Anti-Obesity Effect of Polygalin C Isolated from Polygala japonica Houtt. via Suppression of the Adipogenic and Lipogenic Factors in 3T3-L1 Adipocytes
Abstract
1. Introduction
2. Results
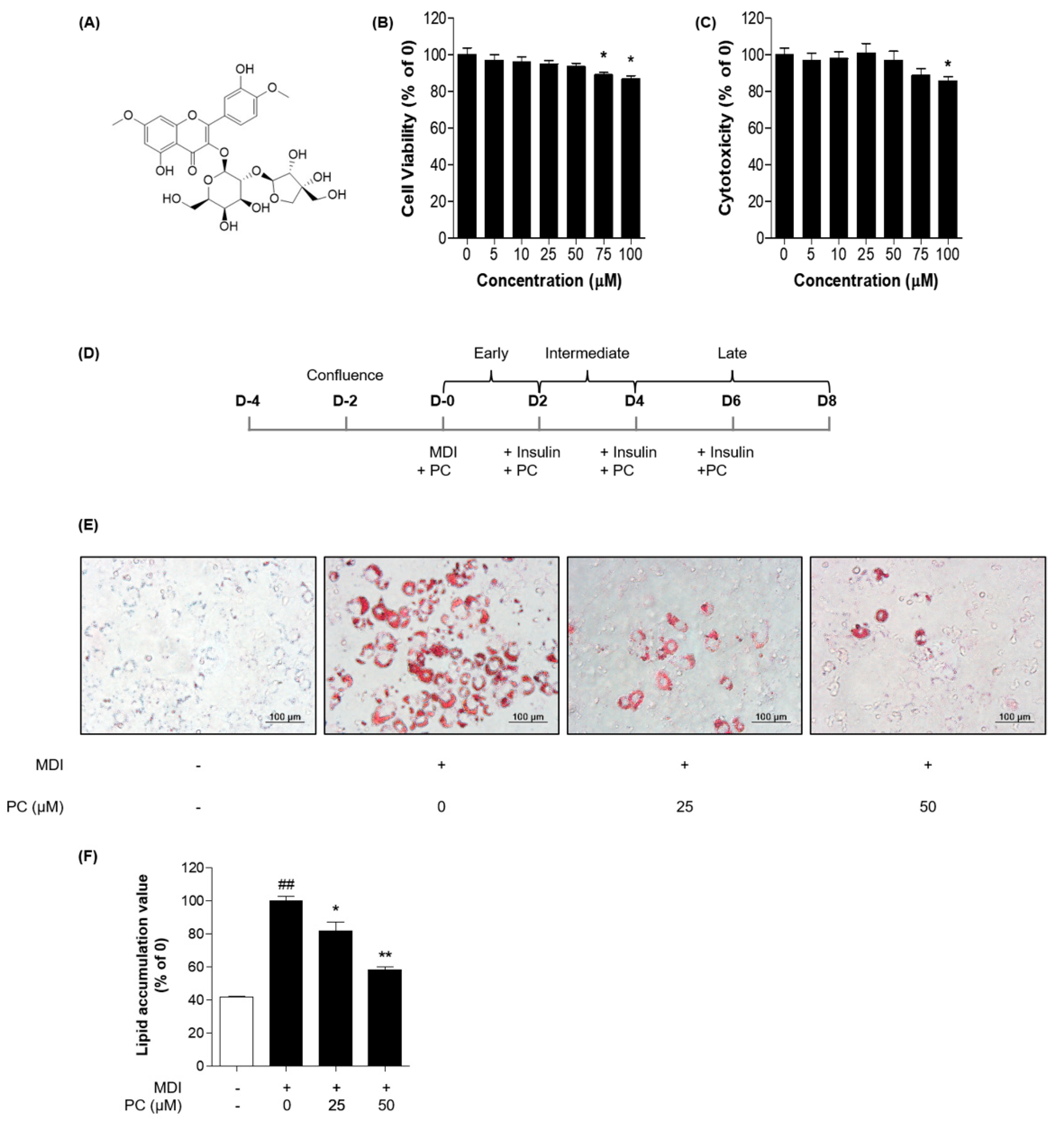
2.1. Cytotoxicity of PC in 3T3-L1 Cells
2.2. Effects of PC on Adipocyte Differentiation in 3T3-L1 Preadipocytes
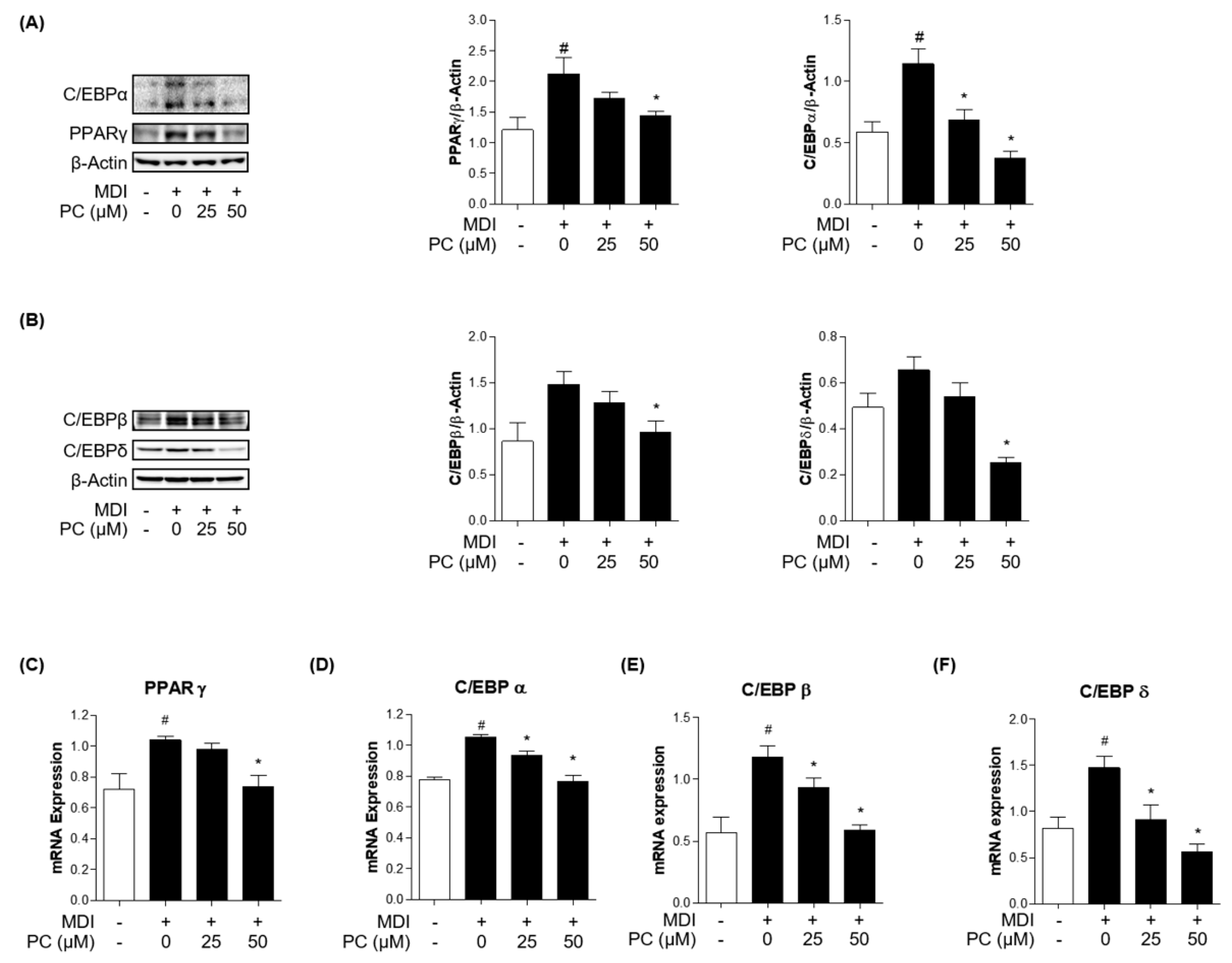
2.3. Effect of PC on Adipogenic Transcription Factor Expression Levels
2.4. Effect of PC on Lipogenic Factor Expression Levels
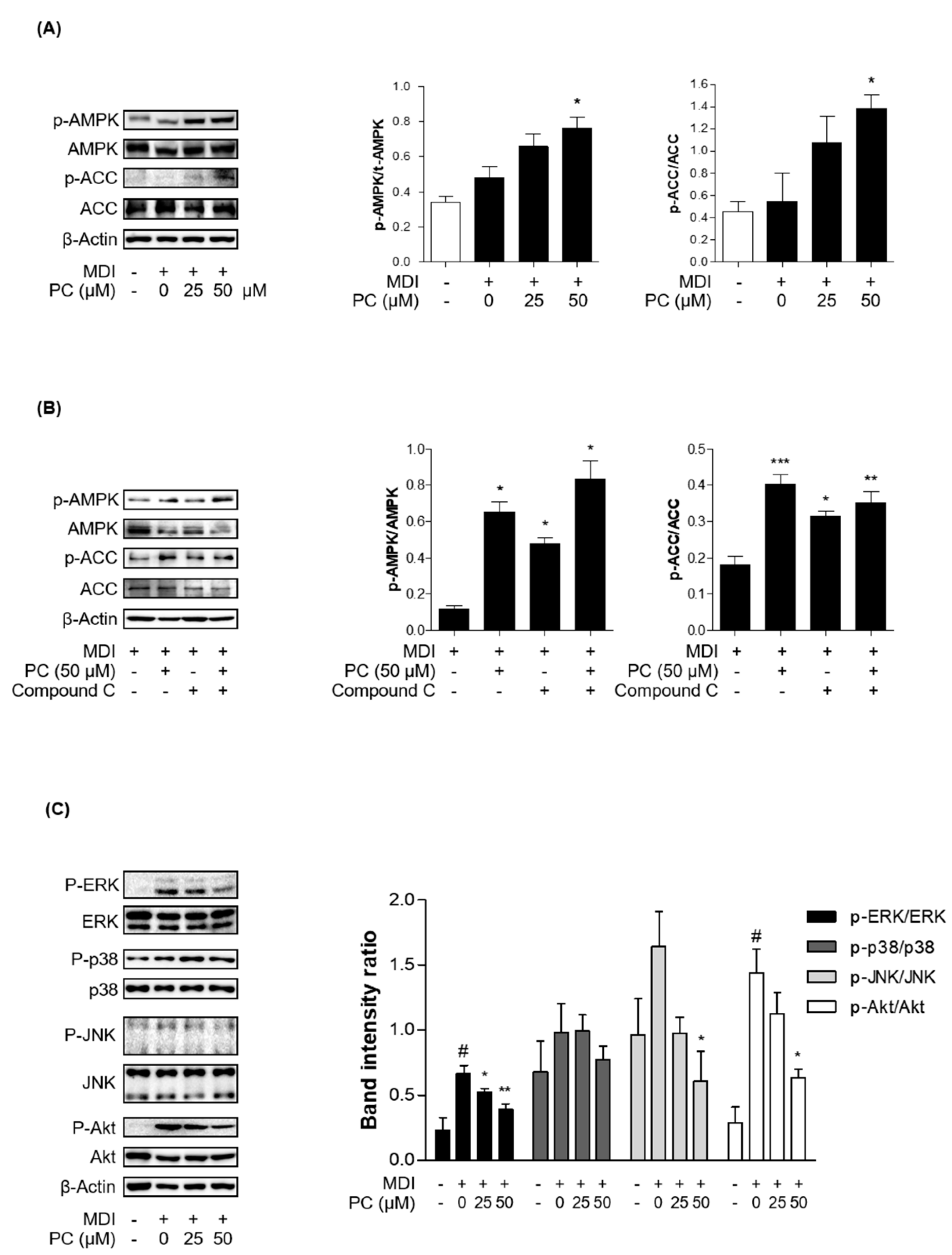
2.5. Effect of PC on AMPK/ACC and MAPK/Akt Signaling Pathway Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Reagents
4.3. Cell Culture and Experimental Conditions
4.4. Cell Viability
4.5. Immunoblotting
4.6. Oil Red O Assay
4.7. Immunofluorescence Assay
4.8. Isolation of Total RNA and Real-Time Polymerase Chain Reaction (PCR)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase/acetyl-CoA |
| BSA | Bovine serum albumin |
| C/EBPs | CCAAT/enhancer-binding proteins |
| DAPI | 4,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified Eagle’s medium |
| ERK | Extracellular signal-regulated kinase |
| FAS | Fatty acid synthase |
| FBS | Fetal bovine serum |
| HRP | Horseradish peroxidase |
| IBMX | 3-isobutyl-1-methylxanthine |
| JNK | Phospho-c-Jun N-terminal kinase |
| LDH | Lactate dehydrogenase |
| MAPK | Mitogen-activated protein kinase |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NASH | Non-alcoholic steatohepatitis |
| P. japonica Houtt | Polygala japonica Houtt. |
| p38 | p38 mitogen-activated protein kinases |
| PBS | Phosphate-buffered saline |
| PC | Polygalin C |
| PCR | Polymerase chain reaction |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| SREBP1c | Sterol regulatory element-binding protein 1c |
| TBS-T | Tris-buffered saline/Tween 20 |
| NICE | National Institute for Health and Care Excellence |
References
- Kitade, H.; Chen, G.; Ni, Y.; Ota, T. Nonalcoholic Fatty Liver Disease and Insulin Resistance: New Insights and Potential New Treatments. Nutrients 2017, 9, 387. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-obesity Effects and Different Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef]
