Geranylgeraniol Inhibits Lipopolysaccharide-Induced Inflammation in Mouse-Derived MG6 Microglial Cells via NF-κB Signaling Modulation
Abstract
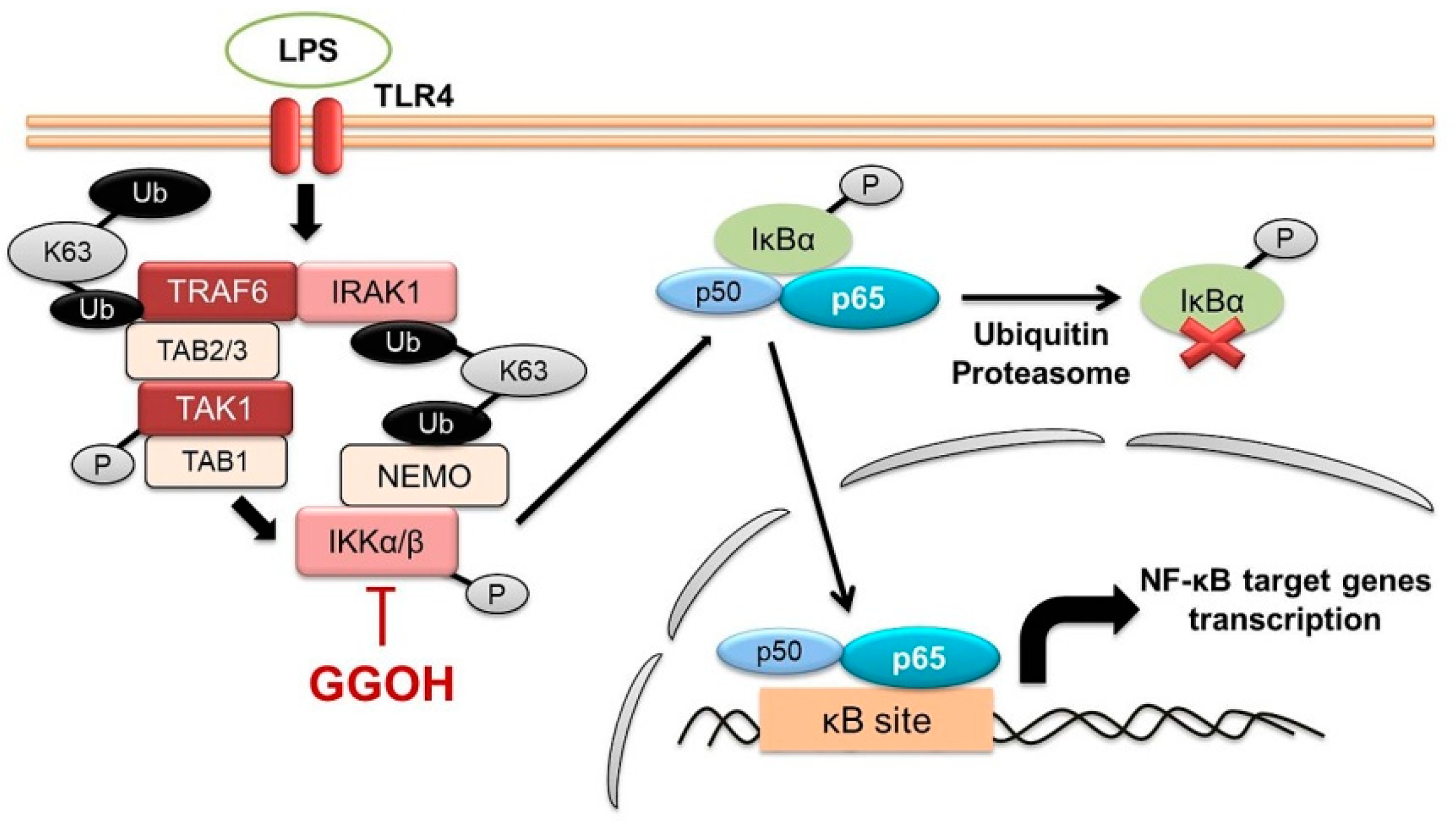
1. Introduction
2. Results
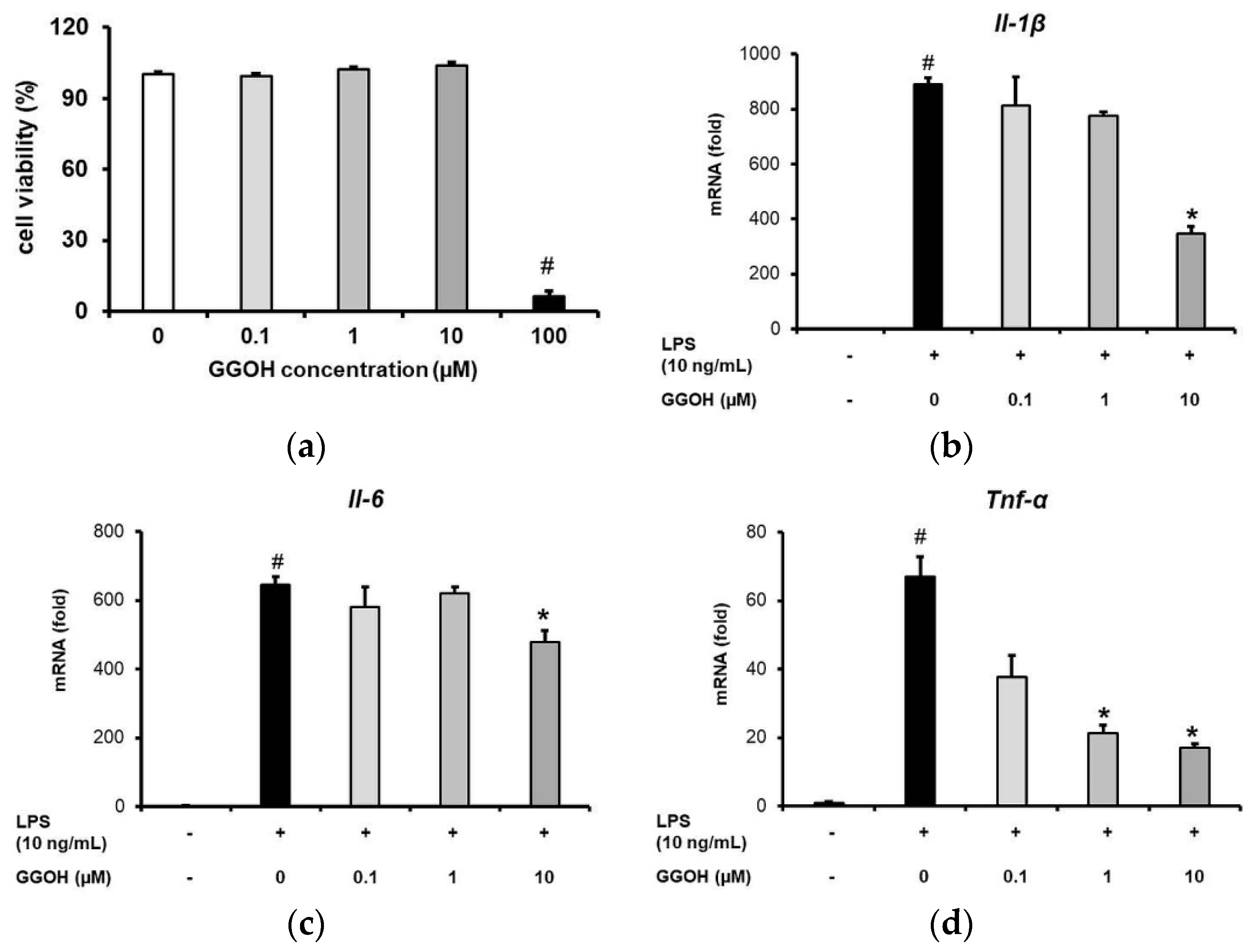
2.1. GGOH Inhibited the Upregulation of Pro-Inflammatory Cytokine Expression in MG6 Cells
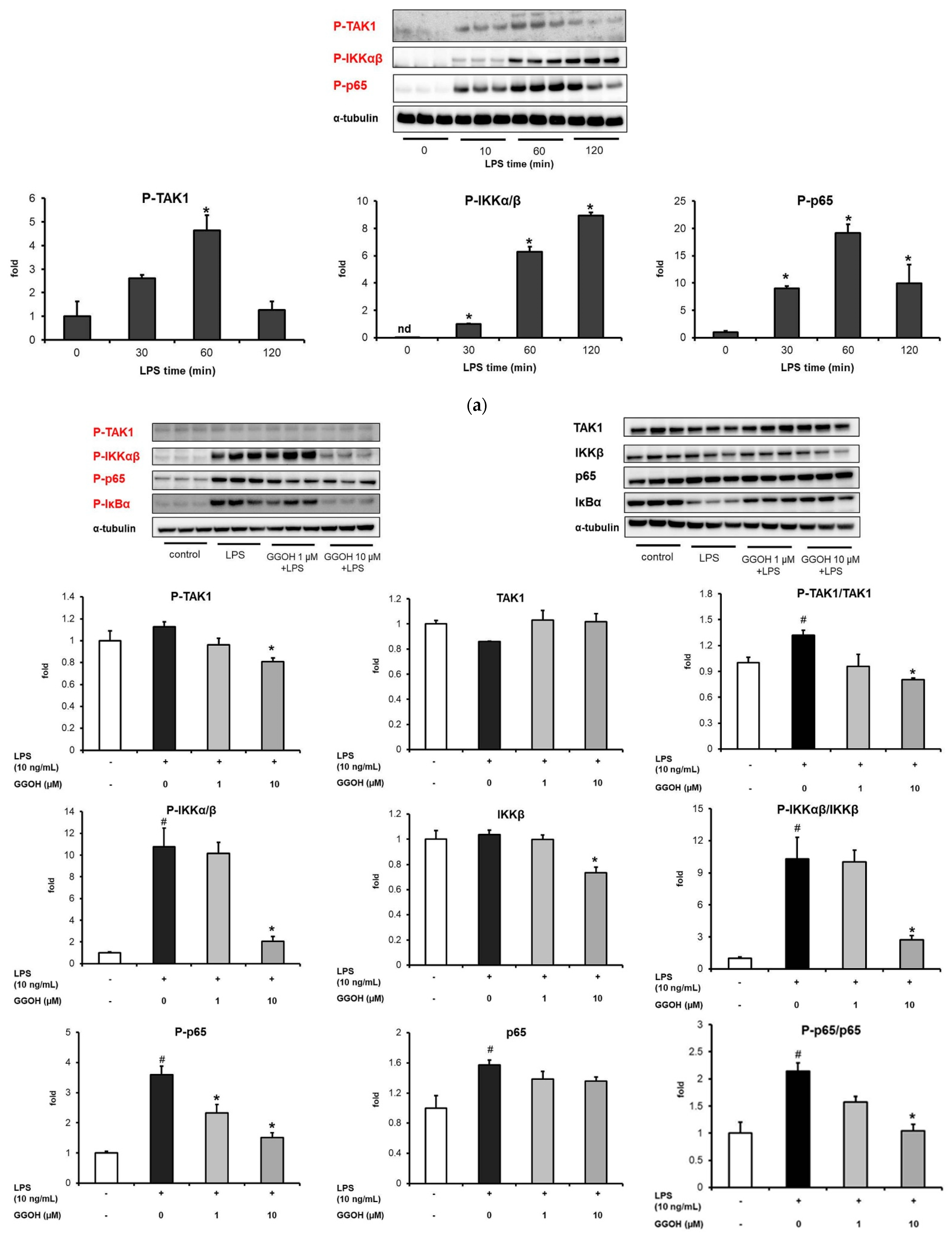
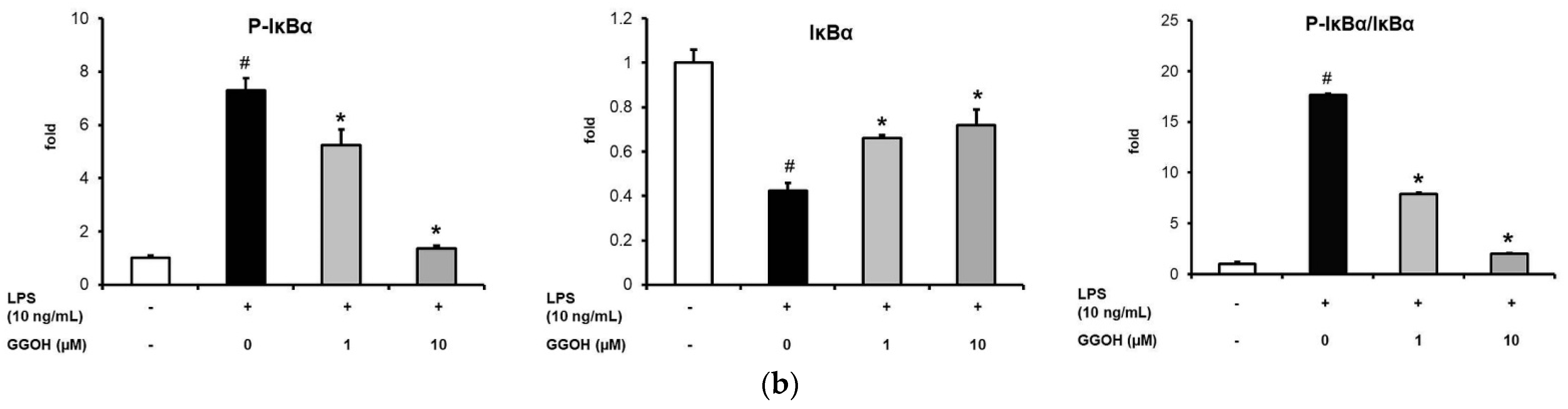
2.2. Mitigation of NF-κB Activation in MG6 Cells by GGOH
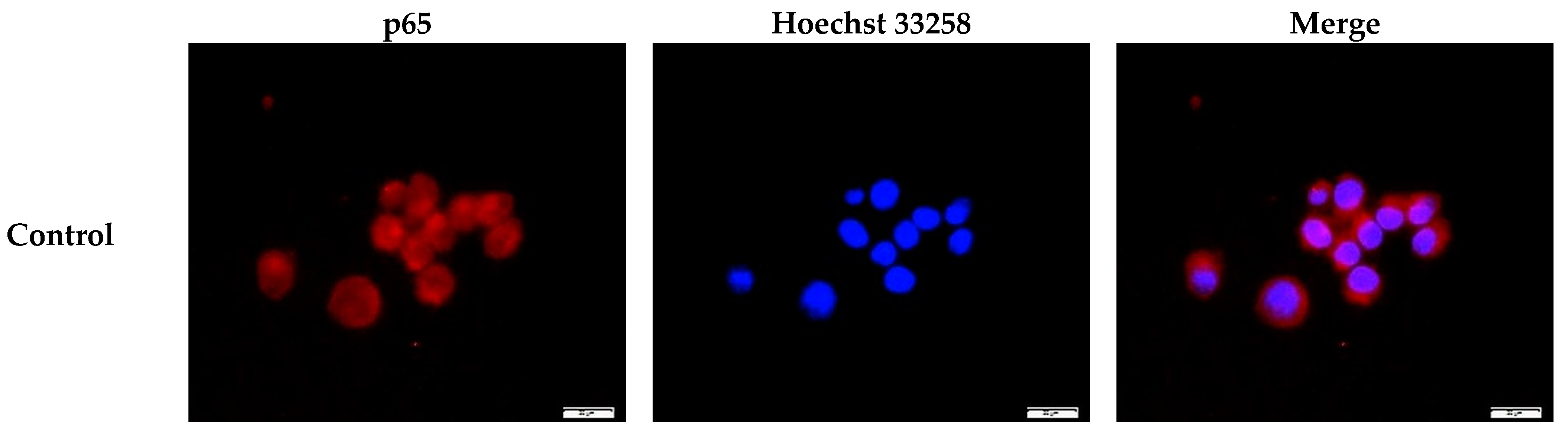
2.3. Inhibition of the Nuclear Translocation of NF-κB by GGOH
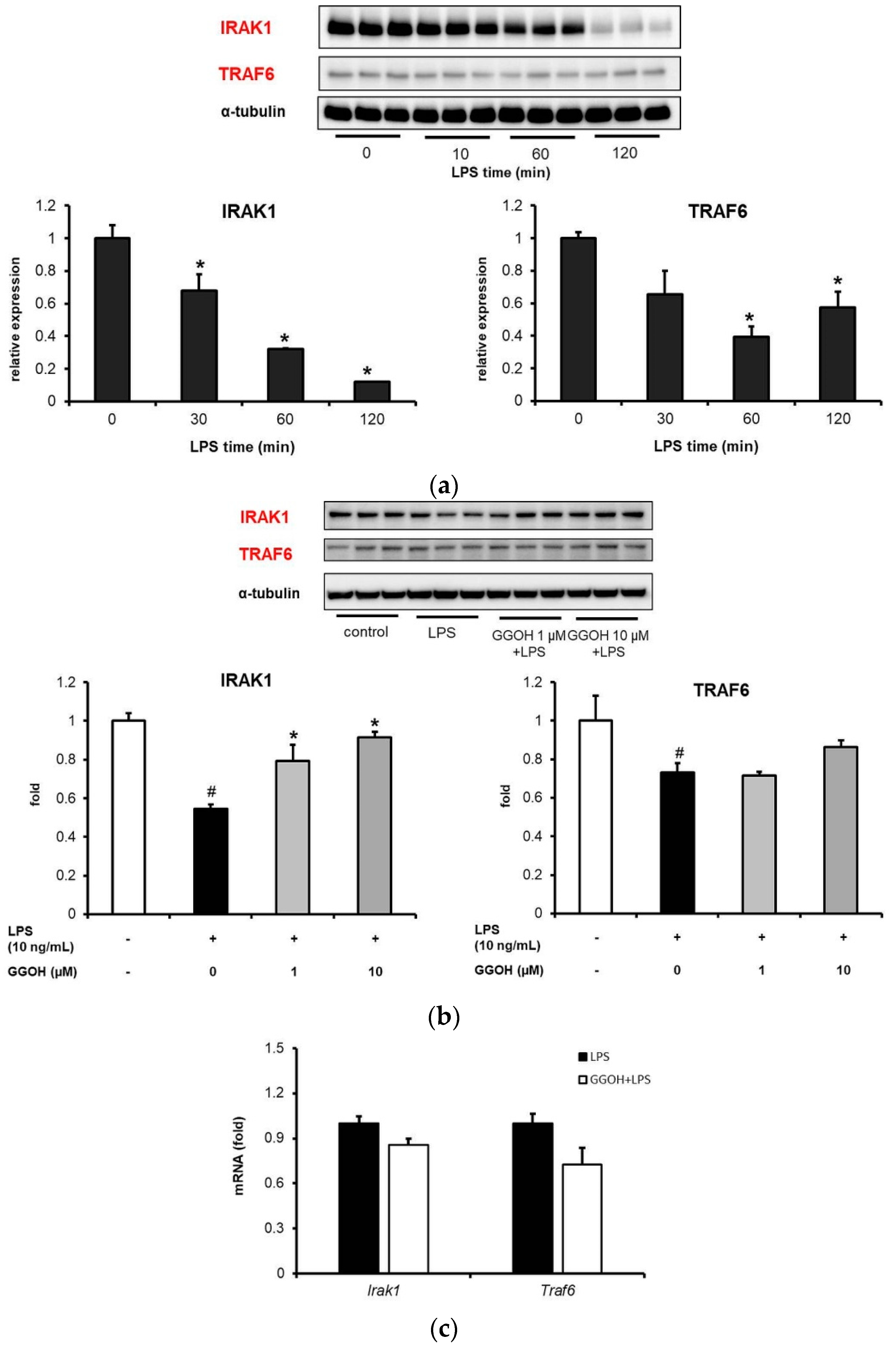
2.4. LPS-Induced Disappearance of IRAK1 in MG6 Cells and Its Reversal by GGOH
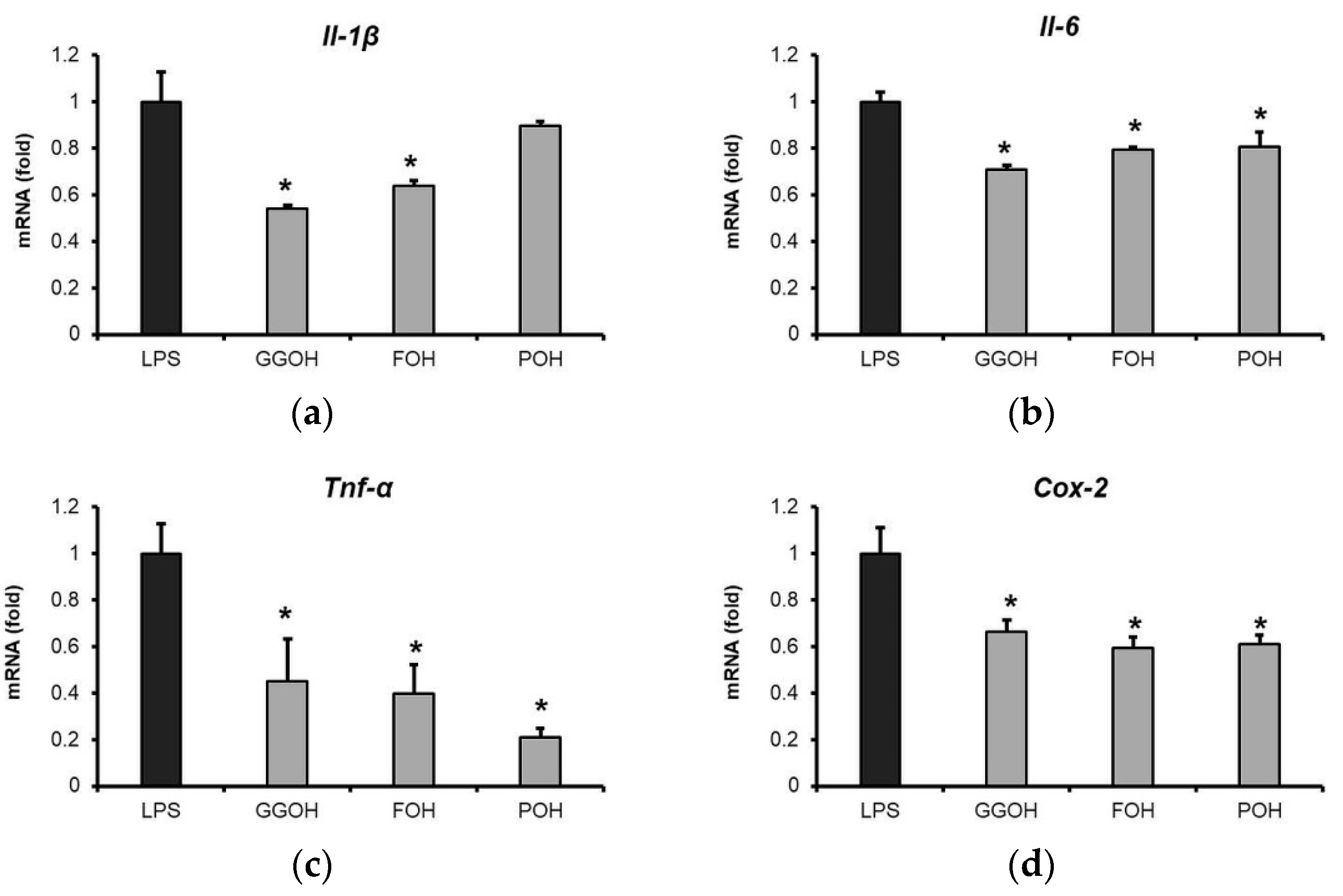
2.5. Attenuation of LPS-Induced Pro-Inflammatory Cytokine Expression by GGOH and Other Isoprenoid Analogues
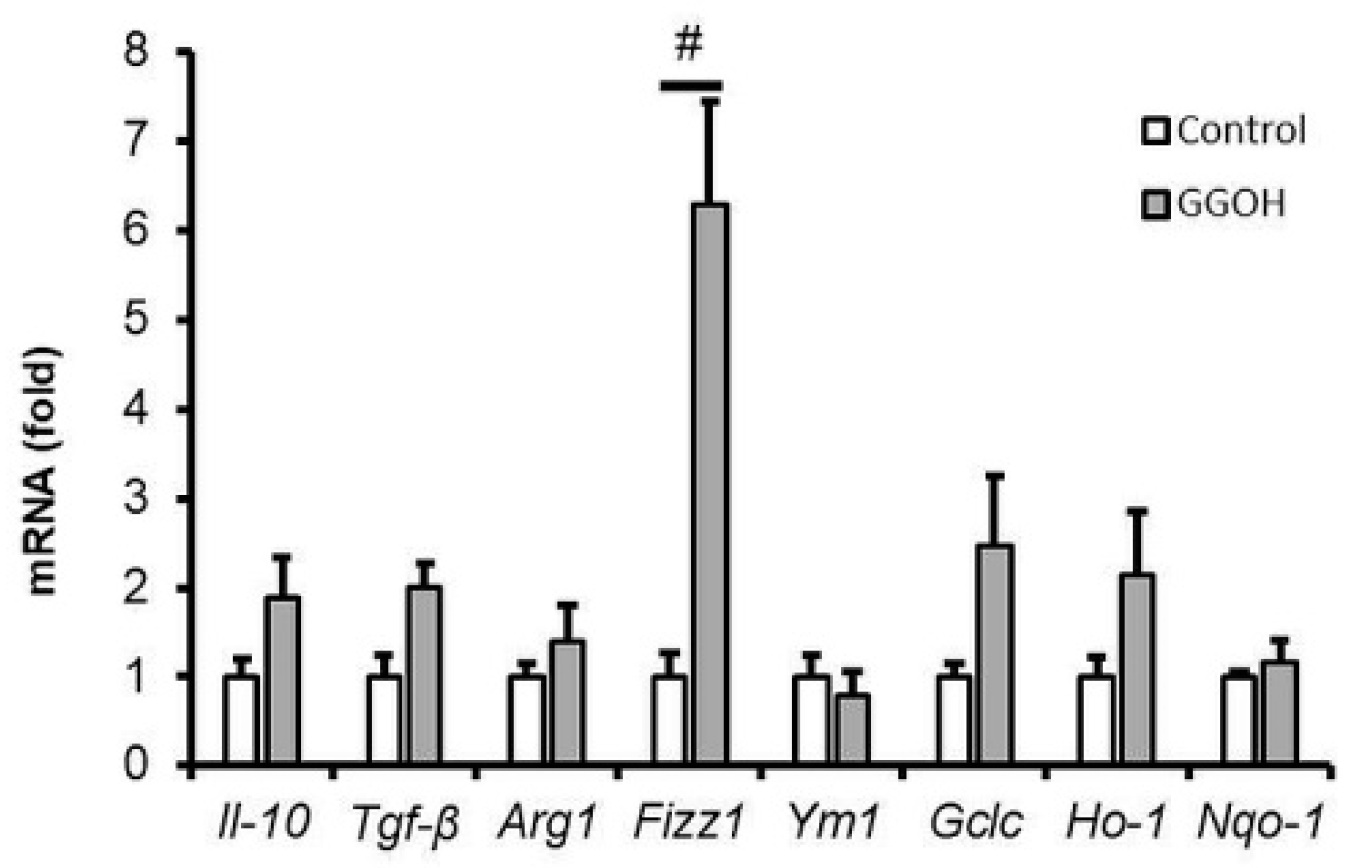
2.6. Effect of GGOH Treatment on M2 Phenotype Polarization in MG6 Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures and Treatment
4.3. Cell Viability Assay
4.4. RNA Extraction and Quantitative RT-PCR Assay
4.5. Immunoblot Analysis
4.6. Cytoplasmic-Nuclear Fractionation
4.7. Immunocytofluorescence
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Streit, W.J.; Mrak, R.E.; Griffin, W.S.T. Microglia and Neuroinflammation: A Pathological Perspective. J. Neuroinflamm. 2004, 1, 14. [Google Scholar] [CrossRef][Green Version]
- Salter, M.W.; Stevens, B. Microglia Emerge as Central Players in Brain Disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Fernández-Suárez, D. Alternatively Activated Microglia and Macrophages in the Central Nervous System. Prog. Neurobiol. 2015, 131, 65–86. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of Pro-Inflammatory Cytokines Released from Microglia in Alzheimer’s Disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, S.; Landreth, G.E. Microglia and Inflammation in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 Microglia: The Good, the Bad, and the Inflamed. J. Neuroinflamm. 2014, 11, 98. [Google Scholar] [CrossRef]
