Detecting Diabetic Retinal Neuropathy Using Fundus Perimetry
Abstract
:1. Rationale of Fundus Perimetry and Macular Disease
1.1. Visual Field and Retinal Sensitivity
1.2. What Is the Difference between Fundus Perimetry and Visual Acuity?
1.3. Why Examine Retinal Sensitivity?
1.4. What Is the Difference between Fundus Perimetry and a Conventional Visual Field Test?
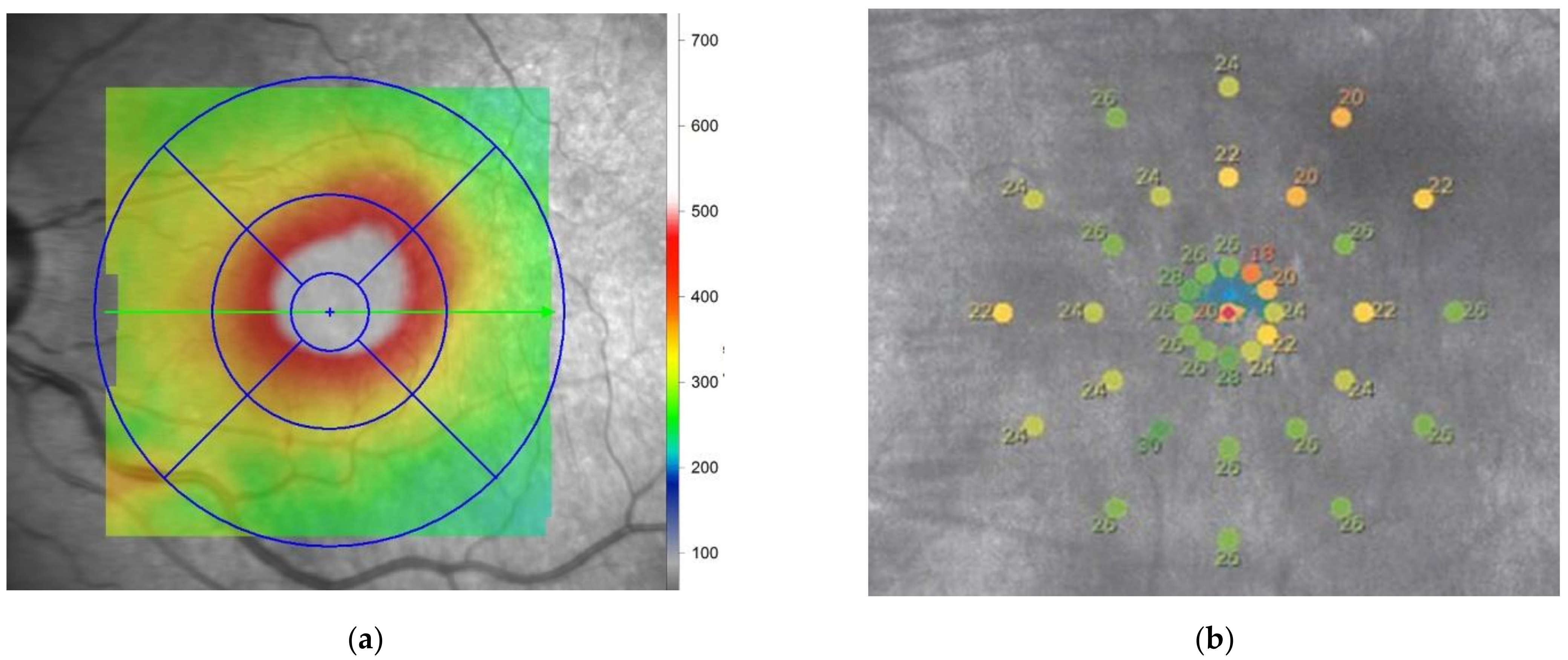
1.5. Measurement Principle of Fundus Perimetry
1.6. Relationship between the Measurement of Retinal Sensitivity and SLO
1.7. Types of Fundus Perimetry
1.8. Difference between Dark and Bright Fields
1.9. Retinal Sensitivity in Normal and Diseased Eyes
2. Role of Fundus Perimetry to Detect Early Functional Loss in Diabetic Retinopathy
2.1. Diabetic Retinopathy
2.2. Functional Macular Changes before any Morphological Changes Occur in Patients with Diabetes
2.3. Evaluation of Significant Diabetic Retinopathy
3. Importance of the Functional Evaluation of Retinal Sensitivity in Clinical Trials for Diabetic Retinopathy
4. Conclusions: The Importance of Fundus Perimetry in the Evaluation of Diabetic Retinopathy
Author Contributions
Funding
Conflicts of Interest
References
- Spector, R.H. Visual Fields. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Gramer, E.; Proll, M.; Krieglstein, G.K. The perimetry of the blind spot. A comparison of kinetic and static, computerized stratgies. Ophthalmologica 1979, 179, 201–208. [Google Scholar] [CrossRef]
- Wild, J.M. Techniques and developments in automated perimetry: A review. Ophthalmic Physiol. Opt. 1988, 8, 295–308. [Google Scholar] [CrossRef]
- Portney, G.L.; Krohn, M.A. Automated perimetry: Background, instruments and methods. Surv. Ophthalmol. 1978, 22, 271–278. [Google Scholar] [CrossRef]
- Schneider, U.; Kuck, H.; Inhoffen, W.; Kreissig, I. Fundus-controlled microperimetry with the scanning laser ophthalmoscope in macular diseases. Klin. Monbl. Augenheilkd. 1993, 203, 212–218. [Google Scholar] [CrossRef]
- Schneider, U.; Kuck, H.; Kreissig, I. Fixation and central visual field after perifoveal krypton laser treatment of subfoveal neovascularizations. Eur. J. Ophthalmol. 1993, 3, 193–200. [Google Scholar] [CrossRef]
- Shpak, A.A.; Kachalina, G.F.; Pedanova, E.K. Comparative analysis of the results of microperimetry and conventional computed perimetry in health. Vestn. Oftalmol. 2009, 125, 31–34. [Google Scholar]
- Springer, C.; Bultmann, S.; Volcker, H.E.; Rohrschneider, K. Fundus perimetry with the Micro Perimeter 1 in normal individuals: Comparison with conventional threshold perimetry. Ophthalmology 2005, 112, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Radin, P.P.; Convento, E.; Cavarzeran, F. Macular automatic fundus perimetry threshold versus standard perimetry threshold. Eur. J. Ophthalmol. 2007, 17, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Tuten, W.S.; Tiruveedhula, P.; Roorda, A. Adaptive Optics Scanning Laser Ophthalmoscope-Based Microperimetry. Optom. Vis. Sci. 2012, 89, 563–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrschneider, K.; Springer, C.; Bultmann, S.; Volcker, H.E. Microperimetry--comparison between the micro perimeter 1 and scanning laser ophthalmoscope--fundus perimetry. Am. J. Ophthalmol. 2005, 139, 125–134. [Google Scholar] [CrossRef]
