Localization of Neurotrophin Specific Trk Receptors in Mechanosensory Systems of Killifish (Nothobranchius guentheri)
Abstract
1. Introduction
2. Results
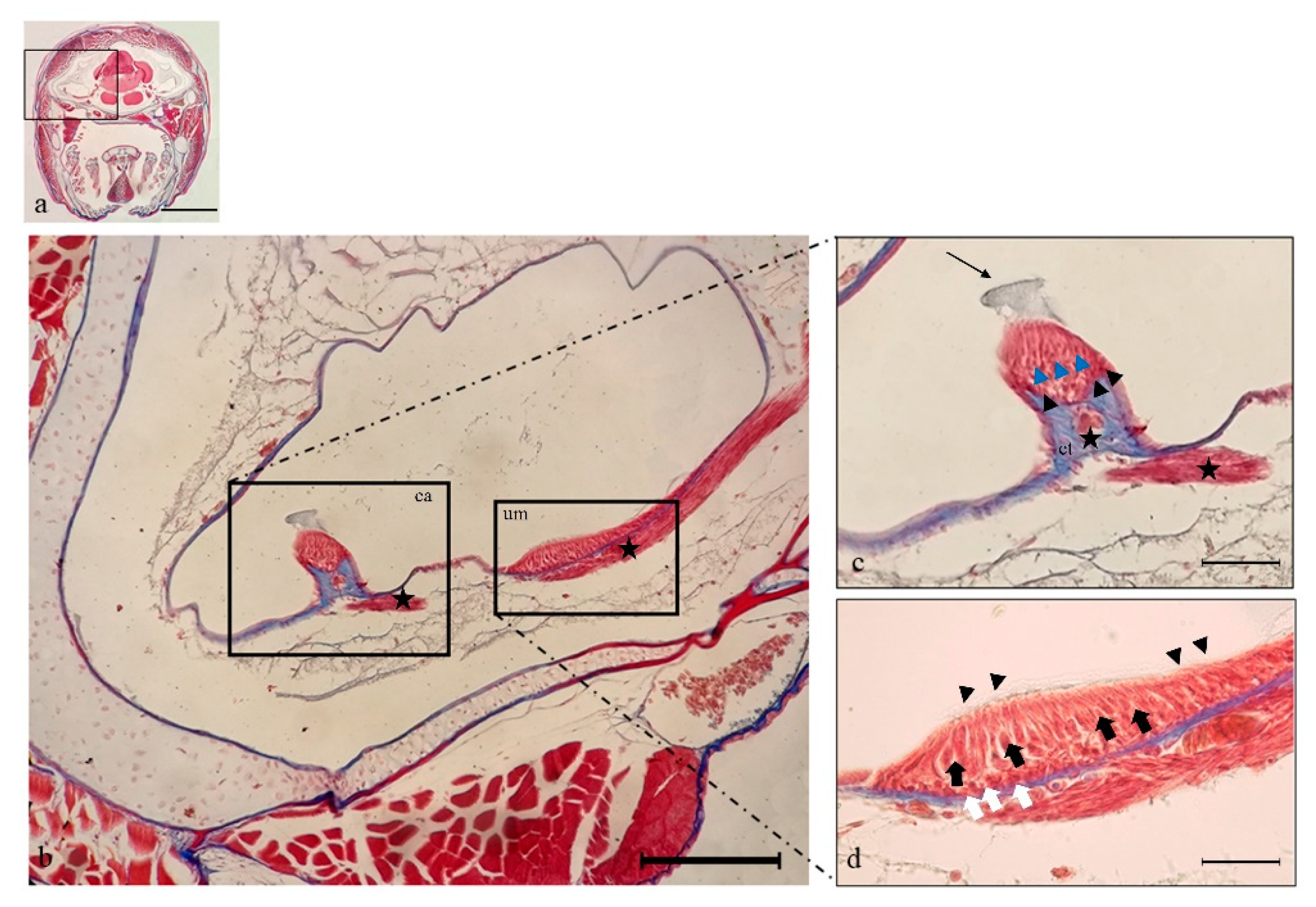
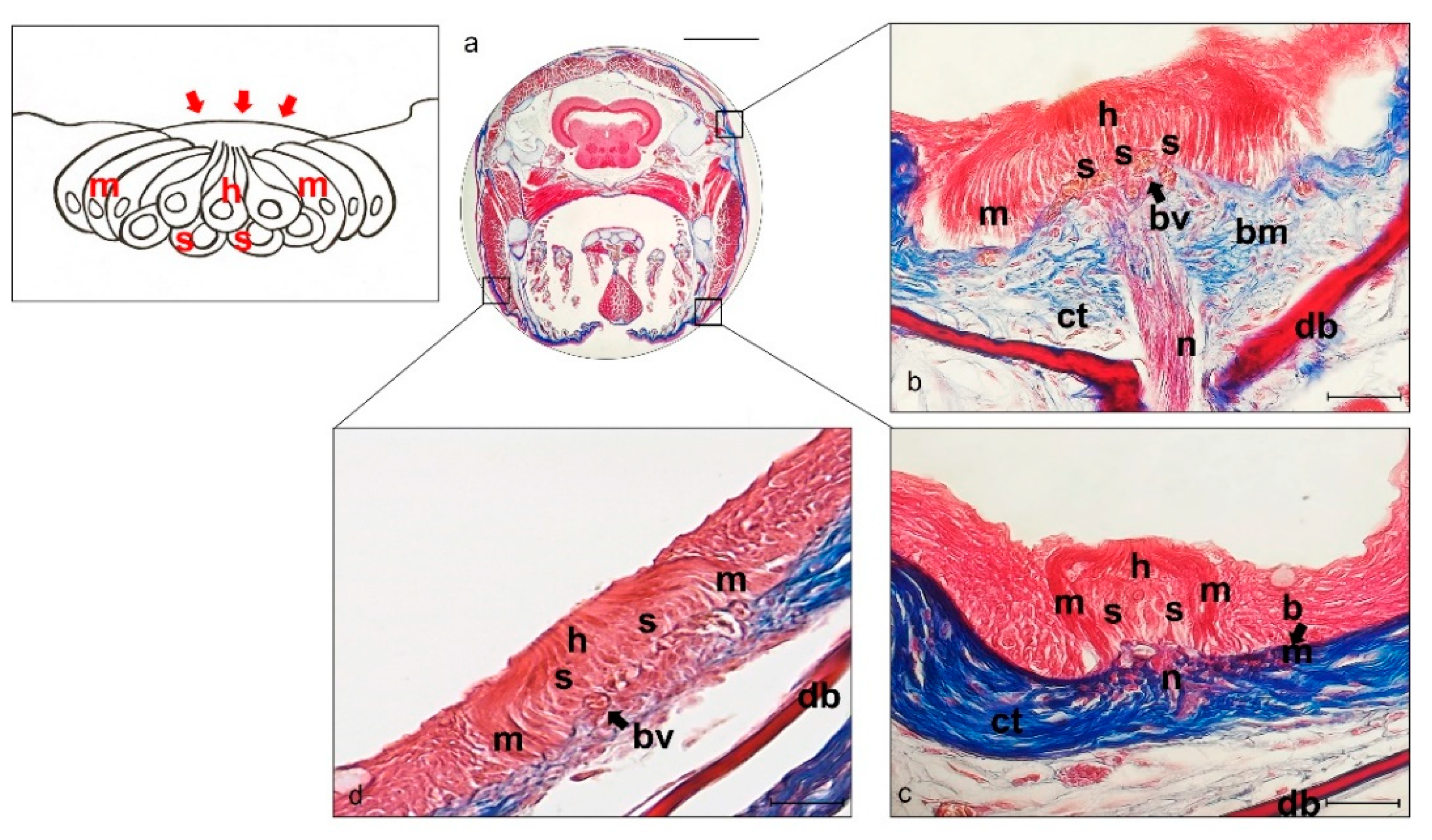
2.1. Histology
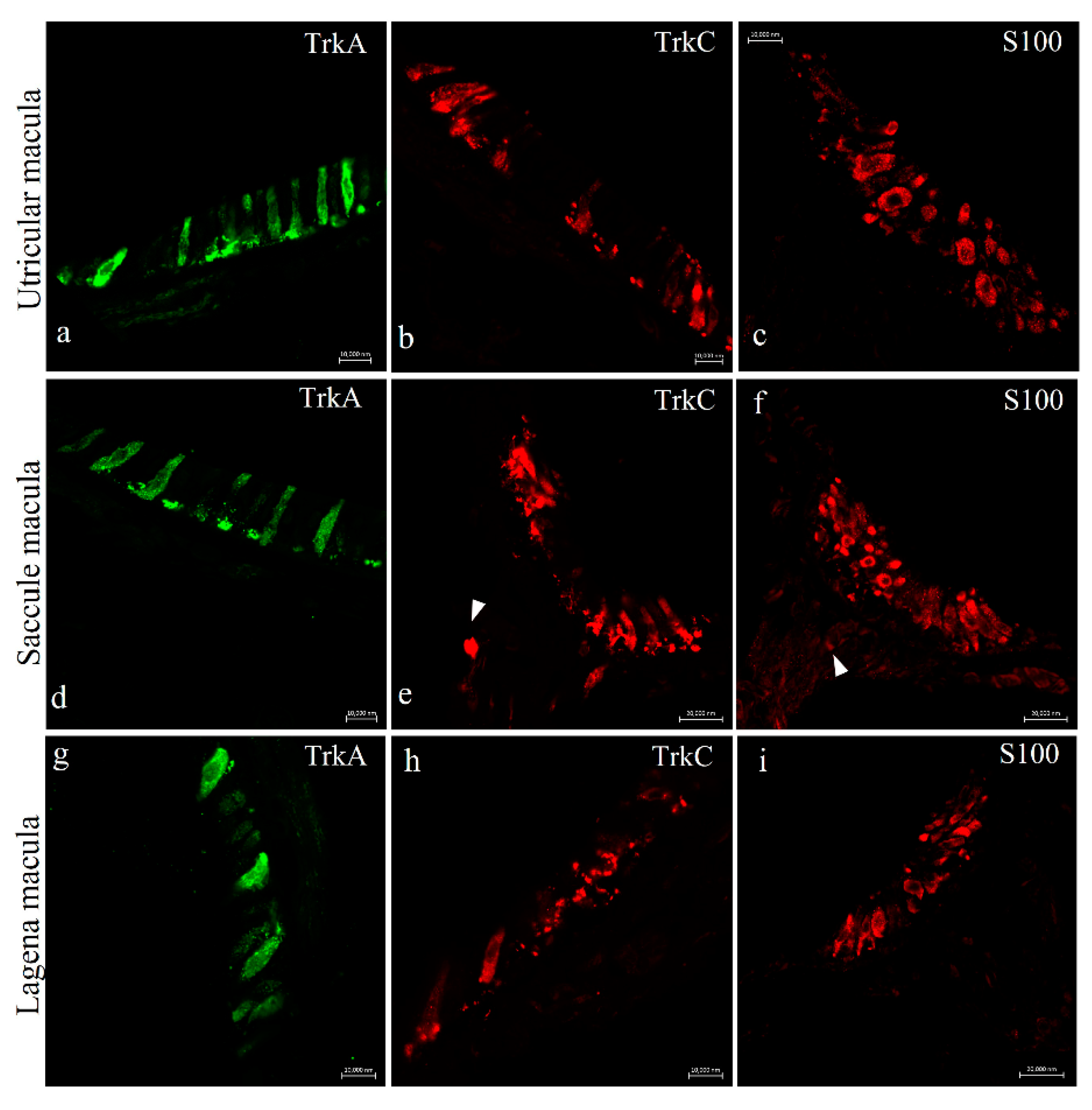
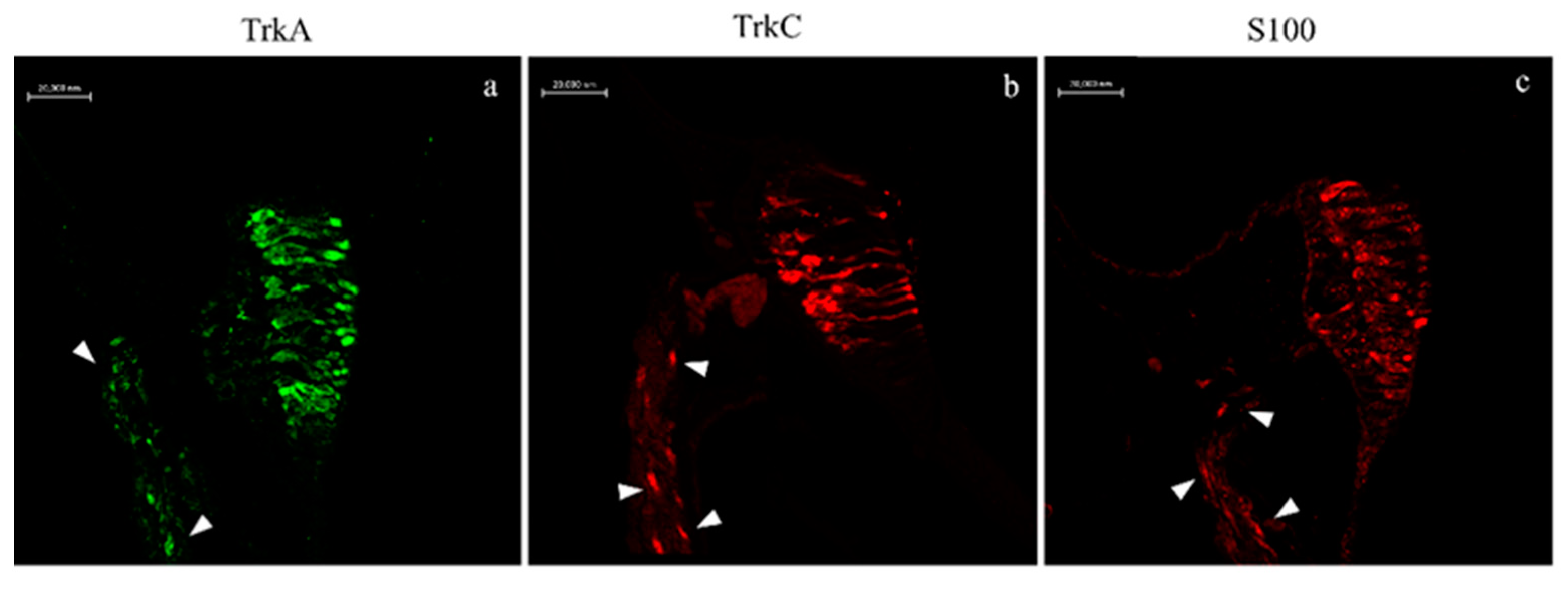
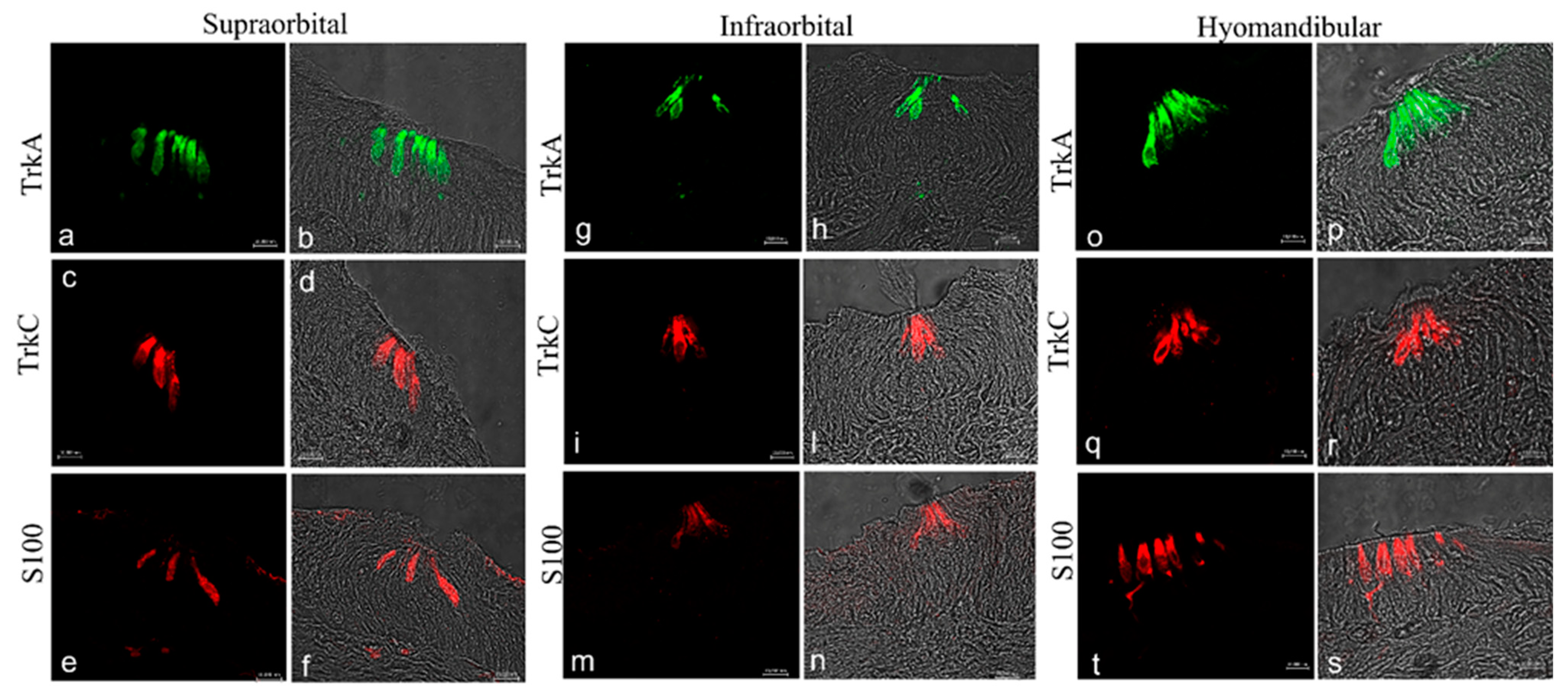
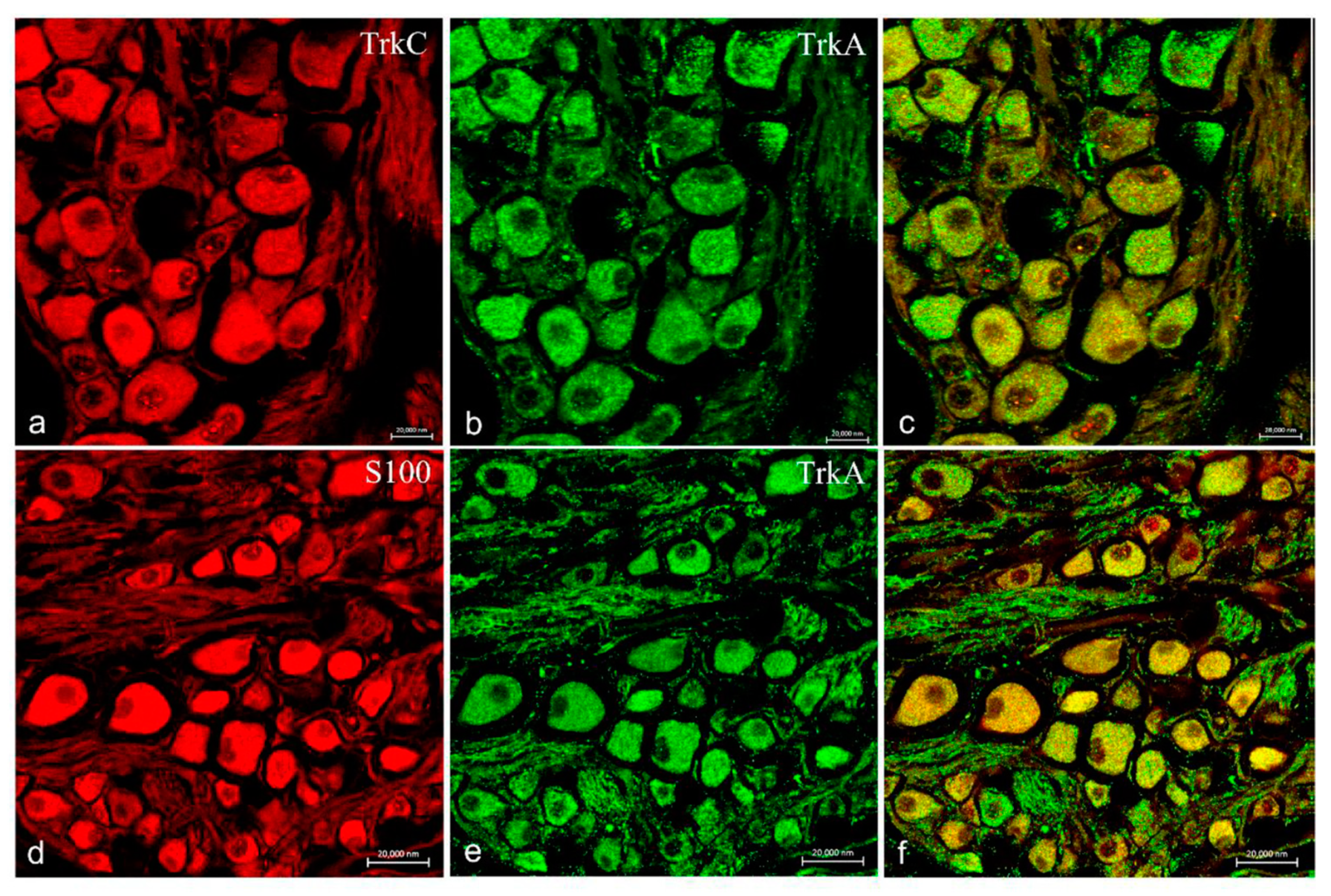
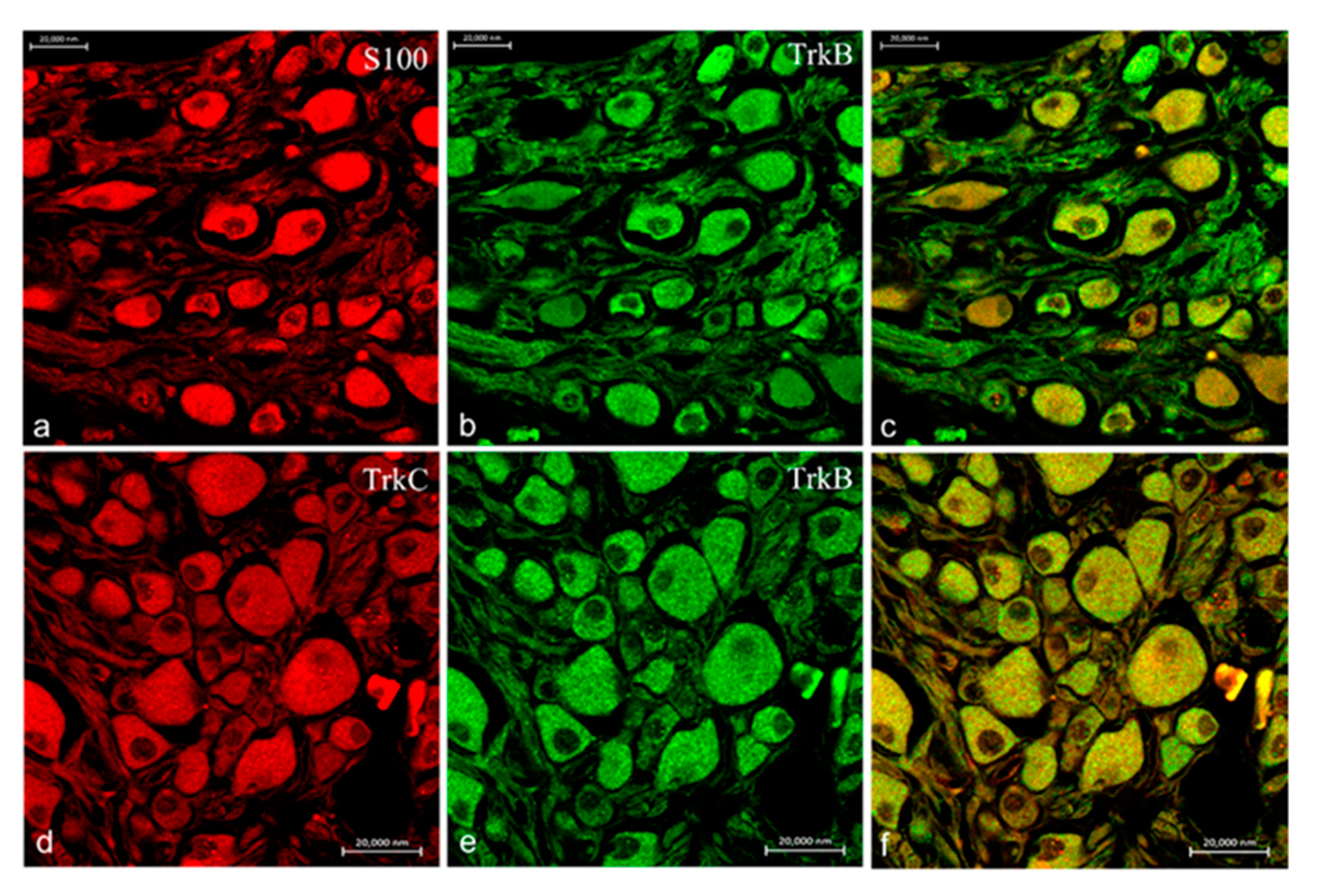
2.2. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Fish and Tissue Treatment
4.2. Localization of TrkS and S100 Protein Using Single and Double Immunofluorescence Staining
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henderson:, C.E. Role of neurotrophic factors in neuronal development. Curr. Opin. Neurobiol. 1996, 6, 64–70. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Hallböök, F. Evolution of the vertebrate neurotrophin and Trk receptor gene families. Curr. Opin. Neurobiol. 1999, 9, 616–621. [Google Scholar] [CrossRef]
- Heinrich, G.; Lum, T. Fish neurotrophins and Trk receptors. Int. J. Dev. Neurosci. 2000, 18, 1–27. [Google Scholar] [CrossRef]
- Martin, S.C.; Marazzi, G.; Sandell, J.H.; Heinrich, G. Five Trk Receptors in the Zebrafish. Dev. Biol. 1995, 169, 745–758. [Google Scholar] [CrossRef]
- Caminos, E.; Becker, E.; Martín-Zanca, D.; Vecino, E. Neurotrophins and their receptors in the tench retina during optic nerve regeneration. J. Comp. Neurol. 1999, 404, 321–331. [Google Scholar] [CrossRef]
- García-Suárez, O.; Germanà, A.; Hannestad, J.; Pérez-Pérez, M.; Esteban, I.; J Naves, F.; Vega, J.A. Changes in the expression of the nerve growth factor receptors TrkA and p75LNGR in the rat thymus with ageing and increased nerve growth factor plasma levels. Cell Tissue Res. 2000, 301, 225–234. [Google Scholar] [CrossRef]
- Götz, R.; Köster, R.; Winkler, C.; Raulf, F.; Lottspeich, F.; Schartl, M.; Thoenen, H. Neurotrophin-6 is a new member of the nerve growth factor family. Nature 1994, 372, 266. [Google Scholar] [CrossRef]
- Nilsson, A.-S.; Fainzilber, M.; Falck, P.; Ibáñez, C.F. Neurotrophin-7: A novel member of the neurotrophin family from the zebrafish. FEBS Lett. 1998, 424, 285–290. [Google Scholar] [CrossRef]
- Lai, K.-O.; Fu, W.-Y.; Ip, F.C.F.; Ip, N.Y. Cloning and Expression of a Novel Neurotrophin, NT-7, from Carp. Mol. Cell. Neurosci. 1998, 11, 64–76. [Google Scholar] [CrossRef]
- Hannestad, J.; Marino, F.; Germanà, A.; Catania, S.; Abbate, F.; Ciriaco, E.; Vega, J. Trk neurotrophin receptor-like proteins in the teleost Dicentrarchus labrax. Cell Tissue Res. 2000, 300, 1–9. [Google Scholar] [CrossRef]
