Restoration of HDAC1 Enzymatic Activity after Stroke Protects Neurons from Ischemia/Reperfusion Damage and Attenuates Behavioral Deficits in Rats
Abstract
1. Introduction
2. Results
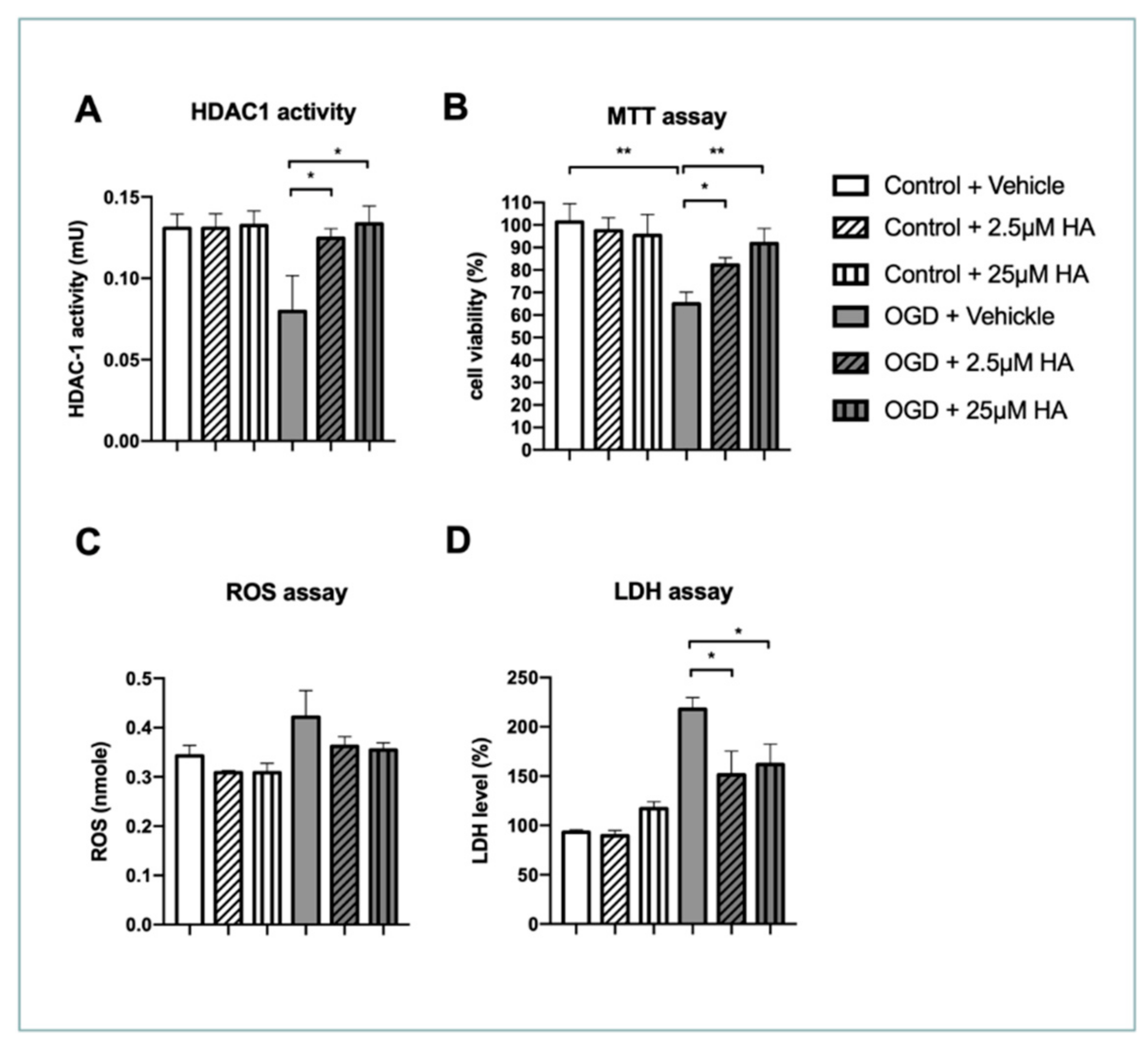
2.1. Increased HDAC1 Enzymatic Activity Preserves Neuronal Survival and Ameliorates Reactive Oxygen Species and LDH Production In Vitro
2.2. Increased HDAC1 Enzymatic Activity Conserves the Complexity of Neurite Outgrowth in OGD Neurons
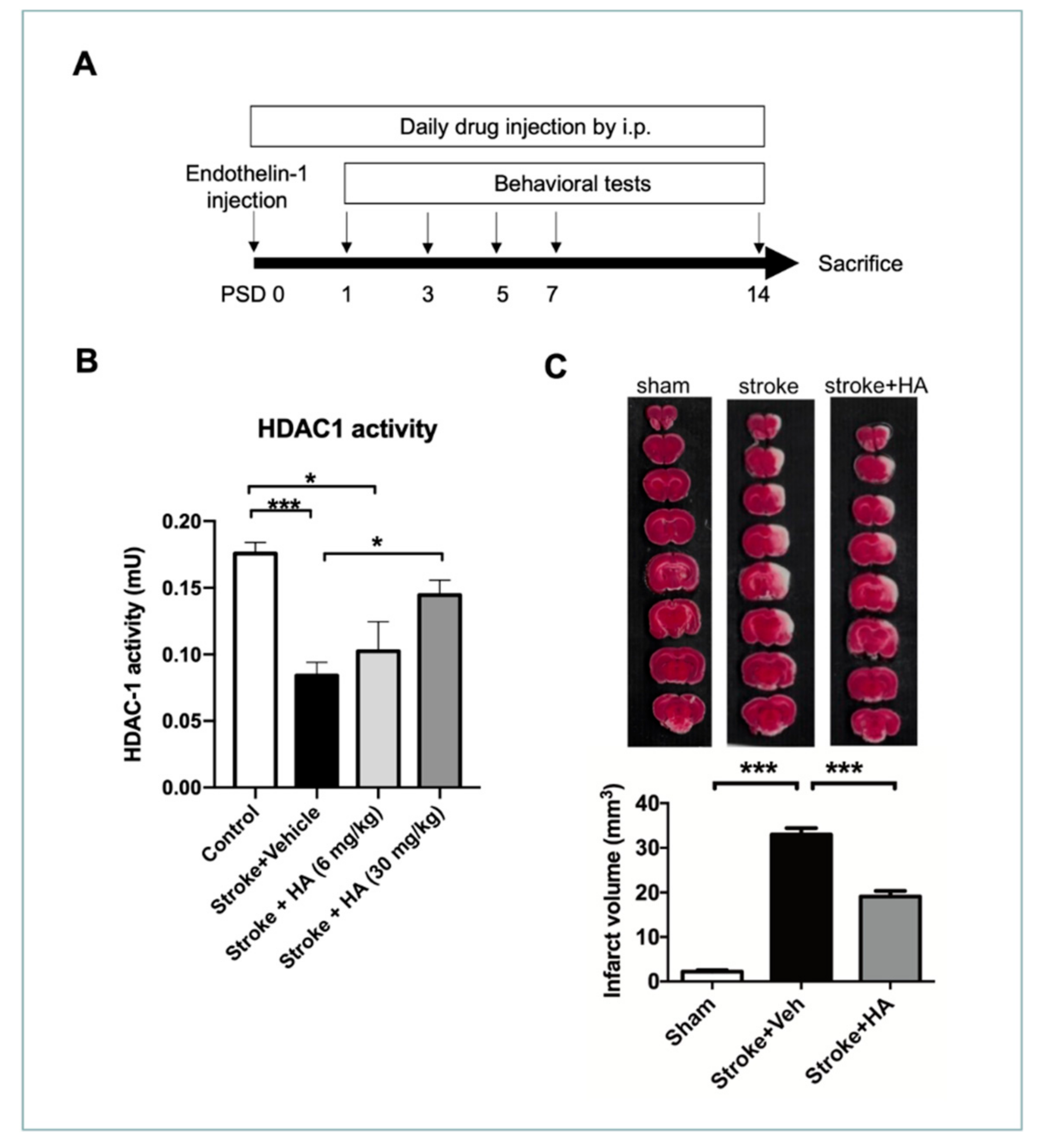
2.3. HA Treatment Restores HDAC1 Activity and Attenuates Brain Damage after Cerebral Ischemia
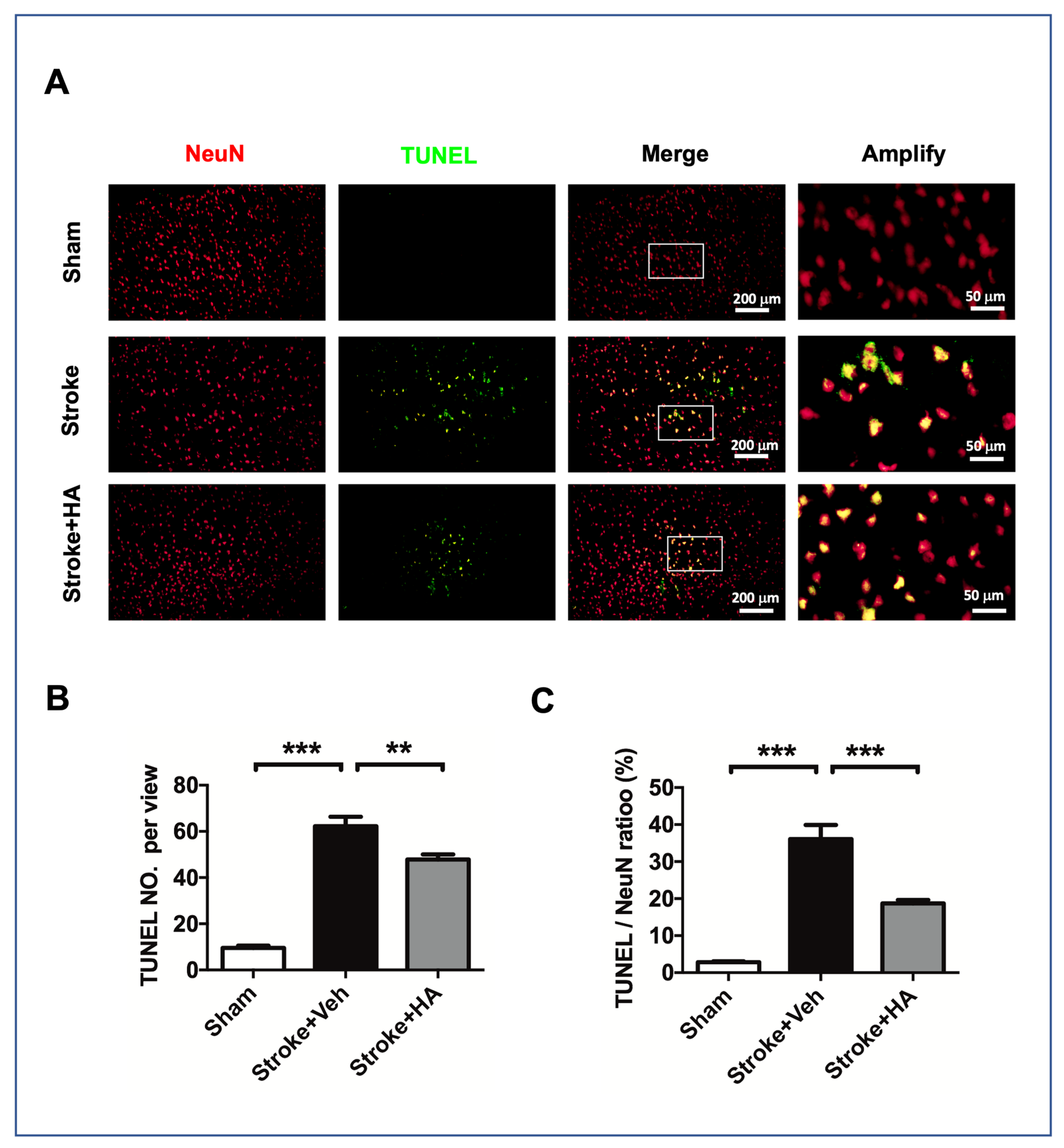
2.4. HA Decreases Neuronal Apoptosis after a Stroke
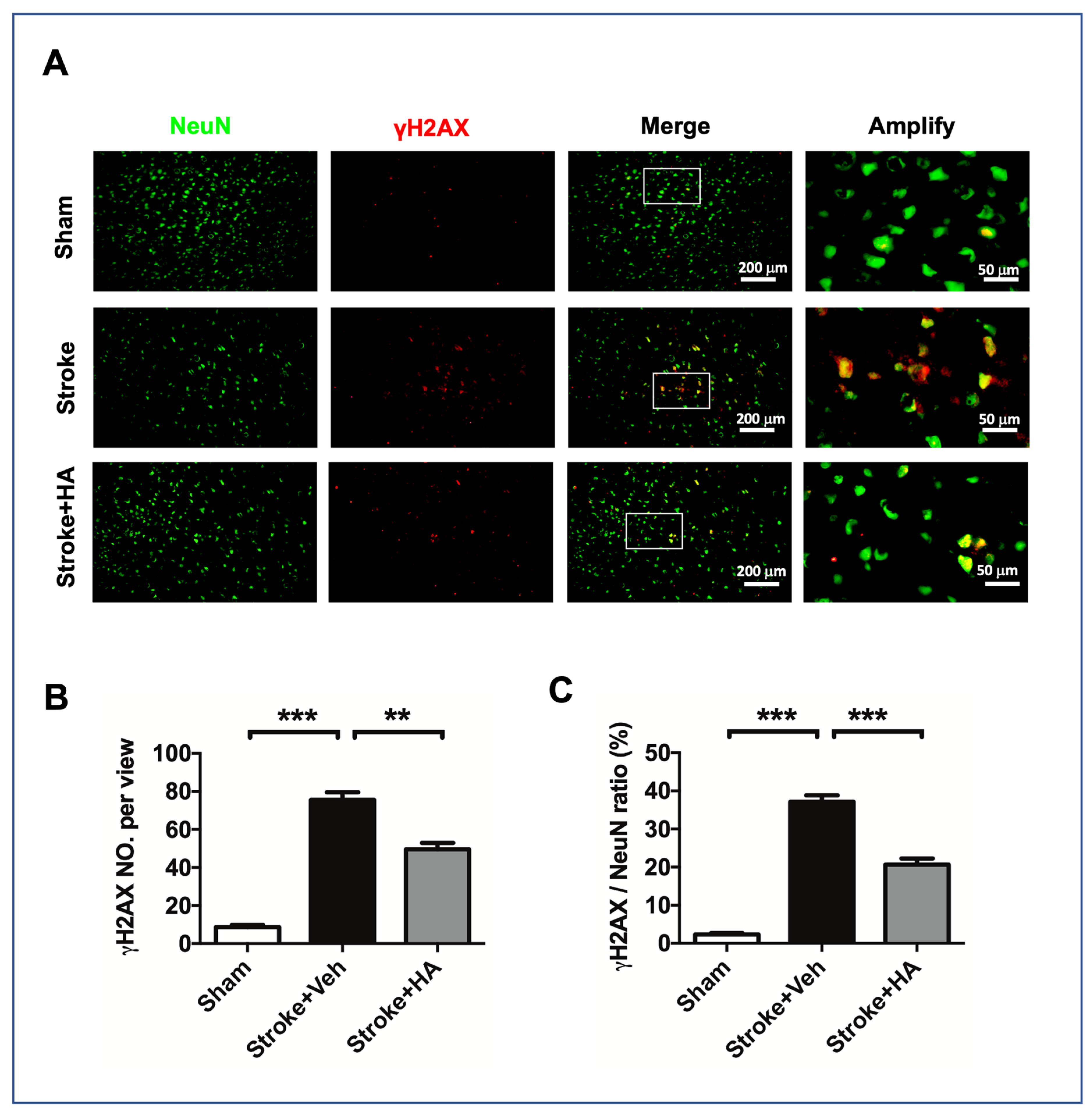
2.5. HA Attenuates DNA Damage after a Stroke
2.6. HA Alleviates DNA Breaks after a Stroke
2.7. HA Promotes Recovery of Behavioral Deficits in Rats with Ischemic Stroke
2.8. HA Improves Recover of Synaptic Density in Rats with Ischemic Stroke
3. Discussion
4. Materials and Methods
4.1. Animal Experiments and Drug Administration
4.2. Primary Neuronal Culture, Oxygen Deprivation, and Neurite Outgrowth Assay
4.3. Behavioral Tests
4.4. ROS Assay, LDH Assay, HDAC1 Activity Assay
4.5. Infarct Volume Assessment
4.6. Immunofluorescent Staining
4.7. Neuron Primary Culture, Neurite Outgrowth Assay
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Neurological Disorders; Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Fieschi, C.; Toni, D.; Lesaffre, E.; von Kummer, R.; Boysen, G.; Bluhmki, E.; Hoxter, G.; Mahagne, M.H.; et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995, 274, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Fieschi, C.; von Kummer, R.; Davalos, A.; Meier, D.; Larrue, V.; Bluhmki, E.; Davis, S.; Donnan, G.; et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998, 352, 1245–1251. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Sandercock, P.A.; Berge, E. Thrombolytic therapy with recombinant tissue plasminogen activator for acute ischemic stroke: Where do we go from here? A cumulative meta-analysis. Stroke 2003, 34, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Berkhemer, O.A.; Fransen, P.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef]
