The Combination of Abscisic Acid (ABA) and Water Stress Regulates the Epicuticular Wax Metabolism and Cuticle Properties of Detached Citrus Fruit
Abstract
:1. Introduction
2. Results
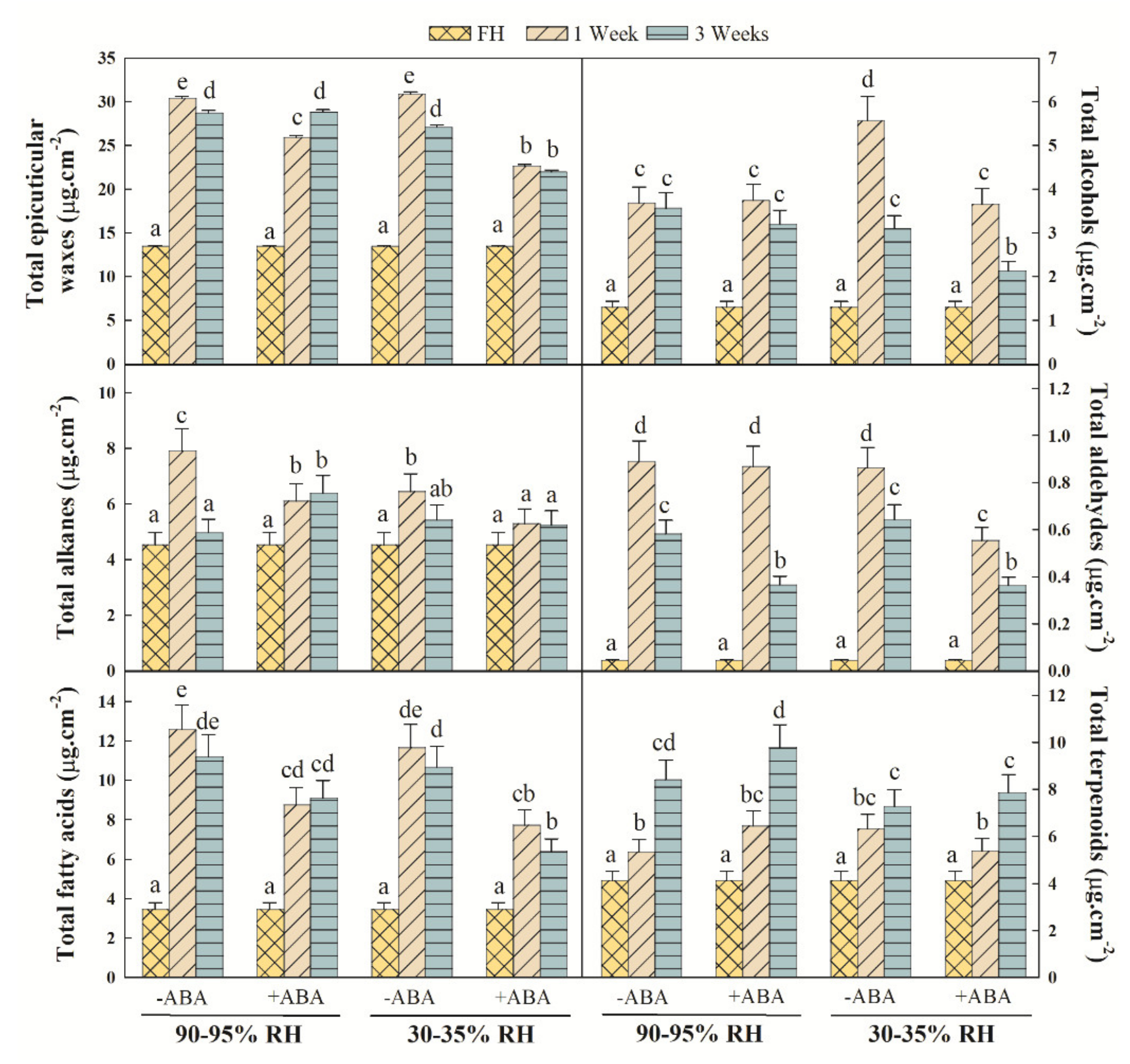
2.1. Effects of ABA and Water Stress on Wax Content and Wax Fraction Distribution
2.2. Epicuticular Wax Composition Analysis
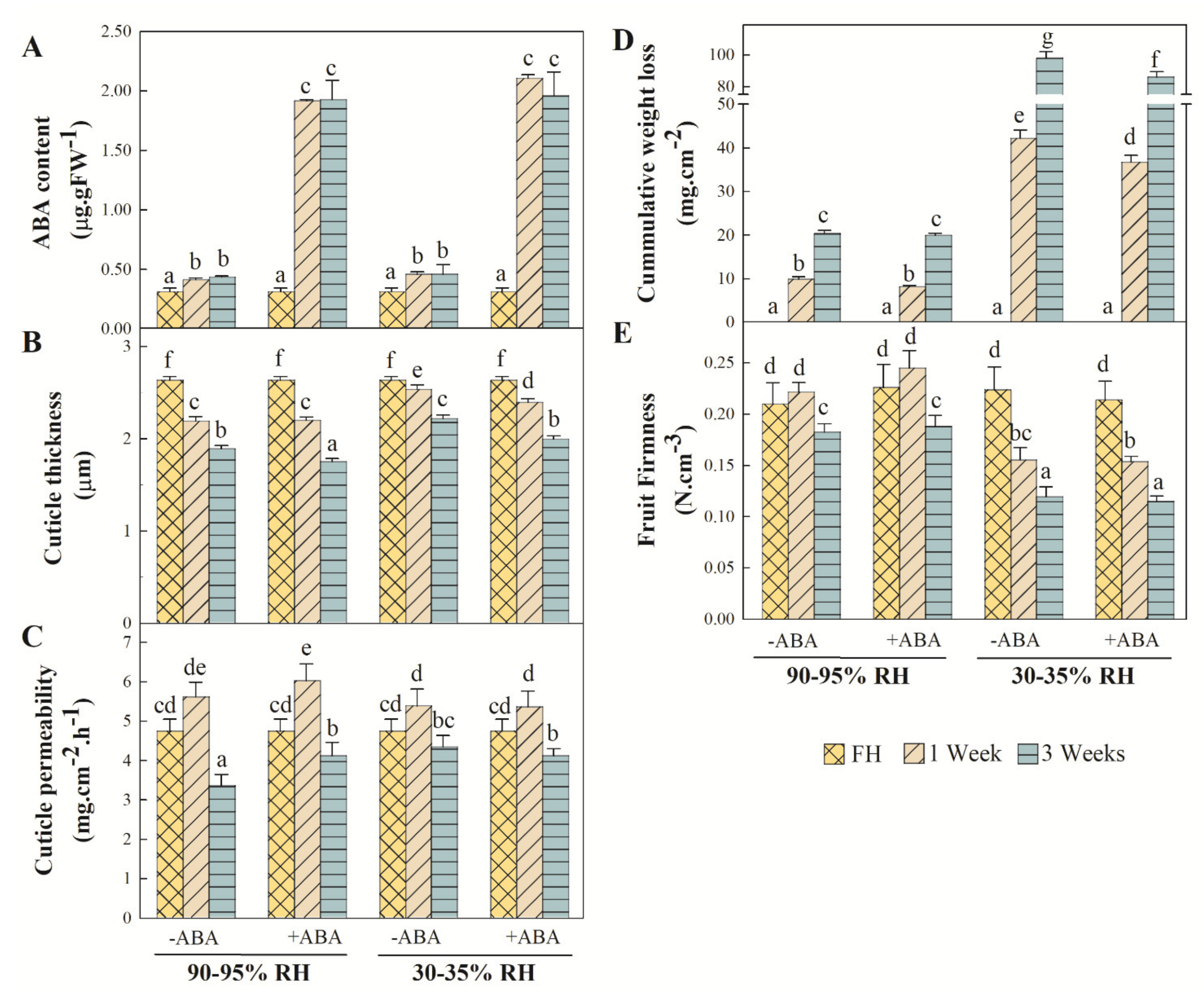
2.3. Variations in Cuticle Properties and Fruit ABA Content, Firmness and Weight Loss
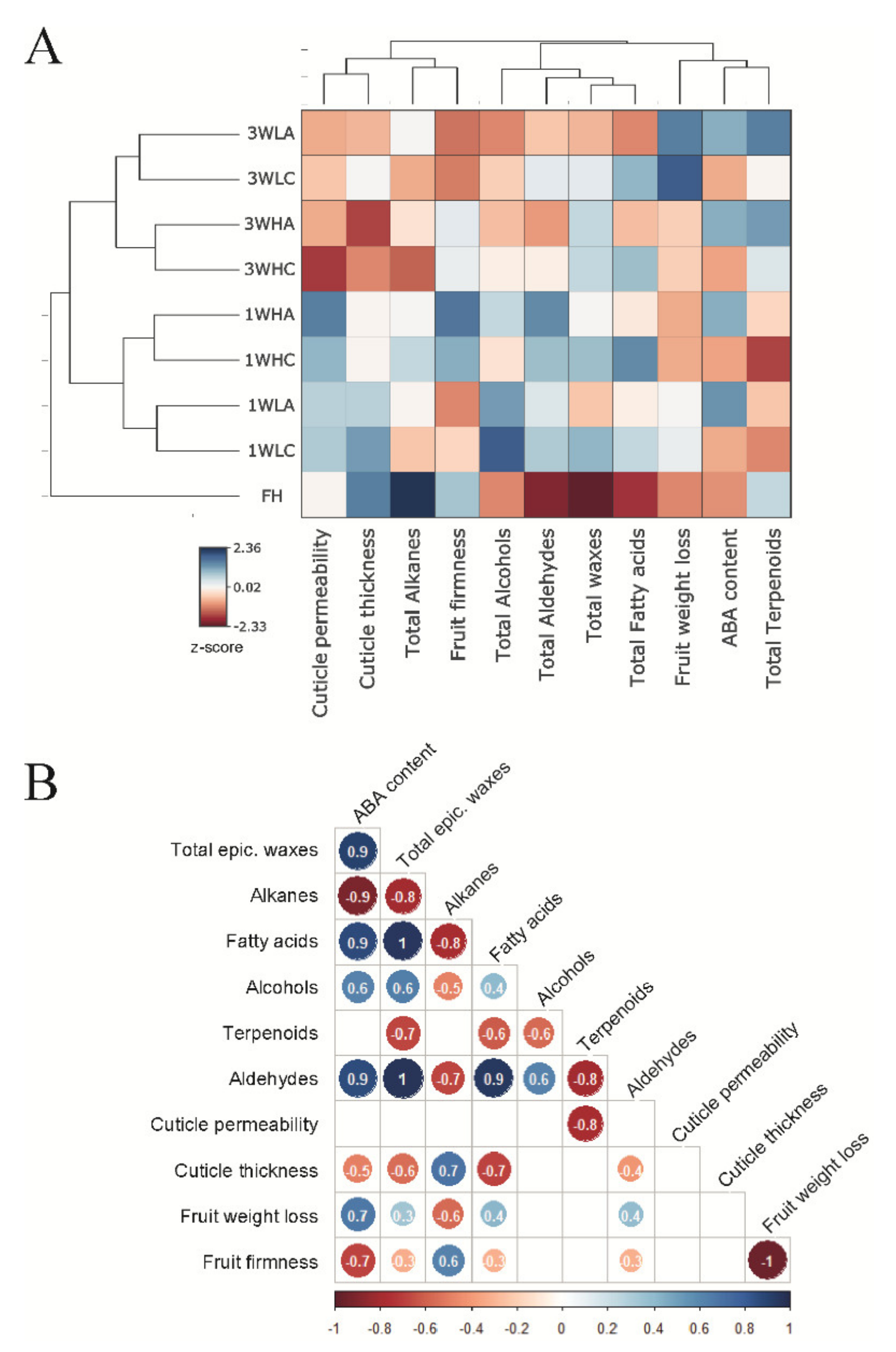
2.4. Relationships between ABA Content, Cuticle Composition and Properties and Fruit Physiology
2.5. Effects of ABA and Water Stress on the Transcriptional Regulation of Epicuticular Wax-Related Genes
3. Discussion
4. Materials and Methods
4.1. Fruit Materials and Experimental Design
4.2. Cuticular Wax Analysis
4.3. Cuticle Permeability and Thickness
4.4. ABA Analysis
4.5. Fruit Weight Loss and Firmness Determinations
4.6. Clustering Analysis
4.7. RNA Extraction, cDNA Synthesis and RT-qPCR
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohkuma, K.; Lyon, J.L.; Addicott, F.T.; Smith, O.E. Abscisin II, an abscission-accelerating substance from young cotton fruit. Science 1963, 142, 1592–1593. [Google Scholar] [CrossRef]
- Addicott, F.T.; Lyon, J.L.; Ohkuma, K.; Thiessen, W.E.; Carns, H.R.; Smith, O.E.; Cornforth, J.W.; Milborrow, B.V.; Ryback, G.; Wareing, P.F. Abscisic acid: A new name for Abscisin II (Dormin). Science 1968, 159, 1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, P.; Yuan, B.; Guo, Y. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2014, 65, 4577–4588. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curvers, K.; Seifi, H.; Mouille, G.; de Rycke, R.; Asselbergh, B.; Van Hecke, A.; Vanderschaeghe, D.; Hofte, H.; Callewaert, N.; Van Breusegem, F.; et al. Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea. Plant Physiol. 2010, 154, 847–860. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, L.; Xie, L.; He, Y.; Luo, T.; Sheng, L.; Luo, Y.; Zeng, Y.; Xu, J.; Deng, X.; et al. Regulation of cuticle formation during fruit development and ripening in ‘Newhall’ navel orange (Citrus sinensis Osbeck) revealed by transcriptomic and metabolomic profiling. Plant Sci. 2016, 243, 131–144. [Google Scholar] [CrossRef]
- Romero, P.; Lafuente, M.T. Abscisic acid deficiency alters epicuticular wax metabolism and morphology that leads to increased cuticle permeability during sweet orange (Citrus sinensis) fruit ripening. Front. Plant Sci. 2020, 11, 1914. [Google Scholar] [CrossRef]
