A Study to Explore the Suitability of LiNi0.8Co0.15Al0.05O2/Silicon@Graphite Cells for High-Power Lithium-Ion Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Characterization
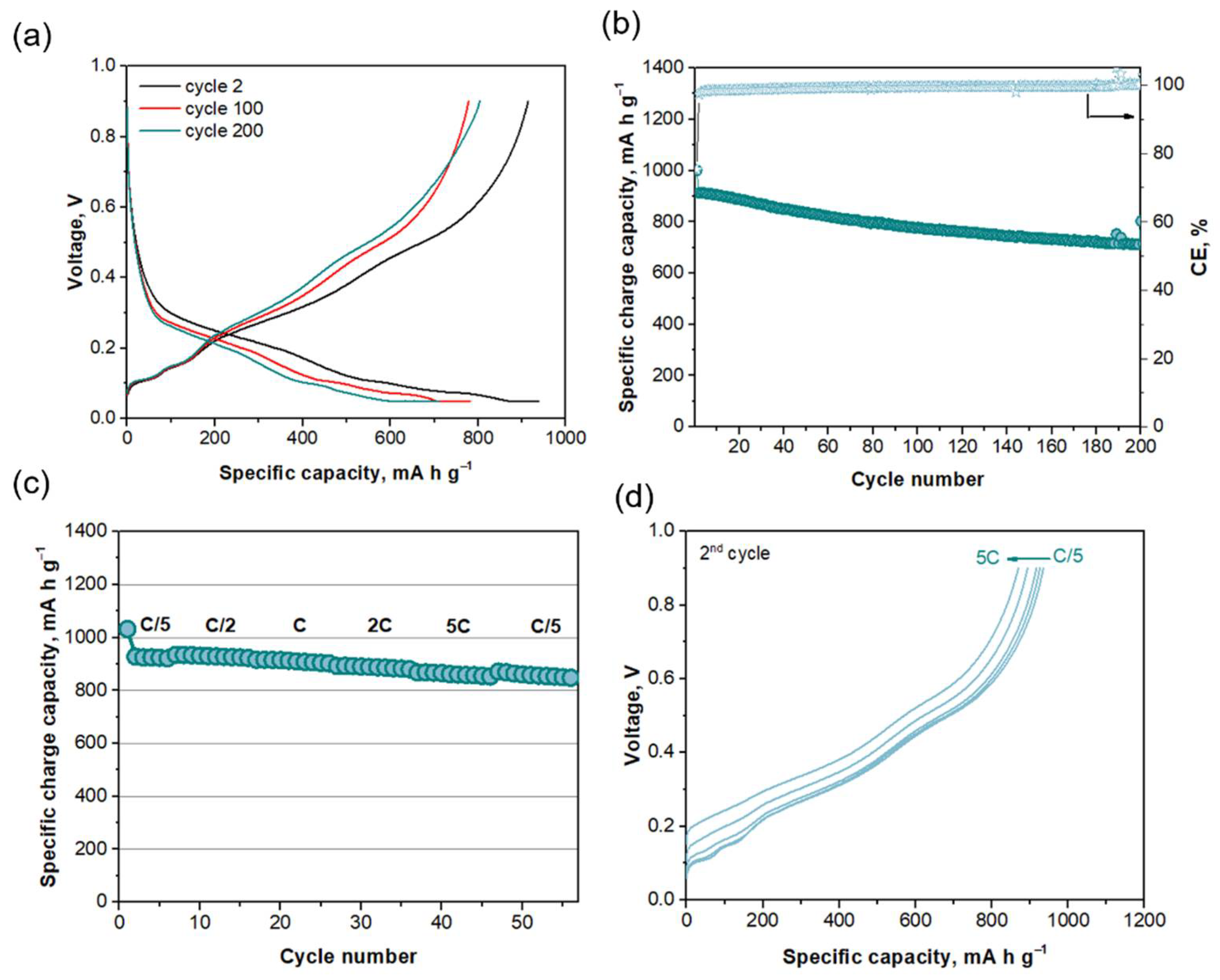
2.2. Electrochemical Performance of Individual Electrodes: Half Cell
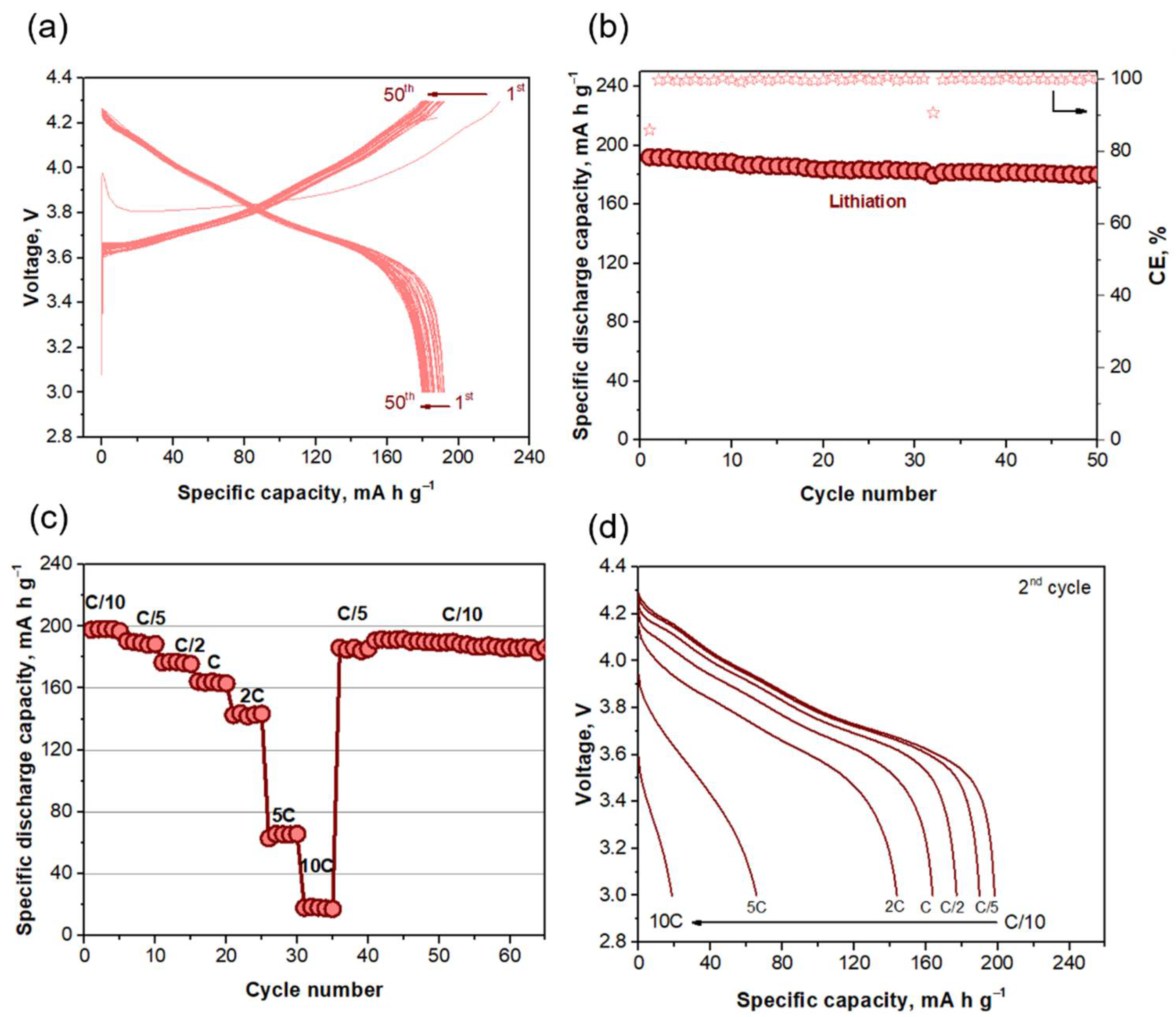
