Candida albicans Sfp1 Is Involved in the Cell Wall and Endoplasmic Reticulum Stress Responses Induced by Human Antimicrobial Peptide LL-37
Abstract
1. Introduction
2. Results
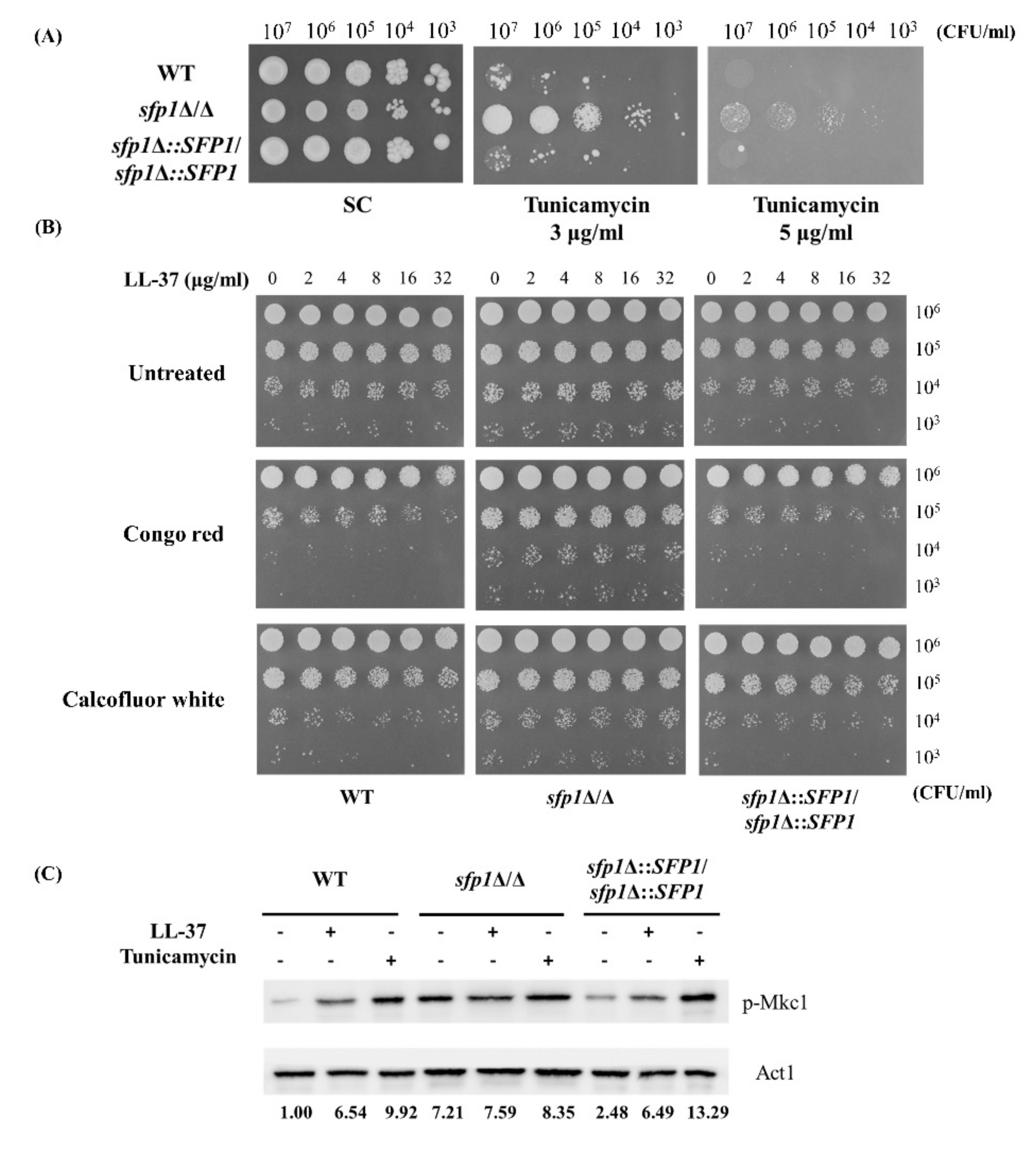
2.1. Deletion of SFP1 Reduces Susceptibility to LL-37
2.2. The Effect of SFP1 Deletion on Cell Susceptibility to LL-37 Is Cell Wall-Related
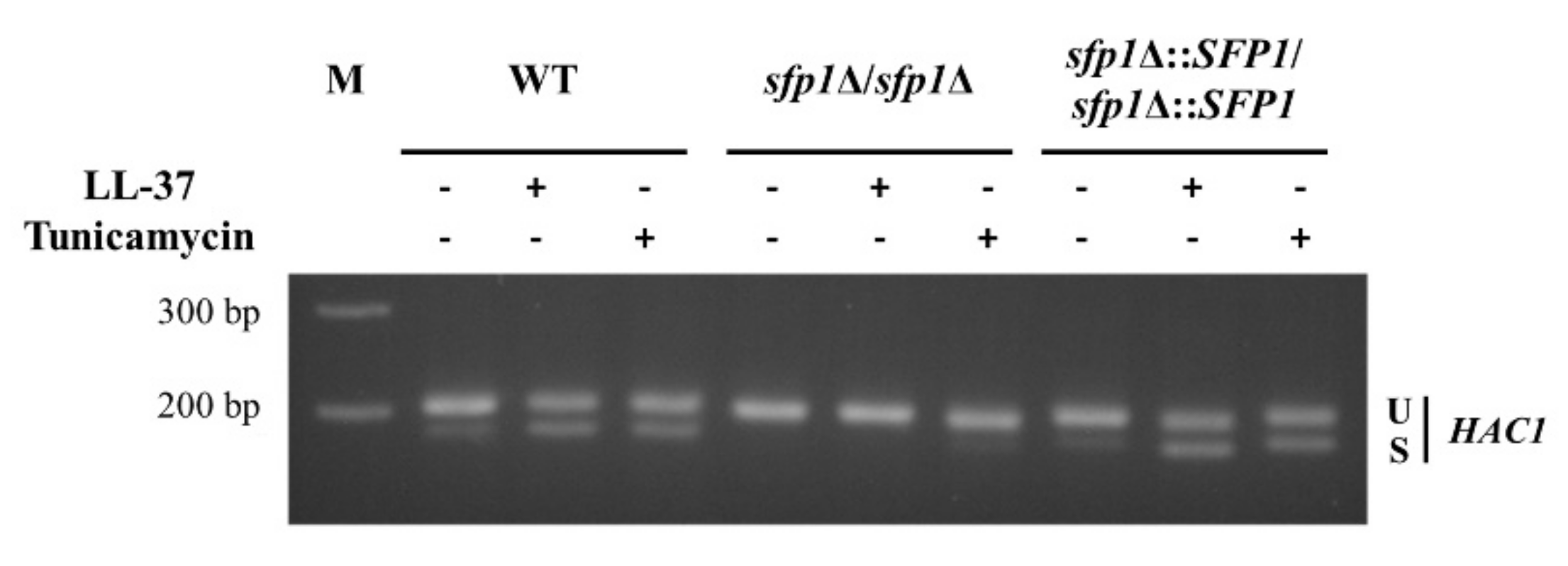
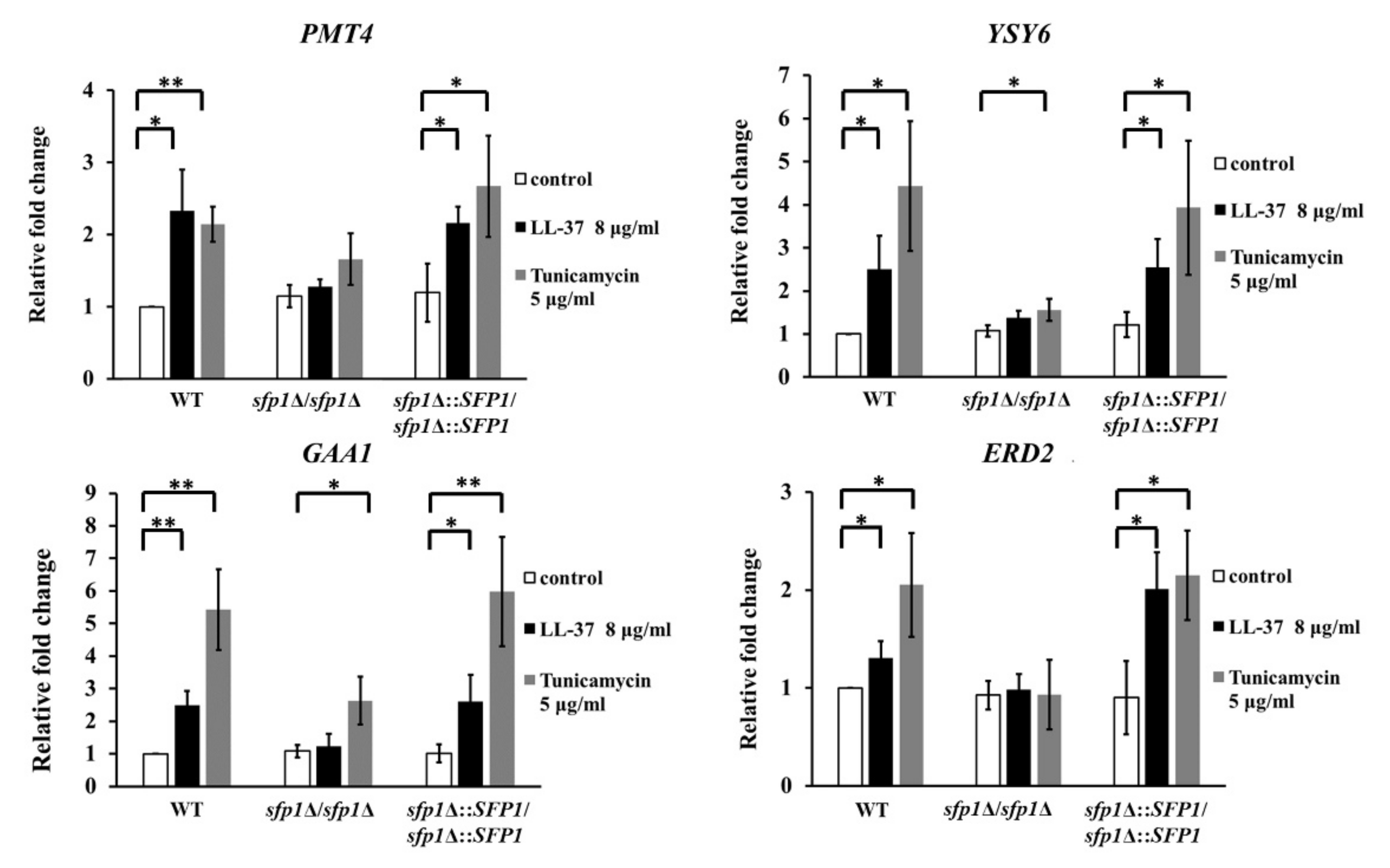
2.3. Deletion of SFP1 Attenuates HAC1 mRNA Splicing and UPR-Responsive Gene Activation upon LL-37 Treatment
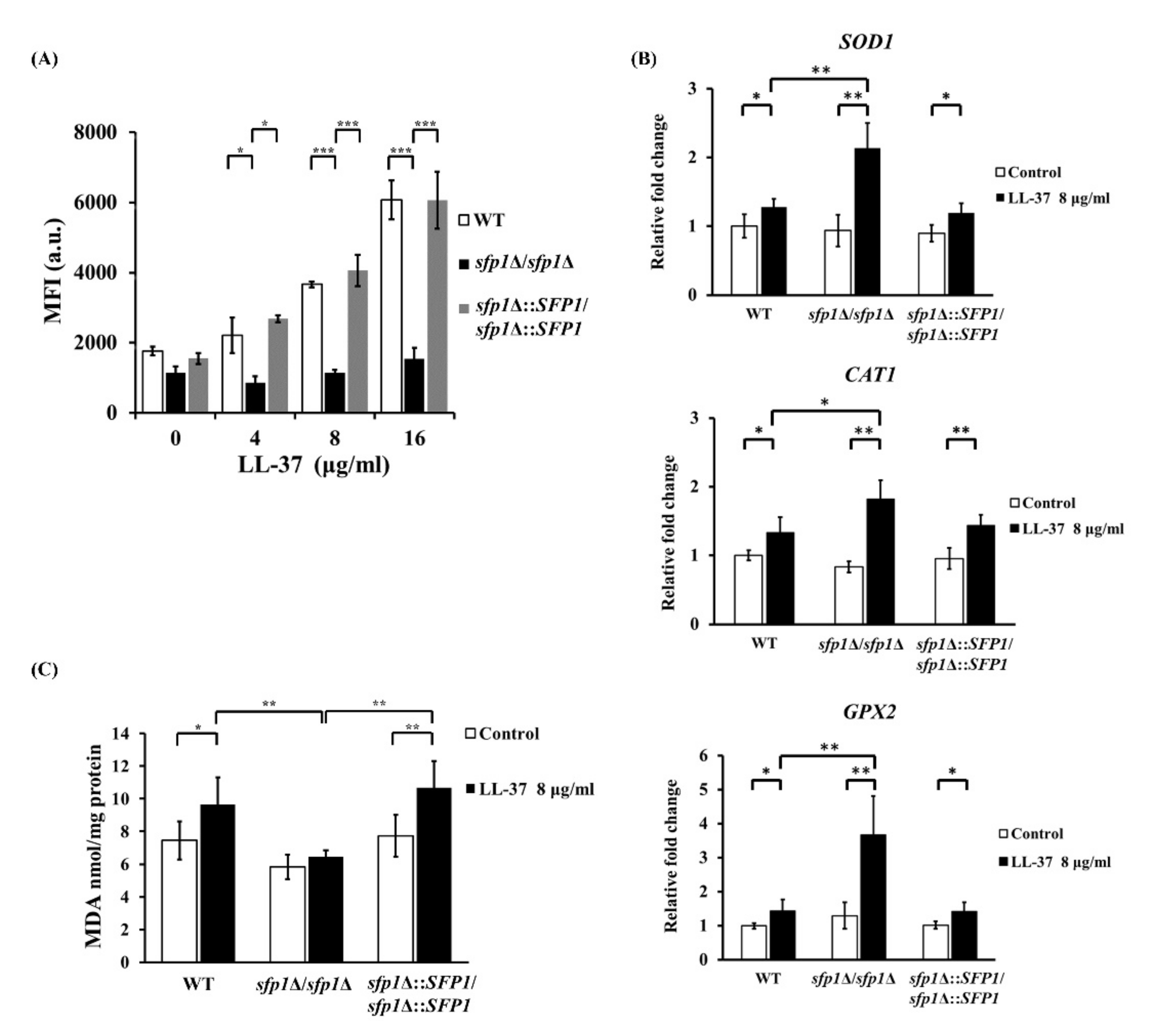
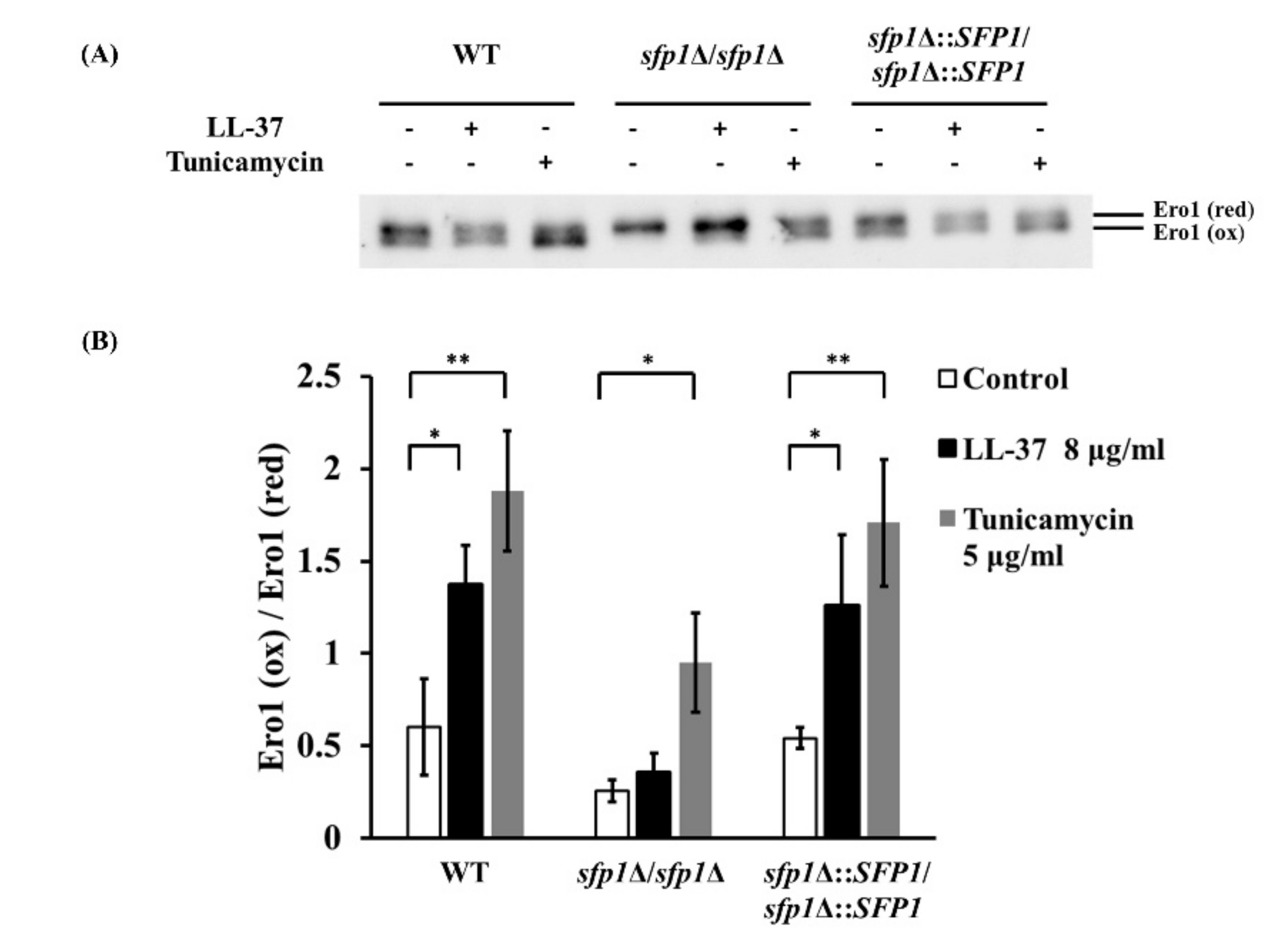
2.4. Deletion of SFP1 also Attenuates ROS-Induced Toxicity and Oxidation of Ero1 Promoted by LL-37
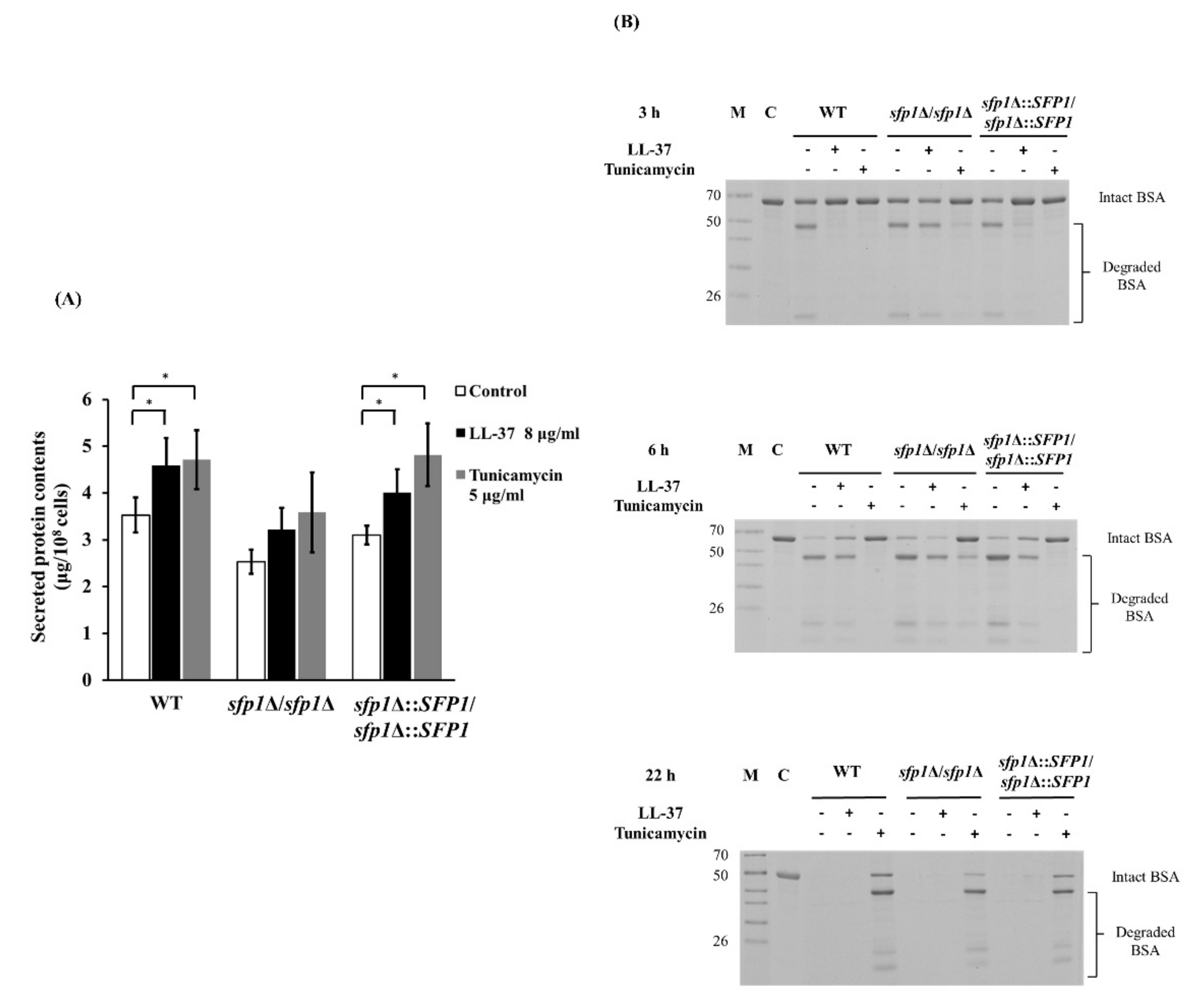
2.5. Deletion of SFP1 Affects Protein Secretion and BSA Degradation in Cells Treated with LL-37
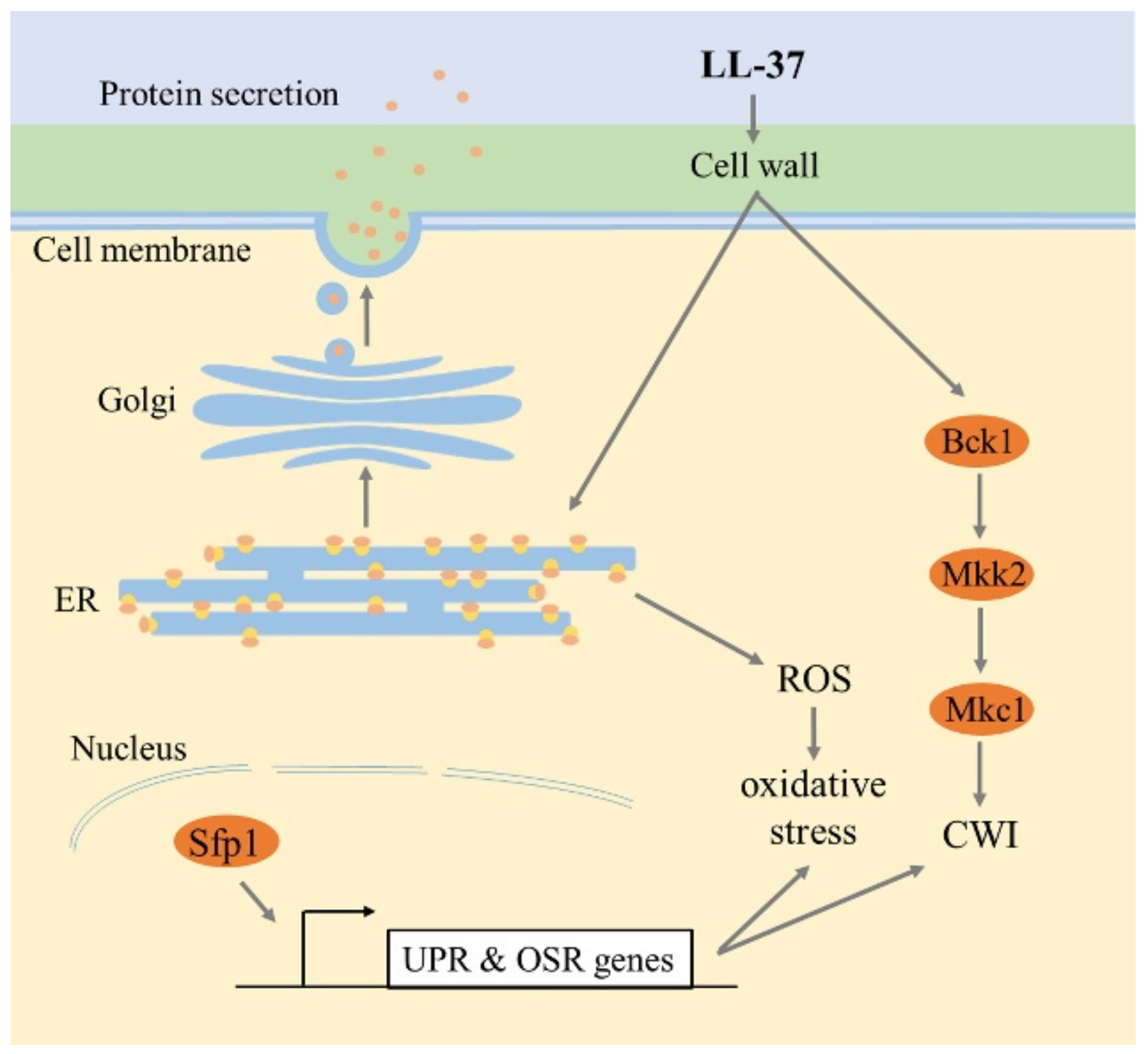
3. Discussion
4. Materials and Methods
4.1. Peptide and Reagents
4.2. C. albicans Strains and Growth Conditions
4.3. Cell Susceptibility to LL-37 and Other Agents
4.4. Assay for Mkc1 Phosphorylation
4.5. Assay for HAC1 mRNA Splicing
4.6. Real-Time Quantitative PCR (qPCR)
4.7. Measurement of Intracellular Reactive Oxygen Species (ROS)
4.8. Lipid Peroxidation Assay
4.9. Construction of the Ero1-Flag Expressing Strains
4.10. Detection of the Redox State of Ero1
4.11. Protein Secretion and BSA Degradation Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levita, S.M.; Netea, M.G.; White, T.C. Hidden killers: Human fungal infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Chaffin, W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008, 72, 495–544. [Google Scholar] [CrossRef] [PubMed]
- Cottier, F.; Hall, R.A. Face/off: The interchangeable side of Candida albicans. Front. Cell Infect. Microbiol. 2020, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Hube, B. Importance of the Candida albicans cell wall during commensalism and infection. Curr. Opin. Microbiol. 2012, 15, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Hatinguais, R.; Willment, J.A.; Brown, G.D. PAMPs of the fungal cell wall and mammalian PRRs. Curr. Top. Microbiol. Immunol. 2021, 425, 187–223. [Google Scholar] [CrossRef]
- Muszewska, A.; Pilsyk, S.; Perlińska-Lenart, U.; Kruszewska, J.S. Diversity of cell wall related proteins in human pathogenic fungi. J. Fungi 2017, 4, 6. [Google Scholar] [CrossRef]