- Son, J.W.; Kim, S. Comprehensive Review of Current and Upcoming Anti-Obesity Drugs. Diabetes Metab. J. 2020, 44, 802–818. [Google Scholar] [CrossRef]
- Balaji, M.; Ganjayi, M.S.; Hanuma Kumar, G.E.; Parim, B.N.; Mopuri, R.; Dasari, S. A review on possible therapeutic targets to contain obesity: The role of phytochemicals. Obes. Res. Clin. Pract. 2016, 10, 363–380. [Google Scholar] [CrossRef]
- Mopuri, R.; Islam, M.S. Medicinal plants and phytochemicals with anti-obesogenic potentials: A review. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 89, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Bahmad, H.F.; Daouk, R.; Azar, J.; Sapudom, J. Modeling Adipogenesis: Current and Future Perspective. Cells 2020, 9, 2326. [Google Scholar] [CrossRef] [PubMed]
- Hausman, D.B.; DiGirolamo, M.; Bartness, T.J.; Hausman, G.J.; Martin, R.J. The biology of white adipocyte proliferation. Obes. Rev. An Off. J. Int. Assoc. Study Obes. 2001, 2, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Kim, C.Y. Natural Products and Obesity: A Focus on the Regulation of Mitotic Clonal Expansion during Adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. Molecular Regulation of Adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yoshida, N.; Kishimoto, T.; Akira, S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997, 16, 7432–7443. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Cao, Y.; Xiao, J.; Song, M.; Ho, C.T. Molecular mechanisms of the anti-obesity effect of bioactive ingredients in common spices: A review. Food Funct. 2018, 9, 4569–4581. [Google Scholar] [CrossRef] [PubMed]
- Lefterova, M.I.; Lazar, M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009, 20, 107–114. [Google Scholar] [CrossRef]
- Hardie, D.G. AMP-activated protein kinase: An energy sensor that regulates all aspects of cell function. Genes Dev. 2011, 25, 1895–1908. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Lee, M.H.; Hsu, C.C.; Wei, C.L.; Tsai, Y.C. Methyl cinnamate inhibits adipocyte differentiation via activation of the CaMKK2-AMPK pathway in 3T3-L1 preadipocytes. J. Agric. Food Chem. 2012, 60, 955–963. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, H.D.; Kim, D.Y.; Quan, H.Y.; Chung, S.H. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK) pathways. J. Agric. Food Chem. 2011, 59, 3666–3673. [Google Scholar] [CrossRef]