- Campbell, B.C.; Mitchell, P.J.; Kleinig, T.J.; Dewey, H.M.; Churilov, L.; Yassi, N.; Yan, B.; Dowling, R.J.; Parsons, M.W.; Oxley, T.J.; et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015, 372, 1009–1018. [Google Scholar] [CrossRef]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willinsky, R.A.; Sapkota, B.L.; et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef]
- Baron, J.C. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nat. Rev. Neurol. 2018, 14, 325–337. [Google Scholar] [CrossRef]
- Abel, T.; Zukin, R.S. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr. Opin. Pharmacol. 2008, 8, 57–64. [Google Scholar] [CrossRef]
- Chuang, D.M.; Leng, Y.; Marinova, Z.; Kim, H.J.; Chiu, C.T. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009, 32, 591–601. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.A.; D’Mello, S.R. Complex neuroprotective and neurotoxic effects of histone deacetylases. J. Neurochem. 2018, 145, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Frank, C.L.; Dobbin, M.M.; Tsunemoto, R.K.; Tu, W.; Peng, P.L.; Guan, J.S.; Lee, B.H.; Moy, L.Y.; Giusti, P.; et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron 2008, 60, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Pan, L.; Su, S.C.; Quinn, E.J.; Sasaki, M.; Jimenez, J.C.; Mackenzie, I.R.; Huang, E.J.; Tsai, L.H. Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat. Neurosci. 2013, 16, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Faraco, G.; Pittelli, M.; Cavone, L.; Fossati, S.; Porcu, M.; Mascagni, P.; Fossati, G.; Moroni, F.; Chiarugi, A. Histone deacetylase (HDAC) inhibitors reduce the glial inflammatory response in vitro and in vivo. Neurobiol. Dis. 2009, 36, 269–279. [Google Scholar] [CrossRef]
- Bardai, F.H.; Price, V.; Zaayman, M.; Wang, L.; D’Mello, S.R. Histone deacetylase-1 (HDAC1) is a molecular switch between neuronal survival and death. J. Biol. Chem. 2012, 287, 35444–35453. [Google Scholar] [CrossRef]
- Chen, J.S.; Wang, H.K.; Hsu, C.Y.; Su, Y.T.; Chen, J.S.; Liang, C.L.; Hsieh, P.C.; Wu, C.C.; Kwan, A.L. HDAC1 deregulation promotes neuronal loss and deficit of motor function in stroke pathogenesis. Sci. Rep. 2021, 11, 16354. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.D.