- Boxx, G.M.; Kozel, T.R.; Nishiya, C.T.; Zhang, M.X. Influence of mannan and glucan on complement activation and C3 binding by Candida albicans. Infect. Immun. 2010, 78, 1250–1259. [Google Scholar] [CrossRef]
- Childers, D.S.; Avelar, G.M.; Bain, J.M.; Larcombe, D.E.; Pradhan, A.; Budge, S.; Heaney, H.; Brown, A.J.P. Impact of the environment upon the Candida albicans cell wall and resultant effects upon immune surveillance. Curr. Top. Microbiol. Immunol. 2020, 425, 297–330. [Google Scholar] [CrossRef]
- Cottier, F.; Sherrington, S.; Cockerill, S.; Del Olmo Toledo, V.; Kissane, S.; Tournu, H.; Orsini, L.; Palmer, G.E.; Pérez, J.C. Remasking of Candida albicans β-glucan in response to environmental pH is regulated by quorum sensing. mBio 2019, 10, e02347. [Google Scholar] [CrossRef] [PubMed]
- McCall, A.D.; Pathirana, R.U.; Prabhakar, A.; Cullen, P.J.; Edgerton, M. Candida albicans biofilm development is governed by cooperative attachment and adhesion maintenance proteins. NPJ Biofilms Microbiomes 2019, 5, 21. [Google Scholar] [CrossRef]
- Walker, L.A.; Munro, C.A.; de Bruijn, I.; Lenardon, M.D.; McKinnon, A.; Gow, N.A.R. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog. 2008, 4, e1000040. [Google Scholar] [CrossRef]
- Alves, R.; Barata-Antunes, C.; Casal, M.; Brown, A.J.P.; Van Dijck, P.; Paiva, S. Adapting to survive: How Candida overcomes host-imposed constraints during human colonization. PLoS Pathog. 2020, 16, e1008478. [Google Scholar] [CrossRef] [PubMed]
- Hopke, A.; Brown, A.J.P.; Hall, R.A.; Wheeler, R.T. Dynamic fungal cell wall architecture in stress adaptation and immune evasion. Trends Microbiol. 2018, 26, 284–295. [Google Scholar] [CrossRef]
- Heilmann, C.J.; Sorgo, A.G.; Mohammadi, S.; Sosinska, G.J.; de Koster, C.G.; Brul, S.; de Koning, L.J.; Klis, F.M. Surface stress induces a conserved cell wall stress response in the pathogenic fungus Candida albicans. Eukaryot. Cell 2013, 12, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Dürr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Den Hertog, A.L.; van Marle, J.; van Veen, H.A.; Van’t Hof, W.; Bolscher, J.G.; Veerman, E.C.; Nieuw Amerongen, A.V. Candidacidal effects of two antimicrobial peptides: Histatin 5 causes small membrane defects, but LL-37 causes massive disruption on the cell membrane. Biochem. J. 2005, 388, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.W.; Yang, C.Y.; Chang, H.T.; Lan, C.Y. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS ONE 2011, 6, e17755. [Google Scholar] [CrossRef]
