Mesenchymal Stem Cell Transplantation for the Treatment of Age-Related Musculoskeletal Frailty
Abstract
:1. Introduction
2. Aging in Muscle
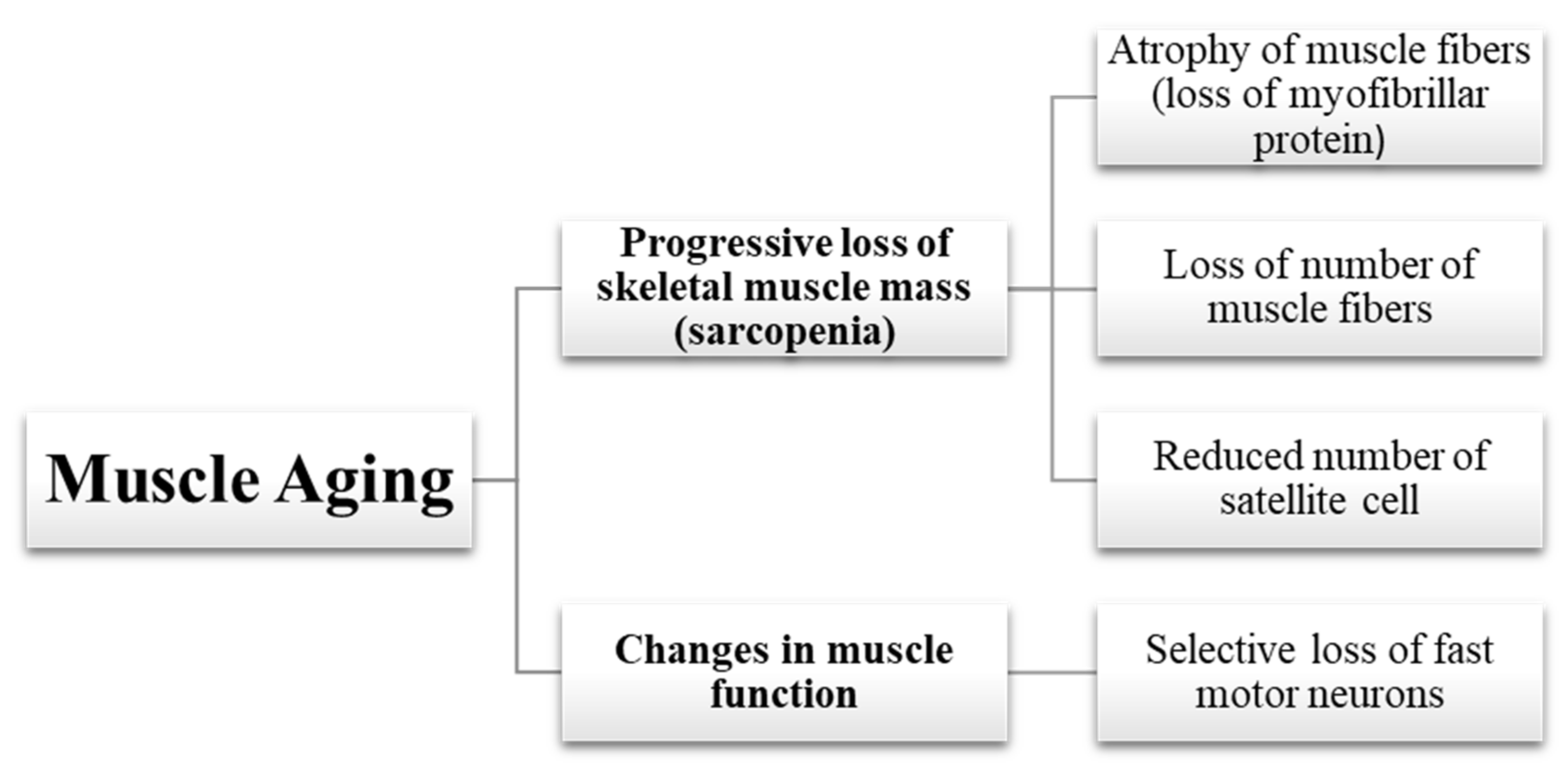
2.1. Loss of Muscle Mass
2.1.1. Atrophy of Muscle Fibers
2.1.2. Loss of Muscle Fibers
2.1.3. Reduced Number of Satellite Cells
2.2. Changes in Muscle Function
2.3. The Muscle-Bone Relationship and Aging
3. Aging in Bone
3.1. Gonadocorticoids and Age-Associated Hypogonadism
3.2. Diminished Osteoblast Viability
3.3. Increased Osteoclast Activity
4. Mesenchymal Stem Cells Transplantation for Musculoskeletal Aging Frailty
4.1. Mesenchymal Stem Cells (MSCs)
4.2. Mechanism of Actions of MSCs
4.2.1. Immunomodulatory Properties of MSC Facilitate Allogeneic Transplantation
4.2.2. Exosomes and Extracellular Vesicles
4.2.3. Mitochondrial Transfer
4.3. MSCs to Treat Musculoskeletal Frailty
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Department of Economic and Social Affairs of the United Nations. World Population Ageing 2019; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2019; ISBN 9789211483253. [Google Scholar]
- Crimmins, E.M.; Beltrán-Sánchez, H. Mortality and Morbidity Trends: Is There Compression of Morbidity? J. Gerontol. Ser. B 2011, 66B, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Florea, V.; Bagno, L.; Rieger, A.C.; Hare, J.M. Attenuation of frailty in older adults with mesenchymal stem cells. Mech. Ageing Dev. 2019, 181, 47–58. [Google Scholar] [CrossRef]
- Church, S.; Rogers, E.; Rockwood, K.; Theou, O. A scoping review of the Clinical Frailty Scale. BMC Geriatr. 2020, 20, 393. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Soysal, P.; Stubbs, B.; Lucato, P.; Luchini, C.; Solmi, M.; Peluso, R.; Sergi, G.; Isik, A.T.; Manzato, E.; Maggi, S.; et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 2016, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Deschenes, M.R. Effects of Aging on Muscle Fibre Type and Size. Sports Med. 2004, 34, 809–824. [Google Scholar] [CrossRef]
- Szulc, P.; Duboeuf, F.; Marchand, F.; Delmas, P.D. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: The MINOS study. Am. J. Clin. Nutr. 2004, 80, 496–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, P.L.; Dias, D.P.M.; Silva, L.E.V.; Virtuoso-Junior, J.S.; Marocolo, M. Cardiac autonomic modulation in non-frail, pre-frail and frail elderly women: A pilot study. Aging Clin. Exp. Res. 2015, 27, 621–629. [Google Scholar] [CrossRef]
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med Dir. Assoc. 2013, 14, 877–882. [Google Scholar] [CrossRef]
- Oliva, A.A.; McClain-Moss, L.; Pena, A.; Drouillard, A.; Hare, J.M. Allogeneic mesenchymal stem cell therapy: A regenerative medicine approach to geroscience. Aging Med. 2019, 2, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Golpanian, S.; DiFede, D.L.; Pujol, M.V.; Lowery, M.H.; Levis-Dusseau, S.; Goldstein, B.J.; Schulman, I.H.; Longsomboon, B.; Wolf, A.; Khan, A.; et al. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase I/II, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty. Oncotarget 2016, 7, 11899–11912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Liu, J.; Liao, G.; Zhang, J.; Chen, Y.; Li, L.; Li, L.; Liu, F.; Chen, B.; Guo, G.; et al. Early intervention with mesenchymal stem cells prevents nephropathy in diabetic rats by ameliorating the inflammatory microenvironment. Int. J. Mol. Med. 2018, 41, 2629–2639. [Google Scholar] [CrossRef] [Green Version]
- Liau, L.L.; Looi, Q.H.; Eng, S.P.; Yazid, M.D.; Sulaiman, N.; Busra, M.F.M.; Ng, M.H.; Law, J.X. Mesenchymal stem cells for the treatment of immune-mediated diseases. In Stem Cells; World Scientific: Singapore, 2020; pp. 178–210. [Google Scholar]
- Milosavljevic, N.; Gazdic, M.; Simovic Markovic, B.; Arsenijevic, A.; Nurkovic, J.; Dolicanin, Z.; Djonov, V.; Lukic, M.L.; Volarevic, V. Mesenchymal stem cells attenuate acute liver injury by altering ratio between interleukin 17 producing and regulatory natural killer T cells. Liver Transplant. 2017, 23, 1040–1050. [Google Scholar] [CrossRef] [Green Version]
- Bartunek, J.; Wojakowski, W. Intracoronary autologous bone marrow cell transfer after acute myocardial infarction: Abort and refocus. Eur. Heart J. 2017, 38, 2944–2947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncalli, J.; Mouquet, F.; Piot, C.; Trochu, J.-N.; Le Corvoisier, P.; Neuder, Y.; Le Tourneau, T.; Agostini, D.; Gaxotte, V.; Sportouch, C.; et al. Intracoronary autologous mononucleated bone marrow cell infusion for acute myocardial infarction: Results of the randomized multicenter BONAMI trial. Eur. Heart J. 2010, 32, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Law, P.K.; Goodwin, T.G.; Fang, Q.; Deering, M.B.; Duggirala, V.; Larkin, C.; Florendo, J.A.; Kirby, D.S.; Li, H.J.; Chen, M.; et al. Cell Transplantation as an Experimental Treatment for Duchenne Muscular Dystrophy. Cell Transplant. 1993, 2, 485–505. [Google Scholar] [CrossRef]