2.3. Electrochemical Performance: Full Cell
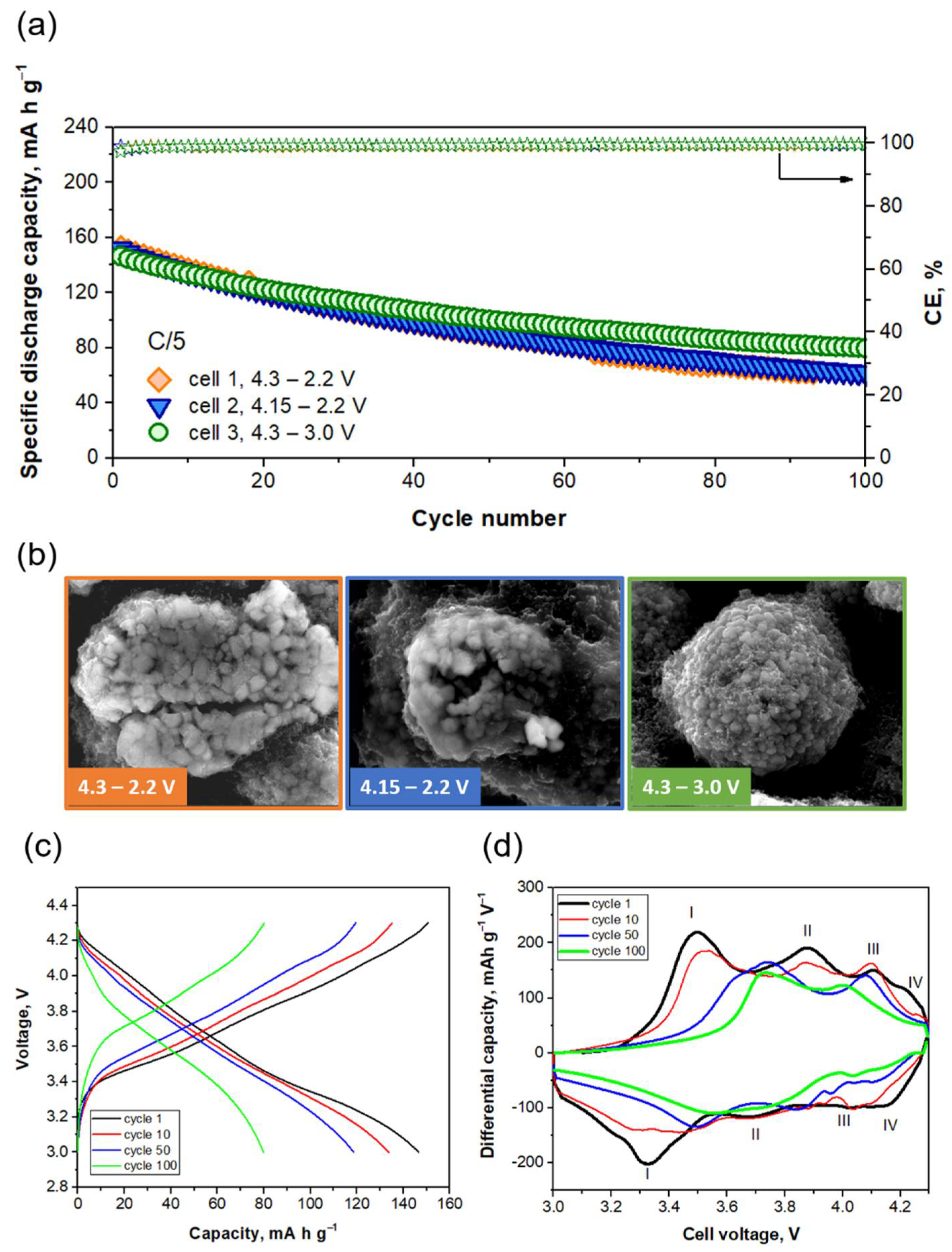
2.3.1. Evaluation of the Voltage Window
2.3.2. Rate Capability Test
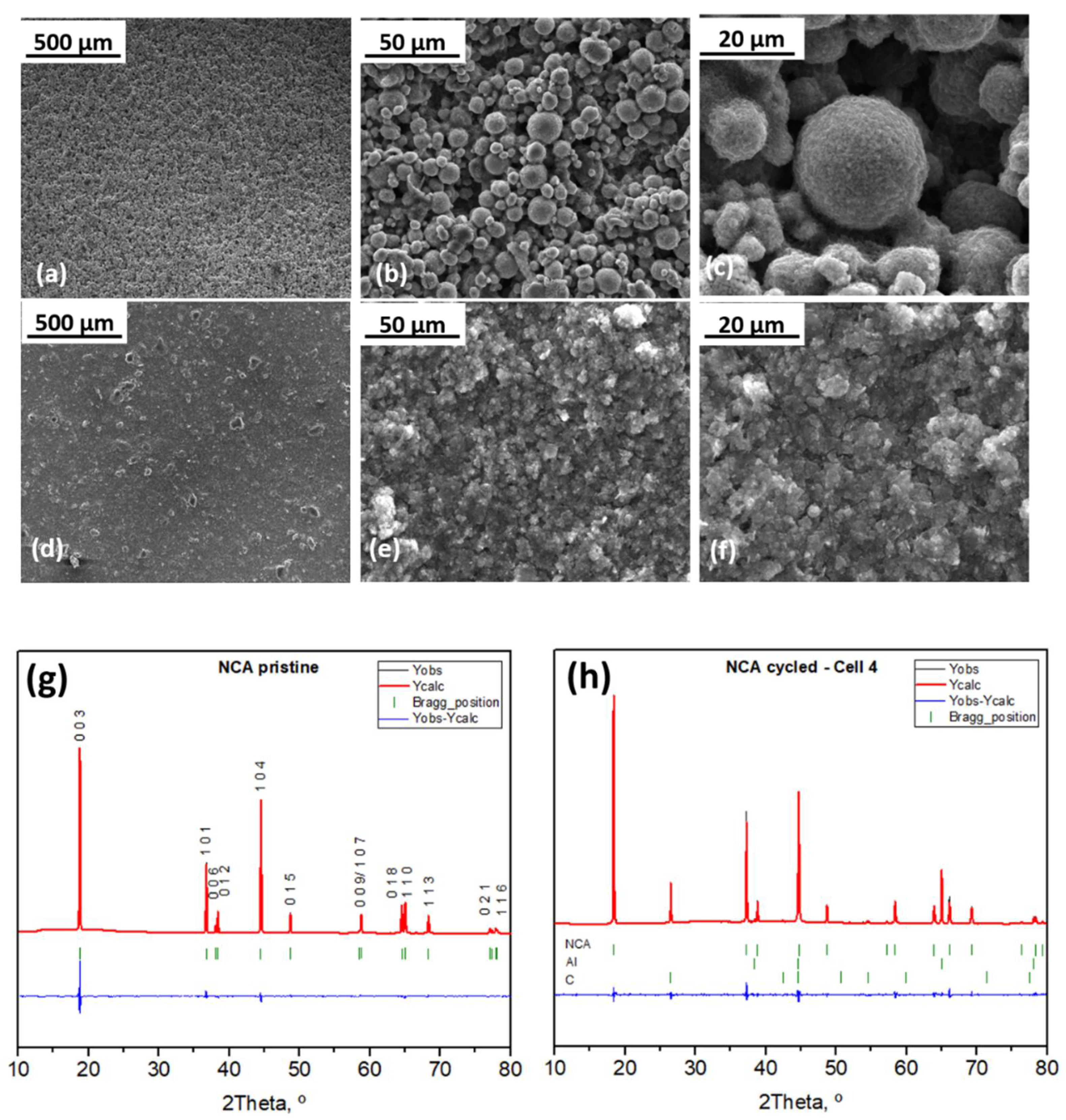
2.4. Characterization after Cycling
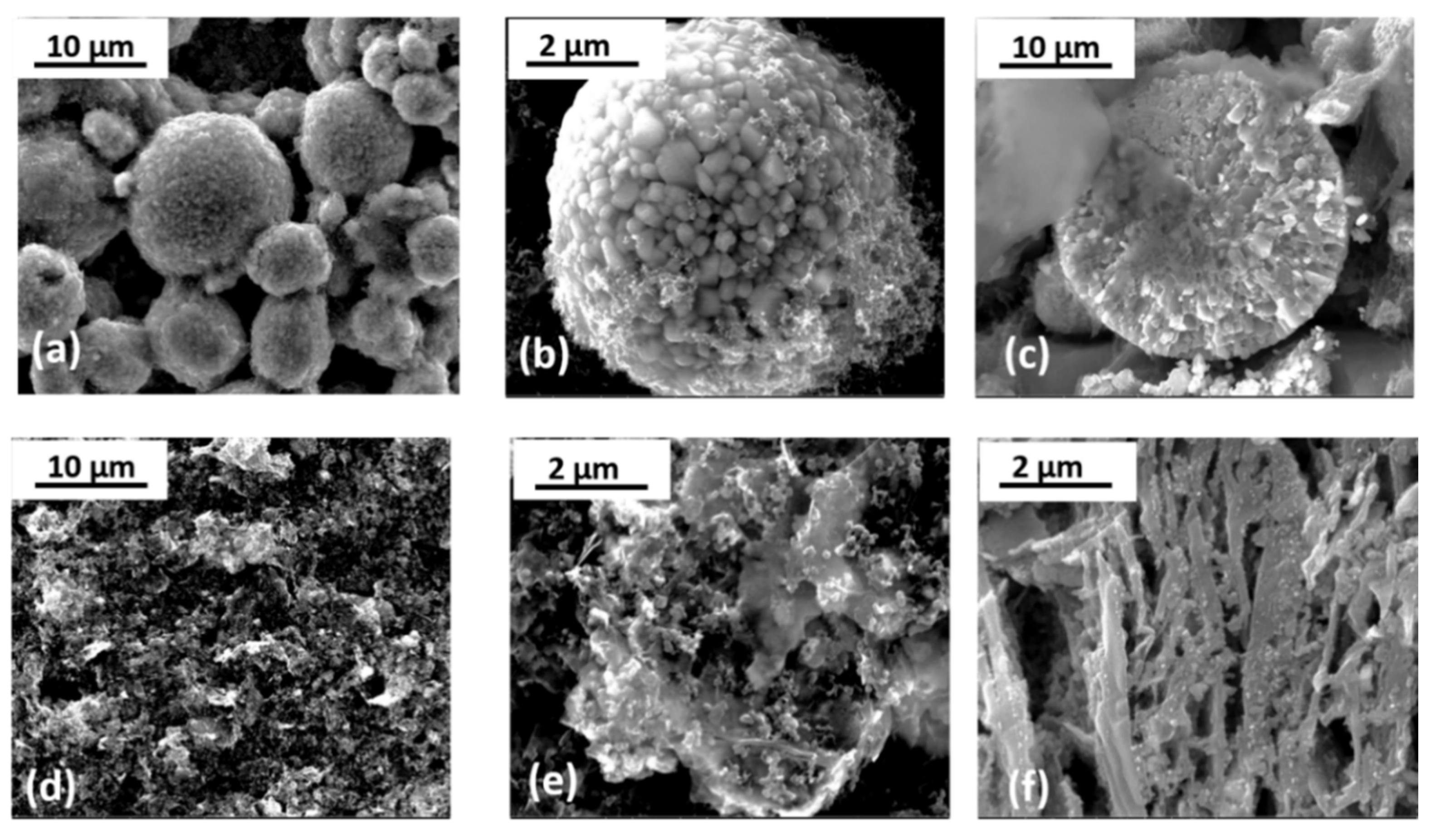
2.4.1. Electrode Morphology and Structure
2.4.2. Electrolyte Composition
2.4.3. Impedance of the Full Cell
3. Materials and Methods
3.1. Anode Preparation
3.2. Cathode Preparation
3.3. Electrochemical Testing
3.4. Physicochemical Characterization of Electrodes and Electrolyte
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Myung, S.T.; Maglia, F.; Park, K.J.; Yoon, C.S.; Lamp, P.; Kim, S.J.; Sun, Y.K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Tesla Motors Panasonic and Tesla Expand Supply Agreement for Li-ion Cells; Nearly 2 Billion through 2017. Panasonic Corporation, 2013. Available online: https://www.greencarcongress.com/2013/10/20131030-tesla-1.html (accessed on 15 September 2021).