- Sharp, P.F.; Manivannan, A. The scanning laser ophthalmoscope. Phys. Med. Biol. 1997, 42, 951–966. [Google Scholar] [CrossRef]
- Sharp, P.F.; Manivannan, A.; Xu, H.; Forrester, J.V. The scanning laser ophthalmoscope--a review of its role in bioscience and medicine. Phys. Med. Biol. 2004, 49, 1085–1096. [Google Scholar] [CrossRef]
- MacKeben, M.; Gofen, A. Gaze-contingent display for retinal function testing by scanning laser ophthalmoscope. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2007, 24, 1402–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Bittencourt, M.G.; Sophie, R.; Sepah, Y.J.; Hanout, M.; Rentiya, Z.; Annam, R.; Scholl, H.P.N.; Nguyen, Q.D. Fixation Stability Measurement Using Two Types of Microperimetry Devices. Transl. Vis. Sci. Technol. 2015, 4, 3. [Google Scholar] [CrossRef]
- Tepelus, T.C.; Hariri, A.H.; Al-Sheikh, M.; Sadda, S.R. Correlation Between Mesopic Retinal Sensitivity and Optical Coherence Tomographic Metrics of the Outer Retina in Patients with Non-Atrophic Dry Age-Related Macular Degeneration. Ophthalmic Surg. Lasers Imaging Retin. 2017, 48, 312–318. [Google Scholar] [CrossRef]
- Kakisu, M.; Baba, T.; Iwase, T.; Yokouchi, H.; Yamamoto, S. Relationship between retinal sensitivities and optical coherence tomographic findings in eyes with myopic chorioretinal atrophy. Eur. J. Ophthalmol. 2021, 11206721211008038. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.; Lindner, M.; Müller, P.L.; Birtel, J.; Finger, R.P.; Harmening, W.M.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Effective Dynamic Range and Retest Reliability of Dark-Adapted Two-Color Fundus-Controlled Perimetry in Patients with Macular Diseases. Investig. Opthalmology Vis. Sci. 2017, 58, BIO158. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.S.; Saßmannshausen, M.; Pfau, M.; Fleckenstein, M.; Finger, R.P.; Holz, F.G.; Schmitz-Valckenberg, S. Evaluation of Two Systems for Fundus-Controlled Scotopic and Mesopic Perimetry in Eye with Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2017, 6, 7. [Google Scholar] [CrossRef]
- Von Der Emde, L.; Pfau, M.; Thiele, S.; Möller, P.T.; Hassenrik, R.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Mesopic and Dark-Adapted Two-Color Fundus-Controlled Perimetry in Choroidal Neovascularization Secondary to Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2019, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfau, M.; Lindner, M.; Gliem, M.; Steinberg, J.S.; Thiele, S.; Finger, R.P.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Mesopic and dark-adapted two-color fundus-controlled perimetry in patients with cuticular, reticular, and soft drusen. Eye 2018, 32, 1819–1830. [Google Scholar] [CrossRef] [Green Version]
- Nizawa, T.; Baba, T.; Kitahashi, M.; Oshitari, T.; Yamamoto, S. Different fixation targets affect retinal sensitivity obtained by microperimetry in normal individuals. Clin. Ophthalmol. 2017, 11, 2011–2015. [Google Scholar] [CrossRef] [Green Version]
