Peptide Location Fingerprinting Reveals Tissue Region-Specific Differences in Protein Structures in an Ageing Human Organ
Abstract
1. Introduction
2. Results and Discussion
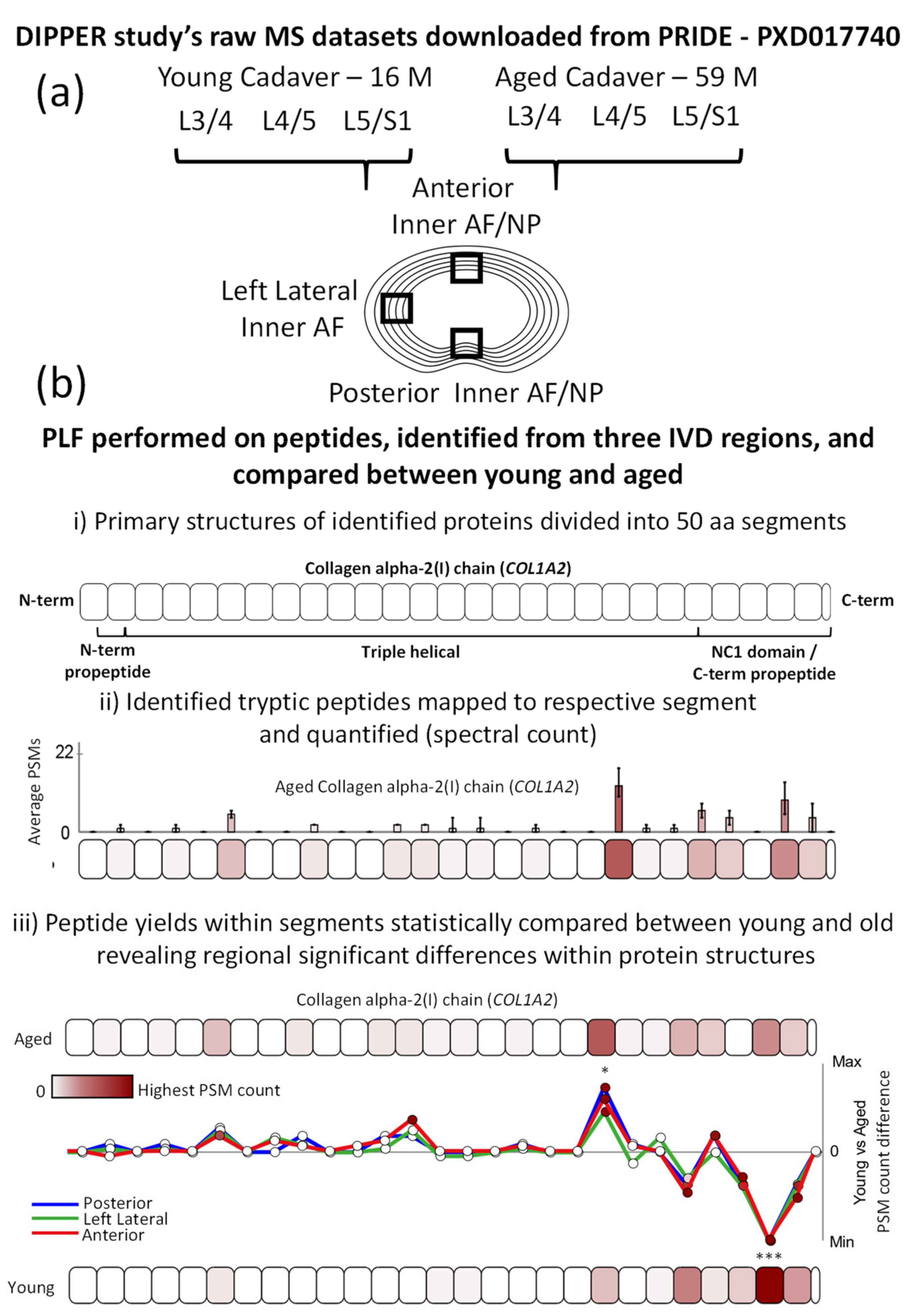
2.1. Peptide Location Fingerprinting Reveals Proteins with Region-Specific Differences between Aged and Young IVDs
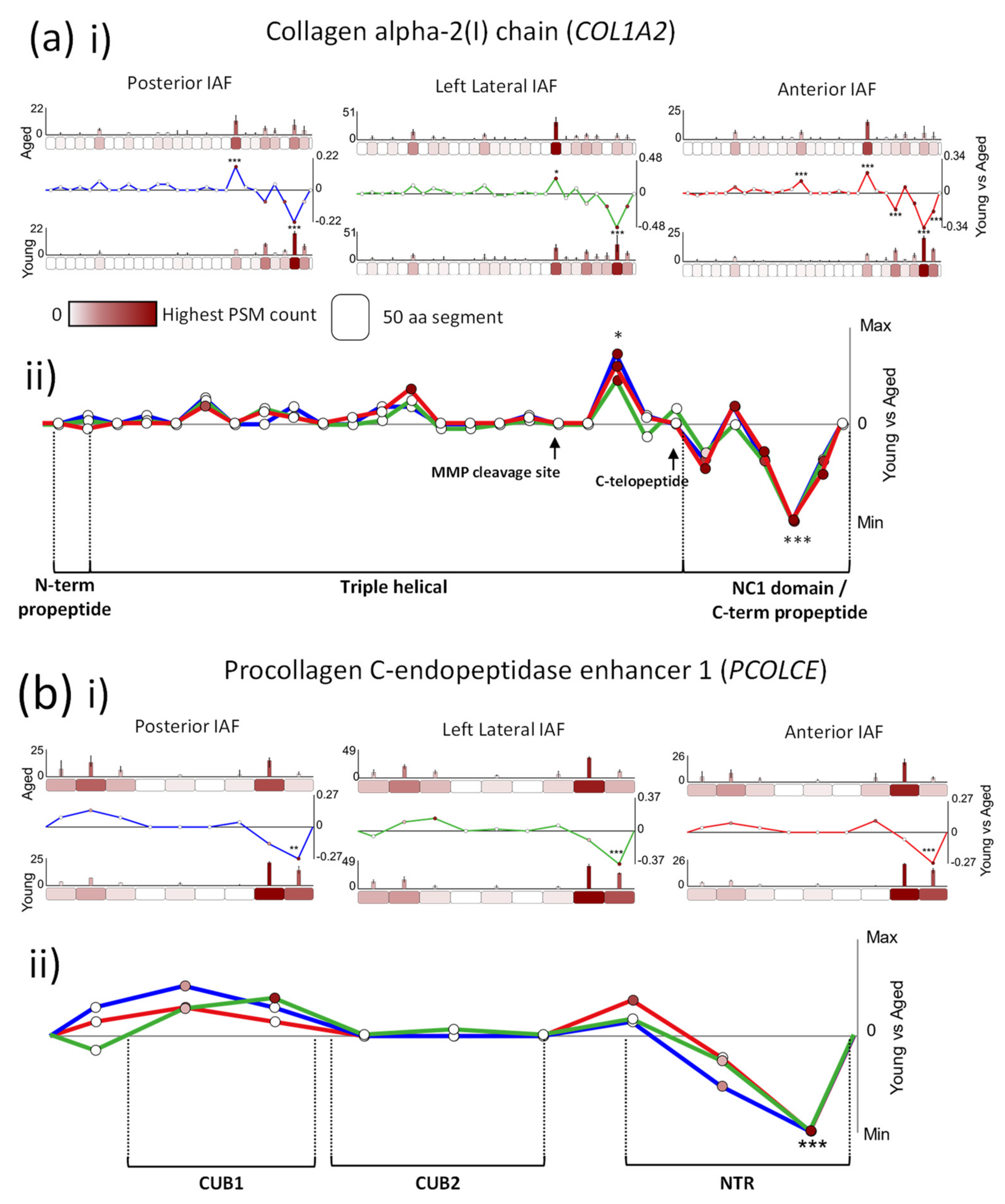
2.2. Age-Associated Fluctuations in Peptide Yield along the Primary Structures of COL1A2 and PCOLCE Are Markedly Conserved between Posterior, Lateral and Anterior IVD Regions
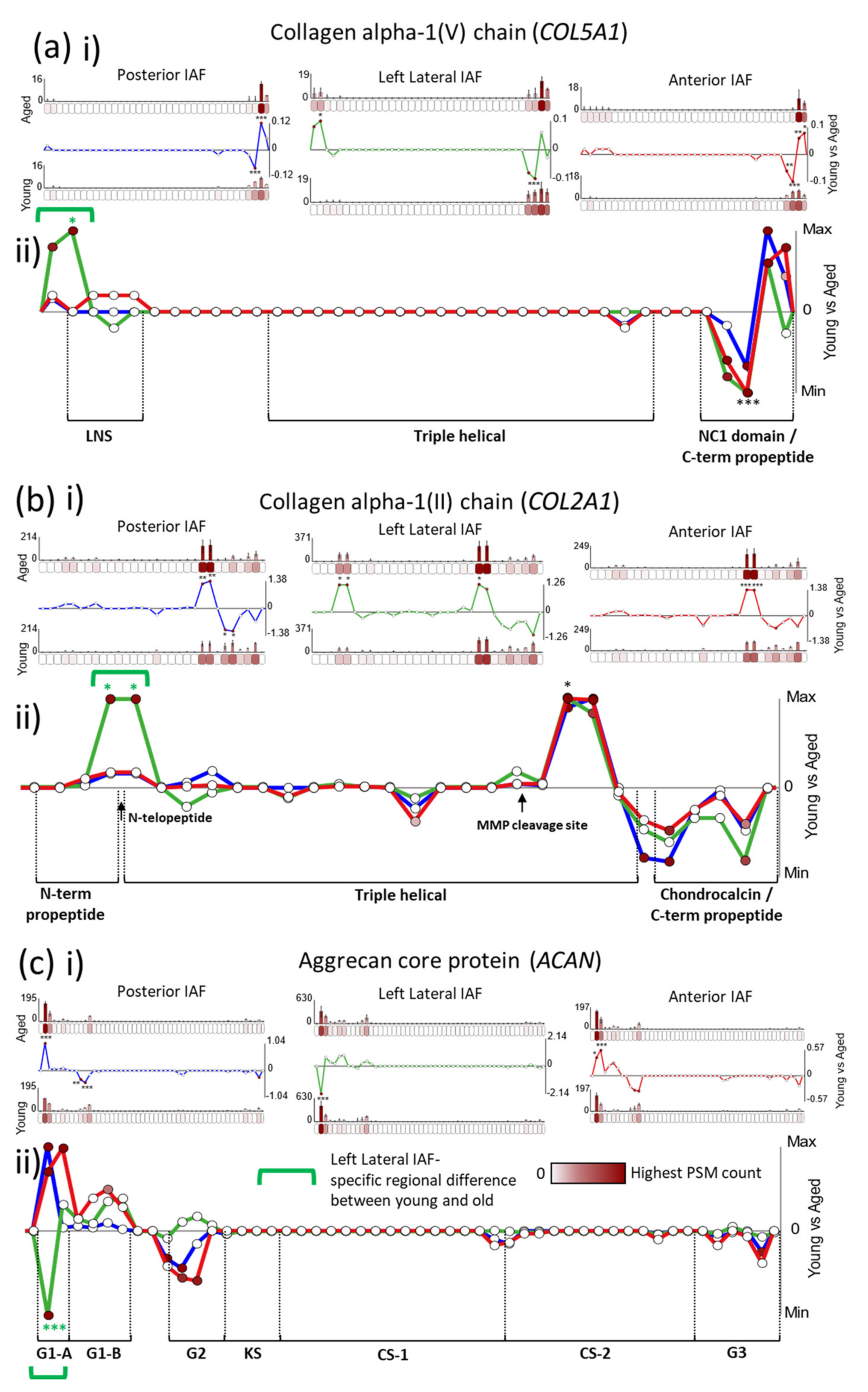
2.3. Age-Associated Peptide Yield Patterns along the Primary Structures of the α1 Chains of Collagens II and V and Aggrecan Exhibit Regional Tissue Specificity within the IVD
3. Materials and Methods
3.1. Dataset Collection
3.2. Peptide Identification
3.3. Peptide Location Fingerprinting
3.4. Interaction Network Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso, F.; Hart, D.J. Intervertebral Disk. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: Oxford, UK, 2014; pp. 724–729. ISBN 978-0-12-385158-1. [Google Scholar]
- Hoy, D.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Bain, C.; Williams, G.; Smith, E.; Vos, T.; Barendregt, J. The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 968. [Google Scholar] [CrossRef]
- Mirza, S.K. Intervertebral Disk. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: New York, NY, USA, 2003; pp. 683–689. ISBN 978-0-12-226870-0. [Google Scholar]
- Ohnishi, T.; Novais, E.J.; Risbud, M.V. Alterations in ECM signature underscore multiple sub-phenotypes of intervertebral disc degeneration. Matrix Biol. Plus 2020, 6–7, 100036. [Google Scholar] [CrossRef]
- Kepler, C.K.; Ponnappan, R.K.; Tannoury, C.A.; Risbud, M.V.; Anderson, D.G. The molecular basis of intervertebral disc degeneration. Spine J. 2013, 13, 318–330. [Google Scholar] [CrossRef]