- Camp, H.S.; Tafuri, S.R. Regulation of peroxisome proliferator-activated receptor gamma activity by mitogen-activated protein kinase. J. Biol. Chem. 1997, 272, 10811–10816. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Muise, A.M.; Lyons, P.J.; Ro, H.S. Regulation of adipogenesis by a transcriptional repressor that modulates MAPK activation. J. Biol. Chem. 2001, 276, 10199–10206. [Google Scholar] [CrossRef] [PubMed]
- Sale, E.M.; Atkinson, P.G.; Sale, G.J. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995, 14, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Pizzolatti, M.G.; Koga, A.H.; Grisard, E.C.; Steindel, M. Trypanocidal activity of extracts from Brazilian Atlantic Rain Forest plant species. Phytomedicine 2003, 10, 422–426. [Google Scholar] [CrossRef]
- Cheong, M.H.; Lee, S.R.; Yoo, H.S.; Jeong, J.W.; Kim, G.Y.; Kim, W.J.; Jung, I.C.; Choi, Y.H. Anti-inflammatory effects of Polygala tenuifolia root through inhibition of NF-κB activation in lipopolysaccharide-induced BV2 microglial cells. J. Ethnopharmacol. 2011, 137, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Nucci-Martins, C.; Nascimento, L.F.; Venzke, D.; Brethanha, L.C.; Sako, A.V.; Oliveira, A.S.; Brighente, I.M.; Micke, G.A.; Pizzolatti, M.G.; Santos, A.R. Antinociceptive effect of hydroalcoholic extract and isoflavone isolated from Polygala molluginifolia in mice: Evidence for the involvement of opioid receptors and TRPV1 and TRPA1 channels. Phytomedicine 2016, 23, 429–440. [Google Scholar] [CrossRef]
- Johann, S.; Mendes, B.G.; Missau, F.C.; de Resende, M.A.; Pizzolatti, M.G. Antifungal activity of five species of Polygala. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2011, 42, 1065–1075. [Google Scholar] [CrossRef]
- Wang, C.C.; Yen, J.H.; Cheng, Y.C.; Lin, C.Y.; Hsieh, C.T.; Gau, R.J.; Chiou, S.J.; Chang, H.Y. Polygala tenuifolia extract inhibits lipid accumulation in 3T3-L1 adipocytes and high-fat diet-induced obese mouse model and affects hepatic transcriptome and gut microbiota profiles. Food Nutr. Res. 2017, 61, 1379861. [Google Scholar] [CrossRef]
