57, 1523–1528. [Google Scholar] [CrossRef] [Green Version]
- Laforest, R.; Lapi, S.E.; Oyama, R.; Bose, R.; Tabchy, A.; Marquez-Nostra, B.V.; Burkemper, J.; Wright, B.D.; Frye, J.; Frye, S.; et al. [(89)Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Mol. Imaging Biol. 2016, 18, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.K.; Jang, S.J.; Seo, M.J.; Park, J.H.; Kim, B.S.; Kim, E.J.; Lee, Y.J.; Lee, T.S.; An, G.I.; Song, I.H.; et al. Development of (64)Cu-NOTA-Trastuzumab for HER2 Targeting: A Radiopharmaceutical with Improved Pharmacokinetics for Human Studies. J. Nucl. Med. 2019, 60, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Lim, I.; Byun, B.H.; Kim, B.I.; Choi, C.W.; Woo, S.-K.; Kim, K.I.; Lee, K.C.; Kang, J.H.; Seong, M.-K.; et al. A preliminary clinical trial to evaluate 64Cu-NOTA-Trastuzumab as a positron emission tomography imaging agent in patients with breast cancer. EJNMMI Res. 2021, 11, 8. [Google Scholar] [CrossRef]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Altunay, B.; Morgenroth, A.; Beheshti, M.; Vogg, A.; Wong, N.C.L.; Ting, H.H.; Biersack, H.J.; Stickeler, E.; Mottaghy, F.M. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1371–1389. [Google Scholar] [CrossRef]
- Bhusari, P.; Vatsa, R.; Singh, G.; Parmar, M.; Bal, A.; Dhawan, D.K.; Mittal, B.R.; Shukla, J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int. J. Cancer 2017, 140, 938–947. [Google Scholar] [CrossRef]
- Dijkers, E.; Oude Munnink, T.; Kosterink, J.; Brouwers, A.; Jager, P.; De Jong, J.; Van Dongen, G.; Schröder, C.; Lub-de Hooge, M.; De Vries, E. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Even, A.J.; Hamming-Vrieze, O.; van Elmpt, W.; Winnepenninckx, V.J.; Heukelom, J.; Tesselaar, M.E.; Vogel, W.V.; Hoeben, A.; Zegers, C.M.; Vugts, D.J. Quantitative assessment of Zirconium-89 labeled cetuximab using PET/CT imaging in patients with advanced head and neck cancer: A theragnostic approach. Oncotarget 2017, 8, 3870. [Google Scholar] [CrossRef] [Green Version]
- Gaykema, S.B.; Brouwers, A.H.; Lub-de Hooge, M.N.; Pleijhuis, R.G.; Timmer-Bosscha, H.; Pot, L.; van Dam, G.M.; van der Meulen, S.B.; de Jong, J.R.; Bart, J. 89Zr-bevacizumab PET imaging in primary breast cancer. J. Nucl. Med. 2013, 54, 1014–1018. [Google Scholar] [CrossRef] [Green Version]
- Dammes, N.; Peer, D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 2020, 10, 938. [Google Scholar] [CrossRef]
- Xenaki, K.T.; Oliveira, S.; van Bergen En Henegouwen, P.M. Antibody or antibody fragments: Implications for molecular imaging and targeted therapy of solid tumors. Front. Immunol. 2017, 8, 1287. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Puttemans, J.; Lahoutte, T.; D’Huyvetter, M.; Devoogdt, N. Beyond the Barrier: Targeted Radionuclide Therapy in Brain Tumors and Metastases. Pharmaceutics 2019, 11, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garousi, J.; Orlova, A.; Frejd, F.Y.; Tolmachev, V. Imaging using radiolabelled targeted proteins: Radioimmunodetection and beyond. EJNMMI Radiopharm. Chem. 2020, 5, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, S.; Orlova, A.; Wållberg, H.; Hansson, M.; Sandström, M.; Lewsley, R.; Wennborg, A.; Abrahmsén, L.; Tolmachev, V.; Feldwisch, J. Targeting of HER2-expressing tumors using 111In-ABY-025, a second-generation affibody molecule with a fundamentally reengineered scaffold. J. Nucl. Med. 2010, 51, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [Green Version]