- Eyre, D.R.; Muir, H. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 1976, 157, 267. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, P.-P.A.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.W.; Welting, T.J.; van Royen, B.J.; van Dieën, J.H.; Smit, T.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.-J.; Kienle, A.; Maile, S.; Rasche, V.; Berger-Roscher, N. A new dynamic six degrees of freedom disc-loading simulator allows to provoke disc damage and herniation. Eur. Spine J. 2016, 25, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.M.C.; Karppinen, J.; Chan, D.; Ho, D.W.H.; Song, Y.-Q.; Sham, P.; Cheah, K.S.E.; Leong, J.C.Y.; Luk, K.D.K. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Hoelscher, G.L.; Ingram, J.A.; Bethea, S.; Zinchenko, N.; Hanley, E.N. Variations in aggrecan localization and gene expression patterns characterize increasing stages of human intervertebral disk degeneration. Exp. Mol. Pathol. 2011, 91, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Collin, E.C.; Carroll, O.; Kilcoyne, M.; Peroglio, M.; See, E.; Hendig, D.; Alini, M.; Grad, S.; Pandit, A. Ageing affects chondroitin sulfates and their synthetic enzymes in the intervertebral disc. Signal Transduct. Target. Ther. 2017, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.; Lam, M.P.Y.; Tam, V.; Chan, W.C.W.; Chu, I.K.; Cheah, K.S.E.; Cheung, K.M.C.; Chan, D. Fibrotic-like changes in degenerate human intervertebral discs revealed by quantitative proteomic analysis. Osteoarthr. Cartil. 2016, 24, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.; Chen, P.; Yee, A.; Solis, N.; Klein, T.; Kudelko, M.; Sharma, R.; Chan, W.C.W.; Overall, C.M.; Haglund, L. DIPPER, a spatiotemporal proteomics atlas of human intervertebral discs for exploring ageing and degeneration dynamics. eLife 2020, 9, e64940. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef]
- Sivan, S.-S.; Wachtel, E.; Tsitron, E.; Sakkee, N.; van der Ham, F.; DeGroot, J.; Roberts, S.; Maroudas, A. Collagen turnover in normal and degenerate human intervertebral discs as determined by the racemization of aspartic acid. J. Biol. Chem. 2008, 283, 8796. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Hanley, E.N. Observations on morphologic changes in the aging and degenerating human disc: Secondary collagen alterations. BMC Musculoskelet. Disord. 2002, 3, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Craddock, R.J.; Hodson, N.W.; Ozols, M.; Shearer, T.; Hoyland, J.A.; Sherratt, M.J. Extracellular matrix fragmentation in young, healthy cartilaginous tissues. Eur. Cells Mater. 