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Xuan, A.; Long, D.; Li, J.; Ji, W.; Hong, L.; Zhang, M.; Zhang, W. Neuroprotective effects of valproic acid following transient global ischemia in rats. Life Sci. 2012, 90, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, Z.; Jia, L.Q.; Zhan, K.X.; Wang, L.J.; Song, N.; Liu, Y.; Cheng, Y.Y.; Yang, Y.J.; Guan, L.; et al. Valproic acid attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-pyroptosis pathways. Neurochem. Int. 2019, 124, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, X.; Shao, Q.; Chen, Z.; Huang, Q.; Jiao, Z.; Huang, X.; Yue, M.; Peng, J.; Zhou, X.; et al. Early Histone Deacetylase Inhibition Mitigates Ischemia/Reperfusion Brain Injury by Reducing Microglia Activation and Modulating Their Phenotype. Front. Neurol. 2019, 10, 893. [Google Scholar] [CrossRef]
- Larsson, P.; Alwis, I.; Niego, B.; Sashindranath, M.; Fogelstrand, P.; Wu, M.C.; Glise, L.; Magnusson, M.; Daglas, M.; Bergh, N.; et al. Valproic acid selectively increases vascular endothelial tissue-type plasminogen activator production and reduces thrombus formation in the mouse. J. Thromb. Haemost. 2016, 14, 2496–2508. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Jaworska, J.; Sypecka, J.; Polowy, R.; Filipkowski, R.K.; Zalewska, T. Sodium Butyrate, a Histone Deacetylase Inhibitor, Exhibits Neuroprotective/Neurogenic Effects in a Rat Model of Neonatal Hypoxia-Ischemia. Mol. Neurobiol. 2017, 54, 5300–5318. [Google Scholar] [CrossRef]
- Kim, H.J.; Rowe, M.; Ren, M.; Hong, J.S.; Chen, P.S.; Chuang, D.M. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. J. Pharm. Exp. 2007, 321, 892–901. [Google Scholar] [CrossRef]
- Dietz, K.C.; Casaccia, P. HDAC inhibitors and neurodegeneration: At the edge between protection and damage. Pharmacol. Res. 2010, 62, 11–17. [Google Scholar] [CrossRef]
- Zhao, H.; Han, Z.; Ji, X.; Luo, Y. Epigenetic Regulation of Oxidative Stress in Ischemic Stroke. Aging Dis. 2016, 7, 295–306. [Google Scholar] [CrossRef]
- Ansari, S.; Azari, H.; Caldwell, K.J.; Regenhardt, R.W.; Hedna, V.S.; Waters, M.F.; Hoh, B.L.; Mecca, A.P. Endothelin-1 induced middle cerebral artery occlusion model for ischemic stroke with laser Doppler flowmetry guidance in rat. J. Vis. Exp. 2013, 72, e50014. [Google Scholar] [CrossRef]