- Martin, L.B.B.; Romero, P.; Fich, E.A.; Domozych, D.S.; Rose, J.K.C. Cuticle biosynthesis in tomato leaves is developmentally regulated by abscisic acid. Plant Physiol. 2017, 174, 1384–1398. [Google Scholar] [CrossRef] [Green Version]
- Correia, S.; Santos, M.; Glińska, S.; Gapińska, M.; Matos, M.; Carnide, V.; Schouten, R.; Silva, A.P.; Gonçalves, B. Effects of exogenous compound sprays on cherry cracking: Skin properties and gene expression. J. Sci. Food Agric. 2020, 100, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.B.B.; Rose, J.K.C. There’s more than one way to skin a fruit: Formation and functions of fruit cuticles. J. Exp. Bot. 2014, 65, 4639–4651. [Google Scholar] [CrossRef] [Green Version]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [Green Version]
- Bhanot, V.; Fadanavis, S.V.; Panwar, J. Revisiting the architecture, biosynthesis and functional aspects of the plant cuticle: There is more scope. Environ. Exp. Bot. 2021, 183, 104364. [Google Scholar] [CrossRef]
- Joubès, J.; Domergue, F. Biosynthesis of the Plant Cuticle. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; Springer International Publishing: Berlin, Germany, 2018; pp. 1–19. [Google Scholar]
- Lara, I.; Heredia, A.; Domínguez, E. Shelf life potential and the fruit cuticle: The unexpected player. Front. Plant Sci. 2019, 10, 770. [Google Scholar] [CrossRef] [Green Version]
- Tafolla-Arellano, J.C.; Báez-Sañudo, R.; Tiznado-Hernández, M.E. The cuticle as a key factor in the quality of horticultural crops. Sci. Hortic. 2018, 232, 145–152. [Google Scholar] [CrossRef]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Lü, S.; Alkalai-Tuvia, S.; Perzelan, Y.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and fruit post-harvest water loss in an advanced backcross generation of pepper (Capsicum sp.). Physiol. Plant. 2012, 146, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Gao, H.; Chen, H.; Fang, X.; Zheng, Y. Effects of cuticular wax on the postharvest quality of blueberry fruit. Food Chem. 2018, 239, 68–74. [Google Scholar] [CrossRef]
- Chai, Y.; Li, A.; Chit Wai, S.; Song, C.; Zhao, Y.; Duan, Y.; Zhang, B.; Lin, Q. Cuticular wax composition changes of 10 apple cultivars during postharvest storage. Food Chem. 2020, 324, 126903. [Google Scholar] [CrossRef]
- Saladié, M.; Matas, A.J.; Isaacson, T.; Jenks, M.A.; Goodwin, S.M.; Niklas, K.J.; Xiaolin, R.; Labavitch, J.M.; Shackel, K.A.; Fernie, A.R.; et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol. 2007, 144, 1012–1028. [Google Scholar] [CrossRef] [Green Version]