- Rougier, A.; Saadoune, I.; Gravereau, P.; Willmann, P.; Delmas, C. Effect of cobalt substitution on cationic distribution in LiNi1-yCOyO2 electrode materials. Solid State Ion. 1996, 90, 83–90. [Google Scholar] [CrossRef]
- Ohzuku, T.; Yanagawa, T.; Kouguchi, M.; Ueda, A. Innovative insertion material of LiAl1/4Ni3/4O2 (Rm) for lithium-ion (shuttlecock) batteries. J. Power Sources 1997, 68, 131–134. [Google Scholar] [CrossRef]
- Hwang, S.; Chang, W.; Kim, S.M.; Su, D.; Kim, D.H.; Lee, J.Y.; Chung, K.Y.; Stach, E.A. Investigation of changes in the surface structure of LixNi0.8Co0.15Al0.05O2 cathode materials induced by the initial charge. Chem. Mater. 2014, 26, 1084–1092. [Google Scholar] [CrossRef]
- Robert, R.; Novák, P. Structural changes and microstrain generated on LiNi0.8Co0.15Al0.05O2 during cycling: Effects on the electrochemical performance. J. Electrochem. Soc. 2015, 162, A1823–A1828. [Google Scholar] [CrossRef]
- Bak, S.-M.; Nam, K.-W.; Chang, W.; Yu, X.; Hu, E.; Hwang, S.; Stach, E.A.; Kim, K.B.; Chung, K.Y.; Yang, X.Q. Correlating structural changes and gas evolution during the thermal decomposition of charged LixNi0.8Co0.15Al0.05O2 cathode materials. Chem. Mater. 2013, 25, 337–351. [Google Scholar] [CrossRef]
- Liu, B.-S.; Wang, Z.-B.; Yu, F.D.; Xue, Y.; Wang, G.-J.; Zhang, Y.; Zhou, Y.-X. Facile strategy of NCA cation mixing regulation and its effect on electrochemical performance. RSC Adv. 2016, 6, 108558–108565. [Google Scholar] [CrossRef]
- Dose, W.M.; Blauwkamp, J.; Piernas-Muñoz, M.J.; Bloom, I.; Rui, X.; Klie, R.F.; Senguttuvan, P.; Johnson, C.S. Liquid ammonia chemical lithiation: An approach for high-energy and high-voltage si–graphite|Li1+xNi0.5Mn1.5O4 Li-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 5019–5028. [Google Scholar] [CrossRef]
- Wagner, N.P.; Tron, A.; Tolchard, J.R.; Noia, G.; Bellmann, M.P. Silicon anodes for lithium-ion batteries produced from recovered kerf powders. J. Power Sources 2019, 414, 486–494. [Google Scholar] [CrossRef]
- Marinaro, M.; Yoon, D.H.; Gabrielli, G.; Stegmaier, P.; Figgemeier, E.; Spurk, P.C.; Nelis, D.; Schmidt, G.; Chauveau, J.; Axmann, P.; et al. High performance 1.2 Ah Si-alloy/Graphite|LiNi0.5Mn0.3Co0.2O2prototype Li-ion battery. J. Power Sources 2017, 357, 188–197. [Google Scholar] [CrossRef]
- Hasa, I.; Hassoun, J.; Passerini, S. Nanostructured Na-ion and Li-ion anodes for battery application: A comparative overview. Nano Res. 2017, 10, 3942–3969. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural changes in silicon anodes during lithium insertion/extraction. Electrochem. Solid-State Lett. 2004, 7, A93–A96. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible cycling of crystalline silicon powder. J. Electrochem. Soc. 2007, 154, A103–A108. [Google Scholar] [CrossRef]
- Jin, Y.; Li, S.; Kushima, A.; Zheng, X.; Sun, Y.; Xie, J.; Sun, J.; Xue, W.; Zhou, G.; Wu, J.; et al. Self-healing SEI enables full-cell cycling of a silicon-majority anode with a coulombic efficiency exceeding 99.9%. Energy Environ. Sci. 2017, 10, 580–592. [Google Scholar] [CrossRef]
- Qi, W.; Shapter, J.G.; Wu, Q.; Yin, T.; Gao, G.; Cui, D. Nanostructured anode materials for lithium-ion batteries: Principle, recent progress and future perspectives. J. Mater. Chem. A 2017, 5, 19521–19540. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.; Lan, J.-L.; Xue, M.; Yu, Y.; Yang, X. Two-step ball-milling synthesis of a Si/SiOx/C composite electrode for lithium ion batteries with excellent long-term cycling stability. RSC Adv. 2017, 7, 36697–36704. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhou, J.; Guan, Y.; Cai, W.; Zhao, Y.; Zhu, Y.; Zhu, L.; Zhu, Y.; Qian, Y. Hierarchical graphene-scaffolded silicon/graphite composites as high performance anodes for lithium-ion batteries. Small 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Zheng, Y.; Seifert, H.J.; Shi, H.; Zhang, Y.; Kübel, C.; Pfleging, W. 3D silicon/graphite composite electrodes for high-energy lithium-ion batteries. Electrochim. Acta 2019, 317, 502–508. [Google Scholar] [CrossRef]