- Frank, R.N. Diabetic retinopathy: Current concepts of evaluation and treatment. Clin. Endocrinol. Metab. 1986, 15, 933–969. [Google Scholar] [CrossRef]
- Kohner, E.M.; Porta, M. Protocols for screening and treatment of diabetic retinopathy in Europe. Eur. J. Ophthalmol. 1991, 1, 45–54. [Google Scholar] [CrossRef]
- Sokol, S.; Moskowitz, A.; Skarf, B.; Evans, R.; Molitch, M.; Senior, B. Contrast sensitivity in diabetics with and without background retinopathy. Arch. Ophthalmol. 1985, 103, 51–54. [Google Scholar] [CrossRef]
- Di Leo, M.A.; Caputo, S.; Falsini, B.; Porciatti, V.; Minnella, A.; Greco, A.V.; Ghirlanda, G. Nonselective loss of contrast sensitivity in visual system testing in early type I diabetes. Diabetes Care 1992, 15, 620–625. [Google Scholar] [CrossRef]
- Bresnick, G.H.; Korth, K.; Groo, A.; Palta, M. Electroretinographic oscillatory potentials predict progression of diabetic retinopathy. Preliminary report. Arch. Ophthalmol. 1984, 102, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Kizawa, J.; Machida, S.; Kobayashi, T.; Gotoh, Y.; Kurosaka, D. Changes of Oscillatory Potentials and Photopic Negative Response in Patients with Early Diabetic Retinopathy. Jpn. J. Ophthalmol. 2006, 50, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.S.; Gunkel, R.D.; Podgor, M.J. Color vision defects in early diabetic retinopathy. Arch. Ophthalmol. 1986, 104, 225–228. [Google Scholar] [CrossRef]
- Li, T.; Jia, Y.; Wang, S.; Wang, A.; Gao, L.; Yang, C.; Zou, H. Retinal Microvascular Abnormalities in Children with Type 1 Diabetes Mellitus Without Visual Impairment or Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2019, 60, 990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, D.C.; Leal, I.; Moreira, S.; Do Vale, S.; Silva-Herdade, A.R.; Dionísio, P.; Castanho, M.A.R.B.; Abegão Pinto, L.; Marques-Neves, C. Optical coherence tomography angiography study of the retinal vascular plexuses in type 1 diabetes without retinopathy. Eye 2020, 34, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Nittala, M.G.; Gella, L.; Raman, R.; Sharma, T. Measuring retinal sensitivity with the microperimeter in patients with diabetes. Retina 2012, 32, 1302–1309. [Google Scholar] [CrossRef]
- Verma, A.; Rani, P.K.; Raman, R.; Pal, S.S.; Laxmi, G.; Gupta, M.; Sahu, C.; Vaitheeswaran, K.; Sharma, T. Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and spectral domain optical coherence tomography (SD-OCT) study in individuals with diabetes, but no diabetic retinopathy. Eye 2009, 23, 1824–1830. [Google Scholar] [CrossRef]
- Verma, A.; Raman, R.; Vaitheeswaran, K.; Pal, S.S.; Laxmi, G.; Gupta, M.; Shekar, S.C.; Sharma, T. Does neuronal damage precede vascular damage in subjects with type 2 diabetes mellitus and having no clinical diabetic retinopathy? Ophthalmic Res. 2012, 47, 202–207. [Google Scholar] [CrossRef]
- Montesano, G.; Gervasoni, A.; Ferri, P.; Allegrini, D.; Migliavacca, L.; De Cilla, S.; Rossetti, L. Structure-function relationship in early diabetic retinopathy: A spatial correlation analysis with OCT and microperimetry. Eye 2017, 31, 931–939. [Google Scholar] [CrossRef]
