Imatinib Regulates miR-483-3p and Mitochondrial Respiratory Complexes in Gastrointestinal Stromal Tumors
Abstract
:1. Introduction
2. Results
2.1. Differentially Expressed miRNAs between Imatinib-Treated and Untreated GISTs
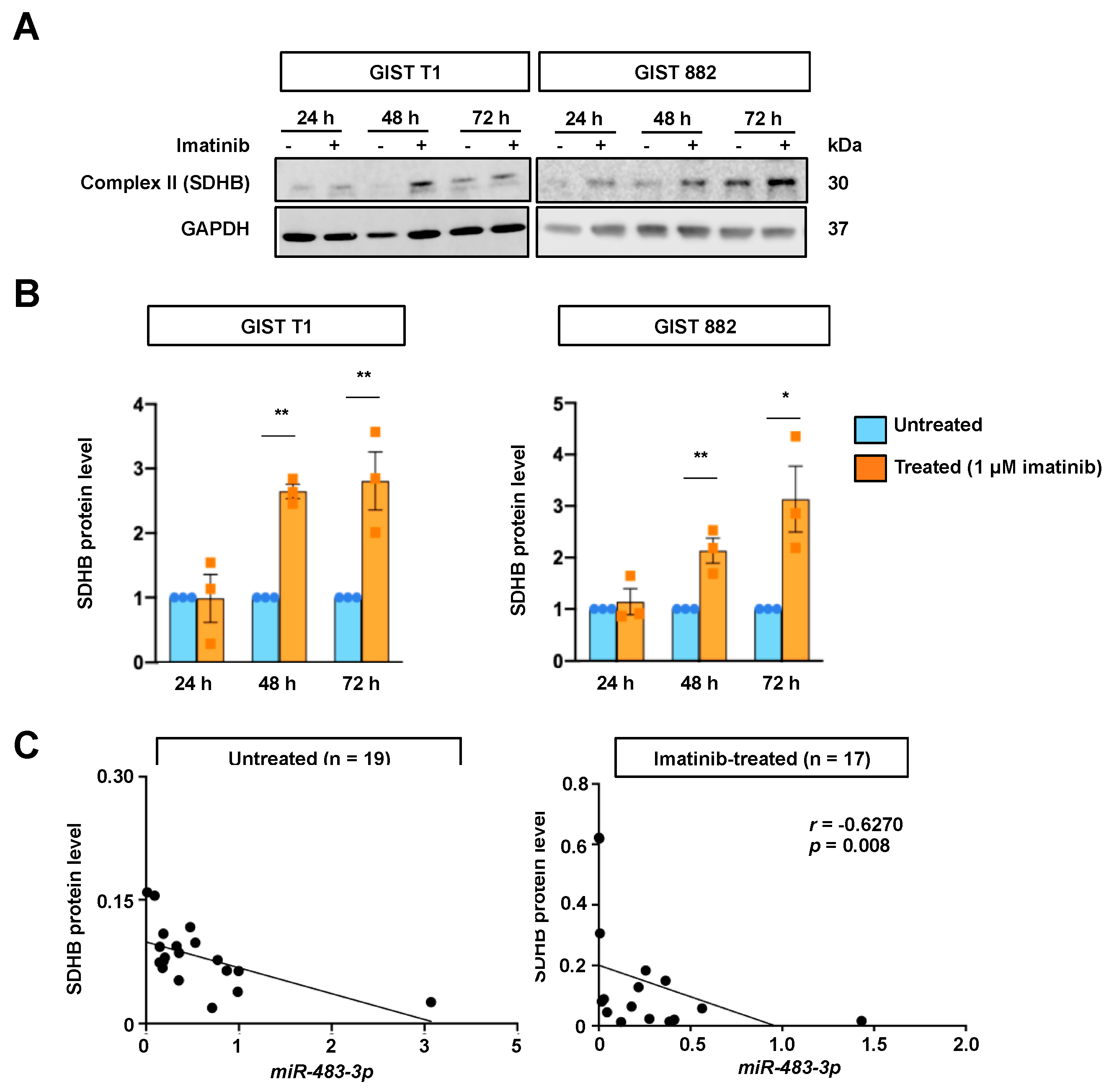
2.2. miR-320b and miR-483-3p Expression in GIST Cells upon Imatinib Treatment
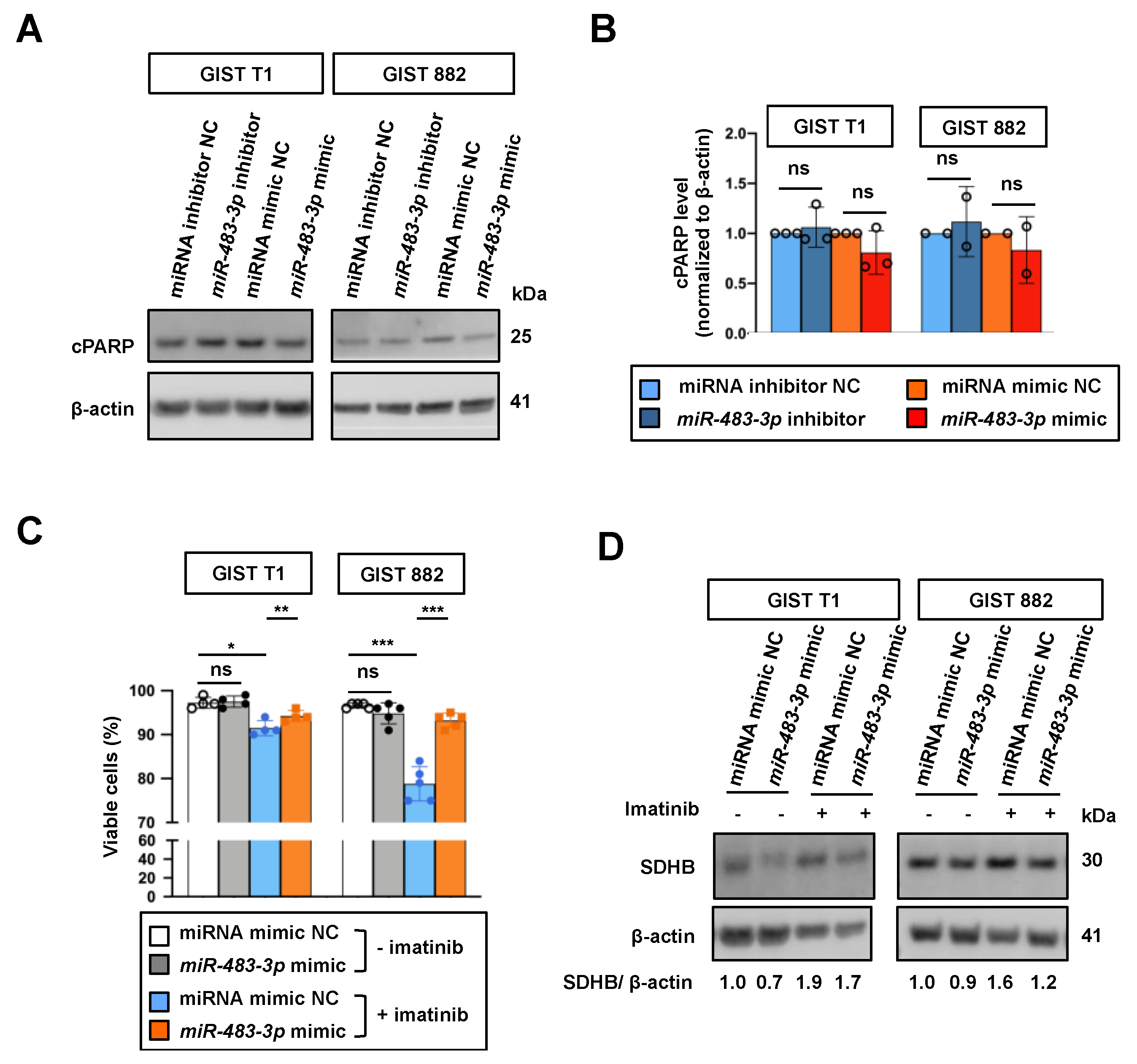
2.3. Effects of miR-483-3p on OXPHOS Protein Expressions
2.4. Relationship between miR-483-3p and SDHB Expression upon Imatinib Treatment
2.5. Functional Role of miR-483-3p in Regulation of Cell Viability upon Imatinib Treatment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Clinical Samples
5.2. GIST Cell Lines and Imatinib Treatment
5.3. RNA Extraction and Quantification
5.4. miRNA Expression Profiling
5.5. Reverse Transcription Quantitative PCR (RT-qPCR)
5.6. In Vitro Transfection of GIST Cells and Cell Viability Analysis
5.7. Western Blot Analysis
5.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Von Mehren, M.; Joensuu, H. Gastrointestinal Stromal Tumors. J. Clin. Oncol. 2018, 36, 136–143. [Google Scholar] [CrossRef]
- Blanke, C.D.; Demetri, G.D.; von Mehren, M.; Heinrich, M.C.; Eisenberg, B.; Fletcher, J.A.; Corless, C.L.; Fletcher, C.D.; Roberts, P.J.; Heinz, D.; et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J. Clin. Oncol. 2008, 26, 620–625. [Google Scholar] [CrossRef]
- Patrikidou, A.; Chabaud, S.; Ray-Coquard, I.; Bui, B.N.; Adenis, A.; Rios, M.; Bertucci, F.; Duffaud, F.; Chevreau, C.; Cupissol, D.; et al. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: Results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann. Oncol. 2013, 24, 1087–1093. [Google Scholar] [CrossRef]
- Gold, J.S.; van der Zwan, S.M.; Gonen, M.; Maki, R.G.; Singer, S.; Brennan, M.F.; Antonescu, C.R.; De Matteo, R.P. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann. Surg. Oncol. 2007, 14, 134–142. [Google Scholar] [CrossRef]