- Lownds, N.; Banaras, M.; Bosland, P.W. Relationships between postharvest water loss and physical properties of pepper fruit (Capsicum annuum L.). HortScience 1993, 28, 1182–1184. [Google Scholar] [CrossRef] [Green Version]
- Tafolla-Arellano, J.C.; Zheng, Y.; Sun, H.; Jiao, C.; Ruiz-May, E.; Hernández-Oñate, M.A.; González-León, A.; Báez-Sañudo, R.; Fei, Z.; Domozych, D.; et al. Transcriptome analysis of mango (Mangifera indica L.) fruit epidermal peel to identify putative cuticle-associated genes. Sci. Rep. 2017, 7, 46163. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Burghardt, M.; Schuster, A.-C.; Leide, J.; Lara, I.; Riederer, M. Chemical composition and water permeability of fruit and leaf cuticles of Olea europaea L. J. Agric. Food Chem. 2017, 65, 8790–8797. [Google Scholar] [CrossRef]
- Diarte, C.; Xavier de Souza, A.; Staiger, S.; Deininger, A.-C.; Bueno, A.; Burghardt, M.; Graell, J.; Riederer, M.; Lara, I.; Leide, J. Compositional, structural and functional cuticle analysis of Prunus laurocerasus L. sheds light on cuticular barrier plasticity. Plant Physiol. Biochem. 2021, 158, 434–445. [Google Scholar] [CrossRef]
- Vichi, S.; Cortés-Francisco, N.; Caixach, J.; Barrios, G.; Mateu, J.; Ninot, A.; Romero, A. Epicuticular wax in developing olives (Olea europaea) is highly dependent upon cultivar and fruit ripeness. J. Agric. Food Chem. 2016, 64, 5985–5994. [Google Scholar] [CrossRef] [PubMed]
- Belge, B.; Llovera, M.; Comabella, E.; Graell, J.; Lara, I. Fruit cuticle composition of a melting and a nonmelting peach cultivar. J. Agric. Food Chem. 2014, 62, 3488–3495. [Google Scholar] [CrossRef]
- Parsons, E.P.; Popopvsky, S.; Lohrey, G.T.; Alkalai-Tuvia, S.; Perzelan, Y.; Bosland, P.; Bebeli, P.J.; Paran, I.; Fallik, E.; Jenks, M.A. Fruit cuticle lipid composition and water loss in a diverse collection of pepper (Capsicum). Physiol. Plant. 2013, 149, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Sala, J.M. Content, chemical composition and morphology of epicuticular wax of Fortune mandarin fruits in relation to peel pitting. J. Sci. Food Agric. 2000, 80, 1887–1894. [Google Scholar] [CrossRef]
- Domínguez, E.; Fernández, M.D.; Hernández, J.C.L.; Parra, J.P.; España, L.; Heredia, A.; Cuartero, J. Tomato fruit continues growing while ripening, affecting cuticle properties and cracking. Physiol. Plant. 2012, 146, 473–486. [Google Scholar] [CrossRef]