- Jung, S.-Y.; Park, S.-H.; Nam, C.-H.; Lee, H.-J.; Lee, Y.-M.; Chang, K.-S. The Distribution of Vascular Plants in Ulleungdo and Nearby Island Regions (Gwaneumdo, Jukdo), Korea. J. Asia-Pac. Biodivers. 2013, 6, 123–156. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Gao, J.; Kou, J.; Zhu, D.; Yu, B. Anti-inflammatory activities of triterpenoid saponins from Polygala japonica. Phytomedicine 2008, 15, 321–326. [Google Scholar] [CrossRef]
- Wang, H.; Gao, J.; Zhu, D.; Yu, B. Quality evaluation of Polygala japonica through simultaneous determination of six bioactive triterpenoid saponins by HPLC-ELSD. J. Pharm. Biomed. Anal. 2007, 43, 1552–1556. [Google Scholar] [CrossRef] [PubMed]
- Pacy, P.J.; Webster, J.; Garrow, J.S. Exercise and obesity. Sports Med. 1986, 3, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Haththotuwa, R.N.; Wijeyaratne, C.N.; Senarath, U. Chapter 1—Worldwide epidemic of obesity. In Obesity and Obstetrics, 2nd ed.; Mahmood, T.A., Arulkumaran, S., Chervenak, F.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–8. [Google Scholar]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Korakas, E.; Ikonomidis, I.; Kousathana, F.; Balampanis, K.; Kountouri, A.; Raptis, A.; Palaiodimou, L.; Kokkinos, A.; Lambadiari, V. Obesity and COVID-19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E105–E109. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- The National Institute for Health and Care Excellence. Obesity: Identification, Assessment and Management. Clinical Guideline CG189; The National Institute for Health and Care Excellence: London, UK, 2014. [Google Scholar]
- Zhi, J.; Melia, A.T.; Guerciolini, R.; Chung, J.; Kinberg, J.; Hauptman, J.B.; Patel, I.H. Retrospective population-based analysis of the dose-response (fecal fat excretion) relationship of orlistat in normal and obese volunteers. Clin. Pharmacol. Ther. 1994, 56, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Meloni, A.R.; DeYoung, M.B.; Lowe, C.; Parkes, D.G. GLP-1 receptor activated insulin secretion from pancreatic β-cells: Mechanism and glucose dependence. Diabetes Obes. Metab. 2013, 15, 15–27. [Google Scholar] [CrossRef]