- Arai, H.; Furuya, T.; Yasuda, T.; Miura, M.; Mizuno, Y.; Mochizuki, H. Neurotoxic Effects of Lipopolysaccharide on Nigral Dopaminergic Neurons Are Mediated by Microglial Activation, Interleukin-1β, and Expression of Caspase-11 in Mice. J. Biol. Chem. 2004, 279, 51647–51653. [Google Scholar] [CrossRef]
- Biesmans, S.; Meert, T.F.; Bouwknecht, J.A.; Acton, P.D.; Davoodi, N.; De Haes, P.; Kuijlaars, J.; Langlois, X.; Matthews, L.J.R.; Ver Donck, L.; et al. Systemic Immune Activation Leads to Neuroinflammation and Sickness Behavior in Mice. Mediat. Inflamm. 2013, 2013, e271359. [Google Scholar] [CrossRef]
- Maimone, D.; Gregory, S.; Arnason, B.G.W.; Reder, A.T. Cytokine Levels in the Cerebrospinal Fluid and Serum of Patients with Multiple Sclerosis. J. Neuroimmunol. 1991, 32, 67–74. [Google Scholar] [CrossRef]
- Khaibullin, T.; Ivanova, V.; Martynova, E.; Cherepnev, G.; Khabirov, F.; Granatov, E.; Rizvanov, A.; Khaiboullina, S. Elevated Levels of Proinflammatory Cytokines in Cerebrospinal Fluid of Multiple Sclerosis Patients. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation Induced by Lipopolysaccharide Causes Cognitive Impairment in Mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-ΚB Regulation in the Immune System. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-ΚB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Purcaro, G.; Barp, L.; Beccaria, M.; Conte, L.S. Characterisation of Minor Components in Vegetable Oil by Comprehensive Gas Chromatography with Dual Detection. Food Chem. 2016, 212, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aracama, A.; Goicoechea, E.; Guillén, M.D. Direct Study of Minor Extra-Virgin Olive Oil Components without Any Sample Modification. 