- DeMatteo, R.P.; Lewis, J.J.; Leung, D.; Mudan, S.S.; Woodruff, J.M.; Brennan, M.F. Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann. Surg. 2000, 231, 51–58. [Google Scholar] [CrossRef]
- Chen, P.; Zong, L.; Zhao, W.; Shi, L. Efficacy evaluation of imatinib treatment in patients with gastrointestinal stromal tumors: A meta-analysis. World J. Gastroenterol. 2010, 16, 4227–4232. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic reprogramming and cancer progression. Science 2020, 368, eaaw5473. [Google Scholar] [CrossRef]
- Farge, T.; Saland, E.; de Toni, F.; Aroua, N.; Hosseini, M.; Perry, R.; Bosc, C.; Sugita, M.; Stuani, L.; Fraisse, M.; et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017, 7, 716–735. [Google Scholar] [CrossRef] [Green Version]
- Hirpara, J.; Eu, J.Q.; Tan, J.K.M.; Wong, A.L.; Clement, M.V.; Kong, L.R.; Ohi, N.; Tsunoda, T.; Qu, J.; Goh, B.C.; et al. Metabolic reprogramming of oncogene-addicted cancer cells to OXPHOS as a mechanism of drug resistance. Redox Biol. 2019, 25, 101076. [Google Scholar] [CrossRef]
- Huang, W.K.; Gao, J.; Chen, Z.; Shi, H.; Yuan, J.; Cui, H.L.; Yeh, C.N.; Branstrom, R.; Larsson, C.; Li, S.; et al. Heterogeneity of Metabolic Vulnerability in Imatinib -Resistant Gastrointestinal Stromal Tumor. Cells 2020, 9, 1333. [Google Scholar] [CrossRef]
- Vitiello, G.A.; Medina, B.D.; Zeng, S.; Bowler, T.G.; Zhang, J.Q.; Loo, J.K.; Param, N.J.; Liu, M.; Moral, A.J.; Zhao, J.N.; et al. Mitochondrial Inhibition Augments the Efficacy of Imatinib by Resetting the Metabolic Phenotype of Gastrointestinal Stromal Tumor. Clin. Cancer Res. 2018, 24, 972–984. [Google Scholar] [CrossRef] [Green Version]
