The Preeclamptic Environment Promotes the Activation of Transcription Factor Kappa B by P53/RSK1 Complex in a HTR8/SVneo Trophoblastic Cell Line
Abstract
1. Introduction
2. Results
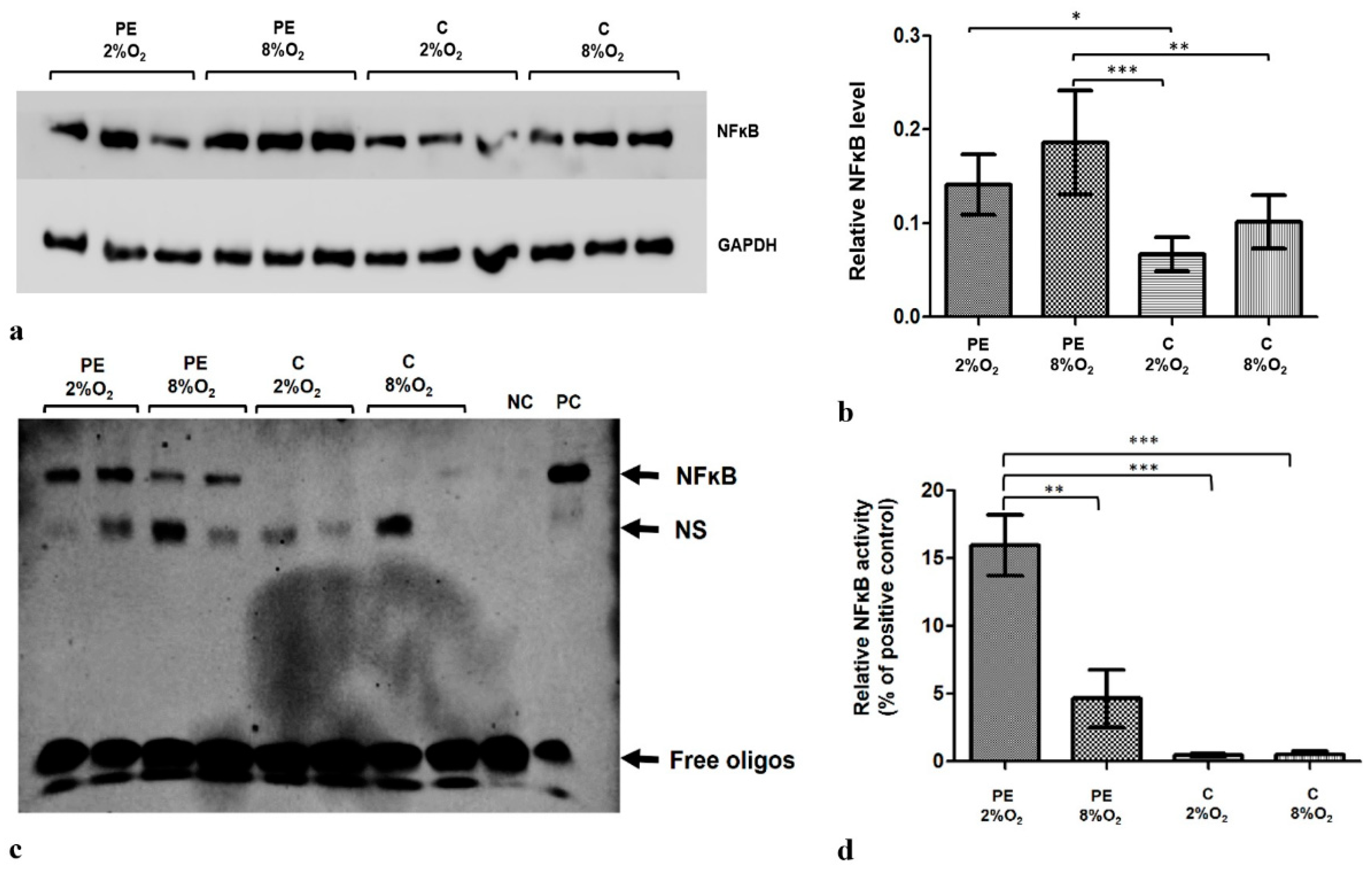
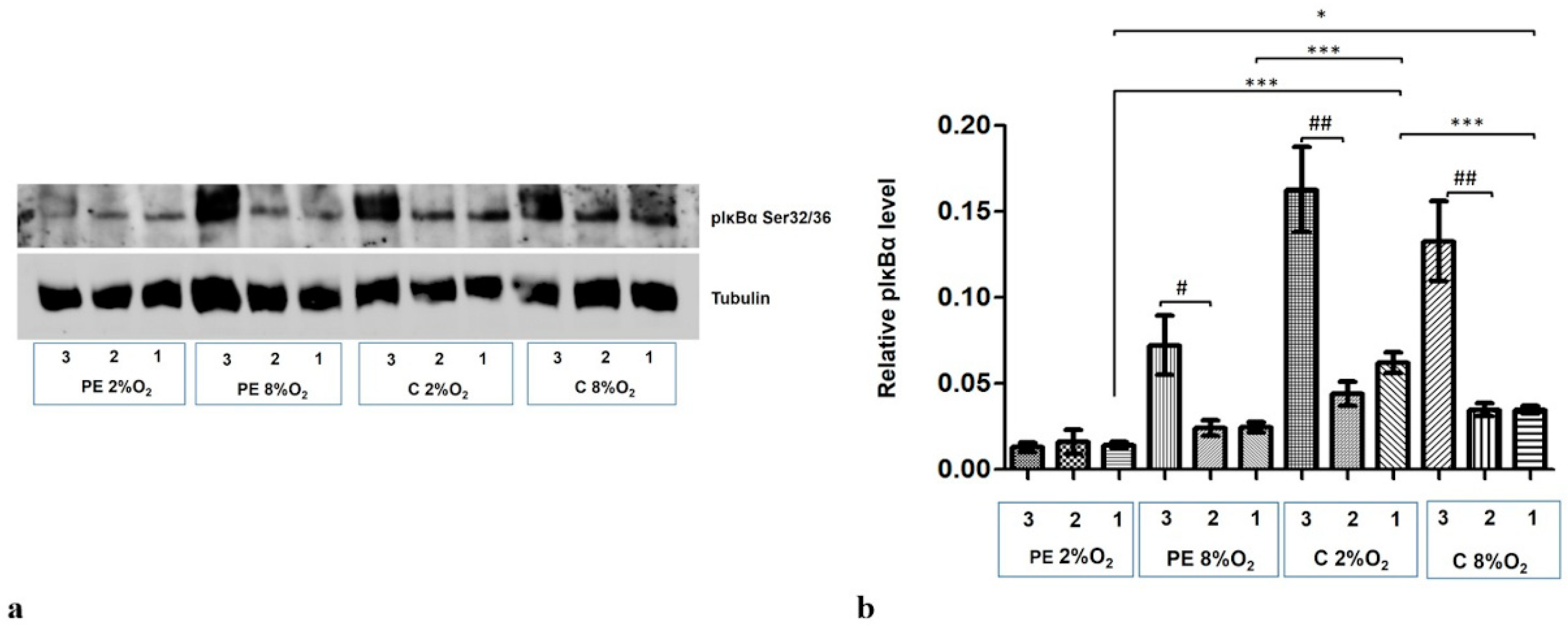
2.1. Both the Maternal Serum Type and Oxygen Tension Influence