- Peschel, S.; Franke, R.; Schreiber, L.; Knoche, M. Composition of the cuticle of developing sweet cherry fruit. Phytochemistry 2007, 68, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Knoche, M.; Beyer, M.; Peschel, S.; Oparlakov, B.; Bukovac, M.J. Changes in strain and deposition of cuticle in developing sweet cherry fruit. Physiol. Plant. 2004, 120, 667–677. [Google Scholar] [CrossRef] [PubMed]
- D’Angeli, S.; Altamura, M. Unsaturated lipids change in olive tree drupe and seed during fruit development and in response to cold-stress and acclimation. Int. J. Mol. Sci. 2016, 17, 1889. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.; Bukovac, M.; Hunt, G. Composition of tomato fruit cuticle as related to fruit growth and development. In The Plant Cuticle; Cutler, D., Alvin, K., Price, C., Eds.; London Academic Press: London, UK, 1982; pp. 33–44. [Google Scholar]
- Oliveira Lino, L.; Quilot-Turion, B.; Dufour, C.; Corre, M.-N.; Lessire, R.; Génard, M.; Poëssel, J.-L. Cuticular waxes of nectarines during fruit development in relation to surface conductance and susceptibility to Monilinia laxa. J. Exp. Bot. 2020, 71, 5521–5537. [Google Scholar] [CrossRef] [PubMed]
- Cajuste, J.F.; González-Candelas, L.; Veyrat, A.; García-Breijo, F.J.; Reig-Armiñana, J.; Lafuente, M.T. Epicuticular wax content and morphology as related to ethylene and storage performance of ‘Navelate’ orange fruit. Postharvest Biol. Technol. 2010, 55, 29–35. [Google Scholar] [CrossRef] [Green Version]
- El-Otmani, M.; Coggins, C.W.J. Fruit age and growth regulator effects on the quantity and structure of the epicuticular wax of Washington Navel orange fruit. J. Am. Soc. Hortic. Sci. 1985, 110, 371–378. [Google Scholar]
- Li, F.; Min, D.; Song, B.; Shao, S.; Zhang, X. Ethylene effects on apple fruit cuticular wax composition and content during cold storage. Postharvest Biol. Technol. 2017, 134, 98–105. [Google Scholar] [CrossRef]
- Curry, E. Effects of 1-MCP applied postharvest on epicuticular wax of apples (Malus domestica Borkh.) during storage. J. Sci. Food Agric. 2008, 88, 996–1006. [Google Scholar] [CrossRef]
- Klein, B.; Ribeiro, Q.M.; Thewes, F.R.; de OliveiraAnese, R.; de Candido deOliveira, F.; Santos, I.D.; Ribeiro, S.R.; Donadel, J.Z.; Brackmann, A.; Barin, J.S.; et al. The isolated or combined effects of dynamic controlled atmosphere (DCA) and 1-MCP on the chemical composition of cuticular wax and metabolism of ‘Maxi Gala’ apples after long-term storage. Food Res. Int. 2020, 140, 109900. [Google Scholar] [CrossRef]