1H NMR Multisupression Experiment: A Powerful Tool. Food Chem. 2017, 228, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Muraguchi, T.; Okamoto, K.; Mitake, M.; Ogawa, H.; Shidoji, Y. Polished Rice as Natural Sources of Cancer-Preventing Geranylgeranoic Acid. J. Clin. Biochem. Nutr. 2011, 49, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Pontillo, A.; Leo, L.D.; Tommasini, A.; Decorti, G.; Not, T.; Ventura, A. Natural Isoprenoids Are Able to Reduce Inflammation in a Mouse Model of Mevalonate Kinase Deficiency. Pediatr. Res. 2008, 64, 177–182. [Google Scholar] [CrossRef]
- de Moura Espíndola, R.; Mazzantini, R.P.; Ong, T.P.; de Conti, A.; Heidor, R.; Moreno, F.S. Geranylgeraniol and β-Ionone Inhibit Hepatic Preneoplastic Lesions, Cell Proliferation, Total Plasma Cholesterol and DNA Damage during the Initial Phases of Hepatocarcinogenesis, but Only the Former Inhibits NF-ΚB Activation. Carcinogenesis 2005, 26, 1091–1099. [Google Scholar] [CrossRef]
- Giriwono, P.E.; Shirakawa, H.; Ohsaki, Y.; Hata, S.; Kuriyama, H.; Sato, S.; Goto, T.; Komai, M. Dietary Supplementation with Geranylgeraniol Suppresses Lipopolysaccharide-Induced Inflammation via Inhibition of Nuclear Factor-ΚB Activation in Rats. Eur. J. Nutr. 2013, 52, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Giriwono, P.E.; Shirakawa, H.; Ohsaki, Y.; Sato, S.; Aoyama, Y.; Ho, H.-J.; Goto, T.; Komai, M. Geranylgeraniol Suppresses the Expression of IRAK1 and TRAF6 to Inhibit NFκB Activation in Lipopolysaccharide-Induced Inflammatory Responses in Human Macrophage-Like Cells. Int. J. Mol. Sci. 2019, 20, 2320. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, A.; Tommasini, A.; Crovella, S.; Pontillo, A. Natural Isoprenoids Inhibit LPS-Induced-Production of Cytokines and Nitric Oxide in Aminobisphosphonate-Treated Monocytes. Int. Immunopharmacol. 2010, 10, 639–642. [Google Scholar] [CrossRef]
- Frenkel, J.; Rijkers, G.T.; Mandey, S.H.L.; Buurman, S.W.M.; Houten, S.M.; Wanders, R.J.A.; Waterham, H.R.; Kuis, W. Lack of Isoprenoid Products Raises Ex Vivo Interleukin-1β Secretion in Hyperimmunoglobulinemia D and Periodic Fever Syndrome. Arthritis Rheum. 2002, 46, 2794–2803. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.N.; Ye, J.; Hao, R.; DeBose-Boyd, R.; Ye, J. Sufficient Production of Geranylgeraniol Is Required to Maintain Endotoxin Tolerance in Macrophages. J. Lipid Res. 2013, 54, 3430–3437. [Google Scholar] [CrossRef]