- Uccelli, A.; Laroni, A.; Brundin, L.; Clanet, M.; Fernandez, O.; Nabavi, S.M.; Muraro, P.A.; Oliveri, R.S.; Radue, E.W.; Sellner, J.; et al. MEsenchymal StEm cells for Multiple Sclerosis (MESEMS): A randomized, double blind, cross-over phase I/II clinical trial with autologous mesenchymal stem cells for the therapy of multiple sclerosis. Trials 2019, 20, 263. [Google Scholar] [CrossRef] [Green Version]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Metter, E.J.; Talbot, L.A.; Schrager, M.; Conwit, R. Skeletal Muscle Strength as a Predictor of All-Cause Mortality in Healthy Men. J. Gerontol. Ser. A 2002, 57, B359–B365. [Google Scholar] [CrossRef] [PubMed]
- Suetta, C.; Hvid, L.G.; Justesen, L.; Christensen, U.; Neergaard, K.; Simonsen, L.; Ortenblad, N.; Magnusson, S.P.; Kjaer, M.; Aagaard, P. Effects of aging on human skeletal muscle after immobilization and retraining. J. Appl. Physiol. 2009, 107, 1172–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetta, C.; Aagaard, P.; Magnusson, S.P.; Andersen, L.L.; Sipilä, S.; Rosted, A.; Jakobsen, A.K.; Duus, B.; Kjaer, M. Muscle size, neuromuscular activation, and rapid force characteristics in elderly men and women: Effects of unilateral long-term disuse due to hip-osteoarthritis. J. Appl. Physiol. 2007, 102, 942–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontera, W.R.; Reid, K.F.; Phillips, E.M.; Krivickas, L.S.; Hughes, V.A.; Roubenoff, R.; Fielding, R.A. Muscle fiber size and function in elderly humans: A longitudinal study. J. Appl. Physiol. 2008, 105, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Nair, K.S. Aging muscle. Am. J. Clin. Nutr. 2005, 81, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Aniansson, A.; Hedberg, M.; Henning, G.-B.; Grimby, G. Muscle morphology, enzymatic activity, and muscle strength in elderly men: A follow-up study. Muscle Nerve 1986, 9, 585–591. [Google Scholar] [CrossRef]
- Coggan, A.R.; Spina, R.J.; King, D.S.; Rogers, M.A.; Rogers, M.A.; Brown, M.; Nemeth, P.M.; Holloszy, J.O. Histochemical and Enzymatic Comparison of the Gastrocnemius Muscle of Young and Elderly Men and Women. J. Gerontol. 1992, 47, B71–B76. [Google Scholar] [CrossRef]
- Goldspink, G.; Harridge, S.D.R. Growth factors and muscle ageing. Exp. Gerontol. 2004, 39, 1433–1438. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ’anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Dardevet, D.; Sornet, C.; Taillandier, D.; Savary, I.; Attaix, D.; Grizard, J. Sensitivity and protein turnover response to glucocorticoids are different in skeletal muscle from adult and old rats. Lack of regulation of the ubiquitin-proteasome proteolytic pathway in aging. J. Clin. Investig. 1995, 96, 2113–2119. [Google Scholar] [CrossRef] [Green Version]
- Nedergaard, A.; Henriksen, K.; Karsdal, M.A.; Christiansen, C. Musculoskeletal ageing and primary prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 673–688. [Google Scholar] [CrossRef]
- Delbono, O. Neural control of aging skeletal muscle. Aging Cell 2003, 2, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Biressi, S.; Rando, T.A. Heterogeneity in the muscle satellite cell population. Semin. Cell Dev. Biol. 2010, 21, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Victor, P.; García-Prat, L.; Serrano, A.L.; Perdiguero, E.; Muñoz-Cánoves, P. Muscle stem cell aging: Regulation and rejuvenation. Trends Endocrinol. Metab. 2015, 26, 287–296. [Google Scholar] [CrossRef] [PubMed]
- García-Prat, L.; Sousa-Victor, P.; Muñoz-Cánoves, P. Functional dysregulation of stem cells during aging: A focus on skeletal muscle stem cells. FEBS J. 2013, 280, 4051–4062. [Google Scholar] [CrossRef] [PubMed]
- Jejurikar, S.S.; Henkelman, E.A.; Cederna, P.S.; Marcelo, C.L.; Urbanchek, M.G.; Kuzon, W.M. Aging increases the susceptibility of skeletal muscle derived satellite cells to apoptosis. Exp. Gerontol. 2006, 41, 828–836. [Google Scholar] [CrossRef]