- Tsai, P.W.; Yang, C.Y.; Chang, H.T.; Lan, C.Y. Characterizing the role of cell-wall beta-1,3-exoglucanase Xog1p in Candida albicans adhesion by the human antimicrobial peptide LL-37. PLoS ONE 2011, 6, e21394. [Google Scholar] [CrossRef]
- Chang, H.T.; Tsai, P.W.; Huang, H.H.; Liu, Y.S.; Chien, T.S.; Chien, T.S.; Lan, C.Y. LL37 and hBD-3 elevate the β-1,3-exoglucanase activity of Candida albicans Xog1p, resulting in reduced fungal adhesion to plastic. Biochem. J. 2012, 441, 963–970. [Google Scholar] [CrossRef]
- Tsai, P.W.; Cheng, Y.L.; Hsieh, W.P.; Lan, C.Y. Responses of Candida albicans to the human antimicrobial peptide LL-37. J. Microbiol. 2014, 52, 581–589. [Google Scholar] [CrossRef]
- Kimata, Y.; Kohno, K. Endoplasmic reticulum stress-sensing mechanisms in yeast and mammalian cells. Curr. Opin. Cell Biol. 2011, 23, 135–142. [Google Scholar] [CrossRef]
- Hernández-Elvira, M.; Torres-Quiroz, F.; Escamilla-Ayala, A.; Domínguez-Martin, E.; Escalante, R.; Kawasaki, L.; Ongay-Larios, L.; Coria, R. The unfolded protein response pathway in the yeast Kluyveromyces lactis. A comparative view among yeast species. Cells 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Read, A.; Schröder, M. The unfolded protein response: An overview. Biology 2021, 10, 384. [Google Scholar] [CrossRef]
- Mori, K.; Ma, W.; Gething, M.J.; Sambrook, J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 1993, 74, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.S.; Ehamu, C.E.; Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 1993, 73, 1197–1206. [Google Scholar] [CrossRef]
- Sidrauski, C.; Walter, P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 1997, 90, 1031–1039. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Okamura, K.; Kimata, Y.; Higashio, H.; Tsuru, A.; Kohno, K. Dissociation of Kar2p/BiP from an ER sensory molecule, Ire1p, triggers the unfolded protein response in yeast. Biochem. Biophys. Res. Commun. 2000, 20, 445–450. [Google Scholar] [CrossRef]
- Credle, J.J.; Finer-Moore, J.S.; Rapa, F.R.; Stroud, R.M.; Walter, P. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 2005, 102, 18773–18784. [Google Scholar] [CrossRef] [PubMed]
- Kimata, Y.; Ishiwata-Kimata, Y.; Ito, T.; Hirata, A.; Suzuki, T.; Oikawa, D.; Takeuchi, M.; Kohno, K. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J. Cell Biol. 2007, 179, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ng, B.S.; Thibault, G. Endoplasmic reticulum stress response in yeast and humans. Biosci. Rep. 2014, 34, e00118. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.S.; Walter, P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 1996, 87, 391–404. [Google Scholar] [CrossRef]
