Orchestration of Force Generation and Nuclear Collapse in Apoptotic Cells
Abstract
:1. Introduction
2. Apoptosis: A Stepwise Dynamic Process
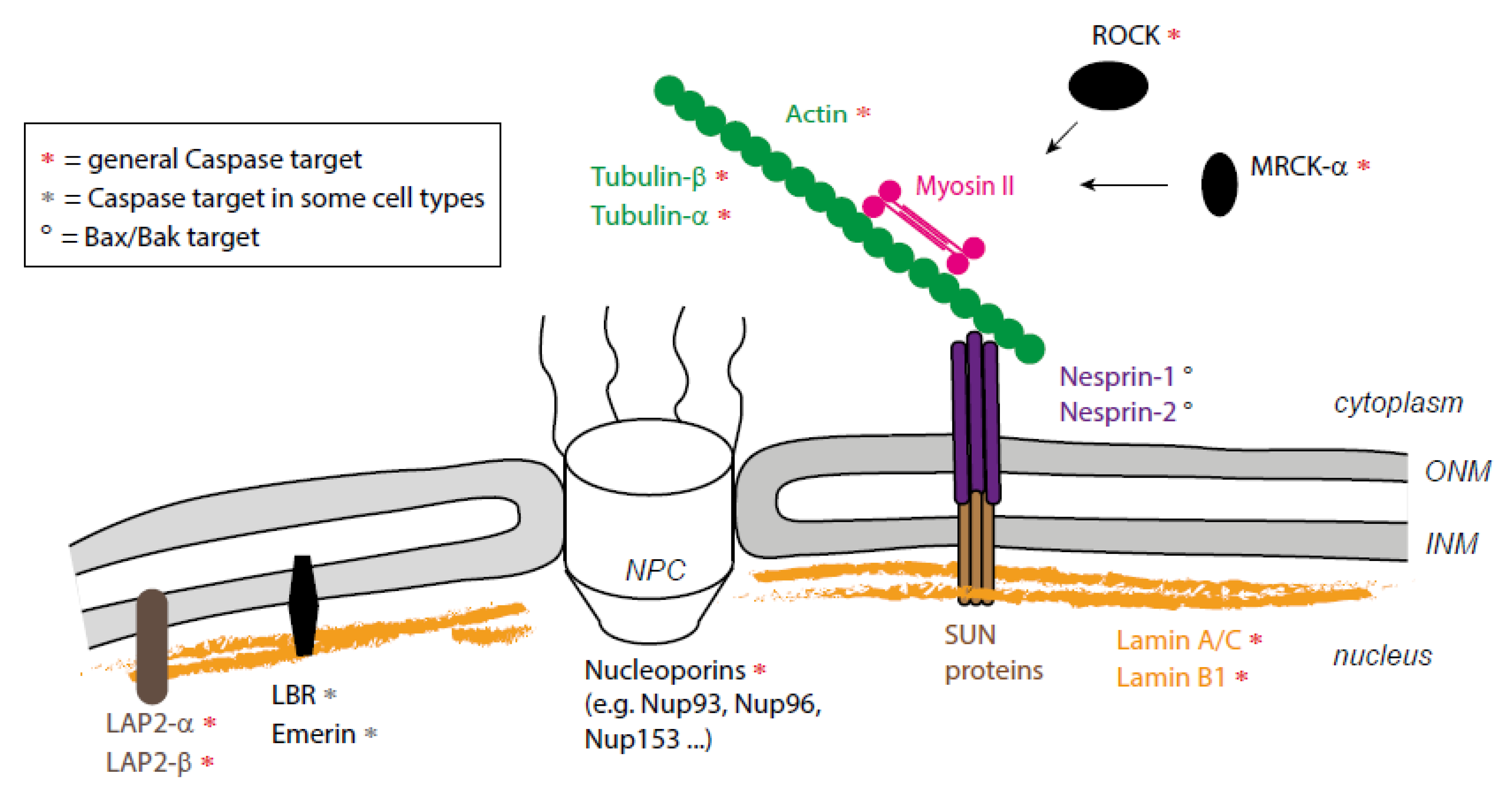
3. A combination of Proteolytic and Mechanical Events Leads to Nucleus Destruction
3.1. Nuclear Blebbing, an Early Step of Apoptotic Nucleus Dynamics
3.2. Nuclear Dismantling
4. Actomyosin-Nucleus Coupling before Fragmentation
5. Could Apoptotic Microtubules Also Contribute to Nuclear Fragmentation?
6. What Mechanism for Nucleus/Cytoskeleton Interaction during Cell Death?
7. A Necessity to Understand Nuclear Mechanotransduction during Cell Death
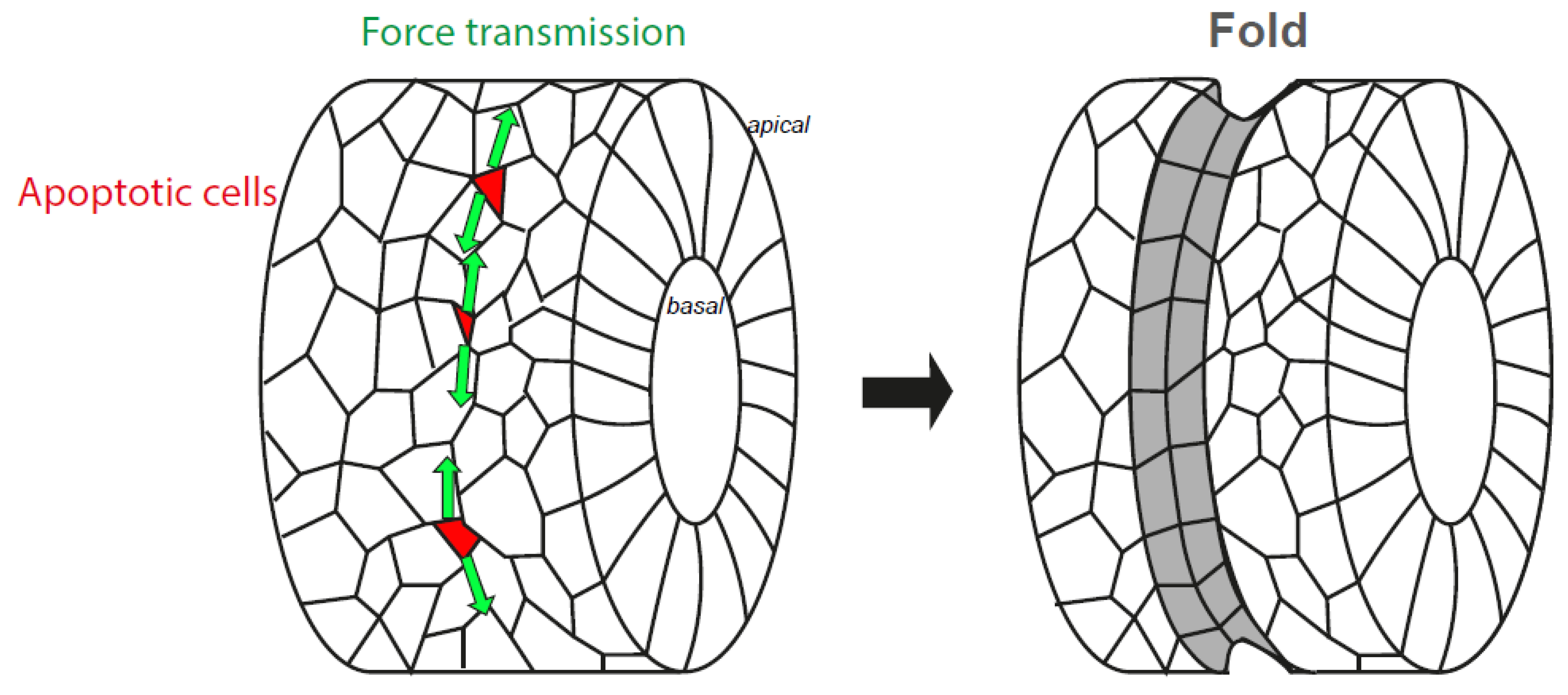
8. The Nucleus, from an Organelle under Force to an Organelle Necessary to Generate Morphogenetic Forces
9. Conclusions
Funding
Conflicts of Interest
References
- De Leeuw, R.; Gruenbaum, Y.; Medalia, O. Nuclear Lamins: Thin Filaments with Major Functions. Trends Cell Biol. 2018, 28, 34–45. [Google Scholar] [CrossRef]
- Turgay, Y.; Eibauer, M.; Goldman, A.E.; Shimi, T.; Khayat, M.; Ben-Harush, K.; Dubrovsky-Gaupp, A.; Sapra, K.T.; Goldman, R.D.; Medalia, O. The molecular architecture of lamins in somatic cells. Nature 2017, 543, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Vashisth, M.; Abbas, A.; Majkut, S.; Vogel, K.; Xia, Y.; Ivanovska, I.L.; Irianto, J.; Tewari, M.; Zhu, K.; et al. Mechanosensing by the Lamina Protects against Nuclear Rupture, DNA Damage, and Cell-Cycle Arrest. Dev. Cell 2019, 49, 920–935. [Google Scholar] [CrossRef]
- Xia, Y.; Pfeifer, C.R.; Cho, S.; Discher, D.E.; Irianto, J. Nuclear mechanosensing. Emerg. Top Life Sci. 2018, 2, 713–725. [Google Scholar] [CrossRef]
- Kim, J.K.; Louhghalam, A.; Lee, G.; Schafer, B.W.; Wirtz, D.; Kim, D.H. Nuclear lamin A/C harnesses the perinuclear apical actin cables to protect nuclear morphology. Nat. Commun. 2017, 8, 2123. [Google Scholar] [CrossRef] [PubMed]
- Horn, H.F. LINC complex proteins in development and disease. Curr. Top Dev. Biol. 2014, 109, 287–321. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, M.R.; Ravichandran, K.S. The Dynamics of Apoptotic Cell Clearance. Dev. Cell 2016, 38, 147–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghose, P.; Shaham, S. Cell death in animal development. Development 2020, 147, dev191882. [Google Scholar] [CrossRef]
- Crawford, E.D.; Wells, J.A. Caspase substrates and cellular remodeling. Annu. Rev. Biochem. 2011, 80, 1055–1087. [Google Scholar] [CrossRef]