- Hughes, P.M.; Anthony, D.C.; Ruddin, M.; Botham, M.S.; Rankine, E.L.; Sablone, M.; Baumann, D.; Mir, A.K.; Perry, V.H. Focal lesions in the rat central nervous system induced by endothelin-1. J. Neuropathol. Exp. Neurol. 2003, 62, 1276–1286. [Google Scholar] [CrossRef]
- Wu, C.C.; Jin, L.W.; Wang, I.F.; Wei, W.Y.; Ho, P.C.; Liu, Y.C.; Tsai, K.J. HDAC1 dysregulation induces aberrant cell cycle and DNA damage in progress of TDP-43 proteinopathies. EMBO Mol. Med. 2020, 12, e10622. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, H.; Hu, Z.; Fan, K.; Wang, J.; Janoschka, S.; Wang, X.; Ge, S.; Feng, J. Parkin mutations reduce the complexity of neuronal processes in iPSC-derived human neurons. Stem Cells 2015, 33, 68–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Franco-Villanueva, A.; Wandosell, F.; Anton, I.M. Neuritic complexity of hippocampal neurons depends on WIP-mediated mTORC1 and Abl family kinases activities. Brain Behav. 2015, 5, e00359. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Varlamova, E.G.; Plotnikov, E.Y. Mechanisms Underlying the Protective Effect of the Peroxiredoxin-6 Are Mediated via the Protection of Astrocytes during Ischemia/Reoxygenation. Int. J. Mol. Sci. 2021, 22, 8805. [Google Scholar] [CrossRef] [PubMed]
- Turovskaya, M.V.; Gaidin, S.G.; Vedunova, M.V.; Babaev, A.A.; Turovsky, E.A. BDNF Overexpression Enhances the Preconditioning Effect of Brief Episodes of Hypoxia, Promoting Survival of GABAergic Neurons. Neurosci. Bull. 2020, 36, 733–760. [Google Scholar] [CrossRef] [PubMed]
- Gaidin, S.G.; Turovskaya, M.V.; Gavrish, M.S.; Babaev, A.A.; Mal’tseva, V.N.; Blinova, E.V.; Turovsky, E.A. The selective BDNF overexpression in neurons protects neuroglial networks against OGD and glutamate-induced excitotoxicity. Int. J. Neurosci. 2020, 130, 363–383. [Google Scholar] [CrossRef]
- Didier, M.; Bursztajn, S.; Adamec, E.; Passani, L.; Nixon, R.A.; Coyle, J.T.; Wei, J.Y.; Berman, S.A. DNA strand breaks induced by sustained glutamate excitotoxicity in primary neuronal cultures. J. Neurosci. 1996, 16, 2238–2250. [Google Scholar] [CrossRef]