- Travers, K.J.; Patil, C.K.; Wodicka, D.J.; Lockhart, D.J.; Weissman, J.S.; Walter, P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 2000, 101, 249–258. [Google Scholar] [CrossRef]
- Guerra-Moreno, A.; Ang, J.; Welsch, H.; Jochem, M.; Hanna, J. Regulation of the unfolded protein response in yeast by oxidative stress. FEBS Lett. 2019, 593, 1080–1088. [Google Scholar] [CrossRef]
- Scrimale, T.; Didone, L.; de Mesy Bentley, K.L.; Krysan, D.J. The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae. Mol. Biol. Cell 2009, 20, 164–175. [Google Scholar] [CrossRef]
- Sircaik, S.; Román, E.; Bapat, P.; Lee, K.K.; Andes, D.R.; Gow, N.A.R.; Nobile, C.J.; Pla, J.; Panwar, S.L. The protein kinase Ire1 impacts pathogenicity of Candida albicans by regulating homeostatic adaptation to endoplasmic reticulum stress. Cell Microbiol. 2021, 23, e13307. [Google Scholar] [CrossRef]
- Wimalasena, T.T.; Enjalbert, B.; Guillemette, T.; Plumridge, A.; Budge, S.; Yin, Z.; Brown, A.J.P.; Archer, D.B. Impact of the unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in the polarized growth, of Candida albicans. Fungal Genet. Biol. 2008, 45, 1235–1247. [Google Scholar] [CrossRef]
- Mori, K.; Kawahara, T.; Yoshida, H.; Yanagi, H.; Yura, T. Signaling from endoplasmic reticulum to nucleus: Transcription factor with a basic-leucine zipper motif is required for the unfolded protein-response pathway. Genes Cells. 1996, 1, 803–817. [Google Scholar] [CrossRef]
- Chen, H.F.; Lan, C.Y. Role of SFP1 in the regulation of Candida albicans biofilm formation. PLoS ONE 2015, 10, e0129903. [Google Scholar] [CrossRef] [PubMed]
- Kastora, S.L.; Herrero-de-Dios, C.; Avelar, G.M.; Munro, C.A.; Brown, A.J.P. Sfp1 and Rtg3 reciprocally modulate carbon source-conditional stress adaptation in the pathogenic yeast Candida albicans. Mol. Microbiol. 2017, 105, 620–636. [Google Scholar] [CrossRef]
- Lee, S.Y.; Chen, H.F.; Yeh, Y.C.; Xue, Y.P.; Lan, C.Y. The transcription factor Sfp1 regulates the oxidative stress response in Candida albicans. Microorganisms 2019, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Thomas, D.P.; López-Ribot, J.L. Effect of tunicamycin on Candida albicans biofilm formation and maintenance. J. Antimicrob. Chemother. 2009, 63, 473–479. [Google Scholar] [CrossRef][Green Version]