- Catania, S.; Germana, A.; Cabo, R.; Ochoa-Erena, F.; Guerrera, M.; Hannestad, J.; Represa, J.; Vega, J. Neurotrophin and Trk neurotrophin receptors in the inner ear of Salmo salar and Salmo trutta. J. Anat. 2007, 210, 78–88. [Google Scholar] [CrossRef]
- Germanà, A.; Sánchez-Ramos, C.; Guerrera, M.C.; Calavia, M.; Navarro, M.; Zichichi, R.; García-Suárez, O.; Pérez-Piñera, P.; Vega, J.A. Expression and cell localization of brain-derived neurotrophic factor and TrkB during zebrafish retinal development. J. Anat. 2010, 217, 214–222. [Google Scholar] [CrossRef]
- Germanà, A.; Laurà, R.; Montalbano, G.; Guerrera, M.C.; Amato, V.; Zichichi, R.; Campo, S.; Ciriaco, E.; Vega, J.A. Expression of Brain-Derived Neurotrophic Factor and TrkB in the Lateral Line System of Zebrafish During Development. Cell. Mol. Neurobiol. 2010, 30, 787–793. [Google Scholar] [CrossRef]
- Germanà, A.; Guerrera, M.C.; Laurà, R.; Levanti, M.; Aragona, M.; Mhalhel, K.; Germanà, G.; Montalbano, G.; Abbate, F. Expression and Localization of BDNF/TrkB System in the Zebrafish Inner Ear. Int. J. Mol. Sci. 2020, 21, 5787. [Google Scholar] [CrossRef]
- Germana, A.; Catania, S.; Cavallaro, M.; González-Martínez, T.; Ciriaco, E.; Hannestad, J.; Vega, J.A. Immunohistochemical localization of BDNF-, TrkB- and TrkA-like proteins in the teleost lateral line system. J. Anat. 2002, 200, 477–485. [Google Scholar] [CrossRef]
- Lucini, C.; D’Angelo, L.; Cacialli, P.; Palladino, A.; De Girolamo, P. BDNF, Brain, and Regeneration: Insights from Zebrafish. Int. J. Mol. Sci. 2018, 19, 3155. [Google Scholar] [CrossRef]
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Abbate, F.; Laurà, R.; Navarra, M.; Vega, J.A.; Ciriaco, E.; Germanà, A. Morphological differences in adipose tissue and changes in BDNF/Trkb expression in brain and gut of a diet induced obese zebrafish model. Ann. Anat.-Anat. Anz. 2016, 204, 36–44. [Google Scholar] [CrossRef]
- Cacialli, P.; Gueguen, M.-M.; Coumailleau, P.; D’Angelo, L.; Kah, O.; Lucini, C.; Pellegrini, E. BDNF expression in larval and adult zebrafish brain: Distribution and cell identification. PLoS ONE 2016, 11, e0158057. [Google Scholar] [CrossRef]
- De Felice, E.; Porreca, I.; Alleva, E.; De Girolamo, P.; Ambrosino, C.; Ciriaco, E.; Germanà, A.; Sordino, P. Localization of BDNF expression in the developing brain of zebrafish. J. Anat. 2014, 224, 564–574. [Google Scholar] [CrossRef]