- Botas, C.; Carriazo, D.; Zhang, W.; Rojo, T.; Singh, G. Silicon-reduced graphene oxide self-standing composites suitable as binder-free anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 28800–28808. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Alvarado, J.; Yersak, T.A.; Li, J.; Dudney, N.; Webb, L.J.; Meng, Y.S.; Stevenson, K.J. The effect of fluoroethylene carbonate as an additive on the solid electrolyte interphase on silicon lithium-ion electrodes. Chem. Mater. 2015, 27, 5531–5542. [Google Scholar] [CrossRef]
- Zhang, S.; He, M.; Su, C.C.; Zhang, Z. Advanced electrolyte/additive for lithium-ion batteries with silicon anode. Curr. Opin. Chem. Eng. 2016, 13, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Farmakis, F.; Elmasides, C.; Selinis, P.; Georgoulas, N. Impact of electrolyte on the electrochemical performance of Lithium-ion half and full cells with Silicon film anodes. Electrochim. Acta 2017, 245, 99–106. [Google Scholar] [CrossRef]
- Shin, H.; Park, J.; Sastry, A.M.; Lu, W. Effects of fluoroethylene carbonate (FEC) on anode and cathode interfaces at elevated temperatures. J. Electrochem. Soc. 2015, 162, A1683–A1692. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Feng, M.; Luo, D.; Chen, Z. Fast charging li-ion batteries for a new era of electric vehicles. Cell Rep. Phys. Sci. 2020, 1, 100212. [Google Scholar] [CrossRef]
- Colclasure, A.M.; Dunlop, A.R.; Trask, S.E.; Polzin, B.J.; Jansen, A.N.; Smith, K. Requirements for enabling extreme fast charging of high energy density Li-ion cells while avoiding lithium plating. J. Electrochem. Soc. 2019, 166, A1412–A1424. [Google Scholar] [CrossRef]
- Cabello, M.; Gucciardi, E.; Herrán, A.; Carriazo, D.; Villaverde, A.; Rojo, T. Towards a high-power si @ graphite anode for lithium ion batteries through a wet ball process. Molecules 2020, 25, 2494. [Google Scholar] [CrossRef] [PubMed]
- Ohzuku, T.; Iwakoshi, Y.; Sawai, K. Formation of lithium-graphite intercalation compounds in nonaqueous electrolytes and their application as a negative electrode for a lithium ion (Shuttlecock) cell. J. Electrochem. Soc. 1993, 140, 2490–2498. [Google Scholar] [CrossRef]
- Ogata, K.; Salager, E.; Kerr, C.J.; Fraser, A.E.; Ducati, C.; Morris, A.J.; Hofmann, S.; Grey, C.P. Revealing lithium-silicide phase transformations in nano-structured silicon-based lithium ion batteries via in situ NMR spectroscopy. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Sung, J.; Lee, Y.; Son, Y.; Chae, S.; Kim, N.; Choi, S.H.; Cho, J. Strategic pore architecture for accommodating volume change from high si content in lithium-ion battery anodes. Adv. Energy Mater. 2019, 10, 1903400. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Hsieh, C.C.; Liu, W.R. Synthesis of double core-shell carbon/silicon/graphite composite anode materials for lithium-ion batteries. Surf. Coat. Technol. 2020, 387, 125528. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, J.; Luo, J.; Li, W.; Wu, Z.; Shi, M.; Li, X.; Li, N.; Chang, B.; Wang, X. Spherical Gr/Si/GO/C Composite as high-performance anode material for lithium-ion batteries. Energy Fuels 2020, 34, 7639–7647. [Google Scholar] [CrossRef]
- Sui, D.; Xie, Y.; Zhao, W.; Zhang, H.; Zhou, Y.; Qin, X.; Ma, Y.; Yang, Y.; Chen, Y. A high-performance ternary Si composite anode material with crystal graphite core and amorphous carbon shell. J. Power Sources 2018, 384, 328–333. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Zhao, S.; Zhuang, Y.; Zhao, S.; Zhou, Q.; Zheng, J. Influence of integrated microstructure on the performance of LiNi0.8Co0.15Al0.05O2 as a cathodic material for lithium ion batteries. RSC Adv. 2017, 7, 29233–29239. [Google Scholar] [CrossRef] [Green Version]
- Grenier, A.; Liu, H.; Wiaderek, K.M.; Lebens-Higgins, Z.W.; Borkiewicz, O.J.; Piper, L.F.J.; Chupas, P.J.; Chapman, K.W. Reaction heterogeneity in LiNi0.8Co0.15Al0.05O2 induced by surface layer. Chem. Mater. 2017, 29, 7345–7352. [Google Scholar] [CrossRef]
- Mirolo, M.; Vaz, C.A.F.; Novák, P.; El Kazzi, M. Multi-length-scale x-ray spectroscopies for determination of surface reactivity at high voltages of LiNi0.8Co0.15Al0.05O2vs Li4Ti5O12. J. Chem. Phys. 2020, 152, 184705. [Google Scholar] [CrossRef]