- Ram, A.F.; Klis, F.M. Identification of fungal cell wall mutants using susceptibility assays based on calcofluor white and congo red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef]
- Román, E.; Alonso-Monge, R.; Miranda, A.; Pla, J. The Mkk2 MAPKK regulates cell wall biogenesis in cooperation with the Cek1-pathway in Candida albicans. PLoS ONE 2015, 10, e0133476. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, B.; Li, J.; Zhang, B.; Wang, H.; Li, M. Endoplasmic reticulum-derived reactive oxygen species (ROS) is involved in toxicity of cell wall stress to Candida albicans. Free Radic. Biol. Med. 2016, 99, 572–583. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Zhang, B.; Xiao, C.; Ma, T.; Yi, X.; Liang, C.; Li, M. Stress-associated endoplasmic reticulum protein 1 (SERP1) and Atg8 synergistically regulate unfolded protein response (UPR) that is independent on autophagy in Candida albicans. Int. J. Med. Microbiol. 2018, 308, 378–386. [Google Scholar] [CrossRef]
- Prill, S.K.-H.; Klinkert, B.; Timpel, C.; Gale, C.A.; Schröppel, K.; Ernst, J.F. PMT family of Candida albicans: Five protein mannosyltransferase isoforms affect growth, morphogenesis and antifungal resistance. Mol. Microbiol. 2005, 55, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Eisenhaber, B.; Eisenhaber, S.; Kwang, T.Y.; Grüber, G.; Eisenhaber, F. Transamidase subunit GAA1/GPAA1 is a M28 family metallo-peptide-synthetase that catalyzes the peptide bond formation between the substrate protein’s omega-site and the GPI lipid anchor’s phosphoethanolamine. Cell Cycle 2014, 13, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Hardwick, K.G.; Dean, N.; Pelham, H.R. ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 1990, 61, 1349–1357. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Biliński, T.; Litwińska, J.; Błaszczyński, M.; Bajus, A. Superoxide dismutase deficiency and the toxicity of the products of autooxidation of polyunsaturated fatty acids in yeast. Biochem. Biophys. Acta 1989, 1001, 102–106. [Google Scholar] [CrossRef]
- Frand, A.R.; Kaiser, C.A. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell 1998, 1, 161–170. [Google Scholar] [CrossRef]
- Pollard, M.G.; Travers, K.J.; Weissman, J.S. Ero1p: A novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell 1998, 1, 171–182. [Google Scholar] [CrossRef]
- Frand, A.R.; Kaiser, C.A. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol. Cell 1999, 4, 469–477. [Google Scholar] [CrossRef]