- Chakkalakal, J.V.; Jones, K.M.; Basson, M.A.; Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 2012, 490, 355–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadrach, J.L.; Wagers, A.J. Stem cells for skeletal muscle repair. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2297–2306. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Ashton-Miller, J.A.; Alexander, N.B. Age-related changes in speed and accuracy during rapid targeted center of pressure movements near the posterior limit of the base of support. Clin. Biomech. 2012, 27, 910–916. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, A.; Muller, F.L.; Liu, Y.; Ng, R.; Faulkner, J.; Hamilton, M.; Richardson, A.; Huang, T.-T.; Epstein, C.J.; Van Remmen, H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech. Ageing Dev. 2006, 127, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Models Mech. 2013, 6, 1339–1352. [Google Scholar] [CrossRef] [Green Version]
- Houtkooper, R.H.; Argmann, C.; Houten, S.M.; Cantó, C.; Jeninga, E.H.; Andreux, P.A.; Thomas, C.; Doenlen, R.; Schoonjans, K.; Auwerx, J. The metabolic footprint of aging in mice. Sci. Rep. 2011, 1, 134. [Google Scholar] [CrossRef] [PubMed]
- Doherty, K.R.; McNally, E.M. Repairing the tears: Dysferlin in muscle membrane repair. Trends Mol. Med. 2003, 9, 327–330. [Google Scholar] [CrossRef]
- Altun, M.; Besche, H.C.; Overkleeft, H.S.; Piccirillo, R.; Edelmann, M.J.; Kessler, B.M.; Goldberg, A.L.; Ulfhake, B. Muscle Wasting in Aged, Sarcopenic Rats Is Associated with Enhanced Activity of the Ubiquitin Proteasome Pathway. J. Biol. Chem. 2010, 285, 39597–39608. [Google Scholar] [CrossRef] [Green Version]
- Min, J.-N.; Whaley, R.A.; Sharpless, N.E.; Lockyer, P.; Portbury, A.L.; Patterson, C. CHIP Deficiency Decreases Longevity, with Accelerated Aging Phenotypes Accompanied by Altered Protein Quality Control. Mol. Cell. Biol. 2008, 28, 4018–4025. [Google Scholar] [CrossRef] [Green Version]
- Accili, D.; Arden, K.C. FoxOs at the Crossroads of Cellular Metabolism, Differentiation, and Transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Forwood, M.R.; Burr, D.B. Physical activity and bone mass: Exercises in futility? Bone Miner. 1993, 21, 89–112. [Google Scholar] [CrossRef]
- Pearson, O.M.; Lieberman, D.E. The aging of Wolff’s “law”: Ontogeny and responses to mechanical loading in cortical bone. Am. J. Phys. Anthropol. 2004, 125, 63–99. [Google Scholar] [CrossRef]
- Novotny, S.A.; Warren, G.L.; Hamrick, M.W. Aging and the Muscle-Bone Relationship. Physiology 2015, 30, 8–16. [Google Scholar] [CrossRef]
- Hamrick, M.W. A role for myokines in muscle-bone interactions. Exerc. Sport Sci. Rev. 2011, 39, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Marie, P.J.; Kassem, M. Extrinsic Mechanisms Involved in Age-Related Defective Bone Formation. J. Clin. Endocrinol. Metab. 2011, 96, 600–609. [Google Scholar] [CrossRef] [Green Version]
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2012, 4, 61–76. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, K.; Neutzsky-Wulff, A.V.; Bonewald, L.F.; Karsdal, M.A. Local communication on and within bone controls bone remodeling. Bone 2009, 44, 1026–1033. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Martin, T.J.; Bollerslev, J.; Christiansen, C.; Henriksen, K. Are Nonresorbing Osteoclasts Sources of Bone Anabolic Activity? J. Bone Miner. Res. 2007, 22, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Delmas, P.D. Bone Quality—The Material and Structural Basis of Bone Strength and Fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast Activity and Subtypes as a Function of Physiology and Pathology—Implications for Future Treatments of Osteoporosis. Endocr. Rev. 2011, 32, 31–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeming, D.J.; Henriksen, K.; Byrjalsen, I.; Qvist, P.; Madsen, S.H.; Garnero, P.; Karsdal, M.A. Is bone quality associated with collagen age? Osteoporos. Int. 2009, 20, 1461. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Birth and Death of Bone Cells: Basic Regulatory Mechanisms and Implications for the Pathogenesis and Treatment of Osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriksen, K.; Leeming, D.J.; Byrjalsen, I.; Nielsen, R.H.; Sorensen, M.G.; Dziegiel, M.H.; Martin, T.J.; Christiansen, C.; Qvist, P.; Karsdal, M.A. Osteoclasts prefer aged bone. Osteoporos. Int. 2007, 18, 751–759. [Google Scholar] [CrossRef]
- Fledelius, C.; Johnsen, A.H.; Cloos, P.A.C.; Bonde, M.; Qvist, P. Characterization of Urinary Degradation Products Derived from Type I Collagen: Identification of a β-isomerized asp-gly sequence within the c-terminal telopeptide (α1) region. J. Biol. Chem. 1997, 272, 9755–9763. [Google Scholar] [CrossRef] [Green Version]