- Li, P.; Stetler, R.A.; Leak, R.K.; Shi, Y.; Li, Y.; Yu, W.; Bennett, M.V.L.; Chen, J. Oxidative stress and DNA damage after cerebral ischemia: Potential therapeutic targets to repair the genome and improve stroke recovery. Neuropharmacology 2018, 134, 208–217. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Fillingham, J.; Keogh, M.-C.; Krogan, N.J. γH2AX and its role in DNA double-strand break repairThis paper is one of a selection of papers published in this Special Issue, entitled 27th International West Coast Chromatin and Chromosome Conference, and has undergone the Journal’s usual peer review process. Biochem. Cell Biol. 2006, 84, 568–577. [Google Scholar] [CrossRef]
- Melillo, G. HIF-1: A Target For Cancer, Ischemia and Inflammation—Too Good to be True? Cell Cycle 2004, 3, 149–150. [Google Scholar] [CrossRef]
- Miller, K.M.; Tjeertes, J.V.; Coates, J.; Legube, G.; Polo, S.E.; Britton, S.; Jackson, S.P. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat. Struct. Mol. Biol. 2010, 17, 1144–1151. [Google Scholar] [CrossRef]
- Bhaskara, S. Histone deacetylases 1 and 2 regulate DNA replication and DNA repair: Potential targets for genome stability-mechanism-based therapeutics for a subset of cancers. Cell Cycle 2015, 14, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Carter, K.M.; Bautista, R.-M.; He, D.; Wang, C.; D’Orazio, J.A. Sirtuin 1-mediated deacetylation of XPA DNA repair protein enhances its interaction with ATR protein and promotes cAMP-induced DNA repair of UV damage. J. Biol. Chem. 2018, 293, 19025–19037. [Google Scholar] [CrossRef] [PubMed]
- Baechtold, H.; Kuroda, M.; Sok, J.; Ron, D.; Lopez, B.S.; Akhmedov, A.T. Human 75-kDa DNA-pairing Protein Is Identical to the Pro-oncoprotein TLS/FUS and Is Able to Promote D-loop Formation. J. Biol. Chem. 1999, 274, 34337–34342. [Google Scholar] [CrossRef] [PubMed]
- Thurn, K.T.; Thomas, S.; Raha, P.; Qureshi, I.; Munster, P.N. Histone deacetylase regulation of ATM-mediated DNA damage signaling. Mol. Cancer 2013, 12, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Goder, A.; Emmerich, C.; Nikolova, T.; Kiweler, N.; Schreiber, M.; Kuhl, T.; Imhof, D.; Christmann, M.; Heinzel, T.; Schneider, G.; et al. HDAC1 and HDAC2 integrate checkpoint kinase phosphorylation and cell fate through the phosphatase-2A subunit PR130. Nat. Commun. 2018, 9, 764. [Google Scholar] [CrossRef]
- Huttner, H.B.; Bergmann, O.; Salehpour, M.; Racz, A.; Tatarishvili, J.; Lindgren, E.; Csonka, T.; Csiba, L.; Hortobagyi, T.; Mehes, G.; et al. The age and genomic integrity of neurons after cortical stroke in humans. Nat. Neurosci. 2014, 17, 801–803. [Google Scholar] [CrossRef]