- Fischer, U.; Jänicke, R.U.; Schulze-Osthoff, K. Many cuts to ruin: A comprehensive update of caspase substrates. Cell Death Differ. 2003, 10, 76–100. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Sahai, E.A.; Yeo, M.; Bosch, M.; Dewar, A.; Olson, M.F. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 2001, 3, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Sebbagh, M.; Renvoizé, C.; Hamelin, J.; Riché, N.; Bertoglio, J.; Bréard, J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 2001, 3, 346–352. [Google Scholar] [CrossRef]
- Tixeira, R.; Phan, T.K.; Caruso, S.; Shi, B.; Atkin-Smith, G.K.; Nedeva, C.; Chow, J.D.Y.; Puthalakath, H.; Hulett, M.D.; Herold, M.J.; et al. ROCK1 but not LIMK1 or PAK2 is a key regulator of apoptotic membrane blebbing and cell disassembly. Cell Death Differ. 2020, 27, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Satoi, S.; Harada, S.; Uchida, S.; Iwasa, Y.; Ikenouchi, J. Coordinated changes in cell membrane and cytoplasm during maturation of apoptotic bleb. Mol. Biol. Cell 2020, 31, 833–844. [Google Scholar] [CrossRef]
- Julian, L.; Naylor, G.; Wickman, G.R.; Rath, N.; Castino, G.; Stevenson, D.; Bryson, S.; Munro, J.; McGarry, L.; Mullin, M.; et al. Defective apoptotic cell contractility provokes sterile inflammation, leading to liver damage and tumour suppression. eLife 2021, 10, e61983. [Google Scholar] [CrossRef]
- Lubkov, V.; Bar-Sagi, D. E-cadherin-mediated cell coupling is required for apoptotic cell extrusion. Curr. Biol. 2014, 24, 868–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, S.J.; Rosenblatt, J. Early mechanical selection of cell extrusion and extrusion signaling in cancer. Curr. Opin. Cell Biol. 2021, 72, 36–40. [Google Scholar] [CrossRef]
- Gagliardi, P.A.; Somale, D.; Puliafito, A.; Chiaverina, G.; di Blasio, L.; Oneto, M.; Bianchini, P.; Bussolino, F.; Primo, L. MRCKα is activated by caspase cleavage to assemble an apical actin ring for epithelial cell extrusion. J. Cell Biol. 2018, 217, 231–249. [Google Scholar] [CrossRef]
- Le, A.P.; Rupprecht, J.F.; Mège, R.M.; Toyama, Y.; Lim, C.T.; Ladoux, B. Adhesion-mediated heterogeneous actin organization governs apoptotic cell extrusion. Nat. Commun. 2021, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Lindenboim, L.; Zohar, H.; Worman, H.J.; Stein, R. The nuclear envelope: Target and mediator of the apoptotic process. Cell Death Discov. 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, E.A.; Zamaraev, A.V.; Kopeina, G.S.; Zhivotovsky, B.; Lavrik, I.N. Role of the nucleus in apoptosis: Signaling and execution. Cell Mol. Life Sci. 2015, 72, 4593–4612. [Google Scholar] [CrossRef] [PubMed]