- Lara, I. The fruit cuticle: Actively tuning postharvest quality. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality; Siddiqui, M.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 93–120. ISBN 978-0-12-809807-3. [Google Scholar]
- Trivedi, P.; Nguyen, N.; Hykkerud, A.L.; Häggman, H.; Martinussen, I.; Jaakola, L.; Karppinen, K. Developmental and environmental regulation of cuticular wax biosynthesis in fleshy fruits. Front. Plant Sci. 2019, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Jetter, R.; Riederer, M. Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: Water transport resistances are associated with fatty acyl rather than alicyclic components. Plant Physiol. 2016, 170, 921–934. [Google Scholar] [CrossRef] [Green Version]
- Romero, P.; Rose, J.K.C. A relationship between tomato fruit softening, cuticle properties and water availability. Food Chem. 2019, 295, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Brizzolara, S.; Minnocci, A.; Yembaturova, E.; Tonutti, P. Ultrastructural analysis of berry skin from four grapes varieties at harvest and in relation to postharvest dehydration. OENO One 2020, 54, 1121–1131. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, H.; Lv, Y.; Chen, G.; Jiang, Y. Comparative analysis of total wax content, chemical composition and crystal morphology of cuticular wax in Korla pear under different relative humidity of storage. Food Chem. 2021, 339, 128097. [Google Scholar] [CrossRef]
- Tadeo, F.R.; Cercós, M.; Colmenero-Flores, J.M.; Iglesias, D.J.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; et al. Molecular physiology of development and quality of citrus. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2008; Volume 47, pp. 147–223. ISBN 9780123743275. [Google Scholar]
- Zhang, Y.-J.; Wang, X.-J.; Wu, J.-X.; Chen, S.-Y.; Chen, H.; Chai, L.-J.; Yi, H.-L. Comparative transcriptome analyses between a spontaneous late-ripening sweet orange mutant and its wild type suggest the functions of ABA, sucrose and JA during citrus fruit ripening. PLoS ONE 2014, 9, e116056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Terol, J.; Nueda, M.J.; Ventimilla, D.; Tadeo, F.; Talon, M. Transcriptomic analysis of Citrus clementina mandarin fruits maturation reveals a MADS-box transcription factor that might be involved in the regulation of earliness. BMC Plant Biol. 2019, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Lafuente, M.T.; Rodrigo, M.J. A sweet orange mutant impaired in carotenoid biosynthesis and reduced ABA levels results in altered molecular responses along peel ripening. Sci. Rep. 2019, 9, 9813. [Google Scholar] [CrossRef] [Green Version]