2018, 35, 34. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Mei, Q.; Xu, B.; Liu, G.; Zhao, J. Expression of matrix metalloproteinases is positively related to the severity of disc degeneration and growing age in the East Asian lumbar disc herniation patients. Cell Biochem. Biophys. 2014, 70, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Hoy, R.C.; D’Erminio, D.N.; Krishnamoorthy, D.; Natelson, D.M.; Laudier, D.M.; Illien-Jünger, S.; Iatridis, J.C. Advanced glycation end products cause RAGE-dependent annulus fibrosus collagen disruption and loss identified using in situ second harmonic generation imaging in mice intervertebral disk in vivo and in organ culture models. JOR Spine 2020, 3, e1126. [Google Scholar] [CrossRef]
- Alkhatib, B.; Gawri, R.; Önnerfjord, P.; Ouellet, J.; Roughley, P.; Steffen, T.; Haglund, L.A. Chondroadherin Fragmentation as a Biochemical Marker for Early Stage Disk Degeneration. Glob. Spine J. 2012, 2, s-0032. [Google Scholar] [CrossRef]
- Feng, C.; Yang, M.; Lan, M.; Liu, C.; Zhang, Y.; Huang, B.; Liu, H.; Zhou, Y. ROS: Crucial intermediators in the pathogenesis of intervertebral disc degeneration. Oxid. Med. Cell. Longev. 2017, 2017, 5601593. [Google Scholar] [CrossRef]
- Babu, N.S.; Krishnan, S.; Swamy, C.V.B.; Subbaiah, G.P.V.; Reddy, A.V.G.; Idris, M.M. Quantitative proteomic analysis of normal and degenerated human intervertebral disc. Spine J. 2016, 16, 989. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Tangavel, C.; Soundararajan, D.C.R.; Nayagam, S.M.; Matchado, M.S.; Muthurajan, R.; Anand, K.S.S.V.; Rajendran, S.; Shetty, A.P.; Kanna, R.M. Proteomic Signatures of Healthy Intervertebral Discs From Organ Donors: A Comparison With Previous Studies on Discs From Scoliosis, Animals, and Trauma. Neurospine 2020, 17, 426. [Google Scholar] [CrossRef]
- Ye, D.; Liang, W.; Dai, L.; Zhou, L.; Yao, Y.; Zhong, X.; Chen, H.; Xu, J. Comparative and quantitative proteomic analysis of normal and degenerated human annulus fibrosus cells. Clin. Exp. Pharmacol. Physiol. 2015, 42, 530–536. [Google Scholar] [CrossRef]