- Marcuzzi, A.; Piscianz, E.; Zweyer, M.; Bortul, R.; Loganes, C.; Girardelli, M.; Baj, G.; Monasta, L.; Celeghini, C. Geranylgeraniol and Neurological Impairment: Involvement of Apoptosis and Mitochondrial Morphology. Int. J. Mol. Sci. 2016, 17, 365. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tatsuno, I.; Uchida, D.; Moroo, I.; Morio, H.; Nakamura, S.; Noguchi, Y.; Yasuda, T.; Kitagawa, M.; Saito, Y.; et al. Geranylgeranyl-Pyrophosphate, an Isoprenoid of Mevalonate Cascade, Is a Critical Compound for Rat Primary Cultured Cortical Neurons to Protect the Cell Death Induced by 3-Hydroxy-3-Methylglutaryl-CoA Reductase Inhibition. J. Neurosci. 2000, 20, 2852–2859. [Google Scholar] [CrossRef]
- Cole, S.L.; Grudzien, A.; Manhart, I.O.; Kelly, B.L.; Oakley, H.; Vassar, R. Statins Cause Intracellular Accumulation of Amyloid Precursor Protein, β-Secretase-Cleaved Fragments, and Amyloid β-Peptide via an Isoprenoid-Dependent Mechanism *. J. Biol. Chem. 2005, 280, 18755–18770. [Google Scholar] [CrossRef]
- Cole, S.L.; Vassar, R. Isoprenoids and Alzheimer’s Disease: A Complex Relationship. Neurobiol. Dis. 2006, 22, 209–222. [Google Scholar] [CrossRef]
- Bi, X.; Baudry, M.; Liu, J.; Yao, Y.; Fu, L.; Brucher, F.; Lynch, G. Inhibition of Geranylgeranylation Mediates the Effects of 3-Hydroxy-3-Methylglutaryl (HMG)-CoA Reductase Inhibitors on Microglia. J. Biol. Chem. 2004, 279, 48238–48245. [Google Scholar] [CrossRef]
- Kotti, T.J.; Ramirez, D.M.O.; Pfeiffer, B.E.; Huber, K.M.; Russell, D.W. Brain Cholesterol Turnover Required for Geranylgeraniol Production and Learning in Mice. Proc. Natl. Acad. Sci. USA 2006, 103, 3869–3874. [Google Scholar] [CrossRef]
- Saputra, W.D.; Aoyama, N.; Komai, M.; Shirakawa, H. Menaquinone-4 Suppresses Lipopolysaccharide-Induced Inflammation in MG6 Mouse Microglia-Derived Cells by Inhibiting the NF-ΚB Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 2317. [Google Scholar] [CrossRef]