- Lafuente, M.T.; Martínez-Téllez, M.A.; Zacarías, L. Abscisic acid in the response of ‘Fortune’ mandarins to chilling. Effect of maturity and high-temperature conditioning. J. Sci. Food Agric. 1997, 73, 494–502. [Google Scholar] [CrossRef]
- Romero, P.; Lafuente, M.; Rodrigo, M.J. The Citrus ABA signalosome: Identification and transcriptional regulation during sweet orange fruit ripening and leaf dehydration. J. Exp. Bot. 2012, 63, 4931–4945. [Google Scholar] [CrossRef] [Green Version]
- Romero, P.; Rodrigo, M.J.; Alférez, F.; Ballester, A.-R.; González-Candelas, L.; Zacarías, L.; Lafuente, M.T. Unravelling molecular responses to moderate dehydration in harvested fruit of sweet orange (Citrus sinensis L. Osbeck) using a fruit-specific ABA-deficient mutant. J. Exp. Bot. 2012, 63, 2753–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, P.; Lafuente, M.T.; Alférez, F. A transcriptional approach to unravel the connection between phospholipases A2 and D and ABA signal in citrus under water stress. Plant Physiol. Biochem. 2014, 80, 23–32. [Google Scholar] [CrossRef]
- Romero, P.; Gandía, M.; Alférez, F. Interplay between ABA and phospholipases A2 and D in the response of citrus fruit to postharvest dehydration. Plant Physiol. Biochem. 2013, 70, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, M.J.; Alquezar, B.; Zacarías, L. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J. Exp. Bot. 2006, 57, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agustí, J.; Zapater, M.; Iglesias, D.J.; Cercós, M.; Tadeo, F.R.; Talón, M. Differential expression of putative 9-cis-epoxycarotenoid dioxygenases and abscisic acid accumulation in water stressed vegetative and reproductive tissues of citrus. Plant Sci. 2007, 172, 85–94. [Google Scholar] [CrossRef]
- Xian, L.; Sun, P.; Hu, S.; Wu, J.; Liu, J.-H. Molecular cloning and characterization of CrNCED1, a gene encoding 9-cis-epoxycarotenoid dioxygenase in Citrus reshni, with functions in tolerance to multiple abiotic stresses. Planta 2014, 239, 61–77. [Google Scholar] [CrossRef]
- Romero, P.; Rodrigo, M.J.; Lafuente, M.T. Differential expression of the Citrus sinensis ABA perception system genes during postharvest fruit dehydration. Postharvest Biol. Technol. 2013, 76, 65–73. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Marcos, J.F.; Alférez, F.; Mallent, M.D.; Zacarías, L. Characterization of Pinalate, a novel Citrus sinensis mutant with a fruit-specific alteration that results in yellow pigmentation and decreased ABA content. J. Exp. Bot. 2003, 54, 727–738. [Google Scholar] [CrossRef] [Green Version]