- Ozols, M.; Eckersley, A.; Mellody, K.T.; Mallikarjun, V.; Warwood, S.; O’Cualain, R.; Knight, D.; Watson, R.E.B.; Griffiths, C.E.M.; Swift, J.; et al. Peptide location fingerprinting reveals modification-associated biomarker candidates of ageing in human tissue proteomes. Aging Cell 2021, 20, e13355. [Google Scholar] [CrossRef]
- Eckersley, A.; Ozols, M.; O’Cualain, R.; Keevill, E.J.; Foster, A.; Pilkington, S.; Knight, D.; Griffiths, C.E.M.; Watson, R.E.B.; Sherratt, M.J. Proteomic fingerprints of damage in extracellular matrix assemblies. Matrix Biol. Plus 2020, 5, 100027. [Google Scholar] [CrossRef] [PubMed]
- Eckersley, A.; Mellody, K.T.; Pilkington, S.; Griffiths, C.E.M.; Watson, R.E.B.; O’Cualain, R.; Baldock, C.; Knight, D.; Sherratt, M.J. Structural and compositional diversity of fibrillin microfibrils in human tissues. J. Biol. Chem. 2018, 293, 5117. [Google Scholar] [CrossRef] [PubMed]
- Tsuruha, J.; Masuko-Hongo, K.; Kato, T.; Sakata, M.; Nakamura, H.; Nishioka, K. Implication of cartilage intermediate layer protein in cartilage destruction in subsets of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 2001, 44, 838–845. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Belluoccio, D.; Rowley, L.; Little, C.B.; Hansen, U.; Bateman, J.F. Cartilage intermediate layer protein 2 (CILP-2) is expressed in articular and meniscal cartilage and down-regulated in experimental osteoarthritis. J. Biol. Chem. 2011, 286, 37758–37767. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P.; Agah, A.; Kyriakides, T.R. The role of thrombospondins 1 and 2 in the regulation of cell–matrix interactions, collagen fibril formation, and the response to injury. Int. J. Biochem. Cell Biol. 2004, 36, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Schilling, T.F. Thrombospondin-4 controls matrix assembly during development and repair of myotendinous junctions. eLife 2014, 3, e02372. [Google Scholar] [CrossRef]
- Yuan, B.; Ji, W.; Fan, B.; Zhang, B.; Zhao, Y.; Li, J. Association analysis between thrombospondin-2 gene polymorphisms and intervertebral disc degeneration in a Chinese Han population. Medicine 2018, 97, e9586. [Google Scholar] [CrossRef]