- Puissegur, M.P.; Mazure, N.M.; Bertero, T.; Pradelli, L.; Grosso, S.; Robbe-Sermesant, K.; Maurin, T.; Lebrigand, K.; Cardinaud, B.; Hofman, V.; et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011, 18, 465–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakada, C.; Hijiya, N.; Tsukamoto, Y.; Yano, S.; Kai, T.; Uchida, T.; Kimoto, M.; Takahashi, M.; Daa, T.; Matsuura, K.; et al. A transgenic mouse expressing miR-210 in proximal tubule cells shows mitochondrial alteration: Possible association of miR-210 with a shift in energy metabolism. J. Pathol. 2020, 251, 12–25. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Zhang, H.; Huang, P.; Luthra, R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 2010, 29, 4362–4368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Ferlito, M.; Kent, O.A.; Fox-Talbot, K.; Wang, R.; Liu, D.; Raghavachari, N.; Yang, Y.; Wheelan, S.J.; Murphy, E.; et al. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ. Res. 2012, 110, 1596–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aschrafi, A.; Schwechter, A.D.; Mameza, M.G.; Natera-Naranjo, O.; Gioio, A.E.; Kaplan, B.B. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J. Neurosci. 2008, 28, 12581–12590. [Google Scholar] [CrossRef] [PubMed]
- Willers, I.M.; Martinez-Reyes, I.; Martinez-Diez, M.; Cuezva, J.M. miR-127-5p targets the 3’UTR of human beta-F1-ATPase mRNA and inhibits its translation. Biochim. Biophys. Acta 2012, 1817, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Baseler, W.A.; Thapa, D.; Jagannathan, R.; Dabkowski, E.R.; Croston, T.L.; Hollander, J.M. miR-141 as a regulator of the mitochondrial phosphate carrier (Slc25a3) in the type 1 diabetic heart. Am. J. Physiol. Cell Physiol. 2012, 303, C1244–C1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepe, F.; Visone, R.; Veronese, A. The Glucose-Regulated MiR-483-3p Influences Key Signaling Pathways in Cancer. Cancers 2018, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Sarmento-Ribeiro, A.B.; Scorilas, A.; Goncalves, A.C.; Efferth, T.; Trougakos, I.P. The emergence of drug resistance to targeted cancer therapies: Clinical evidence. Drug Resist. Updates 2019, 47, 100646. [Google Scholar] [CrossRef]