- Lindenboim, L.; Sasson, T.; Worman, H.J.; Borner, C.; Stein, R. Cellular stress induces Bax-regulated nuclear bubble budding and rupture followed by nuclear protein release. Nucleus 2014, 5, 527–541. [Google Scholar] [CrossRef] [Green Version]
- Raab, M.; Gentili, M.; de Belly, H.; Thiam, H.R.; Vargas, P.; Jimenez, A.J.; Lautenschlaeger, F.; Voituriez, R.; Lennon-Duménil, A.M.; Manel, N.; et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 2016, 352, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Denais, C.M.; Gilbert, R.M.; Isermann, P.; McGregor, A.L.; te Lindert, M.; Weigelin, B.; Davidson, P.M.; Friedl, P.; Wolf, K.; Lammerding, J. Nuclear envelope rupture and repair during cancer cell migration. Science 2016, 352, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Hatch, E.M.; Hetzer, M.W. Nuclear envelope rupture is induced by actin-based nucleus confinement. J. Cell Biol. 2016, 215, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Ungricht, R.; Kutay, U. Mechanisms and functions of nuclear envelope remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 229–245. [Google Scholar] [CrossRef]
- Pfeifer, C.R.; Vashisth, M.; Xia, Y.; Discher, D.E. Nuclear failure, DNA damage, and cell cycle disruption after migration through small pores: A brief review. Essays Biochem. 2019, 63, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Mei, Y.; Schipma, M.J.; Roth, E.W.; Bleher, R.; Rappoport, J.Z.; Wickrema, A.; Yang, J.; Ji, P. Nuclear Condensation during Mouse Erythropoiesis Requires Caspase-3-Mediated Nuclear Opening. Dev. Cell 2016, 36, 498–510. [Google Scholar] [CrossRef] [Green Version]
- Neamati, N.; Fernandez, A.; Wright, S.; Kiefer, J.; McConkey, D.J. Degradation of lamin B1 precedes oligonucleosomal DNA fragmentation in apoptotic thymocytes and isolated thymocyte nuclei. J. Immunol. 1995, 154, 3788–3795. [Google Scholar]
- Rao, L.; Perez, D.; White, E. Lamin proteolysis facilitates nuclear events during apoptosis. J. Cell Biol. 1996, 135, 1441–1455. [Google Scholar] [CrossRef] [PubMed]