- Shyangdan, D.S.; Royle, P.; Clar, C.; Sharma, P.; Waugh, N.; Snaith, A. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2011, 2011, Cd006423. [Google Scholar] [CrossRef]
- Siebenhofer, A.; Winterholer, S.; Jeitler, K.; Horvath, K.; Berghold, A.; Krenn, C.; Semlitsch, T. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database Syst. Rev. 2021, 1, Cd007654. [Google Scholar] [CrossRef] [PubMed]
- García-Barrado, M.J.; Iglesias-Osma, M.C.; Pérez-García, E.; Carrero, S.; Blanco, E.J.; Carretero-Hernández, M. Role of Flavonoids in The Interactions among Obesity, Inflammation, and Autophagy. Pharmaceuticals 2020, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Wu, Y.-X.; Hwang, D.-I.; Bae, S.-J.; Kim, T. Anti-obesity effect of Polygala tenuifolia. Korean J. Food Preserv. 2014, 21, 97–106. [Google Scholar] [CrossRef]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef]
- Yeh, W.C.; Cao, Z.; Classon, M.; McKnight, S.L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995, 9, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Antunes, B.; Velo-Antón, G.; Buckley, D.; Pereira, R.J.; Martínez-Solano, I. An edible fungi Pleurotus ostreatus inhibits adipogenesis via suppressing expression of PPAR γ and C/EBP α in 3T3-L1 cells: In vitro validation of gene knock out of RNAs in PPAR γ using CRISPR spcas9. Biomed. Pharmacother. 2019, 116, 109030. [Google Scholar]
- Kim, Y.M.; Jang, M.S. Anti-obesity effects of Laminaria japonica fermentation on 3T3-L1 adipocytes are mediated by the inhibition of C/EBP-α/β and PPAR-γ. Cell. Mol. Biol. 2018, 64, 71–77. [Google Scholar] [CrossRef]
- Long, Y.C.; Zierath, J.R. AMP-activated protein kinase signaling in metabolic regulation. J. Clin. Invest. 2006, 116, 1776–1783. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef]
- Xiao, J.; Högger, P. Stability of dietary polyphenols under the cell culture conditions: Avoiding erroneous conclusions. J. Agric. Food Chem. 2015, 63, 1547–1557. [Google Scholar] [CrossRef]