- Abbate, F.; Guerrera, M.C.; Montalbano, G.; Levanti, M.B.; Germanà, G.P.; Navarra, M.; Laurà, R.; Vega, J.A.; Ciriaco, E.; Germanà, A. Expression and anatomical distribution of TrkB in the encephalon of the adult zebrafish (Danio rerio). Neurosci. Lett. 2014, 563, 66–69. [Google Scholar] [CrossRef]
- Sánchez-Ramos, C.; Bonnin-Arias, C.; Guerrera, M.C.; Calavia, M.G.; Chamorro, E.; Montalbano, G.; López-Velasco, S.; López-Muñiz, A.; Germanà, A.; Vega, J.A. Light regulates the expression of the BDNF/TrkB system in the adult Zebrafish retina. Microsc. Res. Tech. 2013, 76, 42–49. [Google Scholar] [CrossRef]
- Germana, A.; Montalbano, G.; Laura, R.; Ciriaco, E.; Del Valle, M.; Vega, J.A. S100 protein-like immunoreactivity in the crypt olfactory neurons of the adult zebrafish. Neurosci. Lett. 2004, 371, 196–198. [Google Scholar] [CrossRef]
- Catania, S.; Germana, A.; Laura, R.; Gonzalez-Martinez, T.; Ciriaco, E.; Vega, J. The crypt neurons in the olfactory epithelium of the adult zebrafish express TrkA-like immunoreactivity. Neurosci. Lett. 2003, 350, 5–8. [Google Scholar] [CrossRef]
- Brignull, H.R.; Raible, D.W.; Stone, J.S. Feathers and fins: Non-mammalian models for hair cell regeneration. Brain Res. 2009, 1277, 12–23. [Google Scholar] [CrossRef]
- Esterberg, R.; Coffin, A.B.; Ou, H.; Simon, J.A.; Raible, D.W.; Rubel, E.W. Fish in a dish: Drug discovery for hearing habilitation. Drug Discov. Today: Dis. Models 2013, 10, e23–e29. [Google Scholar] [CrossRef][Green Version]
- Thomas, E.D.; Cruz, I.A.; Hailey, D.W.; Raible, D.W. There and back again: Development and regeneration of the zebrafish lateral line system. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 1–16. [Google Scholar] [CrossRef]
- Montalbano, G.; Capillo, G.; Laurà, R.; Abbate, F.; Levanti, M.; Guerrera, M.; Ciriaco, E.; Germanà, A. Neuromast hair cells retain the capacity of regeneration during heavy metal exposure. Ann. Anat.-Anat. Anz. 2018, 218, 183–189. [Google Scholar] [CrossRef]
- Montalbano, G.; Abbate, F.; Levanti, M.B.; Germanà, G.P.; Laurà, R.; Ciriaco, E.; Vega, J.A.; Germanà, A. Topographical and drug specific sensitivity of hair cells of the zebrafish larvae to aminoglycoside-induced toxicity. Ann. Anat.-Anat. Anz. 2014, 196, 236–240. [Google Scholar] [CrossRef]
- Montalbano, G.; Maugeri, A.; Guerrera, M.C.; Miceli, N.; Navarra, M.; Barreca, D.; Cirmi, S.; Germanà, A. A white grape juice extract reduces fat accumulation through the modulation of ghrelin and leptin expression in an in vivo model of overfed zebrafish. Molecules 2021, 26, 1119. [Google Scholar] [CrossRef]