- Croft, D.R.; Coleman, M.L.; Li, S.; Robertson, D.; Sullivan, T.; Stewart, C.L.; Olson, M.F. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J. Cell Biol. 2005, 168, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambrosini, A.; Rayer, M.; Monier, B.; Suzanne, M. Mechanical Function of the Nucleus in Force Generation during Epithelial Morphogenesis. Dev. Cell 2019, 50, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Suzanne, M. Molecular and cellular mechanisms involved in leg joint morphogenesis. Semin. Cell Dev. Biol. 2016, 55, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Monier, B.; Gettings, M.; Gay, G.; Mangeat, T.; Schott, S.; Guarner, A.; Suzanne, M. Apico-basal forces exerted by apoptotic cells drive epithelium folding. Nature 2015, 518, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Baena-Lopez, L.A.; Arthurton, L.; Bischoff, M.; Vincent, J.P.; Alexandre, C.; McGregor, R. Novel initiator caspase reporters uncover previously unknown features of caspase-activating cells. Development 2018, 145. [Google Scholar] [CrossRef] [Green Version]
- Moss, D.K.; Betin, V.M.; Malesinski, S.D.; Lane, J.D. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J. Cell Sci. 2006, 119, 2362–2374. [Google Scholar] [CrossRef] [Green Version]
- Oropesa-Ávila, M.; Fernández-Vega, A.; de la Mata, M.; Maraver, J.G.; Cordero, M.D.; Cotán, D.; de Miguel, M.; Calero, C.P.; Paz, M.V.; Pavón, A.D.; et al. Apoptotic microtubules delimit an active caspase free area in the cellular cortex during the execution phase of apoptosis. Cell Death Dis. 2013, 4, e527. [Google Scholar] [CrossRef] [Green Version]
- Moss, D.K.; Wilde, A.; Lane, J.D. Dynamic release of nuclear RanGTP triggers TPX2-dependent microtubule assembly during the apoptotic execution phase. J. Cell Sci. 2009, 122, 644–655. [Google Scholar] [CrossRef] [Green Version]
- Povea-Cabello, S.; Oropesa-Ávila, M.; de la Cruz-Ojeda, P.; Villanueva-Paz, M.; de la Mata, M.; Suárez-Rivero, J.M.; Álvarez-Córdoba, M.; Villalón-García, I.; Cotán, D.; Ybot-González, P.; et al. Dynamic Reorganization of the Cytoskeleton during Apoptosis: The Two Coffins Hypothesis. Int. J. Mol. Sci. 2017, 18, 2393. [Google Scholar] [CrossRef] [Green Version]
- Bao, X.; Liu, H.; Liu, X.; Ruan, K.; Zhang, Y.; Zhang, Z.; Hu, Q.; Liu, Y.; Akram, S.; Zhang, J.; et al. Mitosis-specific acetylation tunes Ran effector binding for chromosome segregation. J. Mol. Cell Biol. 2018, 10, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Leal, J.; Amaya, C.; Li, B.; Xu, X.X. Nuclear Lamin A/C Expression Is a Key Determinant of Paclitaxel Sensitivity. Mol. Cell Biol. 2021, 41, e0064820. [Google Scholar] [CrossRef]