- Bhakat, K.K.; Mantha, A.K.; Mitra, S. Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid. Redox Signal 2009, 11, 621–638. [Google Scholar] [CrossRef]
- Haberland, M.; Montgomery, R.L.; Olson, E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009, 10, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Volmar, C.; Wahlestedt, C. Histone deacetylases (HDACs) and brain function. Neuroepigenetics 2015, 1, 20–27. [Google Scholar] [CrossRef]
- Zhu, Y.; Vidaurre, O.G.; Adula, K.P.; Kezunovic, N.; Wentling, M.; Huntley, G.W.; Casaccia, P. Subcellular Distribution of HDAC1 in Neurotoxic Conditions Is Dependent on Serine Phosphorylation. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 7547–7559. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Shen, S.; Dietz, K.; He, Y.; Howell, O.; Reynolds, R.; Casaccia, P. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat. Neurosci. 2010, 13, 180–189. [Google Scholar] [CrossRef]
- Donmez, G.; Outeiro, T.F. SIRT1 and SIRT2: Emerging targets in neurodegeneration. EMBO Mol. Med. 2013, 5, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.E.; Majdzadeh, N.; Zhang, X.; Lyles, A.; Bassel-Duby, R.; Olson, E.N.; D’Mello, S.R. Neuroprotection by histone deacetylase-related protein. Mol. Cell Biol. 2006, 26, 3550–3564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Zang, X.F.; Pan, J.; Zhu, X.L.; Chen, F.; Chen, Z.B.; Xu, Y. Expression patterns of histone deacetylases in experimental stroke and potential targets for neuroprotection. Clin. Exp. Pharmacol. Physiol. 2012, 39, 751–758. [Google Scholar] [CrossRef]
- Tsai, H.D.; Wu, J.S.; Kao, M.H.; Chen, J.J.; Sun, G.Y.; Ong, W.Y.; Lin, T.N. Clinacanthus nutans Protects Cortical Neurons Against Hypoxia-Induced Toxicity by Downregulating HDAC1/6. Neuromol. Med. 2016, 18, 274–282. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Fan, Z.; Li, G.; Ma, Q.; Tao, Z.; Wang, R.; Feng, J.; Luo, Y. Long Noncoding RNA H19 Promotes Neuroinflammation in Ischemic Stroke by Driving Histone Deacetylase 1-Dependent M1 Microglial Polarization. Stroke 2017, 48, 2211–2221. [Google Scholar] [CrossRef]