- Takenouchi, T.; Iwamaru, Y.; Sugama, S.; Tsukimoto, M.; Fujita, M.; Sekigawa, A.; Sekiyama, K.; Sato, M.; Kojima, S.; Conti, B.; et al. The Activation of P2X7 Receptor Induces Cathepsin D-Dependent Production of a 20-KDa Form of IL-1β under Acidic Extracellular PH in LPS-Primed Microglial Cells. J. Neurochem. 2011, 117, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Yanagitai, M.; Itoh, S.; Kitagawa, T.; Takenouchi, T.; Kitani, H.; Satoh, T. Carnosic Acid, a pro-Electrophilic Compound, Inhibits LPS-Induced Activation of Microglia. Biochem. Biophys. Res. Commun. 2012, 418, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Horng, T. Transcriptional Control of the Inflammatory Response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef]
- Ohsaki, Y.; Shirakawa, H.; Miura, A.; Giriwono, P.E.; Sato, S.; Ohashi, A.; Iribe, M.; Goto, T.; Komai, M. Vitamin K Suppresses the Lipopolysaccharide-Induced Expression of Inflammatory Cytokines in Cultured Macrophage-like Cells via the Inhibition of the Activation of Nuclear Factor ΚB through the Repression of IKKα/β Phosphorylation. J. Nutr. Biochem. 2010, 21, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Kadl, A.; Meher Akshaya, K.; Sharma Poonam, R.; Lee Monica, Y.; Doran Amanda, C.; Johnstone Scott, R.; Elliott Michael, R.; Gruber, F.; Han, J.; Chen, W.; et al. Identification of a Novel Macrophage Phenotype That Develops in Response to Atherogenic Phospholipids via Nrf2. Circ. Res. 2010, 107, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-ΚB Function in the Nervous System. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Häcker, H.; Karin, M. Regulation and Function of IKK and IKK-Related Kinases. Sci. STKE 2006, 2006, re13. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xia, Y.; Parker, A.S.; Verma, I.M. IKK Biology. Immunol. Rev. 2012, 246, 239–253. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, J.-Y.; Seo, J.H.; Lee, H.Y.; Ryu, S.Y.; Ahn, B.W.; Lee, C.-K.; Hwang, B.Y.; Han, S.-B.; Kim, Y. Artemisolide Is a Typical Inhibitor of IκB Kinase β Targeting Cysteine-179 Residue and down-Regulates NF-ΚB-Dependent TNF-α Expression in LPS-Activated Macrophages. Biochem. Biophys. Res. Commun. 2007, 361, 593–598. [Google Scholar] [CrossRef]