- Cloos, P.A.C.; Fledelius, C.; Christgau, S.; Christiansen, C.; Engsig, M.; Delmas, P.; Body, J.J.; Garnero, P. Investigation of Bone Disease Using Isomerized and Racemized Fragments of Type I Collagen. Calcif. Tissue Int. 2003, 72, 8–17. [Google Scholar] [CrossRef]
- Cloos, P.A.C.; Lyubimova, N.; Solberg, H.; Qvist, P.; Christiansen, C.; Byrjalsen, I.; Christgau, S. An immunoassay for measuring fragments of newly synthesized collagen type I produced during metastatic invasion of bone. Clin. Lab. 2004, 50, 279–289. [Google Scholar]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos. Int. 2006, 17, 319–336. [Google Scholar] [CrossRef]
- Byrjalsen, I.; Leeming, D.J.; Qvist, P.; Christiansen, C.; Karsdal, M.A. Bone turnover and bone collagen maturation in osteoporosis: Effects of antiresorptive therapies. Osteoporos. Int. 2008, 19, 339–348. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.; Harris, S.T.; Miller, P.D.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; Kendler, D.L.; Yuen, C.K.; Lewiecki, E.M. Bisphosphonate Therapy for Osteoporosis: Benefits, Risks, and Drug Holiday. Am. J. Med. 2013, 126, 13–20. [Google Scholar] [CrossRef]
- Seeman, E. Pathogenesis of bone fragility in women and men. Lancet 2002, 359, 1841–1850. [Google Scholar] [CrossRef]
- Khosla, S. Estrogen and bone: Insights from estrogen-resistant, aromatase-deficient, and normal men. Bone 2008, 43, 414–417. [Google Scholar] [CrossRef] [Green Version]
- Khosla, S.; Melton, L.J., III; Atkinson, E.J.; O’Fallon, W.M. Relationship of Serum Sex Steroid Levels to Longitudinal Changes in Bone Density in Young Versus Elderly Men. J. Clin. Endocrinol. Metab. 2001, 86, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Merlotti, D.; Martini, G.; Gonnelli, S.; Franci, B.; Campagna, S.; Lucani, B.; Dal Canto, N.; Valenti, R.; Gennari, C.; et al. Longitudinal Association between Sex Hormone Levels, Bone Loss, and Bone Turnover in Elderly Men. J. Clin. Endocrinol. Metab. 2003, 88, 5327–5333. [Google Scholar] [CrossRef]
- Carani, C.; Qin, K.; Simoni, M.; Faustini-Fustini, M.; Serpente, S.; Boyd, J.; Korach, K.S.; Simpson, E.R. Effect of Testosterone and Estradiol in a Man with Aromatase Deficiency. N. Engl. J. Med. 1997, 337, 91–95. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Morishima, A.; Bell, J.; Grumbach, M.M. Increased Bone Mass as a Result of Estrogen Therapy in a Man with Aromatase Deficiency. N. Engl. J. Med. 1998, 339, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Tsai, Y.-T.; DiMarco, N.M.; Long, M.A.; Sun, X.; Tang, L. Transplantation of mesenchymal stem cells from young donors delays aging in mice. Sci. Rep. 2011, 1, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.J.; Wronski, T.J.; Iwaniec, U.; Phleger, L.; Kurimoto, P.; Boudignon, B.; Halloran, B.P. Aging Increases Stromal/Osteoblastic Cell-Induced Osteoclastogenesis and Alters the Osteoclast Precursor Pool in the Mouse. J. Bone Miner. Res. 2005, 20, 1659–1668. [Google Scholar] [CrossRef]
- Jevon, M.; Sabokbar, A.; Fujikawa, Y.; Hirayama, T.; Neale, S.; Wass, J.; Athanasou, N. Gender- and age-related differences in osteoclast formation from circulating precursors. J. Endocrinol. 2002, 172, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Chung, P.-L.; Zhou, S.; Eslami, B.; Shen, L.; LeBoff, M.S.; Glowacki, J. Effect of Age on Regulation of Human Osteoclast Differentiation. J. Cell. Biochem. 2014, 115, 1412–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartali, B.; Frongillo, E.A.; Bandinelli, S.; Lauretani, F.; Semba, R.D.; Fried, L.P.; Ferrucci, L. Low Nutrient Intake Is an Essential Component of Frailty in Older Persons. J. Gerontol. Ser. A 2006, 61, 589–593. [Google Scholar] [CrossRef]
- Theou, O.; Stathokostas, L.; Roland, K.P.; Jakobi, J.M.; Patterson, C.; Vandervoort, A.A.; Jones, G.R. The Effectiveness of Exercise Interventions for the Management of Frailty: A Systematic Review. J. Aging Res. 2011, 2011, 569194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clegg, A.P.; Barber, S.E.; Young, J.B.; Forster, A.; Iliffe, S.J. Do home-based exercise interventions improve outcomes for frail older people? Findings from a systematic review. Rev. Clin. Gerontol. 2012, 22, 68–78. [Google Scholar] [CrossRef]
- Pahor, M.; Kritchevsky, S.B.; Waters, D.L.; Villareal, D.T.; Morley, J.; Hare, J.M.; Vellas, B.; The, I.T.F. Designing Drug Trials for Frailty: ICFSR Task Force 2018. J. Frailty Aging 2018, 7, 150–154. [Google Scholar] [CrossRef]