- Tsai, W.T.K.; Wu, A.M. Aligning physics and physiology: Engineering antibodies for radionuclide delivery. J. Label. Compd. Radiopharm. 2018, 61, 693–714. [Google Scholar] [CrossRef]
- Olafsen, T.; Wu, A.M. Antibody vectors for imaging. Semin. Nucl. Med. 2010, 40, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Löfblom, J.; Feldwisch, J.; Tolmachev, V.; Carlsson, J.; Ståhl, S.; Frejd, F.Y. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010, 584, 2670–2680. [Google Scholar] [CrossRef] [Green Version]
- Orlova, A.; Magnusson, M.; Eriksson, T.L.; Nilsson, M.; Larsson, B.; Höidén-Guthenberg, I.; Widström, C.; Carlsson, J.; Tolmachev, V.; Ståhl, S. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006, 66, 4339–4348. [Google Scholar] [CrossRef] [Green Version]
- Kramer-Marek, G.; Shenoy, N.; Seidel, J.; Griffiths, G.; Choyke, P.; Capala, J. 68Ga-Radiolabeled DOTA-affibody molecule for in vivo assessment of HER2 expression with PET. Cancer Res. 2011, 71. [Google Scholar] [CrossRef]
- Qi, S.; Hoppmann, S.; Xu, Y.; Cheng, Z. PET imaging of HER2-positive tumors with Cu-64-labeled affibody molecules. Mol. Imaging Biol. 2019, 21, 907–916. [Google Scholar] [CrossRef]
- Baum, R.P.; Prasad, V.; Müller, D.; Schuchardt, C.; Orlova, A.; Wennborg, A.; Tolmachev, V.; Feldwisch, J. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In-or 68Ga-labeled affibody molecules. J. Nucl. Med. 2010, 51, 892–897. [Google Scholar] [CrossRef] [Green Version]
- Sandström, M.; Lindskog, K.; Velikyan, I.; Wennborg, A.; Feldwisch, J.; Sandberg, D.; Tolmachev, V.; Orlova, A.; Sörensen, J.; Carlsson, J. Biodistribution and radiation dosimetry of the anti-HER2 affibody molecule 68Ga-ABY-025 in breast cancer patients. J. Nucl. Med. 2016, 57, 867–871. [Google Scholar] [CrossRef] [Green Version]
- Fu, R.; Carroll, L.; Yahioglu, G.; Aboagye, E.O.; Miller, P.W. Antibody fragment and affibody immunoPET imaging agents: Radiolabelling strategies and applications. Chem. Med. Chem. 2018, 13, 2466–2478. [Google Scholar] [CrossRef]
- Tolmachev, V.; Orlova, A. Affibody molecules as targeting vectors for PET imaging. Cancers 2020, 12, 651. [Google Scholar] [CrossRef] [Green Version]
- Steeland, S.; Vandenbroucke, R.E.; Libert, C. Nanobodies as therapeutics: Big opportunities for small antibodies. Drug Discov. Today 2016, 21, 1076–1113. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen en Henegouwen, P.M. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 10, 161–174. [Google Scholar] [CrossRef]
- Kolkman, J.A.; Law, D.A. Nanobodies–from llamas to therapeutic proteins. Drug Discov. Today: Technol. 2010, 7, e139–e146. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, C.; Muyldermans, S. Nanobody-based delivery systems for diagnosis and targeted tumor therapy. Front. Immunol. 2017, 8, 1442. [Google Scholar] [CrossRef] [PubMed]