- Gottipati, S.; Rao, N.L.; Fung-Leung, W.-P. IRAK1: A Critical Signaling Mediator of Innate Immunity. Cell. Signal. 2008, 20, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Yamin, T.-T.; Miller, D.K. The Interleukin-1 Receptor-Associated Kinase Is Degraded by Proteasomes Following Its Phosphorylation. J. Biol. Chem. 1997, 272, 21540–21547. [Google Scholar] [CrossRef] [PubMed]
- Conze, D.B.; Wu, C.-J.; Thomas, J.A.; Landstrom, A.; Ashwell, J.D. Lys63-Linked Polyubiquitination of IRAK-1 Is Required for Interleukin-1 Receptor- and Toll-Like Receptor-Mediated NF-ΚB Activation. Mol. Cell. Biol. 2008, 28, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Windheim, M.; Stafford, M.; Peggie, M.; Cohen, P. Interleukin-1 (IL-1) Induces the Lys63-Linked Polyubiquitination of IL-1 Receptor-Associated Kinase 1 To Facilitate NEMO Binding and the Activation of IκBα Kinase. Mol. Cell. Biol. 2008, 28, 1783–1791. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raes, G.; Noël, W.; Beschin, A.; Brys, L.; de Baetselier, P.; Hassanzadeh, G.G. FIZZ1 and Ym as Tools to Discriminate between Differentially Activated Macrophages. Dev. Immunol. 2002, 9, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.G.; Guild, K.J.; Artis, D. Novel Effector Molecules in Type 2 Inflammation: Lessons Drawn from Helminth Infection and Allergy. J. Immunol. 2006, 177, 1393–1399. [Google Scholar] [CrossRef]
- Satoh, T.; Takeuchi, O.; Vandenbon, A.; Yasuda, K.; Tanaka, Y.; Kumagai, Y.; Miyake, T.; Matsushita, K.; Okazaki, T.; Saitoh, T.; et al. The Jmjd3-Irf4 Axis Regulates M2 Macrophage Polarization and Host Responses against Helminth Infection. Nat. Immunol. 2010, 11, 936–944. [Google Scholar] [CrossRef]
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural Inhibitors of NF-ΚB Signaling with Anti-Inflammatory and Anticancer Potential. Cell. Mol. Life Sci. 2008, 65, 2979–2999. [Google Scholar] [CrossRef]