- Valcarcel-Jimenez, L.; Gaude, E.; Torrano, V.; Frezza, C.; Carracedo, A. Mitochondrial Metabolism: Yin and Yang for Tumor Progression. Trends Endocrinol. Metab. 2017, 28, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, W.; Ma, J.; Zhang, H.; Li, Z.; Zhang, L.; Liu, J.; Han, Z.; Wang, H.; Hong, L. Role of miR-483 in digestive tract cancers: From basic research to clinical value. J. Cancer 2018, 9, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Ozata, D.M.; Caramuta, S.; Velazquez-Fernandez, D.; Akcakaya, P.; Xie, H.; Hoog, A.; Zedenius, J.; Backdahl, M.; Larsson, C.; Lui, W.O. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr. Relat. Cancer 2011, 18, 643–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Zhang, S.; Zhou, Y.; Hu, X.; Shao, C. MicroRNA 483-3p suppresses the expression of DPC4/Smad4 in pancreatic cancer. FEBS Lett. 2011, 585, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veronese, A.; Lupini, L.; Consiglio, J.; Visone, R.; Ferracin, M.; Fornari, F.; Zanesi, N.; Alder, H.; D’Elia, G.; Gramantieri, L.; et al. Oncogenic role of miR-483-3p at the IGF2/483 locus. Cancer Res. 2010, 70, 3140–3149. [Google Scholar] [CrossRef] [Green Version]
- Veronese, A.; Visone, R.; Consiglio, J.; Acunzo, M.; Lupini, L.; Kim, T.; Ferracin, M.; Lovat, F.; Miotto, E.; Balatti, V.; et al. Mutated beta-catenin evades a microRNA-dependent regulatory loop. Proc. Natl. Acad. Sci. USA 2011, 108, 4840–4845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korzeniewski, N.; Tosev, G.; Pahernik, S.; Hadaschik, B.; Hohenfellner, M.; Duensing, S. Identification of cell-free microRNAs in the urine of patients with prostate cancer. Urol. Oncol. 2015, 33, 16.e17–16.e22. [Google Scholar] [CrossRef]
- Yi, C.; Wang, Q.; Wang, L.; Huang, Y.; Li, L.; Liu, L.; Zhou, X.; Xie, G.; Kang, T.; Wang, H.; et al. MiR-663, a microRNA targeting p21(WAF1/CIP1), promotes the proliferation and tumorigenesis of nasopharyngeal carcinoma. Oncogene 2012, 31, 4421–4433. [Google Scholar] [CrossRef] [Green Version]