- Sivan, S.S.; Wachtel, E.; Roughley, P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 3181–3189. [Google Scholar] [CrossRef]
- Sherratt, M.J. Tissue elasticity and the ageing elastic fibre. Age 2009, 31, 305–325. [Google Scholar] [CrossRef]
- Watson, R.E.B.; Griffiths, C.E.M.; Craven, N.M.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal-epidermal junction. J. Investig. Dermatol. 1999, 112, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Lovell, C.R.; Smolenski, K.A.; Duance, V.C.; Light, N.D.; Young, S.; Dyson, M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.S.; Tsitron, E.; Wachtel, E.; Roughley, P.; Sakkee, N.; Van Der Ham, F.; Degroot, J.; Maroudas, A. Age-related accumulation of pentosidine in aggrecan and collagen from normal and degenerate human intervertebral discs. Biochem. J. 2006, 399, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Urabe, G.; Hoshina, K.; Shimanuki, T.; Nishimori, Y.; Miyata, T.; Deguchi, J. Structural analysis of adventitial collagen to feature aging and aneurysm formation in human aorta. J. Vasc. Surg. 2016, 63, 1341–1350. [Google Scholar] [CrossRef]
- Vadon-Le Goff, S.; Kronenberg, D.; Bourhis, J.-M.; Bijakowski, C.; Raynal, N.; Ruggiero, F.; Farndale, R.W.; Stöcker, W.; Hulmes, D.J.S.; Moali, C. Procollagen C-proteinase Enhancer Stimulates Procollagen Processing by Binding to the C-propeptide Region Only. J. Biol. Chem. 2011, 286, 38932–38938. [Google Scholar] [CrossRef]
- Lagoutte, P.; Bettler, E.; Vadon-Le Goff, S.; Moali, C. Procollagen C-proteinase enhancer-1 (PCPE-1), a potential biomarker and therapeutic target for fibrosis. Matrix Biol. Plus 2021, 11, 100062. [Google Scholar] [CrossRef]
- Canty, E.G.; Kadler, K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005, 118, 1341–1353. [Google Scholar] [CrossRef]
- Khoshnoodi, J.; Cartailler, J.-P.; Alvares, K.; Veis, A.; Hudson, B.G. Molecular Recognition in the Assembly of Collagens: Terminal Noncollagenous Domains Are Key Recognition Modules in the Formation of Triple Helical Protomers. J. Biol. Chem. 2006, 281, 38117–38121. [Google Scholar] [CrossRef]