the Level and Activity of Kappa B in the HTR8/SVneo Cells
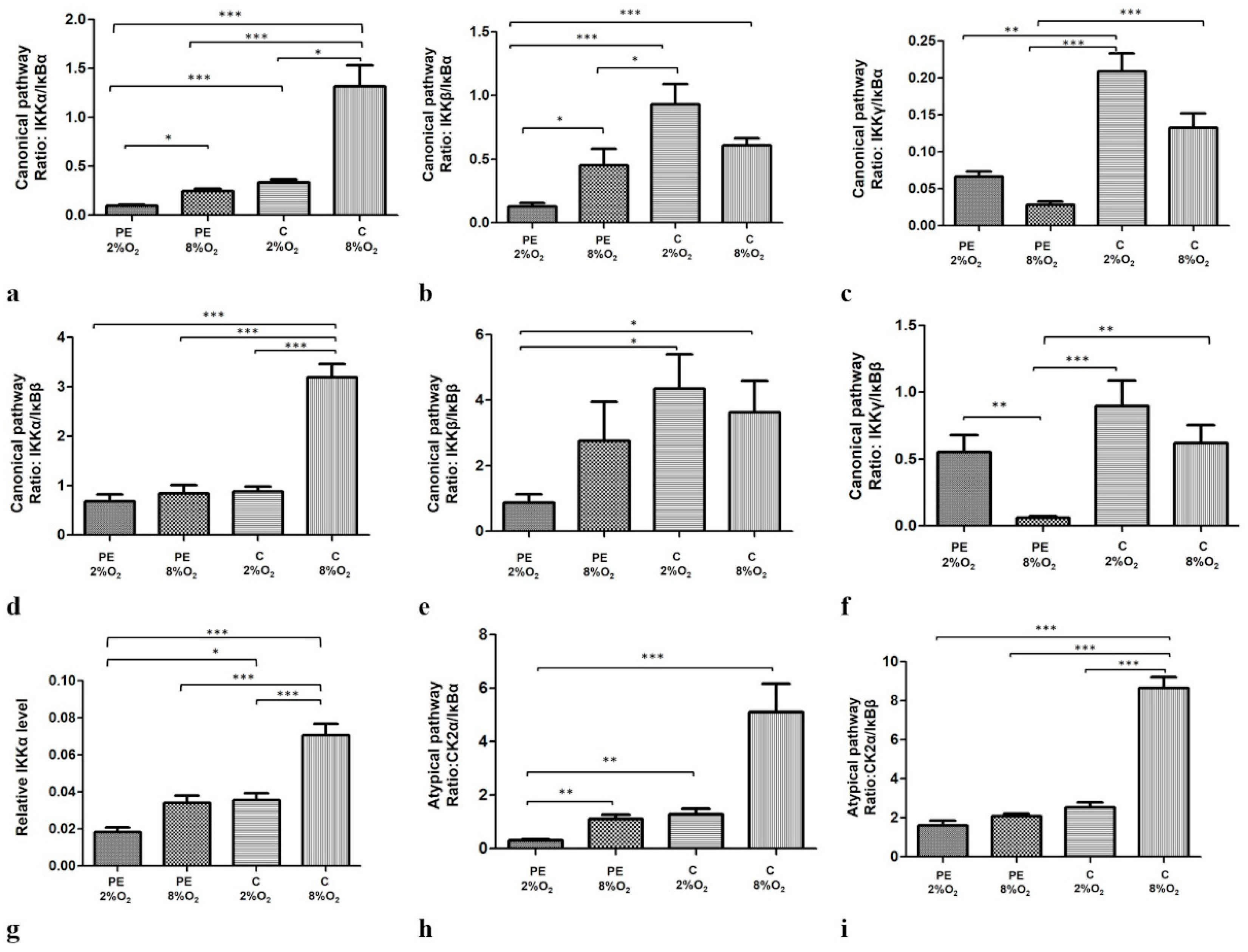
2.2. The Canonical, Non-Canonical and Atypical NFκB Pathways