- Menbari, M.N.; Rahimi, K.; Ahmadi, A.; Mohammadi-Yeganeh, S.; Elyasi, A.; Darvishi, N.; Hosseini, V.; Abdi, M. miR-483-3p suppresses the proliferation and progression of human triple negative breast cancer cells by targeting the HDAC8>oncogene. J. Cell Physiol. 2020, 235, 2631–2642. [Google Scholar] [CrossRef]
- Yu, F.Y.; Zhou, C.Y.; Liu, Y.B.; Wang, B.; Mao, L.; Li, Y. miR-483 is down-regulated in gastric cancer and suppresses cell proliferation, invasion and protein O-GlcNAcylation by targeting OGT. Neoplasma 2018, 65, 406–414. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, J.; Kuang, D.; Wang, X.; Zhu, Y.; Xu, S.; Chen, Y.; Cheng, H.; Zhao, Q.; Duan, Y.; et al. miR-148b-3p functions as a tumor suppressor in GISTs by directly targeting KIT. Cell Commun. Signal. 2018, 16, 16. [Google Scholar] [CrossRef] [Green Version]
- Killian, J.K.; Miettinen, M.; Walker, R.L.; Wang, Y.; Zhu, Y.J.; Waterfall, J.J.; Noyes, N.; Retnakumar, P.; Yang, Z.; Smith, W.I., Jr.; et al. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci. Transl. Med. 2014, 6, 268ra177. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs)-a review. Int. J. Biochem. Cell Biol. 2014, 53, 514–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lasota, J.; Wang, Z.; Kim, S.Y.; Helman, L.; Miettinen, M. Expression of the receptor for type i insulin-like growth factor (IGF1R) in gastrointestinal stromal tumors: An immunohistochemical study of 1078 cases with diagnostic and therapeutic implications. Am. J. Surg. Pathol. 2013, 37, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Pepe, F.; Pagotto, S.; Soliman, S.; Rossi, C.; Lanuti, P.; Braconi, C.; Mariani-Costantini, R.; Visone, R.; Veronese, A. Regulation of miR-483-3p by the O-linked N-acetylglucosamine transferase links chemosensitivity to glucose metabolism in liver cancer cells. Oncogenesis 2017, 6, e328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottschalk, S.; Anderson, N.; Hainz, C.; Eckhardt, S.G.; Serkova, N.J. Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin. Cancer Res. 2004, 10, 6661–6668. [Google Scholar] [CrossRef] [Green Version]