- Alférez, F.; Sala, J.M.; Sanchez-Ballesta, M.T.; Mulas, M.; Lafuente, M.T.; Zacarias, L. A comparative study of the postharvest performance of an ABA-deficient mutant of oranges: I. Physiological and quality aspects. Postharvest Biol. Technol. 2005, 37, 222–231. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Lado, J.; Alós, E.; Alquézar, B.; Dery, O.; Hirschberg, J.; Zacarías, L. A mutant allele of ζ-carotene isomerase (Z-ISO) is associated with the yellow pigmentation of the “Pinalate” sweet orange mutant and reveals new insights into its role in fruit carotenogenesis. BMC Plant Biol. 2019, 19, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, K.; Hartmann, K.D.; Schreiber, L.; Barthlott, W.; Neinhuis, C. Influences of air humidity during the cultivation of plants on wax chemical composition, morphology and leaf surface wettability. Environ. Exp. Bot. 2006, 56, 1–9. [Google Scholar] [CrossRef]
- Lewandowska, M.; Keyl, A.; Feussner, I. Wax biosynthesis in response to danger: Its regulation upon abiotic and biotic stress. New Phytol. 2020, 227, 698–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaheenuzzamn, M.; Shi, S.; Sohail, K.; Wu, H.; Liu, T.; An, P.; Wang, Z.; Hasanuzzaman, M. Regulation of cuticular wax biosynthesis in plants under abiotic stress. Plant Biotechnol. Rep. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Zarrouk, O.; Pinheiro, C.; Misra, C.S.; Fernández, V.; Chaves, M.M. Fleshy fruit epidermis is a protective barrier under water stress. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; Academic Press: Cambridge, MA, USA, 2018; pp. 507–533. ISBN 9780128131640. [Google Scholar]
- Wang, J.; Hao, H.; Liu, R.; Ma, Q.; Xu, J.; Chen, F.; Cheng, Y.; Deng, X. Comparative analysis of surface wax in mature fruits between Satsuma mandarin (Citrus unshiu) and ‘Newhall’ navel orange (Citrus sinensis) from the perspective of crystal morphology, chemical composition and key gene expression. Food Chem. 2014, 153, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, T.; Kosma, D.K.; Matas, A.J.; Buda, G.J.; He, Y.; Yu, B.; Pravitasari, A.; Batteas, J.D.; Stark, R.E.; Jenks, M.A.; et al. Cutin deficiency in the tomato fruit cuticle consistently affects resistance to microbial infection and biomechanical properties, but not transpirational water loss. Plant J. 2009, 60, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Leide, J.; Hildebrandt, U.; Reussing, K.; Riederer, M.; Vogg, G. The developmental pattern of tomato fruit wax accumulation and its impact on cuticular transpiration barrier properties: Effects of a deficiency in a beta-ketoacyl-coenzyme A synthase (LeCER6). Plant Physiol. 