- Debie, P.; Lafont, C.; Defrise, M.; Hansen, I.; van Willigen, D.M.; van Leeuwen, F.W.; Gijsbers, R.; D’huyvetter, M.; Devoogdt, N.; Lahoutte, T. Size and affinity kinetics of nanobodies influence targeting and penetration of solid tumours. J. Control. Release 2020, 317, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ewert, S.; Huber, T.; Honegger, A.; Plückthun, A. Biophysical properties of human antibody variable domains. J. Mol. Biol. 2003, 325, 531–553. [Google Scholar] [CrossRef]
- Dumoulin, M.; Conrath, K.; Van Meirhaeghe, A.; Meersman, F.; Heremans, K.; Frenken, L.G.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002, 11, 500–515. [Google Scholar] [CrossRef]
- De Vos, J.; Devoogdt, N.; Lahoutte, T.; Muyldermans, S. Camelid single-domain antibody-fragment engineering for (pre) clinical in vivo molecular imaging applications: Adjusting the bullet to its target. Expert Opin. Biol. Ther. 2013, 13, 1149–1160. [Google Scholar] [CrossRef]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Puttemans, J.; Dekempeneer, Y.; Eersels, J.L.; Hanssens, H.; Debie, P.; Keyaerts, M.; Windhorst, A.D.; van der Aa, F.; Lecocq, Q.; Breckpot, K. Preclinical Targeted α-and β−-Radionuclide Therapy in HER2-Positive Brain Metastasis Using Camelid Single-Domain Antibodies. Cancers 2020, 12, 1017. [Google Scholar] [CrossRef] [Green Version]
- Jovčevska, I.; Muyldermans, S. The therapeutic potential of nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, G.; Kühn, H.; Sauter, M.; Haberkorn, U.; Mier, W. Radiolabeling strategies for tumor-targeting proteinaceous drugs. Molecules 2014, 19, 2135–2165. [Google Scholar] [CrossRef]
- Massa, S.; Xavier, C.; Muyldermans, S.; Devoogdt, N. Emerging site-specific bioconjugation strategies for radioimmunotracer development. Expert Opin. Drug Deliv. 2016, 13, 1149–1163. [Google Scholar] [CrossRef]
- Schumacher, D.; Helma, J.; Schneider, A.F.L.; Leonhardt, H.; Hackenberger, C.P.R. Nanobodies: Chemical Functionalization Strategies and Intracellular Applications. Angew Chem. Int. Ed. Engl. 2018, 57, 2314–2333. [Google Scholar] [CrossRef]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N.; Lahoutte, T.; Zalutsky, M.R. Targeting breast carcinoma with radioiodinated anti-HER2 Nanobody. Nucl. Med. Biol. 2013, 40, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Pruszynski, M.; Koumarianou, E.; Vaidyanathan, G.; Revets, H.; Devoogdt, N.; Lahoutte, T.; Lyerly, H.K.; Zalutsky, M.R. Improved tumor targeting of anti-HER2 nanobody through N-succinimidyl 4-guanidinomethyl-3-iodobenzoate radiolabeling. J. Nucl. Med. 2014, 55, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Vaidyanathan, G.; McDougald, D.; Choi, J.; Koumarianou, E.; Weitzel, D.; Osada, T.; Lyerly, H.K.; Zalutsky, M.R. Preclinical Evaluation of 18F-Labeled Anti-HER2 Nanobody Conjugates for Imaging HER2 Receptor Expression by Immuno-PET. J. Nucl. Med. 2016, 57, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Vaidyanathan, G.; Koumarianou, E.; Kang, C.M.; Zalutsky, M.R. Astatine-211 