- Sang, S.; Lee, M.J.; Hou, Z.; Ho, C.T.; Yang, C.S. Stability of tea polyphenol (-)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic study of catechin stability: Effects of pH, concentration, and temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef] [PubMed]


| Genes | Primer | Forward Primers |
|---|---|---|
| C/EBPβ | Forward | AGCGGCTGCAGAAGAAGGT |
| Reverse | GGCAGCTGCTTGAACAAGTTC | |
| C/EBPδ | Forward | TTCCAACCCCTTCCCTGAT |
| Reverse | CTGGAGGGTTTGTGTTTTCTGT | |
| PPARγ | Forward | GGTGAAACTCTGGGAGATTC |
| Reverse | CAACCATTGGGTCAGCTCTT | |
| C/EBPα | Forward | AGGTGCTGGAGTTGACCAGT |
| Reverse | CAGCCTAGAGATCCAGCGAC | |
| FAS | Forward | TTGCTGGCACTACAGAATGC |
| Reverse | AACAGCCTCAGAGCGACAAT | |
| SREBP 1c | Forward | ATCGCAAACAAGCTGACCTG |
| Reverse | AGATCCAGGTTTGAGGTGGG | |
| GAPDH | Forward | GCCACATCGCTCAGACACC |
| Reverse | CCCAATACGACCAAATCCGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jee, W.; Lee, S.-H.; Ko, H.M.; Jung, J.H.; Chung, W.-S.; Jang, H.-J. Anti-Obesity Effect of Polygalin C Isolated from Polygala japonica Houtt. via Suppression of the Adipogenic and Lipogenic Factors in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2021, 22, 10405. https://doi.org/10.3390/ijms221910405
Jee W, Lee S-H, Ko HM, Jung JH, Chung W-S, Jang H-J. Anti-Obesity Effect of Polygalin C Isolated from Polygala japonica Houtt. via Suppression of the Adipogenic and Lipogenic Factors in 3T3-L1 Adipocytes. International Journal of Molecular Sciences. 2021; 22(19):10405. https://doi.org/10.3390/ijms221910405
Chicago/Turabian StyleJee, Wona, Seung-Hyeon Lee, Hyun Min Ko, Ji Hoon Jung, Won-Seok Chung, and Hyeung-Jin Jang. 2021. "Anti-Obesity Effect of Polygalin C Isolated from Polygala japonica Houtt. via Suppression of the Adipogenic and Lipogenic Factors in 3T3-L1 Adipocytes" International Journal of Molecular Sciences 22, no. 19: 10405. https://doi.org/10.3390/ijms221910405
APA StyleJee, W., Lee, S.-H., Ko, H. M., Jung, J. H., Chung, W.-S., & Jang, H.-J. (2021). Anti-Obesity Effect of Polygalin C Isolated from Polygala japonica Houtt. via Suppression of the Adipogenic and Lipogenic Factors in 3T3-L1 Adipocytes. International Journal of Molecular Sciences, 22(19), 10405. https://doi.org/10.3390/ijms221910405