2007, 144, 1667–1679. [Google Scholar] [CrossRef] [Green Version]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of the barrier properties of plant cuticles. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Vogg, G.; Fischer, S.; Leide, J.; Emmanuel, E.; Jetter, R.; Levy, A.A.; Riederer, M. Tomato fruit cuticular waxes and their effects on transpiration barrier properties: Functional characterization of a mutant deficient in a very-long-chain fatty acid β-ketoacyl-CoA synthase. J. Exp. Bot. 2004, 55, 1401–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosma, D.K.; Jenks, M.A. Eco-physiological and molecular-genetic determinants of plant cuticle function in drought and salt stress tolerance. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 91–120. [Google Scholar]
- Lafuente, M.T.; Alférez, F.; Romero, P. Postharvest ethylene conditioning as a tool to reduce quality loss of stored mature sweet oranges. Postharvest Biol. Technol. 2014, 94, 104–111. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, P.; Lafuente, M.T. The Combination of Abscisic Acid (ABA) and Water Stress Regulates the Epicuticular Wax Metabolism and Cuticle Properties of Detached Citrus Fruit. Int. J. Mol. Sci. 2021, 22, 10242. https://doi.org/10.3390/ijms221910242
Romero P, Lafuente MT. The Combination of Abscisic Acid (ABA) and Water Stress Regulates the Epicuticular Wax Metabolism and Cuticle Properties of Detached Citrus Fruit. International Journal of Molecular Sciences. 2021; 22(19):10242. https://doi.org/10.3390/ijms221910242
Chicago/Turabian StyleRomero, Paco, and María Teresa Lafuente. 2021. "The Combination of Abscisic Acid (ABA) and Water Stress Regulates the Epicuticular Wax Metabolism and Cuticle Properties of Detached Citrus Fruit" International Journal of Molecular Sciences 22, no. 19: 10242. https://doi.org/10.3390/ijms221910242
APA StyleRomero, P., & Lafuente, M. T. (2021). The Combination of Abscisic Acid (ABA) and Water Stress Regulates the Epicuticular Wax Metabolism and Cuticle Properties of Detached Citrus Fruit. International Journal of Molecular Sciences, 22(19), 10242. https://doi.org/10.3390/ijms221910242