labeled anti-HER2 5F7 single domain antibody fragment conjugates: Radiolabeling and preliminary evaluation. Nucl. Med. Biol. 2018, 56, 10–20. [Google Scholar] [CrossRef] [Green Version]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. (131)I-labeled Anti-HER2 Camelid sdAb as a Theranostic Tool in Cancer Treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Zhou, Z.; McDougald, D.; Meshaw, R.L.; Vaidyanathan, G.; Zalutsky, M.R. Site-specific radioiodination of an anti-HER2 single domain antibody fragment with a residualizing prosthetic agent. Nucl. Med. Biol. 2021, 92, 171–183. [Google Scholar] [CrossRef]
- Xavier, C.; Blykers, A.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Lahoutte, T.; Devoogdt, N.; Caveliers, V. 18F-nanobody for PET imaging of HER2 overexpressing tumors. Nucl. Med. Biol. 2016, 43, 247–252. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; McDougald, D.; Choi, J.; Pruszynski, M.; Koumarianou, E.; Zhou, Z.; Zalutsky, M.R. N-Succinimidyl 3-((4-(4-[18F]fluorobutyl)-1H-1,2,3-triazol-1-yl)methyl)-5-(guanidinomethyl)benzoate ([18F]SFBTMGMB): A residualizing label for 18F-labeling of internalizing biomolecules. Org. Biomol. Chem. 2016, 14, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Xavier, C.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Keyaerts, M.; Vincke, C.; Devoogdt, N.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J. Nucl. Med. 2013, 54, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Vaneycken, I.; Devoogdt, N.; Van Gassen, N.; Vincke, C.; Xavier, C.; Wernery, U.; Muyldermans, S.; Lahoutte, T.; Caveliers, V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. Faseb J. 2011, 25, 2433–2446. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Liu, C.; Xing, Y.; He, J.; O’Doherty, J.; Huang, W.; Zhao, J. Development of a (99m)Tc-Labeled Single-Domain Antibody for SPECT/CT Assessment of HER2 Expression in Breast Cancer. Mol. Pharm. 2021, 18, 3616–3622. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; Aerts, A.; Xavier, C.; Vaneycken, I.; Devoogdt, N.; Gijs, M.; Impens, N.; Baatout, S.; Ponsard, B.; Muyldermans, S.; et al. Development of 177Lu-nanobodies for radioimmunotherapy of HER2-positive breast cancer: Evaluation of different bifunctional chelators. Contrast Media Mol. Imaging 2012, 7, 254–264. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pruszynski, M.; D’Huyvetter, M.; Bruchertseifer, F.; Morgenstern, A.; Lahoutte, T. Evaluation of an Anti-HER2 Nanobody Labeled with (225)Ac for Targeted α-Particle Therapy of Cancer. Mol. Pharm. 2018, 15, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Massa, S.; Xavier, C.; De Vos, J.; Caveliers, V.; Lahoutte, T.; Muyldermans, S.; Devoogdt, N. Site-specific labeling of cysteine-tagged camelid single-domain antibody-fragments for use in molecular imaging. Bioconjug. Chem. 2014, 25, 979–988. [Google Scholar] [CrossRef]