- Smith, E.R.; Capo-Chichi, C.D.; Xu, X.X. Defective Nuclear Lamina in Aneuploidy and Carcinogenesis. Front. Oncol. 2018, 8, 529. [Google Scholar] [CrossRef]
- Capo-Chichi, C.D.; Yeasky, T.M.; Smith, E.R.; Xu, X.X. Nuclear envelope structural defect underlies the main cause of aneuploidy in ovarian carcinogenesis. BMC Cell Biol. 2016, 17, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenboim, L.; Grozki, D.; Amsalem-Zafran, A.R.; Peña-Blanco, A.; Gundersen, G.G.; Borner, C.; Hodzic, D.; Garcia-Sáez, A.J.; Worman, H.J.; Stein, R. Apoptotic stress induces Bax-dependent, caspase-independent redistribution of LINC complex nesprins. Cell Death Discov. 2020, 6, 90. [Google Scholar] [CrossRef]
- Dey, G.; Baum, B. Nuclear envelope remodelling during mitosis. Curr. Opin. Cell Biol. 2021, 70, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Strasser, C.; Grote, P.; Schäuble, K.; Ganz, M.; Ferrando-May, E. Regulation of nuclear envelope permeability in cell death and survival. Nucleus 2012, 3, 540–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.N.; Zastrow, M.S.; Wilson, K.L. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus 2010, 1, 264–272. [Google Scholar] [CrossRef]
- Martin, A.C.; Kaschube, M.; Wieschaus, E.F. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 2009, 457, 495–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Doubrovinski, K.; Polyakov, O.; Wieschaus, E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 2014, 508, 392–396. [Google Scholar] [CrossRef] [Green Version]
- Polyakov, O.; He, B.; Swan, M.; Shaevitz, J.W.; Kaschube, M.; Wieschaus, E. Passive mechanical forces control cell-shape change during Drosophila ventral furrow formation. Biophys. J. 2014, 107, 998–1010. [Google Scholar] [CrossRef] [Green Version]
- Belaadi, N.; Aureille, J.; Guilluy, C. Under Pressure: Mechanical Stress Management in the Nucleus. Cells 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410. [Google Scholar] [CrossRef]
- Guilluy, C.; Osborne, L.D.; Van Landeghem, L.; Sharek, L.; Superfine, R.; Garcia-Mata, R.; Burridge, K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell. Biol. 2014, 16, 376–381. [Google Scholar] [CrossRef]