- Monroe, J.D.; Rajadinakaran, G.; Smith, M.E. Sensory hair cell death and regeneration in fishes. Front. Cell. Neurosci. 2015, 9, 131. [Google Scholar] [CrossRef]
- Cruickshanks, K.J.; Wiley, T.L.; Tweed, T.S.; Klein, B.E.; Klein, R.; Mares-Perlman, J.A.; Nondahl, D.M. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin: The epidemiology of hearing loss study. Am. J. Epidemiol. 1998, 148, 879–886. [Google Scholar] [CrossRef]
- Cruickshanks, K.J.; Tweed, T.S.; Wiley, T.L.; Klein, B.E.; Klein, R.; Chappell, R.; Nondahl, D.M.; Dalton, D.S. The 5-year incidence and progression of hearing loss: The epidemiology of hearing loss study. Arch. Otolaryngol.-Head Neck Surg. 2003, 129, 1041–1046. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kiang, N.Y. Acoustic trauma in cats: Cochlear pathology and auditory-nerve activity. Acta Oto-Laryngol. 1978. [Google Scholar]
- Mcgill, T.J.; Schuknecht, H.F. Human cochlear changes in noise induced hearing loss. Laryngoscope 1976, 86, 1293–1302. [Google Scholar] [CrossRef]
- Schuknecht, H.F.; Gacek, M.R. Cochlear pathology in presbycusis. Ann. Otol. Rhinol. Laryngol. 1993, 102, 1–16. [Google Scholar] [CrossRef]
- Moser, T.; Starr, A. Auditory neuropathy—neural and synaptic mechanisms. Nat. Rev. Neurol. 2016, 12, 135–149. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Cellerino, A.; Valenzano, D.R.; Reichard, M. From the bush to the bench: The annual Nothobranchius fishes as a new model system in biology. Biol. Rev. 2016, 91, 511–533. [Google Scholar] [CrossRef]
- Lucas-Sánchez, A.; Almaida-Pagán, P.F.; Mendiola, P.; de Costa, J. Nothobranchius as a model for aging studies. A review. Aging Dis 2013, 5, 281–291. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Sharp, S.; Brunet, A. Transposon-mediated transgenesis in the short-lived African killifish Nothobranchius furzeri, a vertebrate model for aging. G3 Genes Genomes Genet. 2011, 1, 531–538. [Google Scholar] [CrossRef]
- Hartmann, N.; Englert, C. A microinjection protocol for the generation of transgenic killifish (Species: Nothobranchius furzeri). Dev. Dyn. 2012, 241, 1133–1141. [Google Scholar] [CrossRef]
- Petzold, A.; Reichwald, K.; Groth, M.; Taudien, S.; Hartmann, N.; Priebe, S.; Shagin, D.; Englert, C.; Platzer, M. The transcript catalogue of the short-lived fish Nothobranchius furzeri provides insights into age-dependent changes of mRNA levels. BMC Genom. 2013, 14, 185. [Google Scholar] [CrossRef]