- van Nederveen, F.H.; Gaal, J.; Favier, J.; Korpershoek, E.; Oldenburg, R.A.; de Bruyn, E.M.; Sleddens, H.F.; Derkx, P.; Riviere, J.; Dannenberg, H.; et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: A retrospective and prospective analysis. Lancet Oncol. 2009, 10, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Gill, A.J. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 2018, 72, 106–116. [Google Scholar] [CrossRef]
- Zhang, W.C.; Wells, J.M.; Chow, K.H.; Huang, H.; Yuan, M.; Saxena, T.; Melnick, M.A.; Politi, K.; Asara, J.M.; Costa, D.B.; et al. miR-147b-mediated TCA cycle dysfunction and pseudohypoxia initiate drug tolerance to EGFR inhibitors in lung adenocarcinoma. Nat. Metab. 2019, 1, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.S.; Hajira, A.; Pardo, P.S.; Boriek, A.M. MicroRNA-149 inhibits PARP-2 and promotes mitochondrial biogenesis via SIRT-1/PGC-1alpha network in skeletal muscle. Diabetes 2014, 63, 1546–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akcakaya, P.; Caramuta, S.; Ahlen, J.; Ghaderi, M.; Berglund, E.; Ostman, A.; Branstrom, R.; Larsson, C.; Lui, W.O. microRNA expression signatures of gastrointestinal stromal tumours: Associations with imatinib resistance and patient outcome. Br. J. Cancer 2014, 111, 2091–2102. [Google Scholar] [CrossRef] [Green Version]
- Caramuta, S.; Egyhazi, S.; Rodolfo, M.; Witten, D.; Hansson, J.; Larsson, C.; Lui, W.O. MicroRNA expression profiles associated with mutational status and survival in malignant melanoma. J. Investig. Dermatol. 2010, 130, 2062–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
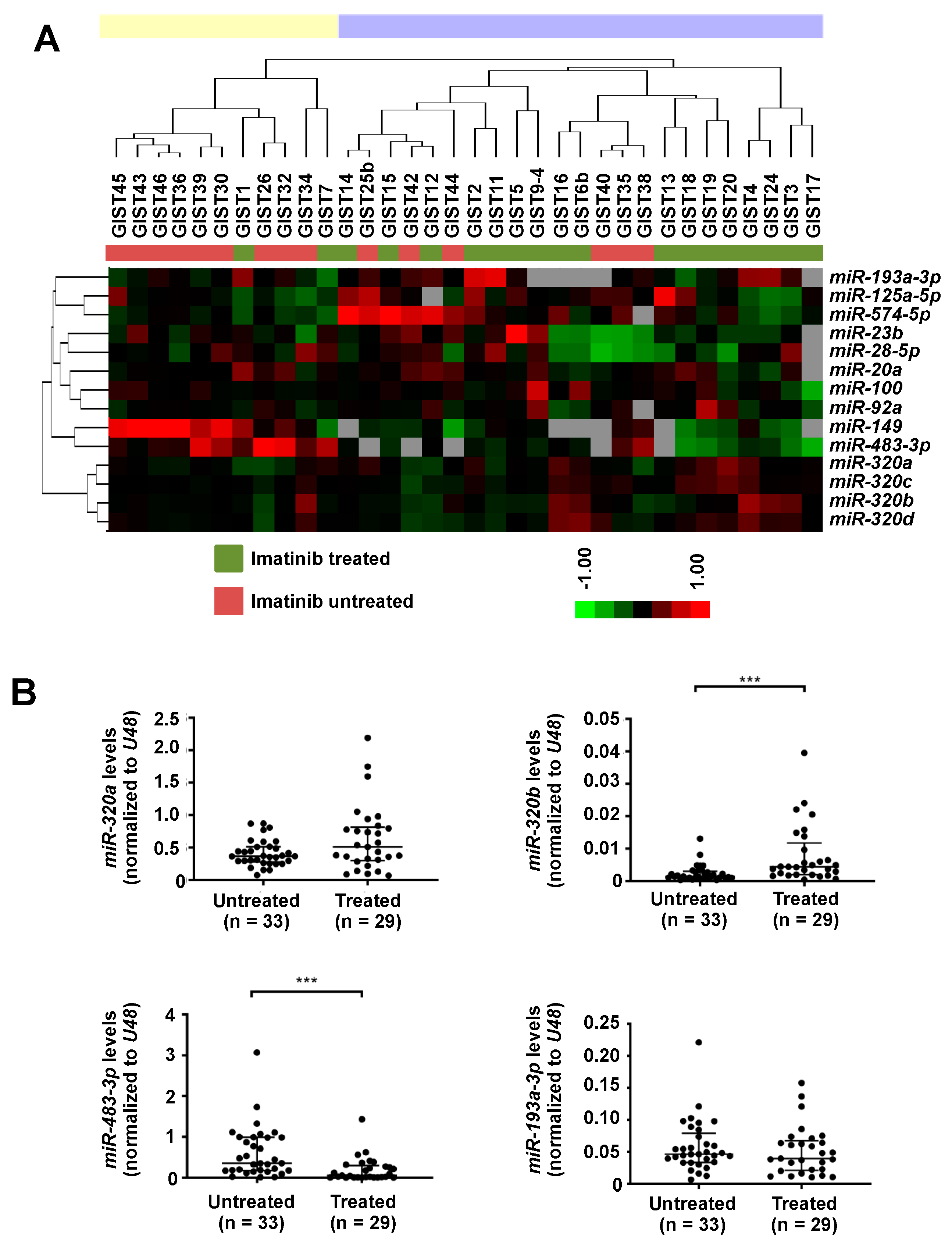


| miRNA | Score (d) | FDR q-Value (%) |
|---|---|---|