- Clark, J.; O’Hagan, D. Strategies for radiolabelling antibody, antibody fragments and affibodies with fluorine-18 as tracers for positron emission tomography (PET). J. Fluor. Chem. 2017, 203, 31–46. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Vaidyanathan, G.; McDougald, D.; Kang, C.M.; Balyasnikova, I.; Devoogdt, N.; Ta, A.N.; McNaughton, B.R.; Zalutsky, M.R. Fluorine-18 Labeling of the HER2-Targeting Single-Domain Antibody 2Rs15d Using a Residualizing Label and Preclinical Evaluation. Mol. Imaging Biol. 2017, 19, 867–877. [Google Scholar] [CrossRef]
- Zhou, Z.; Chitneni, S.K.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. Fluorine-18 labeling of an anti-HER2 VHH using a residualizing prosthetic group via a strain-promoted click reaction: Chemistry and preliminary evaluation. Bioorg. Med. Chem. 2018, 26, 1939–1949. [Google Scholar] [CrossRef]
- Zhou, Z.; McDougald, D.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. Labeling Single Domain Antibody Fragments with Fluorine-18 Using 2,3,5,6-Tetrafluorophenyl 6-[(18)F]Fluoronicotinate Resulting in High Tumor-to-Kidney Ratios. Mol. Pharm. 2019, 16, 214–226. [Google Scholar] [CrossRef]
- Morais, M.; Ma, M.T. Site-specific chelator-antibody conjugation for PET and SPECT imaging with radiometals. Drug Discov. Today Technol. 2018, 30, 91–104. [Google Scholar] [CrossRef]
- Peltek, O.O.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Current outlook on radionuclide delivery systems: From design consideration to translation into clinics. J. Nanobiotechnol. 2019, 17, 90. [Google Scholar] [CrossRef] [Green Version]
- Van Audenhove, I.; Gettemans, J. Nanobodies as Versatile Tools to Understand, Diagnose, Visualize and Treat Cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Keyaerts, M.; Xavier, C.; Everaert, H.; Vaneycken, I.; Fontaine, C.; Decoster, L.; Vanhoeij, M.; Caveliers, V.; Lahoutte, T. Phase II trial of HER2-PET/CT using 68Ga-anti-HER2 VHH1 for characterization of HER2 presence in brain metastases of breast cancer patients. Ann. Oncol. 2019, 30, iii25–iii26. [Google Scholar] [CrossRef]
- Huyvetter, M.; De Vos, J.; Caveliers, V.; Vaneycken, I.; Heemskerk, J.; Duhoux, F.P.; Fontaine, C.; Vanhoeij, M.; Windhorst, A.D.; van der Aa, F.; et al. Phase I trial of 131I-GMIB-Anti-HER2-VHH1, a new promising candidate for HER2-targeted radionuclide therapy in breast cancer patients. J. Nucl. Med. 2020, 62, 1097–1105. [Google Scholar] [CrossRef]
| Molecular Weight (kDa) | Avidity | Clearance Route | Serum Half-Life | Optimal Time for Imaging | Ref. | |
|---|---|---|---|---|---|---|
| IgG | 150 | Bivalent | Liver | 110 h | 4–7 d | [41,47] |
| F(ab)’2 | 110 | Bivalent | Liver/Kidney | 9 h | 72–120 h | [41] |
| Minibody | 80 | Bivalent | Liver | 5–12 h | 24–48 h | [47] |
| Diabody | 50 | Bivalent | Kidney | 2–5 h | 12–24 h | [47] |
| Fab | 55 | Monovalent | Kidney | 28 min | 2–5 h | [41] |
| scFv | 25 | Monovalent | Kidney | 10 min | 1–3 h | [41] |
| Nb | 15 | Monovalent | Kidney | 1–1.5 h | 1–1.5 h | [41] |
| Affibody | 6–7 | Not applicable | Kidney | 4–14 min | 1–2 h | [41,47,48,49] |
| Entry | Radiolabeling Method | Nb | Isotope | Intermediate | Radiolabeling Conditions | RCY (%) | RCP (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Direct | 5F7GGC | 125I | Directly | r.t., 10 min | 83.6 ± 5.0 | >99 | [74] |