- Bekhouche, M.; Kronenberg, D.; Vadon-Le Goff, S.; Bijakowski, C.; Lim, N.H.; Font, B.; Kessler, E.; Colige, A.; Nagase, H.; Murphy, G.; et al. Role of the Netrin-like Domain of Procollagen C-Proteinase Enhancer-1 in the Control of Metalloproteinase Activity. J. Biol. Chem. 2010, 285, 15950–15959. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.M.; Schjerling, P.; Øhlenschlæger, T.F.; Eismark, C.; Olsen, J.; Kjær, M. Carbon-14 bomb pulse dating shows that tendinopathy is preceded by years of abnormally high collagen turnover. FASEB J. 2018, 32, 4763–4775. [Google Scholar] [CrossRef] [PubMed]
- Heinemeier, K.M.; Schjerling, P.; Heinemeier, J.; Magnusson, S.P.; Kjaer, M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J. 2013, 27, 2074–2079. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Garva, R.; Pickard, A.; Yeung, C.Y.C.; Mallikarjun, V.; Swift, J.; Holmes, D.F.; Calverley, B.; Lu, Y.; Adamson, A.; et al. Circadian control of the secretory pathway maintains collagen homeostasis. Nat. Cell Biol. 2020, 22, 74–86. [Google Scholar] [CrossRef]
- Song, L. Chapter One—Calcium and Bone Metabolism Indices. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 82, pp. 1–46. ISBN 0065-2423. [Google Scholar]
- Christgau, S.; Rosenquist, C.; Alexandersen, P.; Bjarnason, N.H.; Ravn, P.; Fledelius, C.; Herling, C.; Qvist, P.; Christiansen, C. Clinical evaluation of the Serum CrossLaps One Step ELISA, a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin. Chem. 1998, 44, 2290–2300. [Google Scholar] [CrossRef] [PubMed]
- Packard, B.Z.; Artym, V.V.; Komoriya, A.; Yamada, K.M. Direct visualization of protease activity on cells migrating in three-dimensions. Matrix Biol. 2009, 28, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-J.; Yu, X.-H.; Wang, C.; Yang, W.; He, W.-S.; Zhang, S.-J.; Yan, Y.-G.; Zhang, J. MMPs and ADAMTSs in intervertebral disc degeneration. Clin. Chim. Acta 2015, 448, 238–246. [Google Scholar] [CrossRef]
- Chung, L.; Dinakarpandian, D.; Yoshida, N.; Lauer-Fields, J.L.; Fields, G.B.; Visse, R.; Nagase, H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004, 23, 3020–3030. [Google Scholar] [CrossRef]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Weiss, T.; Brusel, M.; Rousselle, P.; Kessler, E. The NTR domain of procollagen C-proteinase enhancer-1 (PCPE-1) mediates PCPE-1 binding to syndecans-1,-2 and-4 as well as fibronectin. Int. J. Biochem. Cell Biol. 2014, 57, 45–53. [Google Scholar] [CrossRef]