- Wang, K.; Qin, Y.; Chen, Y. In situ AFM detection of the stiffness of the in situ exposed cell nucleus. BBA Mol. Cell Res. 2021, 5, 118985. [Google Scholar]
- Tajik, A.; Zhang, Y.; Wei, F.; Sun, J.; Jia, Q.; Zhou, W.; Singh, R.; Khanna, N.; Belmont, A.S.; Wang, N. Transcription upregulation via force-induced direct stretching of chromatin. Nat. Mater. 2016, 15, 1287–1296. [Google Scholar] [CrossRef] [Green Version]
- Miroshnikova, Y.A.; Nava, M.M.; Wickström, S.A. Emerging roles of mechanical forces in chromatin regulation. J. Cell Sci. 2017, 130, 2243–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, H.Q.; Ghatak, S.; Yeung, C.Y.; Tellkamp, F.; Günschmann, C.; Dieterich, C.; Yeroslaviz, A.; Habermann, B.; Pombo, A.; Niessen, C.M.; et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat. Cell Biol. 2016, 18, 864–875. [Google Scholar] [CrossRef]
- Kirby, T.J.; Lammerding, J. Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol. 2018, 20, 373–381. [Google Scholar] [CrossRef]
- Wang, Y.; Nagarajan, M.; Uhler, C.; Shivashankar, G.V. Orientation and repositioning of chromosomes correlate with cell geometry-dependent gene expression. Mol. Biol. Cell 2017, 28, 1997–2009. [Google Scholar] [CrossRef]
- Hao, H.; Starr, D.A. SUN/KASH interactions facilitate force transmission across the nuclear envelope. Nucleus 2019, 10, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miroshnikova, Y.A.; Cohen, I.; Ezhkova, E.; Wickström, S.A. Epigenetic gene regulation, chromatin structure, and force-induced chromatin remodelling in epidermal development and homeostasis. Curr. Opin. Genet. Dev. 2019, 55, 46–51. [Google Scholar] [CrossRef]
- Schott, S.; Ambrosini, A.; Barbaste, A.; Benassayag, C.; Gracia, M.; Proag, A.; Rayer, M.; Monier, B.; Suzanne, M. A fluorescent toolkit for spatiotemporal tracking of apoptotic cells in living. Development 2017, 144, 3840–3846. [Google Scholar] [CrossRef] [Green Version]
- Spagnol, S.T.; Armiger, T.J.; Dahl, K.N. Mechanobiology of Chromatin and the Nuclear Interior. Cell Mol. Bioeng. 2016, 9, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnol, S.T.; Dahl, K.N. Spatially Resolved Quantification of Chromatin Condensation through Differential Local Rheology in Cell Nuclei Fluorescence Lifetime Imaging. PLoS ONE 2016, 11, e0146244. [Google Scholar] [CrossRef] [Green Version]