- Florea, V.; Rieger, A.C.; DiFede, D.L.; El-Khorazaty, J.; Natsumeda, M.; Banerjee, M.N.; Tompkins, B.A.; Khan, A.; Schulman, I.H.; Landin, A.M.; et al. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study). Circ. Res. 2017, 121, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J. Am. Coll. Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.N.F.B.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 9529465. [Google Scholar] [CrossRef]
- Lim, J.; Razi, Z.R.M.; Law, J.X.; Nawi, A.M.; Idrus, R.B.H.; Chin, T.G.; Mustangin, M.; Ng, M.H. Mesenchymal Stromal Cells from the Maternal Segment of Human Umbilical Cord is Ideal for Bone Regeneration in Allogenic Setting. Tissue Eng. Regen. Med. 2018, 15, 75–87. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Sanz, A.R.; Carrión, F.S.; Chaparro, A.P. Mesenchymal stem cells from the oral cavity and their potential value in tissue engineering. Periodontology 2000 2015, 67, 251–267. [Google Scholar] [CrossRef]
- Yeo, G.E.C.; Ng, M.H.; Nordin, F.B.; Law, J.X. Potential of Mesenchymal Stem Cells in the Rejuvenation of the Aging Immune System. Int. J. Mol. Sci. 2021, 22, 5749. [Google Scholar] [CrossRef]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan. Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [Green Version]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C.; Noël, D. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumdar, M.K.; Keane-Moore, M.; Buyaner, D.; Hardy, W.B.; Moorman, M.A.; McIntosh, K.R.; Mosca, J.D. Characterization and Functionality of Cell Surface Molecules on Human Mesenchymal Stem Cells. J. Biomed. Sci. 2003, 10, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.L.; Ruszymah, B.H.I.; Ng, M.H.; Law, J.X. Characteristics and clinical applications of Wharton’s jelly-derived mesenchymal stromal cells. Curr. Res. Transl. Med. 2020, 68, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- WM, T.W.P.J.B.; EC, E.M.G. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation Transplantation 75389397. Transplantation 2003, 75, 389. [Google Scholar]
- Prockop, D.J.; Youn Oh, J. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Phinney, D.; Pittenger, M. MSC-derived exosomes for cell-free therapy stem cells. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [Green Version]
- Liau, L.L.; Al-Masawa, M.E.; Koh, B.; Looi, Q.H.; Foo, J.B.; Lee, S.H.; Cheah, F.C.; Law, J.X. The Potential of Mesenchymal Stromal Cell as Therapy in Neonatal Diseases. Front Pediatr. 2020, 8, 591693. [Google Scholar] [CrossRef]
- Nakamura, Y.; Miyaki, S.; Ishitobi, H.; Matsuyama, S.; Nakasa, T.; Kamei, N.; Akimoto, T.; Higashi, Y.; Ochi, M. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015, 589, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Yoon, H.I.; Lee, K.S.; Choi, Y.C.; Yang, S.H.; Kim, I.-S.; Cho, Y.W. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J. Control. Release 2016, 222, 107–115. [Google Scholar] [CrossRef]
- Furuta, T.; Miyaki, S.; Ishitobi, H.; Ogura, T.; Kato, Y.; Kamei, N.; Miyado, K.; Higashi, Y.; Ochi, M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl. Med. 2016, 5, 1620–1630. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.-C.; Kuang, M.-J.; Kang, J.-Y.; Zhao, J.; Ma, J.-X.; Ma, X.-L. Human umbilical cord mesenchymal stem cell-derived exosomes act via the miR-1263/Mob1/Hippo signaling pathway to prevent apoptosis in disuse osteoporosis. Biochem. Biophys. Res. Commun. 2020, 524, 883–889. [Google Scholar] [CrossRef]
- Önfelt, B.; Nedvetzki, S.; Yanagi, K.; Davis, D.M. Cutting Edge: Membrane Nanotubes Connect Immune Cells. J. Immunol. 2004, 173, 1511–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular Highways for Intercellular Organelle Transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spees, J.L.; Olson, S.D.; Whitney, M.J.; Prockop, D.J. Mitochondrial transfer between cells can rescue aerobic respiration. Proc. Natl. Acad. Sci. USA 2006, 103, 1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Khryapenkova, T.G.; Galkina, S.I.; Sukhikh, G.T.; Zorov, D.B. Cytoplasm and organelle transfer between mesenchymal multipotent stromal cells and renal tubular cells in co-culture. Exp. Cell Res. 2010, 316, 2447–2455. [Google Scholar] [CrossRef]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Hafez, P.; Chowdhury, S.R.; Jose, S.; Law, J.X.; Ruszymah, B.H.I.; Mohd Ramzisham, A.R.; Ng, M.H. Development of an In Vitro Cardiac Ischemic Model Using Primary Human Cardiomyocytes. Cardiovasc. Eng. Technol. 2018, 9, 529–538. [Google Scholar] [CrossRef]