- Zeeshan, H.M.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Gross, E.; Kastner, D.B.; Kaiser, C.A.; Fass, D. Structure of Ero1p, source of disulfide bonds for oxidative protein folding in the cell. Cell 2004, 117, 601–610. [Google Scholar] [CrossRef]
- Tu, B.; Weissman, J.S. The FAD- and O2-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell 2002, 10, 983–994. [Google Scholar] [CrossRef]
- Kim, S.; Sideris, D.P.; Sevier, C.S.; Kaiser, C.A. Balanced Ero1 activation and inactivation establishes ER redox homeostasis. J. Cell Biol. 2012, 196, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Rollenhagen, C.; Mamtani, S.; Ma, D.; Dixit, R.; Eszterhas, S.; Lee, S.A. The role of secretory pathways in Candida albicans pathogenesis. J. Fungi 2020, 6, 26. [Google Scholar] [CrossRef]
- Moore, K.A.; Hollien, J. The unfolded protein response in secretory cell function. Annu. Rev. Genet. 2012, 46, 165–183. [Google Scholar] [CrossRef]
- Cassone, A.; Vecchiarelli, A.; Hube, B. Aspartyl proteinases of eukaryotic microbial pathogens: From eating to heating. PLoS Pathog. 2016, 12, e1005992. [Google Scholar] [CrossRef]
- Crandall, M.; Edwards, J.E., Jr. Segregation of proteinase-negative mutants from heterozygous Candida albicans. J. Gen. Microbiol. 1987, 133, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Lin, C.Y.; Tsai, P.W.; Yang, C.Y.; Hsieh, W.P.; Lan, C.Y. Rhb1 regulates the expression of secreted aspartic protease 2 through the TOR signaling pathway in Candida albicans. Eukaryot. Cell 2012, 11, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Heredia, M.Y.; Ikeh, M.A.C.; Gunasekaran, D.; Conrad, K.A.; Filimonava, S.; Marotta, D.A.; Nobile, C.J.; Rauceo, J.M. An expanded cell wall damage signaling network is comprised of the transcription factors Rlm1 and Sko1 in Candida albicans. PLoS Genet. 2020, 16, e1008908. [Google Scholar] [CrossRef] [PubMed]
- Mottola, A.; Ramírez-Zavala, B.; Hünniger, K.; Kurzai, O.; Morschhäuser, J. The zinc cluster transcription factor Czf1 regulates cell wall architecture and integrity in Candida albicans. Mol. Microbiol. 2021, 116, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Navarro-García, F.; Sánchez, M.; Pla, J.; Nombela, C. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell wall integrity. Mol. Cell Biol. 1995, 15, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Navarro-García, F.; Eisman, B.; Fiuza, S.M.; Nombela, C.; Pla, J. The MAPK kinase Mkc1p is activated under different stress conditions in Candida albicans. Microbiology 2005, 151, 2737–2749. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, M.; Ito, F.; Aoyama, T.; Sato-Okamoto, M.; Takahashi-Nakaguchi, A.; Chibana, H.; Shibata, N. KRE5 suppression induces cell wall stress and alternative ER stress response required for maintaining cell wall integrity in Candida glabrata. PLoS ONE 2016, 11, e0161371. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.A.; Jung, K.W.; Chen, Y.L.; Heitman, J.; Bahn, Y.S.; Kang, H.A. Unique evolution of the UPR pathway with a novel bZIP transcription factor, Hxl1, for controlling pathogenicity of Cryptococcus neoformans. PLoS Pathog. 2011, 7, e1002177. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Krishnan, K.; Richis, D.L.; Aimanianda, V.; Hartl, L.; Grahl, N.; Powers-Fletcher, M.V.; Zhang, M.; Fuller, K.K.; Nierman, W.C.; et al. HacA-independent functions of the ER stress sensor IreA synergize with the canonical UPR to influence virulence traits in Aspergillus fumigatus. PLoS Pathog. 2011, 7, e1002330. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The potential of human peptide LL-37 as an antimicrobial and anti-biofilm agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.; Carter, D.E.; Lajoie, P. Hyperactive TORC1 sensitizes yeast cells to endoplasmic reticulum stress by compromising cell wall integrity. FEBS Lett. 2019, 593, 1957–1973. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, M.; Ito, F.; Aoyama, T.; Sato-Okamoto, M.; Takahashi-Nakaguchi, A.; Chibana, H.; Shibata, N. Cooperation between ER stress and calcineurin signaling contributes to the maintenance of cell wall integrity in Candida glabrata. Fungal Biol. 2018, 122, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Sircaik, S.; Roman, E.; Brunel, J.M.; Kohri, A.K.; Pla, J.; Panwar, S.L. The activity of RTA2, a downstream effector of the calcineurin pathway, is required during tunicamycin-induced ER stress response in Candida albicans. FEMS Yeast Res. 2015, 15, fov095. [Google Scholar] [CrossRef] [PubMed]
- Reuss, O.; Vik, A.; Kolter, R.; Morschhäuser, J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 2004, 341, 119–127. [Google Scholar] [CrossRef]
- Rothstein, D.M.; Spacciapoli, P.; Tran, L.T.; Xu, T.; Roberts, F.D.; Serra, M.D.; Buxton, D.K.; Oppenheim, F.G.; Friden, P. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob. Agents Chemother. 2001, 45, 1367–1373. [Google Scholar] [CrossRef]