- Miquel, K.; Pradines, A.; Tercé, F.; Selmi, S.; Favre, G. Competitive Inhibition of Choline Phosphotransferase by Geranylgeraniol and Farnesol Inhibits Phosphatidylcholine Synthesis and Induces Apoptosis in Human Lung Adenocarcinoma A549 Cells. J. Biol. Chem. 1998, 273, 26179–26186. [Google Scholar] [CrossRef]
- Zhou, Y.; Suram, A.; Venugopal, C.; Prakasam, A.; Lin, S.; Su, Y.; Li, B.; Paul, S.M.; Sambamurti, K. Geranylgeranyl Pyrophosphate Stimulates γ-Secretase to Increase the Generation of Aβ and APP-CTFγ. FASEB J. 2008, 22, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hooff, G.P.; Peters, I.; Wood, W.G.; Müller, W.E.; Eckert, G.P. Modulation of Cholesterol, Farnesylpyrophosphate, and Geranylgeranylpyrophosphate in Neuroblastoma SH-SY5Y-APP695 Cells: Impact on Amyloid Beta-Protein Production. Mol. Neurobiol. 2010, 41, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Hooff, G.P.; Wood, W.G.; Kim, J.-H.; Igbavboa, U.; Ong, W.-Y.; Muller, W.E.; Eckert, G.P. Brain Isoprenoids Farnesyl Pyrophosphate and Geranylgeranyl Pyrophosphate Are Increased in Aged Mice. Mol. Neurobiol. 2012, 46, 179–185. [Google Scholar] [CrossRef]
- Takenouchi, T.; Ogihara, K.; Sato, M.; Kitani, H. Inhibitory Effects of U73122 and U73343 on Ca2+ Influx and Pore Formation Induced by the Activation of P2X7 Nucleotide Receptors in Mouse Microglial Cell Line. Biochim. Biophys. Acta BBA-Gen. Subj. 2005, 1726, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, K.; Saiki, M.; Kitani, H.; Kuboyama, Y.; Morimoto, K.; Takayama-Ito, M.; Kurane, I. Suppressive Effect of Simvastatin on Interferon-β-Induced Expression of CC Chemokine Ligand 5 in Microglia. Neurosci. Lett. 2006, 407, 205–210. [Google Scholar] [CrossRef]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Il-1β | CTGTGTCTTTCCCGTGGACC | CAGCTCATATGGGTCCGACA |
| Tnf-α | GACGTGGAACTGGCAGAAGAG | TCTGGAAGCCCCCCATCT |
| Il-6 | AGAGGAGACTTCACAGAGGATACC | AATCAGAATTGCCATTGCACAAC |