- Austefjord, M.W.; Gerdes, H.-H.; Wang, X. Tunneling nanotubes. Commun. Integr. Biol. 2014, 7, e27934. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golpanian, S.; El-Khorazaty, J.; Mendizabal, A.; DiFede, D.L.; Suncion, V.Y.; Karantalis, V.; Fishman, J.E.; Ghersin, E.; Balkan, W.; Hare, J.M. Effect of Aging on Human Mesenchymal Stem Cell Therapy in Ischemic Cardiomyopathy Patients. J. Am. Coll. Cardiol. 2015, 65, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, S.A.; Roobrouck, V.D.; Verfaillie, C.M.; Van Gool, S.W. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol. Cell Biol. 2013, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Larrick, J.W.; Mendelsohn, A.R. Mesenchymal stem cells for frailty? Rejuvenation Res. 2017, 20, 525–529. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Anderson, R.M.; Newman, A.B.; de Cabo, R. Stem Cell Transplantation for Frailty. J. Gerontol. Ser. A 2017, 72, 1503–1504. [Google Scholar] [CrossRef]
- Schulman, I.H.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell Therapy for Aging Frailty. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Jing, X.-M.; Bi, Y.-Z.; Cao, X.-F.; Wang, Y.-Z.; Li, Y.-X.; Qiao, B.-J.; Chen, Y.; Hao, Y.-L.; Hu, J. Human Umbilical Cord Wharton’s Jelly Derived Mesenchymal Stromal Cells May Attenuate Sarcopenia in Aged Mice Induced by Hindlimb Suspension. Med. Sci. Monit. 2018, 24, 9272–9281. [Google Scholar] [CrossRef]
- Li, T.-S.; Shi, H.; Wang, L.; Yan, C. Effect of Bone Marrow Mesenchymal Stem Cells on Satellite Cell Proliferation and Apoptosis in Immobilization-Induced Muscle Atrophy in Rats. Med. Sci. Monit. 2016, 22, 4651–4660. [Google Scholar] [CrossRef] [Green Version]
- Kiernan, J.; Hu, S.; Grynpas, M.D.; Davies, J.E.; Stanford, W.L. Systemic Mesenchymal Stromal Cell Transplantation Prevents Functional Bone Loss in a Mouse Model of Age-Related Osteoporosis. Stem Cells Transl. Med. 2016, 5, 683–693. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.-S.; Lu, C.-H.; Chu, K.-A.; Yeh, C.-C.; Chiang, T.-L.; Ko, T.-L.; Chiu, M.-M.; Chen, C.-F. Xenograft of Human Umbilical Mesenchymal Stem Cells from Wharton’s Jelly Differentiating into Osteocytes and Reducing Osteoclast Activity Reverses Osteoporosis in Ovariectomized Rats. Cell Transplant. 2018, 27, 194–208. [Google Scholar] [CrossRef]
- Engels, M.C.; Rajarajan, K.; Feistritzer, R.; Sharma, A.; Nielsen, U.B.; Schalij, M.J.; de Vries, A.A.F.; Pijnappels, D.A.; Wu, S.M. Insulin-Like Growth Factor Promotes Cardiac Lineage Induction In Vitro by Selective Expansion of Early Mesoderm. Stem Cells 2014, 32, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Schmeckpeper, J.; Verma, A.; Yin, L.; Beigi, F.; Zhang, L.; Payne, A.; Zhang, Z.; Pratt, R.E.; Dzau, V.J.; Mirotsou, M. Inhibition of Wnt6 by Sfrp2 regulates adult cardiac progenitor cell differentiation by differential modulation of Wnt pathways. J. Mol. Cell. Cardiol. 2015, 85, 215–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Study of Intravenous Adult Human Mesenchymal Stem Cells (Prochymal) After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; DiFede Velazquez, D.L.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of Allogeneic vs Autologous Bone Marrow–Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients With Ischemic Cardiomyopathy: The POSEIDON Randomized Trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, C.P.; Bareja, A.; Gomez, J.A.; Dzau, V.J. Emerging Concepts in Paracrine Mechanisms in Regenerative Cardiovascular Medicine and Biology. Circ. Res. 2016, 118, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Hahn, J.-Y.; Cho, H.-J.; Kang, H.-J.; Kim, T.-S.; Kim, M.-H.; Chung, J.-H.; Bae, J.-W.; Oh, B.-H.; Park, Y.-B.; Kim, H.-S. Pre-Treatment of Mesenchymal Stem Cells With a Combination of Growth Factors Enhances Gap Junction Formation, Cytoprotective Effect on Cardiomyocytes, and Therapeutic Efficacy for Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 51, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Tompkins, B.A.; Landin, A.M.; Florea, V.; Natsumeda, M.; Rieger, A.C.; Balkan, W.; Schulman, I.H.; Hare, J.M. Allogeneic Mesenchymal Stem Cells as a Treatment for Aging Frailty. In Frailty and Sarcopenia-Onset, Development and Clinical Challenges; IntechOpen: London, UK, 2017. [Google Scholar]