- Wohlfahrt-Mehrens, M.; Vogler, C.; Garche, J. Aging mechanisms of lithium cathode materials. J. Power Sources 2004, 127, 58–64. [Google Scholar] [CrossRef]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity fading of ni-rich li [NixCoyMn1-x-y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Hosokawa, T.; Morigaki, K.; Nakura, K. Capacity fade of LiAlyNi1-x-yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (surface analysis of LiAlyNi1-x-yCoxO2 cathode after cycle tests in restricted depth of discharge ranges). J. Power Sources 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Hosokawa, T.; Morigaki, K.; Nakura, K. Capacity fading of LiAlyNi1-x-yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (effect of depth of discharge in charge-discharge cycling on the suppression of the micro-crack generation of LiAlyNi 1-x-yCoxO2 parti. J. Power Sources 2014, 260, 50–56. [Google Scholar] [CrossRef]
- Li, J.; Harlow, J.; Stakheiko, N.; Zhang, N.; Paulsen, J.; Dahn, J. Dependence of cell failure on cut-off voltage ranges and observation of kinetic hindrance in LiNi0.8Co0.15Al0.05O2. J. Electrochem. Soc. 2018, 165, A2682–A2695. [Google Scholar] [CrossRef]
- Wagner, N.P.; Asheim, K.; Vullum-Bruer, F.; Svensson, A.M. Performance and failure analysis of full cell lithium ion battery with LiNi0.8Co0.15Al0.05O2 and silicon electrodes. J. Power Sources 2019, 437, 226884. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Holtstiege, F.; Wiemers-Meyer, S.; Nowak, S.; Schappacher, F.; Winter, M. Electropolymerization triggered in situ surface modification of electrode interphases: Alleviating first-cycle lithium loss in silicon anode lithium-ion batteries. ACS Sustain. Chem. Eng. 2020, 8, 12788–12798. [Google Scholar] [CrossRef]
- Eom, K.; Joshi, T.; Bordes, A.; Do, I.; Fuller, T.F. The design of a Li-ion full cell battery using a nano silicon and nano multi-layer graphene composite anode. J. Power Sources 2014, 249, 118–124. [Google Scholar] [CrossRef]
- Mao, C.; Ruther, R.E.; Li, J.; Du, Z.; Belharouak, I. Identifying the limiting electrode in lithium ion batteries for extreme fast charging. Electrochem. Commun. 2018, 97, 37–41. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M. Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J. Electrochem. Soc. 1993, 140, 1862–1870. [Google Scholar] [CrossRef]
- Petibon, R.; Rotermund, L.; Nelson, K.J.; Gozdz, A.S.; Xia, J.; Dahn, J.R. Study of electrolyte components in Li Ion cells using liquid-liquid extraction and gas chromatography coupled with mass spectrometry. J. Electrochem. Soc. 2014, 161, A1167–A1172. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Haering, D.; Solchenbach, S.; Marino, C.; Tsiouvaras, N.; Stinner, C.; Gasteiger, H.A. Consumption of fluoroethylene carbonate (FEC) on Si-C composite electrodes for Li-Ion batteries. J. Electrochem. Soc. 2016, 163, A1705–A1716. [Google Scholar] [CrossRef]
- Petibon, R.; Chevrier, V.L.; Aiken, C.P.; Hall, D.S.; Hyatt, S.R.; Shunmugasundaram, R.; Dahn, J.R. Studies of the capacity fade mechanisms of LiCoO2/Si-Alloy: Graphite cells. J. Electrochem. Soc. 2016, 163, A1146–A1156. [Google Scholar] [CrossRef]
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of fluoroethylene carbonate (FEC) on the performance and surface chemistry of Si-nanowire li-ion battery anodes. Langmuir 2012, 28, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Veith, G.M.; Doucet, M.; Sacci, R.L.; Vacaliuc, B.; Baldwin, J.K.; Browning, J.F. Determination of the solid electrolyte interphase structure grown on a silicon electrode using a fluoroethylene carbonate additive. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, J.; Yao, W.-H.; Yao, Y.-W.; Yang, Y. Fluoroethylene carbonate as an electrolyte additive for improving the performance of mesocarbon microbead electrode. ECS Trans. 2012, 41, 29–40. [Google Scholar] [CrossRef]
- Juarez-Robles, D.; Chen, C.-F.; Barsukov, Y.; Mukherjee, P.P. Impedance evolution characteristics in lithium-ion batteries. J. Electrochem. Soc. 2017, 164, A837–A847. [Google Scholar] [CrossRef]