| Cox-2 | TGAGTACCGCAAACGCTTCT | CAGCCATTTCCTTCTCTCCTGT |
| Irak1 | GCCAGCCAAAGAACTTGATAGAA | TACTCTGCTTGCCTTGCTCACA |
| Traf6 | GGAATCACTTGGCACGACACTT | GGACGCAAAGCAAGGTTAACAT |
| Agr1 | CAGAAGAATGGAAGAGTC | CAGATATGCAGGGAGTCA |
| Fizz1 | CTGATGAGACCATAGAGATTATCGTG | GCACAGGCAGTTGCAAGTATCTCC |
| Gclc | GGCTCTCTGCACCATCAC | TCTGACACGTAGCCTCGG |
| Ho-1 | CCTTCCCGAACATCGACAGCC | GCAGCTCCTCAAACAGCTCAA |
| Nqo-1 | TTCTGTGGCTTCCAGGTCTT | AGGCTGCTTGGAGCAAAATA |
| Ym-1 | AGAAGGGAGTTTCAAACCTGGT | GTCTTGCTCATGTGTGTAAGTGA |
| Il-10 | TGAATTCCCTGGGTGAGAAGCTGA | TGGCCTTGTAGACACCTTGGTCTT |
| Tgf-β | TAAAGAGGTCACCCGCGTGCTAAT | ACTGCTTCCCGAATGTCTGACGTA |
| Eef1a1 | GATGGCCCCAAATTCTTGAAG | GGACCATGTCAACAATTGCAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saputra, W.D.; Shono, H.; Ohsaki, Y.; Sultana, H.; Komai, M.; Shirakawa, H. Geranylgeraniol Inhibits Lipopolysaccharide-Induced Inflammation in Mouse-Derived MG6 Microglial Cells via NF-κB Signaling Modulation. Int. J. Mol. Sci. 2021, 22, 10543. https://doi.org/10.3390/ijms221910543
Saputra WD, Shono H, Ohsaki Y, Sultana H, Komai M, Shirakawa H. Geranylgeraniol Inhibits Lipopolysaccharide-Induced Inflammation in Mouse-Derived MG6 Microglial Cells via NF-κB Signaling Modulation. International Journal of Molecular Sciences. 2021; 22(19):10543. https://doi.org/10.3390/ijms221910543
Chicago/Turabian StyleSaputra, Wahyu Dwi, Hiroki Shono, Yusuke Ohsaki, Halima Sultana, Michio Komai, and Hitoshi Shirakawa. 2021. "Geranylgeraniol Inhibits Lipopolysaccharide-Induced Inflammation in Mouse-Derived MG6 Microglial Cells via NF-κB Signaling Modulation" International Journal of Molecular Sciences 22, no. 19: 10543. https://doi.org/10.3390/ijms221910543
APA StyleSaputra, W. D., Shono, H., Ohsaki, Y., Sultana, H., Komai, M., & Shirakawa, H. (2021). Geranylgeraniol Inhibits Lipopolysaccharide-Induced Inflammation in Mouse-Derived MG6 Microglial Cells via NF-κB Signaling Modulation. International Journal of Molecular Sciences, 22(19), 10543. https://doi.org/10.3390/ijms221910543