- Tsai, C.C.; Kuo, T.Y.; Hong, Z.W.; Yeh, Y.C.; Shih, K.S.; Du, S.Y.; Fu, H.W. Helicobacter pylori neutrophil-activating protein induces release of histamine and interleukin-6 through G protein-mediated MAPKs and PI3K/Akt pathways in HMC-1 cells. Virulence 2015, 6, 755–765. [Google Scholar] [CrossRef]
- Tsao, C.C.; Chen, Y.T.; Lan, C.T. A small G protein Rhb1 and a GTPase-activating protein Tsc2 involved in nitrogen starvation-induced morphogenesis and cell wall integrity of Candida albicans. Fungal Genet. Biol. 2009, 46, 126–136. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Hsu, P.C.; Yang, C.Y.; Lan, C.Y. Candida albicans Hap43 is a repressor induced under low-iron conditions and is essential for iron-responsive transcriptional regulation and virulence. Eukaryot. Cell 2011, 10, 207–225. [Google Scholar] [CrossRef]
- Nailis, H.; Coenya, T.; Van Nieuwerburgh, F.; Deforce, D.; Neils, H.J. Development and evaluation of different normalization strategies for gene expression studies in Candida albicans biofilms by real-time PCR. BMC Mol. Biol. 2006, 7, 25. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Sevier, C.S.; Qu, H.; Heldman, N.; Gross, E.; Fass, D.; Kaiser, C.A. Modulation of cellular disulfide-bond formation and the ER redox environment by feedback regulation of Ero1. Cell 2007, 129, 333–344. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-M.; Liao, Y.-L.; Chang, C.-K.; Lan, C.-Y. Candida albicans Sfp1 Is Involved in the Cell Wall and Endoplasmic Reticulum Stress Responses Induced by Human Antimicrobial Peptide LL-37. Int. J. Mol. Sci. 2021, 22, 10633. https://doi.org/10.3390/ijms221910633
Hsu C-M, Liao Y-L, Chang C-K, Lan C-Y. Candida albicans Sfp1 Is Involved in the Cell Wall and Endoplasmic Reticulum Stress Responses Induced by Human Antimicrobial Peptide LL-37. International Journal of Molecular Sciences. 2021; 22(19):10633. https://doi.org/10.3390/ijms221910633
Chicago/Turabian StyleHsu, Chun-Min, Yi-Ling Liao, Che-Kang Chang, and Chung-Yu Lan. 2021. "Candida albicans Sfp1 Is Involved in the Cell Wall and Endoplasmic Reticulum Stress Responses Induced by Human Antimicrobial Peptide LL-37" International Journal of Molecular Sciences 22, no. 19: 10633. https://doi.org/10.3390/ijms221910633
APA StyleHsu, C.-M., Liao, Y.-L., Chang, C.-K., & Lan, C.-Y. (2021). Candida albicans Sfp1 Is Involved in the Cell Wall and Endoplasmic Reticulum Stress Responses Induced by Human Antimicrobial Peptide LL-37. International Journal of Molecular Sciences, 22(19), 10633. https://doi.org/10.3390/ijms221910633