- Weber, R.; Louli, A.J.; Plucknett, K.P.; Dahn, J.R. Resistance growth in lithium-ion pouch cells with LiNi0.80Co0.15Al0.05O2 positive electrodes and proposed mechanism for voltage dependent charge-transfer resistance. J. Electrochem. Soc. 2019, 166, A1779–A1784. [Google Scholar] [CrossRef]
- Pritzl, D.; Landesfeind, J.; Solchenbach, S.; Gasteiger, H.A. An analysis protocol for three-electrode li-ion battery impedance spectra: Part II. Analysis of a graphite anode Cycled vs. LNMO. J. Electrochem. Soc. 2018, 165, A2145–A2153. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Manthiram, A. Improved electrochemical performance of the 5 V Spinel Cathode LiMn1.5Ni0.42Zn0.08O4 by surface modification. J. Electrochem. Soc. 2009, 156, A66. [Google Scholar] [CrossRef]
- Wise, A.M.; Ban, C.; Weker, J.N.; Misra, S.; Cavanagh, A.S.; Wu, Z.; Li, Z.; Whittingham, M.S.; Xu, K.; George, S.M.; et al. Effect of Al2O3 coating on stabilizing LiNi0.4Mn0.4Co0.2O2 cathodes. Chem. Mater. 2015, 27, 6146–6154. [Google Scholar] [CrossRef]
- Nelson, K.J.; d’Eon, G.L.; Wright, A.T.B.; Ma, L.; Xia, J.; Dahn, J.R. Studies of the effect of high voltage on the impedance and cycling performance of Li [Ni0.4Mn0.4Co0.2]O2/graphite lithium-ion pouch cells. J. Electrochem. Soc. 2015, 162, A1046–A1054. [Google Scholar] [CrossRef]



| Reference | % of Si in the Anode | Voltage Window | Capacity, mAhg−1 | Current Rate |
|---|---|---|---|---|
| Stability Tests | ||||
| This work | 30 wt.% | 0.05–0.9 V | 844 (Cycle 50); 711 (Cycle 200) | C/5 |
| Ma et al. [32] | 17 wt.% | 0.005–1.0 V | 790 (Cycle 50, end of the test) | C/2 |
| Hsu et al. [33] | 30 wt.% | 0.05–1.5 V | 590 (Cycle 50); 600 (Cycle 200) | C/5 |
| Rate Capability Tests | ||||
| This work | 30 wt.% | 0.05–0.9 V | 860 | 5C (5 A g−1) |
| Huang et al. [34] | Not reported | 0.01–1.5 V | 700 | 1.2 A g−1 |
| Sui et al. [35] | 12.8 wt.% | 0.01–1.5 V | 458 | 2 A g−1 |
| Reference | Battery | Configuration | Voltage Window | Capacity, mAh/g | Electrode Thickness | Electrode Loading |
|---|---|---|---|---|---|---|
| This work | Si–graphite composite anode and NCA cathode | Pouch cell | 4.3–3.0 V | 130 @ C/2112 @ 2C87 @ 5C | Cathode: 45 µm Anode: 30 µm | Cathode: 5 mg/cm2 Anode: 1 mg/cm2 |
| Wagner et al. [44] | Prelithiated Si anode and NCA cathode | Coin cell | 4.4–2.2 V | 150 @ C/2110 @ 2C | Not reported | Cathode: 3.5 mg/cm2 Anode: 0.6 mg/cm2 |
| Winter et al. [45] | Si–graphite composite anode and NCA cathode | Coin cell | 4.4–3.0 V | 145 @ C/2 | Not reported | Cathode: 5–5.5 mg/cm2 Anode: not reported |
| Eom et al. [46] | Si–graphene anode and NCA cathode | Pouch cell | 4.2–2.75 V | ca. 125 @ C/2 | Not reported | Cathode: not reported Anode: 1.65 mg/cm2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabello, M.; Gucciardi, E.; Liendo, G.; Caizán-Juananera, L.; Carriazo, D.; Villaverde, A. A Study to Explore the Suitability of LiNi0.8Co0.15Al0.05O2/Silicon@Graphite Cells for High-Power Lithium-Ion Batteries. Int. J. Mol. Sci. 2021, 22, 10331. https://doi.org/10.3390/ijms221910331
Cabello M, Gucciardi E, Liendo G, Caizán-Juananera L, Carriazo D, Villaverde A. A Study to Explore the Suitability of LiNi0.8Co0.15Al0.05O2/Silicon@Graphite Cells for High-Power Lithium-Ion Batteries. International Journal of Molecular Sciences. 2021; 22(19):10331. https://doi.org/10.3390/ijms221910331
Chicago/Turabian StyleCabello, Marta, Emanuele Gucciardi, Guillermo Liendo, Leire Caizán-Juananera, Daniel Carriazo, and Aitor Villaverde. 2021. "A Study to Explore the Suitability of LiNi0.8Co0.15Al0.05O2/Silicon@Graphite Cells for High-Power Lithium-Ion Batteries" International Journal of Molecular Sciences 22, no. 19: 10331. https://doi.org/10.3390/ijms221910331
APA StyleCabello, M., Gucciardi, E., Liendo, G., Caizán-Juananera, L., Carriazo, D., & Villaverde, A. (2021). A Study to Explore the Suitability of LiNi0.8Co0.15Al0.05O2/Silicon@Graphite Cells for High-Power Lithium-Ion Batteries. International Journal of Molecular Sciences, 22(19), 10331. https://doi.org/10.3390/ijms221910331