- Singh Dolt, K.; Hammachi, F.; Kunath, T. Modeling Parkinson’s disease with induced pluripotent stem cells harboring α-synuclein mutations. Brain Pathol. 2017, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Rajan, T.S.; Scionti, D.; Diomede, F.; Grassi, G.; Pollastro, F.; Piattelli, A.; Cocco, L.; Bramanti, P.; Mazzon, E.; Trubiani, O. Gingival Stromal Cells as an In Vitro Model: Cannabidiol Modulates Genes Linked With Amyotrophic Lateral Sclerosis. J. Cell. Biochem. 2017, 118, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Koppitz, M.; Eschenburg, C.; Salzmann, E.; Rosewich, M.; Schubert, R.; Zielen, S. Mucolytic Effectiveness of Tyloxapol in Chronic Obstructive Pulmonary Disease—A Double-Blind, Randomized Controlled Trial. PLoS ONE 2016, 11, e0156999. [Google Scholar] [CrossRef] [PubMed]
- Chambers, D.C.; Enever, D.; Ilic, N.; Sparks, L.; Whitelaw, K.; Ayres, J.; Yerkovich, S.T.; Khalil, D.; Atkinson, K.M.; Hopkins, P.M.A. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology 2014, 19, 1013–1018. [Google Scholar] [CrossRef]
- Thiel, A.; Yavanian, G.; Nastke, M.-D.; Morales, P.; Kouris, N.A.; Kimbrel, E.A.; Lanza, R. Human embryonic stem cell-derived mesenchymal cells preserve kidney function and extend lifespan in NZB/W F1 mouse model of lupus nephritis. Sci. Rep. 2015, 5, 17685. [Google Scholar] [CrossRef]
- Schira, J.; Gasis, M.; Estrada, V.; Hendricks, M.; Schmitz, C.; Trapp, T.; Kruse, F.; Kögler, G.; Wernet, P.; Hartung, H.-P.; et al. Significant clinical, neuropathological and behavioural recovery from acute spinal cord trauma by transplantation of a well-defined somatic stem cell from human umbilical cord blood. Brain 2011, 135, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Moshayedi, P.; Nih, L.R.; Llorente, I.L.; Berg, A.R.; Cinkornpumin, J.; Lowry, W.E.; Segura, T.; Carmichael, S.T. Systematic optimization of an engineered hydrogel allows for selective control of human neural stem cell survival and differentiation after transplantation in the stroke brain. Biomaterials 2016, 105, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt-Clermont, P.J. Loss of bone marrow-derived vascular progenitor cells leads to inflammation and atherosclerosis. Am. Heart J. 2003, 146, S5–S12. [Google Scholar] [CrossRef]
- Rauscher, F.M.; Goldschmidt-Clermont, P.J.; Davis, B.H.; Wang, T.; Gregg, D.; Ramaswami, P.; Pippen, A.M.; Annex, B.H.; Dong, C.; Taylor, D.A. Aging, Progenitor Cell Exhaustion, and Atherosclerosis. Circulation 2003, 108, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golpanian, S.; DiFede, D.L.; Khan, A.; Schulman, I.H.; Landin, A.M.; Tompkins, B.A.; Heldman, A.W.; Miki, R.; Goldstein, B.J.; Mushtaq, M.; et al. Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty. J. Gerontol. Ser. A 2017, 72, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, B.A.; DiFede, D.L.; Khan, A.; Landin, A.M.; Schulman, I.H.; Pujol, M.V.; Heldman, A.W.; Miki, R.; Goldschmidt-Clermont, P.J.; Goldstein, B.J.; et al. Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Gerontol. Ser. A 2017, 72, 1513–1522. [Google Scholar] [CrossRef]
- Kabat, M.; Bobkov, I.; Kumar, S.; Grumet, M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl. Med. 2020, 9, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.-P.; Mohd-Shahrizal, M.-Y.; Liyana, M.-Z.; Then, K.Y.; Cheong, S.K. High Dose of Intravenous Allogeneic Umbilical Cord-Derived Mesenchymal Stem Cells (CLV-100) Infusion Displays Better Immunomodulatory Effect among Healthy Volunteers: A Phase 1 Clinical Study. Stem Cells Int. 2020, 2020, 8877003. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytom. Part A 2018, 93, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Spath, L.; Rotilio, V.; Alessandrini, M.; Gambara, G.; De Angelis, L.; Mancini, M.; Mitsiadis, T.A.; Vivarelli, E.; Naro, F.; Filippini, A.; et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J. Cell. Mol. Med. 2010, 14, 1635–1644. [Google Scholar] [CrossRef] [Green Version]
- Dental Pulp Stem Cell-Derived Conditioned Medium: An Attractive Alternative for Regenerative Therapy. Tissue Eng. Part B Rev. 2019, 25, 78–88. [CrossRef] [PubMed]
- Ueda, N.; Atsuta, I.; Ayukawa, Y.; Yamaza, T.; Furuhashi, A.; Narimatsu, I.; Matsuura, Y.; Kondo, R.; Watanabe, Y.; Zhang, X.; et al. Novel Application Method for Mesenchymal Stem Cell Therapy Utilizing Its Attractant-Responsive Accumulation Property. Appl. Sci. 2019, 9, 4908. [Google Scholar] [CrossRef] [Green Version]
- Castelo-Branco, M.T.L.; Soares, I.D.P.; Lopes, D.V.; Buongusto, F.; Martinusso, C.A.; do Rosario, A., Jr.; Souza, S.A.L.; Gutfilen, B.; Fonseca, L.M.B.; Elia, C.; et al. Intraperitoneal but Not Intravenous Cryopreserved Mesenchymal Stromal Cells Home to the Inflamed Colon and Ameliorate Experimental Colitis. PLoS ONE 2012, 7, e33360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, F.d.C.; Schneider, N.; Pinto, F.O.; Meyer, F.S.; Visioli, F.; Pfaffenseller, B.; Lopez, P.L.d.C.; Passos, E.P.; Cirne-Lima, E.O.; Meurer, L.; et al. Intravenous vs intraperitoneal mesenchymal stem cells administration: What is the best route for treating experimental colitis? World J. Gastroenterol. 2014, 20, 18228–18239. [Google Scholar] [CrossRef]