- Tozzini, E.T.; Cellerino, A. Nothobranchius annual killifishes. EvoDevo 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Genade, T.; Benedetti, M.; Terzibasi, E.; Roncaglia, P.; Valenzano, D.R.; Cattaneo, A.; Cellerino, A. Annual fishes of the genus Nothobranchius as a model system for aging research. Aging Cell 2005, 4, 223–233. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chiu, Y.-C. Ambient temperature influences aging in an annual fish (Nothobranchius rachovii). Aging Cell 2009, 8, 726–737. [Google Scholar] [CrossRef]
- Valenzano, D.R.; Cellerino, A. Resveratrol and the Pharmacology of Aging: A New Vertebrate Model to Validate an Old Molecule. Cell Cycle 2006, 5, 1027–1032. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Feng, W.; Li, G.; Su, F.; Zhang, S. Differential expression of aging biomarkers at different life stages of the annual fish Nothobranchius guentheri. Biogerontology 2012, 13, 501–510. [Google Scholar] [CrossRef]
- Wang, X.; Shang, X.; Luan, J.; Zhang, S. Identification, expression and function of apolipoprotein E in annual fish Nothobranchius guentheri: Implication for an aging marker. Biogerontology 2014, 15, 233–243. [Google Scholar] [CrossRef]
- D’Angelo, L.; De Girolamo, P.; Lucini, C.; Terzibasi, E.T.; Baumgart, M.; Castaldo, L.; Cellerino, A. Brain-derived neurotrophic factor: mRNA expression and protein distribution in the brain of the teleost Nothobranchius furzeri. J. Comp. Neurol. 2014, 522, 1004–1030. [Google Scholar] [CrossRef]
- D’Angelo, L.; de Girolamo, P.; Cellerino, A.; Tozzini, E.T.; Castaldo, L.; Lucini, C. Neurotrophin Trk receptors in the brain of a teleost fish, Nothobranchius furzeri. Microsc. Res. Tech. 2012, 75, 81–88. [Google Scholar] [CrossRef]
- Leggieri, A.; Attanasio, C.; Palladino, A.; Cellerino, A.; Lucini, C.; Paolucci, M.; Terzibasi Tozzini, E.; de Girolamo, P.; D’Angelo, L. Identification and expression of neurotrophin-6 in the brain of Nothobranchius furzeri: One more piece in neurotrophin research. J. Clin. Med. 2019, 8, 595. [Google Scholar] [CrossRef]
- D’angelo, L. Brain Atlas of an Emerging Teleostean Model: Nothobranchius furzeri. Anat. Rec. 2013, 296, 681–691. [Google Scholar] [CrossRef]
- D’Angelo, L.; Lossi, L.; Merighi, A.; de Girolamo, P. Anatomical features for the adequate choice of experimental animal models in biomedicine: I. Fishes. Ann. Anat.-Anat. Anz. 2016, 205, 75–84. [Google Scholar] [CrossRef]
- Abbate, F.; Catania, S.; Germana, A.; González, T.; Diaz-Esnal, B.; Germana, G.; Vega, J. S-100 protein is a selective marker for sensory hair cells of the lateral line system in teleosts. Neurosci. Lett. 2002, 329, 133–136. [Google Scholar] [CrossRef]