- Neriyanuri, S.; Pardhan, S.; Gella, L.; Pal, S.S.; Ganesan, S.; Sharma, T.; Raman, R. Retinal sensitivity changes associated with diabetic neuropathy in the absence of diabetic retinopathy. Br. J. Ophthalmol. 2017, 101, 1174–1178. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Davis, M.D.; Aiello, L.M. Treatment of diabetic retinopathy. N. Engl. J. Med. 1999, 341, 667–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haritoglou, C.; Kook, D.; Neubauer, A.; Wolf, A.; Priglinger, S.; Strauss, R.; Gandorfer, A.; Ulbig, M.; Kampik, A. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina 2006, 26, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Diabetic Retinopathy Clinical Research Network. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch. Ophthalmol. 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology 1987, 94, 761–774. [Google Scholar] [CrossRef]
- Montesano, G.; Ometto, G.; Higgins, B.E.; Das, R.; Graham, K.W.; Chakravarthy, U.; McGuiness, B.; Young, I.S.; Kee, F.; Wright, D.M.; et al. Evidence for Structural and Functional Damage of the Inner Retina in Diabetes with No Diabetic Retinopathy. Investig. Opthalmol. Vis. Sci. 2021, 62, 35. [Google Scholar] [CrossRef]
- Sacconi, R.; Casaluci, M.; Borrelli, E.; Mulinacci, G.; Lamanna, F.; Gelormini, F.; Carnevali, A.; Querques, L.; Zerbini, G.; Bandello, F.; et al. Multimodal Imaging Assessment of Vascular and Neurodegenerative Retinal Alterations in Type 1 Diabetic Patients without Fundoscopic Signs of Diabetic Retinopathy. J. Clin. Med. 2019, 8, 1409. [Google Scholar] [CrossRef] [Green Version]
- Dunbar, H.M.; Crossland, M.D.; Rubin, G.S. Fixation stability: A comparison between the Nidek MP-1 and the Rodenstock scanning laser ophthalmoscope in persons with and without diabetic maculopathy. Invest. Ophthalmol. Vis. Sci. 2010, 51, 4346–4350. [Google Scholar] [CrossRef] [Green Version]
- Vujosevic, S.; Pilotto, E.; Bottega, E.; Benetti, E.; Cavarzeran, F.; Midena, E. Retinal fixation impairment in diabetic macular edema. Retina 2008, 28, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Carpineto, P.; Ciancaglini, M.; Di Antonio, L.; Gavalas, C.; Mastropasqua, L. Fundus microperimetry patterns of fixation in type 2 diabetic patients with diffuse macular edema. Retina 2007, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Midena, E.; Pilotto, E.; Radin, P.P.; Chiesa, L.; Cavarzeran, F. Diabetic macular edema: Correlation between microperimetry and optical coherence tomography findings. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3044–3051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, K.; Yamamoto, S.; Mizunoya, S.; Hoshino, A.; Arai, M.; Takatsuna, Y. Correlation of retinal sensitivity measured with fundus-related microperimetry to visual acuity and retinal thickness in eyes with diabetic macular edema. Eye 2006, 20, 805–809. [Google Scholar] [CrossRef]