- Pickell, Z.; Williams, A.M.; Alam, H.B.; Hsu, C.H. Histone Deacetylase Inhibitors: A Novel Strategy for Neuroprotection and Cardioprotection Following Ischemia/Reperfusion Injury. J. Am. Heart Assoc. 2020, 9, e016349. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Alamri, F.F.; Syeara, N.; Jayaraman, S.; Karamyan, S.T.; Arumugam, T.V.; Karamyan, V.T. The Effect of Histone Deacetylase Inhibitors Panobinostat or Entinostat on Motor Recovery in Mice after Ischemic Stroke. Neuromol. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bonsack, F.; Sukumari-Ramesh, S. Entinostat improves acute neurological outcomes and attenuates hematoma volume after Intracerebral Hemorrhage. Brain Res. 2021, 1752, 147222. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.; Zhang, R.; Wang, G.; Zhang, Y.F. The development prospection of HDAC inhibitors as a potential therapeutic direction in Alzheimer’s disease. Transl. Neurodegener. 2017, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Zahnow, C.A.; Topper, M.; Stone, M.; Murray-Stewart, T.; Li, H.; Baylin, S.B.; Casero, R.A., Jr. Inhibitors of DNA Methylation, Histone Deacetylation, and Histone Demethylation: A Perfect Combination for Cancer Therapy. Adv. Cancer Res. 2016, 130, 55–111. [Google Scholar] [CrossRef]
- Wu, C.C.; Wang, L.C.; Su, Y.T.; Wei, W.Y.; Tsai, K.J. Synthetic alpha5beta1 integrin ligand PHSRN is proangiogenic and neuroprotective in cerebral ischemic stroke. Biomaterials 2018, 185, 142–154. [Google Scholar] [CrossRef]
- Horie, N.; Maag, A.L.; Hamilton, S.A.; Shichinohe, H.; Bliss, T.M.; Steinberg, G.K. Mouse model of focal cerebral ischemia using endothelin-1. J. Neurosci. Methods 2008, 173, 286–290. [Google Scholar] [CrossRef]
- Petullo, D.; Masonic, K.; Lincoln, C.; Wibberley, L.; Teliska, M.; Yao, D.L. Model development and behavioral assessment of focal cerebral ischemia in rats. Life Sci. 1999, 64, 1099–1108. [Google Scholar] [CrossRef]
- Klein, S.M.; Vykoukal, J.; Lechler, P.; Zeitler, K.; Gehmert, S.; Schreml, S.; Alt, E.; Bogdahn, U.; Prantl, L. Noninvasive in vivo assessment of muscle impairment in the mdx mouse model—A comparison of two common wire hanging methods with two different results. J. Neurosci. Methods 2012, 203, 292–297. [Google Scholar] [CrossRef]