- Germana, A.; González-Martínez, T.; Catania, S.; Laura, R.; Cobo, J.; Ciriaco, E.; Vega, J. Neurotrophin receptors in taste buds of adult zebrafish (Danio rerio). Neurosci. Lett. 2004, 354, 189–192. [Google Scholar] [CrossRef]
- Germanà, A.; Paruta, S.; Germanà, G.P.; Ochoa-Erena, F.J.; Montalbano, G.; Cobo, J.; Vega, J.A. Differential distribution of S100 protein and calretinin in mechanosensory and chemosensory cells of adult zebrafish (Danio rerio). Brain Res. 2007, 1162, 48–55. [Google Scholar] [CrossRef]
- Montalbano, G.; Mania, M.; Guerrera, M.C.; Laurà, R.; Abbate, F.; Levanti, M.; Maugeri, A.; Germanà, A.; Navarra, M. Effects of a Flavonoid-Rich Extract from Citrus sinensis Juice on a Diet-Induced Obese Zebrafish. Int. J. Mol. Sci. 2019, 20, 5116. [Google Scholar] [CrossRef]
- Germanà, A.; Marino, F.; Guerrera, M.C.; Campo, S.; de Girolamo, P.; Montalbano, G.; Germanà, G.P.; Ochoa-Erena, F.J.; Ciriaco, E.; Vega, J.A. Expression and distribution of S100 protein in the nervous system of the adult zebrafish (Danio rerio). Microsc. Res. Tech. 2008, 71, 248–255. [Google Scholar] [CrossRef]
- Collazo, A.; Fraser, S.E.; Mabee, P.M. A dual embryonic origin for vertebrate mechanoreceptors. Science 1994, 264, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E. Ferntastsinnesorgane von Oberflächenfischen. Z. Für Morphol. Der Tiere 1970, 67, 40–57. [Google Scholar] [CrossRef]
- Hoin-Radkovsky, I.; Bleckmann, H.; Schwartz, E. Determination of source distance in the surface-feeding fish Pantodon buchholzi Pantodontidae. Anim. Behav. 1984, 32, 840–851. [Google Scholar] [CrossRef]
- Edgley, D.E.; Genner, M.J. Adaptive Diversification of the Lateral Line System during Cichlid Fish Radiation. iScience 2019, 16, 1–11. [Google Scholar] [CrossRef]
- Germana, A.; Abbate, F.; González-Martínez, T.; Del Valle, M.; De Carlos, F.; Germanà, G.; Vega, J. S100 protein is a useful and specific marker for hair cells of the lateral line system in postembryonic zebrafish. Neurosci. Lett. 2004, 365, 186–189. [Google Scholar] [CrossRef]
- Beck, G.; Munno, D.W.; Levy, Z.; Dissel, H.M.; Van-Minnen, J.; Syed, N.I.; Fainzilber, M. Neurotrophic activities of trk receptors conserved over 600 million years of evolution. J. Neurobiol. 2004, 60, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Tessarollo, L.; Coppola, E.; Reichardt, L.F. Neurotrophins in the ear: Their roles in sensory neuron survival and fiber guidance. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2004; Volume 146, pp. 265–278. [Google Scholar]