| 2 | Directly | r.t., 10 min | 86.2 ± 1.6 | >98 | [75] | |||
| 3 | Indirect via prosthetic group | 5F7 | [*I]SGMIB | - | - | - | [76] | |
| 4 | 5F7GGC | 131I | [*I]IB-Mal-D-GEEEK | r.t., 45 min | 91.2 ± 4.4 | >99 | [74] | |
| 5 | 5F7GGC | [*I]IB-Mal-D-GEEEK | r.t., 45 min | 69.6 ± 5.6 | >98 | [75] | ||
| 6 | 5F7GGC | [*I]SGMIB | r.t., 10 min | 50.4 ± 3.6 | >98 | |||
| 7 | 5F7 | [*I]SGMIB | - | 28.9 ± 13.0 | 98.4 | [77] | ||
| 8 | iso-[*I]SGMIB | - | 33.1 ± 7.1 | 98.6 | ||||
| 9 | 2Rs15d | [*I]SGMIB | 20 °C, 30 min | 36.5 ± 12.8 | >97 | [78] | ||
| 10 | 5F7GGC | [*I]MEGMIB | 37 °C, 45 min | 45 ± 7 | >99 | [79] | ||
| 11 | 5F7 | 211At | SAGMB | 20 °C, 20 min | 38.4 ± 15.6 | 97.8 | [77] | |
| 12 | iso-SAGMB | 20 °C, 20 min | 39.5 ± 6.8 | 98.9 | ||||
| 13 | 2Rs15d | 18F | [18F]SFB | r.t., 20 min | 5–15 | >97 | [80] | |
| 14 | 5F7 | 18F-RL-1 | 20 °C, 20 min | - | >95 | [81] | ||
| 15 | 5F7 | 18F-RL-1 | 20 °C, 20 min | - | 95 | [76] | ||
| 16 | [18F]SFB | - | - | 95 | ||||
| 17 | Indirect via complexation | 2Rs15d | 68Ga | p-SCN-Bn-NOTA | r.t., 5 min | >97 | 99 | [82] |
| 18 | 2Rs15d-His6 | r.t., 5 min | >97 | 99 | ||||
| 19 | 2Rs15d | 99mTc | His tag | 50 °C, 90 min | - | >99 | [83] | |
| 20 | NM-02 | His tag | 50 °C, 60 min | - | 97.7 ± 1.2 | [84] | ||
| 21 | 2Rs15d-His | 177Lu | CHX-A”-DTPA | r.t., 30 min | - | 91 ± 1.06 | [85] | |
| 22 | 2Rs15d-His | 1B4M-DTPA | r.t., 30 min | - | 94 ± 2.21 | |||
| 23 | 2Rs15d-His | p-NCS-Bn-DOTA | 50 °C, 45 min | - | 96 ± 2.10 | |||
| 24 | 2Rs15d-His | DOTA-NHS-ester | 50 °C, 45 min | - | 96 ± 1.97 | |||
| 25 | 2Rs15d | 1B4M-DTPA | r.t., 30 min | 97.2 ± 2.5 | >99 | [86] | ||
| 26 | 2Rs15d | 225Ac | p-SCN-Bn-DOTA | 50 °C, 90 min | >90 | >95 | [87] | |
| 27 | 2Rs15d | p-SCN-Bn-DOTA | 55 °C, 90 min | - | 86.8 ± 2.1 | [69] | ||
| 28 | 2Rs15d | 111In | p-SCN-Bn-CHX-A”-DTPA | 55 °C, 30 min | - | 91.3 ± 2.1 | ||
| 29 | 2Rs15d | Malemide-DTPA | 50 °C, 30 min | - | 94.0 ± 4.9 | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrynchak, I.; Santos, L.; Falcão, A.; Gomes, C.M.; Abrunhosa, A.J. Nanobody-Based Theranostic Agents for HER2-Positive Breast Cancer: Radiolabeling Strategies. Int. J. Mol. Sci. 2021, 22, 10745. https://doi.org/10.3390/ijms221910745
Hrynchak I, Santos L, Falcão A, Gomes CM, Abrunhosa AJ. Nanobody-Based Theranostic Agents for HER2-Positive Breast Cancer: Radiolabeling Strategies. International Journal of Molecular Sciences. 2021; 22(19):10745. https://doi.org/10.3390/ijms221910745
Chicago/Turabian StyleHrynchak, Ivanna, Liliana Santos, Amílcar Falcão, Célia M. Gomes, and Antero J. Abrunhosa. 2021. "Nanobody-Based Theranostic Agents for HER2-Positive Breast Cancer: Radiolabeling Strategies" International Journal of Molecular Sciences 22, no. 19: 10745. https://doi.org/10.3390/ijms221910745
APA StyleHrynchak, I., Santos, L., Falcão, A., Gomes, C. M., & Abrunhosa, A. J. (2021). Nanobody-Based Theranostic Agents for HER2-Positive Breast Cancer: Radiolabeling Strategies. International Journal of Molecular Sciences, 22(19), 10745. https://doi.org/10.3390/ijms221910745