- Hua, Y.; Schallert, T.; Keep, R.F.; Wu, J.; Hoff, J.T.; Xi, G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke 2002, 33, 2478–2484. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lien, C.C.; Hou, W.H.; Chiang, P.M.; Tsai, K.J. Gain of BDNF Function in Engrafted Neural Stem Cells Promotes the Therapeutic Potential for Alzheimer’s Disease. Sci. Rep. 2016, 6, 27358. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Huang, C.Y.; Wang, H.K.; Wu, M.H.; Tsai, K.J. Magnesium sulfate and nimesulide have synergistic effects on rescuing brain damage after transient focal ischemia. J. Neurotrauma 2012, 29, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Wang, I.F.; Chiang, P.M.; Wang, L.C.; Shen, C.J.; Tsai, K.J. G-CSF-mobilized Bone Marrow Mesenchymal Stem Cells Replenish Neural Lineages in Alzheimer’s Disease Mice via CXCR4/SDF-1 Chemotaxis. Mol. Neurobiol. 2016, 54, 6198–6212. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-S.; Wang, H.-K.; Su, Y.-T.; Hsu, C.-Y.; Chen, J.-S.; Liang, C.-L.; Wu, C.-C.; Kwan, A.-L. Restoration of HDAC1 Enzymatic Activity after Stroke Protects Neurons from Ischemia/Reperfusion Damage and Attenuates Behavioral Deficits in Rats. Int. J. Mol. Sci. 2021, 22, 10654. https://doi.org/10.3390/ijms221910654
Chen J-S, Wang H-K, Su Y-T, Hsu C-Y, Chen J-S, Liang C-L, Wu C-C, Kwan A-L. Restoration of HDAC1 Enzymatic Activity after Stroke Protects Neurons from Ischemia/Reperfusion Damage and Attenuates Behavioral Deficits in Rats. International Journal of Molecular Sciences. 2021; 22(19):10654. https://doi.org/10.3390/ijms221910654
Chicago/Turabian StyleChen, Jui-Sheng, Hao-Kuang Wang, Yu-Ting Su, Chien-Yu Hsu, Jia-Shing Chen, Cheng-Loong Liang, Cheng-Chun Wu, and Aij-Lie Kwan. 2021. "Restoration of HDAC1 Enzymatic Activity after Stroke Protects Neurons from Ischemia/Reperfusion Damage and Attenuates Behavioral Deficits in Rats" International Journal of Molecular Sciences 22, no. 19: 10654. https://doi.org/10.3390/ijms221910654
APA StyleChen, J.-S., Wang, H.-K., Su, Y.-T., Hsu, C.-Y., Chen, J.-S., Liang, C.-L., Wu, C.-C., & Kwan, A.-L. (2021). Restoration of HDAC1 Enzymatic Activity after Stroke Protects Neurons from Ischemia/Reperfusion Damage and Attenuates Behavioral Deficits in Rats. International Journal of Molecular Sciences, 22(19), 10654. https://doi.org/10.3390/ijms221910654