- Szobota, S.; Mathur, P.D.; Siegel, S.; Black, K.; Saragovi, H.U.; Foster, A.C. BDNF, NT-3 and Trk receptor agonist monoclonal antibodies promote neuron survival, neurite extension, and synapse restoration in rat cochlea ex vivo models relevant for hidden hearing loss. PLoS ONE 2019, 14, e0224022. [Google Scholar] [CrossRef] [PubMed]
- Bang, P.I.; Sewell, W.F.; Malicki, J.J. Morphology and cell type heterogeneities of the inner ear epithelia in adult and juvenile zebrafish (Danio rerio). J. Compart. Neurol. 2001, 438, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Laurà, R.; Abbate, F.; Germanà, G.; Montalbano, G.; Germanà, A.; Levanti, M. Fine structure of the canal neuromasts of the lateral line system in the adult zebrafish. Anat. Histol. Embryol. 2018, 47, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Chai, R.; Li, H. Hair Cell Regeneration. In Hearing Loss: Mechanisms, Prevention and Cure; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–16. [Google Scholar] [CrossRef]
- Coffin, A.B.; Ramcharitar, J. Chemical ototoxicity of the fish inner ear and lateral line. In Fish Hearing and Bioacoustics; Springer: Berlin/Heidelberg, Germany, 2016; Volume 877, pp. 419–437. [Google Scholar]
- Stawicki, T.M.; Esterberg, R.; Hailey, D.W.; Raible, D.W.; Rubel, E.W. Using the zebrafish lateral line to uncover novel mechanisms of action and prevention in drug-induced hair cell death. Front. Cell. Neurosci. 2015, 9, 46. [Google Scholar] [CrossRef]
- Harris, J.A.; Cheng, A.G.; Cunningham, L.L.; MacDonald, G.; Raible, D.W.; Rubel, E.W. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J. Assoc. Res. Otolaryngol. 2003, 4, 219–234. [Google Scholar] [CrossRef]
- Volpe, B.A.; Fotino, T.H.; Steiner, A.B. Confocal Microscope-Based Laser Ablation and Regeneration Assay in Zebrafish Interneuromast Cells. J. Vis. Exp. JoVE 2020. [Google Scholar] [CrossRef]
- Germanà, A.; Montalbano, G.; Guerrera, M.C.; Laura, R.; Levanti, M.; Abbate, F.; de Carlos, F.; Vega, J.A.; Ciriaco, E. Sox-2 in taste bud and lateral line system of zebrafish during development. Neurosci. Lett. 2009, 467, 36–39. [Google Scholar] [CrossRef]
- Pinto-Teixeira, F.; Viader-Llargués, O.; Torres-Mejía, E.; Turan, M.; González-Gualda, E.; Pola-Morell, L.; López-Schier, H. Inexhaustible hair-cell regeneration in young and aged zebrafish. Biol. Open 2015, 4, 903–909. [Google Scholar] [CrossRef]
- Cruz, I.A. Adult Zebrafish Lateral Line: A Well Supported System. 2015. Available online: http://hdl.handle.net/1773/34063 (accessed on 24 September 2021).