- Salza, R.; Peysselon, F.; Chautard, E.; Faye, C.; Moschcovich, L.; Weiss, T.; Perrin-Cocon, L.; Lotteau, V.; Kessler, E.; Ricard-Blum, S. Extended interaction network of procollagen C-proteinase enhancer-1 in the extracellular matrix. Biochem. J. 2014, 457, 137–149. [Google Scholar] [CrossRef]
- Symoens, S.; Renard, M.; Bonod-Bidaud, C.; Syx, D.; Vaganay, E.; Malfait, F.; Ricard-Blum, S.; Kessler, E.; Van Laer, L.; Coucke, P. Identification of binding partners interacting with the α1-N-propeptide of type V collagen. Biochem. J. 2011, 433, 371–381. [Google Scholar] [CrossRef]
- Ozols, M.; Eckersley, A.; Platt, C.I.; Stewart-McGuinness, C.; Hibbert, S.A.; Revote, J.; Li, F.; Griffiths, C.E.M.; Watson, R.E.B.; Song, J. Predicting Proteolysis in Complex Proteomes Using Deep Learning. Int. J. Mol. Sci. 2021, 22, 3071. [Google Scholar] [CrossRef]
- Fallahi, A.; Kroll, B.; Warner, L.R.; Oxford, R.J.; Irwin, K.M.; Mercer, L.M.; Shadle, S.E.; Oxford, J.T. Structural model of the amino propeptide of collagen XI α1 chain with similarity to the LNS domains. Protein Sci. 2005, 14, 1526–1537. [Google Scholar] [CrossRef]
- Hohenester, E. Laminin G-like domains: Dystroglycan-specific lectins. Curr. Opin. Struct. Biol. 2019, 56, 56–63. [Google Scholar] [CrossRef]
- Watanabe, H.; Cheung, S.C.; Itano, N.; Kimata, K.; Yamada, Y. Identification of hyaluronan-binding domains of aggrecan. J. Biol. Chem. 1997, 272, 28057–28065. [Google Scholar] [CrossRef]
- Kiani, C.; Liwen, C.; Wu, Y.J.; Albert, J.Y.E.E.; Burton, B.Y. Structure and function of aggrecan. Cell Res. 2002, 12, 19–32. [Google Scholar] [CrossRef]
- Calippe, B.; Augustin, S.; Beguier, F.; Charles-Messance, H.; Poupel, L.; Conart, J.-B.; Hu, S.J.; Lavalette, S.; Fauvet, A.; Rayes, J. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity 2017, 46, 261–272. [Google Scholar] [CrossRef]
- Klein, R.; Knudtson, M.D.; Klein, B.E.K.; Wong, T.Y.; Cotch, M.F.; Liu, K.; Cheng, C.Y.; Burke, G.L.; Saad, M.F.; Jacobs, D.R., Jr. Inflammation, complement factor h, and age-related macular degeneration: The Multi-ethnic Study of Atherosclerosis. Ophthalmology 2008, 115, 1742–1749. [Google Scholar] [CrossRef]