- Chalut, K.J.; Höpfler, M.; Lautenschläger, F.; Boyde, L.; Chan, C.J.; Ekpenyong, A.; Martinez-Arias, A.; Guck, J. Chromatin decondensation and nuclear softening accompany Nanog downregulation in embryonic stem cells. Biophys. J. 2012, 103, 2060–2070. [Google Scholar] [CrossRef] [Green Version]
- Dahl, K.N.; Engler, A.J.; Pajerowski, J.D.; Discher, D.E. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys. J. 2005, 89, 2855–2864. [Google Scholar] [CrossRef] [Green Version]
- Stephens, A.D.; Banigan, E.J.; Adam, S.A.; Goldman, R.D.; Marko, J.F. Chromatin and lamin A determine two different mechanical response regimes of the cell nucleus. Mol. Biol. Cell 2017, 28, 1984–1996. [Google Scholar] [CrossRef]
- Yevick, H.G.; Miller, P.W.; Dunkel, J.; Martin, A.C. Structural Redundancy in Supracellular Actomyosin Networks Enables Robust Tissue Folding. Dev Cell 2019, 50, 586–598. [Google Scholar] [CrossRef]
- Eritano, A.S.; Bromley, C.L.; Bolea Albero, A.; Schütz, L.; Wen, F.L.; Takeda, M.; Fukaya, T.; Sami, M.M.; Shibata, T.; Lemke, S.; et al. Tissue-Scale Mechanical Coupling Reduces Morphogenetic Noise to Ensure Precision during Epithelial Folding. Dev. Cell 2020, 53, 212–228. [Google Scholar] [CrossRef]
- Martin, E.; Theis, S.; Gay, G.; Monier, B.; Rouvière, C.; Suzanne, M. Arp2/3-dependent mechanical control of morphogenetic robustness in an inherently challenging environment. Dev. Cell 2021, 56, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tamashunas, A.C.; Agrawal, A.; Torbati, M.; Katiyar, A.; Dickinson, R.B.; Lammerding, J.; Lele, T.P. Local, transient tensile stress on the nuclear membrane causes membrane rupture. Mol. Biol. Cell 2019, 30, 899–906. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monier, B.; Suzanne, M. Orchestration of Force Generation and Nuclear Collapse in Apoptotic Cells. Int. J. Mol. Sci. 2021, 22, 10257. https://doi.org/10.3390/ijms221910257
Monier B, Suzanne M. Orchestration of Force Generation and Nuclear Collapse in Apoptotic Cells. International Journal of Molecular Sciences. 2021; 22(19):10257. https://doi.org/10.3390/ijms221910257
Chicago/Turabian StyleMonier, Bruno, and Magali Suzanne. 2021. "Orchestration of Force Generation and Nuclear Collapse in Apoptotic Cells" International Journal of Molecular Sciences 22, no. 19: 10257. https://doi.org/10.3390/ijms221910257
APA StyleMonier, B., & Suzanne, M. (2021). Orchestration of Force Generation and Nuclear Collapse in Apoptotic Cells. International Journal of Molecular Sciences, 22(19), 10257. https://doi.org/10.3390/ijms221910257