- López-Schier, H.; Hudspeth, A. A two-step mechanism underlies the planar polarization of regenerating sensory hair cells. Proc. Natl. Acad. Sci. USA 2006, 103, 18615–18620. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, L.; Castaldo, L.; Cellerino, A.; de Girolamo, P.; Lucini, C. Nerve growth factor in the adult brain of a teleostean model for aging research: Nothobranchius furzeri. Ann. Anat.-Anat. Anz. 2014, 196, 183–191. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, L.; Avallone, L.; Cellerino, A.; de Girolamo, P.; Paolucci, M.; Varricchio, E.; Lucini, C. Neurotrophin-4 in the brain of adult Nothobranchius furzeri. Ann. Anat.-Anat. Anz. 2016, 207, 47–54. [Google Scholar] [CrossRef] [PubMed]


| Primary Antibodies | Supplier | Catalogue Number | Source | Diluition | Antibody ID |
|---|---|---|---|---|---|
| S100 | Dako | Z0311 | rabbit | 1:100 | AB_10013383 |
| TrkA (Y32Ex) | Santa Cruz Biotechnology, Inc. | sc-80398 | mouse | 1:100 | AB_1130726 |
| TrkB (F-1) | Santa Cruz Biotechnology, Inc. | sc-377218 | mouse | 1:100 | AB_2801499 |
| TrkC (798) | Santa Cruz Biotechnology, Inc. | sc-117 | rabbit | 1:100 | AB_632560 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragona, M.; Porcino, C.; Guerrera, M.C.; Montalbano, G.; Levanti, M.; Abbate, F.; Laurà, R.; Germanà, A. Localization of Neurotrophin Specific Trk Receptors in Mechanosensory Systems of Killifish (Nothobranchius guentheri). Int. J. Mol. Sci. 2021, 22, 10411. https://doi.org/10.3390/ijms221910411
Aragona M, Porcino C, Guerrera MC, Montalbano G, Levanti M, Abbate F, Laurà R, Germanà A. Localization of Neurotrophin Specific Trk Receptors in Mechanosensory Systems of Killifish (Nothobranchius guentheri). International Journal of Molecular Sciences. 2021; 22(19):10411. https://doi.org/10.3390/ijms221910411
Chicago/Turabian StyleAragona, Marialuisa, Caterina Porcino, Maria Cristina Guerrera, Giuseppe Montalbano, Maria Levanti, Francesco Abbate, Rosaria Laurà, and Antonino Germanà. 2021. "Localization of Neurotrophin Specific Trk Receptors in Mechanosensory Systems of Killifish (Nothobranchius guentheri)" International Journal of Molecular Sciences 22, no. 19: 10411. https://doi.org/10.3390/ijms221910411
APA StyleAragona, M., Porcino, C., Guerrera, M. C., Montalbano, G., Levanti, M., Abbate, F., Laurà, R., & Germanà, A. (2021). Localization of Neurotrophin Specific Trk Receptors in Mechanosensory Systems of Killifish (Nothobranchius guentheri). International Journal of Molecular Sciences, 22(19), 10411. https://doi.org/10.3390/ijms221910411