- He, L.; Diedrich, J.; Chu, Y.-Y.; Yates, J.R., III. Extracting accurate precursor information for tandem mass spectra by RawConverter. Anal. Chem. 2015, 87, 11361–11367. [Google Scholar] [CrossRef]
- Consortium, U. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2016, 45, D158–D169. [Google Scholar]
- Washburn, M.P.; Wolters, D.; Yates, J.R. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 2001, 19, 242–247. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Launay, G.; Salza, R.; Multedo, D.; Thierry-Mieg, N.; Ricard-Blum, S. MatrixDB, the extracellular matrix interaction database: Updated content, a new navigator and expanded functionalities. Nucleic Acids Res. 2015, 43, D321–D327. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckersley, A.; Ozols, M.; Chen, P.; Tam, V.; Hoyland, J.A.; Trafford, A.; Chan, D.; Sherratt, M.J. Peptide Location Fingerprinting Reveals Tissue Region-Specific Differences in Protein Structures in an Ageing Human Organ. Int. J. Mol. Sci. 2021, 22, 10408. https://doi.org/10.3390/ijms221910408
Eckersley A, Ozols M, Chen P, Tam V, Hoyland JA, Trafford A, Chan D, Sherratt MJ. Peptide Location Fingerprinting Reveals Tissue Region-Specific Differences in Protein Structures in an Ageing Human Organ. International Journal of Molecular Sciences. 2021; 22(19):10408. https://doi.org/10.3390/ijms221910408
Chicago/Turabian StyleEckersley, Alexander, Matiss Ozols, Peikai Chen, Vivian Tam, Judith A. Hoyland, Andrew Trafford, Danny Chan, and Michael J. Sherratt. 2021. "Peptide Location Fingerprinting Reveals Tissue Region-Specific Differences in Protein Structures in an Ageing Human Organ" International Journal of Molecular Sciences 22, no. 19: 10408. https://doi.org/10.3390/ijms221910408
APA StyleEckersley, A., Ozols, M., Chen, P., Tam, V., Hoyland, J. A., Trafford, A., Chan, D., & Sherratt, M. J. (2021). Peptide Location Fingerprinting Reveals Tissue Region-Specific Differences in Protein Structures in an Ageing Human Organ. International Journal of Molecular Sciences, 22(19), 10408. https://doi.org/10.3390/ijms221910408