- Soliman, W.; Hasler, P.; Sander, B.; Larsen, M. Local retinal sensitivity in relation to specific retinopathy lesions in diabetic macular oedema. Acta Ophthalmol. 2012, 90, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Comyn, O.; Sivaprasad, S.; Peto, T.; Neveu, M.M.; Holder, G.E.; Xing, W.; Bunce, C.V.; Patel, P.J.; Egan, C.A.; Bainbridge, J.W.; et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). Am. J. Ophthalmol. 2014, 157, 960–970. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Arden, G.; Prevost, A.T.; Crosby-Nwaobi, R.; Holmes, H.; Kelly, J.; Murphy, C.; Rubin, G.; Vasconcelos, J.; Hykin, P. A multicentre phase III randomised controlled single-masked clinical trial evaluating the clinical efficacy and safety of light-masks at preventing dark-adaptation in the treatment of early diabetic macular oedema (CLEOPATRA): Study protocol for protocol for a randomised controlled trial. Trials 2014, 15, 458. [Google Scholar]
- Gonzalez, V.H.; Boyer, D.S.; Schmidt-Erfurth, U.; Heier, J.S.; Gordon, C.; Benz, M.S.; Marcus, D.M.; Sabates, N.R.; Vitti, R.; Kazmi, H.; et al. Microperimetric assessment of retinal sensitivity in eyes with diabetic macular edema from a phase 2 study of intravitreal aflibercept. Retina 2015, 35, 687–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhablani, J.; Alshareef, R.; Kim, D.T.; Narayanan, R.; Goud, A.; Mathai, A. Comparison of different settings for yellow subthreshold laser treatment in diabetic macular edema. BMC Ophthalmol. 2018, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| MP-1 | Maia | MP-3 | |
|---|---|---|---|
| Fundus image | Infrared | SLO | SLO |
| Measurement area | 40° circle | 20° × 20° | 40° circle |
| Stimulus size | Goldmann I–V | Goldmann III | Goldmann I–V |
| Smallest pupil size | 4 mm | 2.5 mm | 4 mm |
| Background luminance | 4 asb | 4 asb | 4 asb/31.4 asb |
| Display | LCD | LED | LED |
| Maximum stimuli | 400 asb | 1000 asb | 10000 asb |
| Dynamic range | 0–20 dB | 0–36 dB | 0–34 dB |
| Alignment | Manual | Manual | Auto |
| Eye tracking | 25 Hz | 25 Hz | 30 Hz |
| Fundus photo | Color | Grayscale | Color |
| Capture angle | 45° circle | 36° × 36° | 45° circle |
| Resolution of image | 1392 × 1024 pix | 1024 × 1024 pix | 4016 × 3008 pix |
| Focus range | Manual: −15 to +15D | Auto: −15D to +10D | Auto: −12D to +15D; Manual: −25D to +15D |
| Scotopic devices | MP–1S | S–Maia | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baba, T. Detecting Diabetic Retinal Neuropathy Using Fundus Perimetry. Int. J. Mol. Sci. 2021, 22, 10726. https://doi.org/10.3390/ijms221910726
Baba T. Detecting Diabetic Retinal Neuropathy Using Fundus Perimetry. International Journal of Molecular Sciences. 2021; 22(19):10726. https://doi.org/10.3390/ijms221910726
Chicago/Turabian StyleBaba, Takayuki. 2021. "Detecting Diabetic Retinal Neuropathy Using Fundus Perimetry" International Journal of Molecular Sciences 22, no. 19: 10726. https://doi.org/10.3390/ijms221910726
APA StyleBaba, T. (2021). Detecting Diabetic Retinal Neuropathy Using Fundus Perimetry. International Journal of Molecular Sciences, 22(19), 10726. https://doi.org/10.3390/ijms221910726