| Over-expressed in imatinib-treated GISTs | ||
| hsa-miR-320a | 2.02 | 0.00 |
| hsa-miR-193a-3p | 1.87 | 0.00 |
| hsa-miR-320d | 1.80 | 0.00 |
| hsa-miR-320c | 1.76 | 0.00 |
| hsa-miR-320b | 1.44 | 12.33 |
| hsa-miR-574-5p | 1.42 | 12.33 |
| hsa-miR-23b | 1.38 | 12.33 |
| hsa-miR-125a-3p | 1.37 | 12.33 |
| hsa-miR-100 | 1.34 | 12.33 |
| hsa-miR-20a | 1.34 | 12.33 |
| hsa-miR-28-5p | 1.30 | 12.33 |
| hsa-miR-92a | 1.30 | 12.33 |
| Under-expressed in imatinib-treated GISTs | ||
| hsa-miR-149 | −2.69 | 0.00 |
| hsa-miR-483-3p | −2.63 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-K.; Shi, H.; Akçakaya, P.; Zeljic, K.; Gangaev, A.; Caramuta, S.; Yeh, C.-N.; Bränström, R.; Larsson, C.; Lui, W.-O. Imatinib Regulates miR-483-3p and Mitochondrial Respiratory Complexes in Gastrointestinal Stromal Tumors. Int. J. Mol. Sci. 2021, 22, 10600. https://doi.org/10.3390/ijms221910600
Huang W-K, Shi H, Akçakaya P, Zeljic K, Gangaev A, Caramuta S, Yeh C-N, Bränström R, Larsson C, Lui W-O. Imatinib Regulates miR-483-3p and Mitochondrial Respiratory Complexes in Gastrointestinal Stromal Tumors. International Journal of Molecular Sciences. 2021; 22(19):10600. https://doi.org/10.3390/ijms221910600
Chicago/Turabian StyleHuang, Wen-Kuan, Hao Shi, Pinar Akçakaya, Katarina Zeljic, Anastasia Gangaev, Stefano Caramuta, Chun-Nan Yeh, Robert Bränström, Catharina Larsson, and Weng-Onn Lui. 2021. "Imatinib Regulates miR-483-3p and Mitochondrial Respiratory Complexes in Gastrointestinal Stromal Tumors" International Journal of Molecular Sciences 22, no. 19: 10600. https://doi.org/10.3390/ijms221910600
APA StyleHuang, W.-K., Shi, H., Akçakaya, P., Zeljic, K., Gangaev, A., Caramuta, S., Yeh, C.-N., Bränström, R., Larsson, C., & Lui, W.-O. (2021). Imatinib Regulates miR-483-3p and Mitochondrial Respiratory Complexes in Gastrointestinal Stromal Tumors. International Journal of Molecular Sciences, 22(19), 10600. https://doi.org/10.3390/ijms221910600