- Braid, L.R.; Wood, C.A.; Wiese, D.M.; Ford, B.N. Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy 2018, 20, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W.; Huang, S.; Yao, G.; Tang, X.; Sun, L. Mesenchymal stem cells prevent overwhelming inflammation and reduce infection severity via recruiting CXCR3+ regulatory T cells. Clin. Transl. Immunol. 2020, 9, e1181. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.; Saviane, G.; Pini, J.; Belaïd, N.; Dhib, G.; Voha, C.; Ibáñez, L.; Boutin, A.; Mazure, N.M.; Wakkach, A.; et al. Immunosuppressive Mesenchymal Stromal Cells Derived from Human-Induced Pluripotent Stem Cells Induce Human Regulatory T Cells In Vitro and In Vivo. Front. Immunol. 2018, 8, 1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
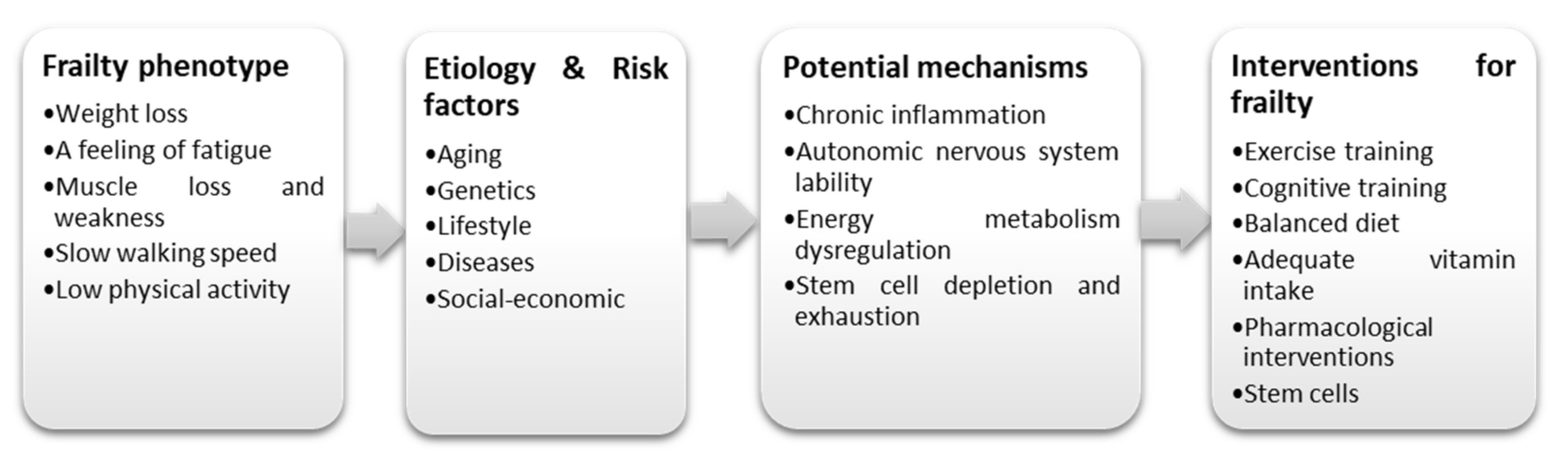


| Frailty Symptoms | Potential MSC Effects | Potential Mechanisms | References |
|---|---|---|---|
| Unintentional weight loss | ↓ chronic inflammation | ↓ chronic inflammation (↓ TNF-α, ↓ CRP, ↓ IL-1, ↓ IL-6, ↑ TGF-β), ↓ onset of sarcopenia | Jacobs et al. (2013) [119] |
| A feeling of fatigue | ↓ chronic inflammation, ↑ pulmonary function | ↓ chronic inflammation (↓ TNF-α, ↓ CRP, ↓ IL-1, ↓ IL-6, ↑ TGF-β), ↑ endothelial function, ↑ pulmonary function (FEV1) | Jacobs et al. (2013) [119] |
| Muscle loss and weakness | ↑ physical activity (six-minute walk distance) | ↑ skeletal muscle performance, ↑ cardiac function performance, ↓ onset of sarcopenia, ↑ endothelial function | Fried et al. (2001) [117] |
| Slow walking speed | ↑ physical activity (six-minute walk distance), ↑ pulmonary function | ↑ skeletal muscle performance, ↑ cardiac function performance, ↑ pulmonary function (FEV1), ↑ endothelial function | Fried et al. (2001) [117] |
| Low levels of physical activity | ↓ chronic inflammation, ↑ physical activity (six-minute walk distance), ↑ quality of life | ↓ chronic inflammation (↓ TNF-α, ↓ CRP, ↓ IL-1, ↓ IL-6, ↑ TGF-β), ↑ skeletal muscle performance, ↑ cognitive status | Jacobs et al. (2013) [119] |
| References | Human Subjects | MSC and Dosage | Results (Related to Musculoskeletal System and Physical Frailty) |
|---|---|---|---|
| Golpanian et al. (2017) [143] | An average age of 78.4 ± 4.7 years and Clinical Frailty Score of 4–6 | Group 1 = 20 × 106 allo-hBM-MSCs, IV injection | No treatment-emergent serious adverse events (TE-SAEs) were reported with any of the doses at 1-month. |
| Group 2 = 100 × 106 allo-hBM-MSCs, IV injection | Six-min walk distance significantly increased at 3 and 6 months in all treatment groups. | ||
| Group 3 = 200 × 106 allo-hBM-MSCs, IV injection | Physical component of the SF-36 quality of life assessment also showed significant improvements in the 100-million dose group at all time points relative to baseline. | ||
| Tompkins et al. (2017) [144] | Age ≥60 and ≤95 years with Clinical Frailty Score of 4–7 | Group 1 = 100 × 106 allo-hBM-MSCs, IV injection | No therapy-related TE-SAEs reported at 1-month post-infusion. |
| Group 2 = 100 × 106 allo-hBM-MSCs, IV injection | Six-min walk test and short physical performance exam improved significantly in the 100-million dose group but not in the 200-million dose or placebo groups. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahindran, E.; Law, J.X.; Ng, M.H.; Nordin, F. Mesenchymal Stem Cell Transplantation for the Treatment of Age-Related Musculoskeletal Frailty. Int. J. Mol. Sci. 2021, 22, 10542. https://doi.org/10.3390/ijms221910542
Mahindran E, Law JX, Ng MH, Nordin F. Mesenchymal Stem Cell Transplantation for the Treatment of Age-Related Musculoskeletal Frailty. International Journal of Molecular Sciences. 2021; 22(19):10542. https://doi.org/10.3390/ijms221910542
Chicago/Turabian StyleMahindran, Elancheleyen, Jia Xian Law, Min Hwei Ng, and Fazlina Nordin. 2021. "Mesenchymal Stem Cell Transplantation for the Treatment of Age-Related Musculoskeletal Frailty" International Journal of Molecular Sciences 22, no. 19: 10542. https://doi.org/10.3390/ijms221910542
APA StyleMahindran, E., Law, J. X., Ng, M. H., & Nordin, F. (2021). Mesenchymal Stem Cell Transplantation for the Treatment of Age-Related Musculoskeletal Frailty. International Journal of Molecular Sciences, 22(19), 10542. https://doi.org/10.3390/ijms221910542