Are Influenced by the Oxygen Tension and Maternal Serum
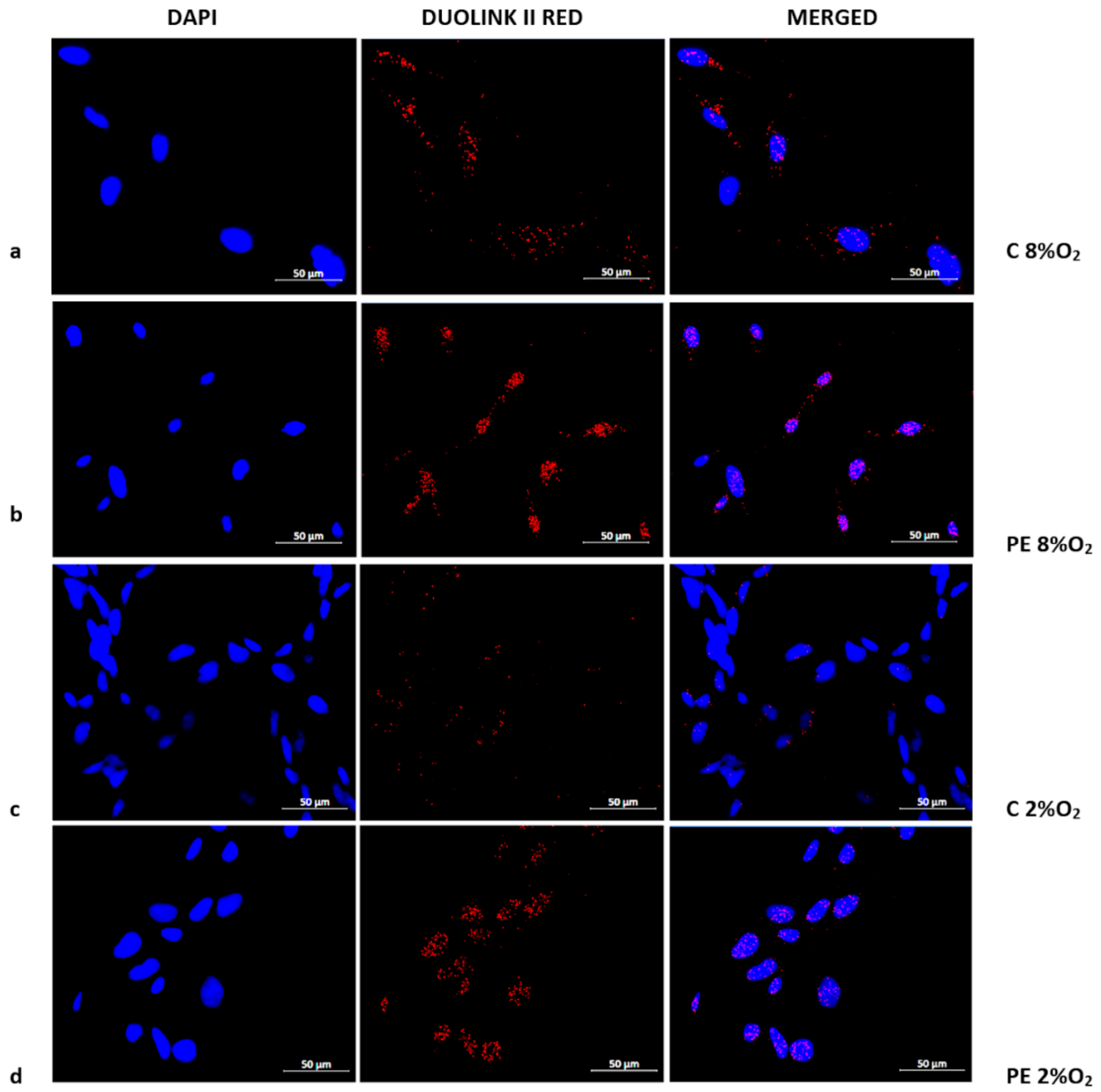
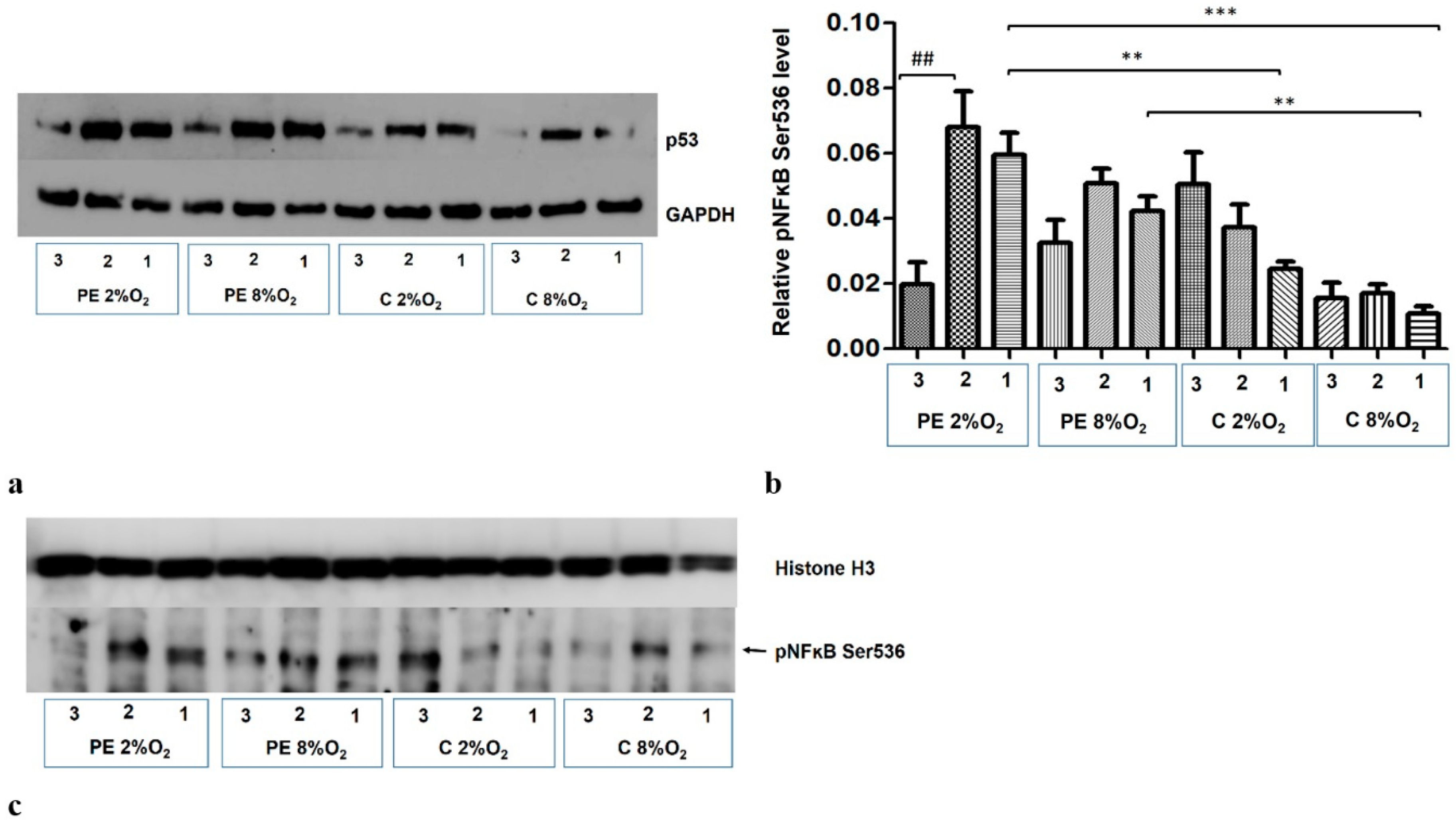
2.3. The Distribution of p53/RSK1 Complex in HTR8/SVneo Cells Depends on Oxygen Tension; the Serum of Preeclamptic Women Favours the Maintenance of p53/RSK1complex Formation in Hypoxia
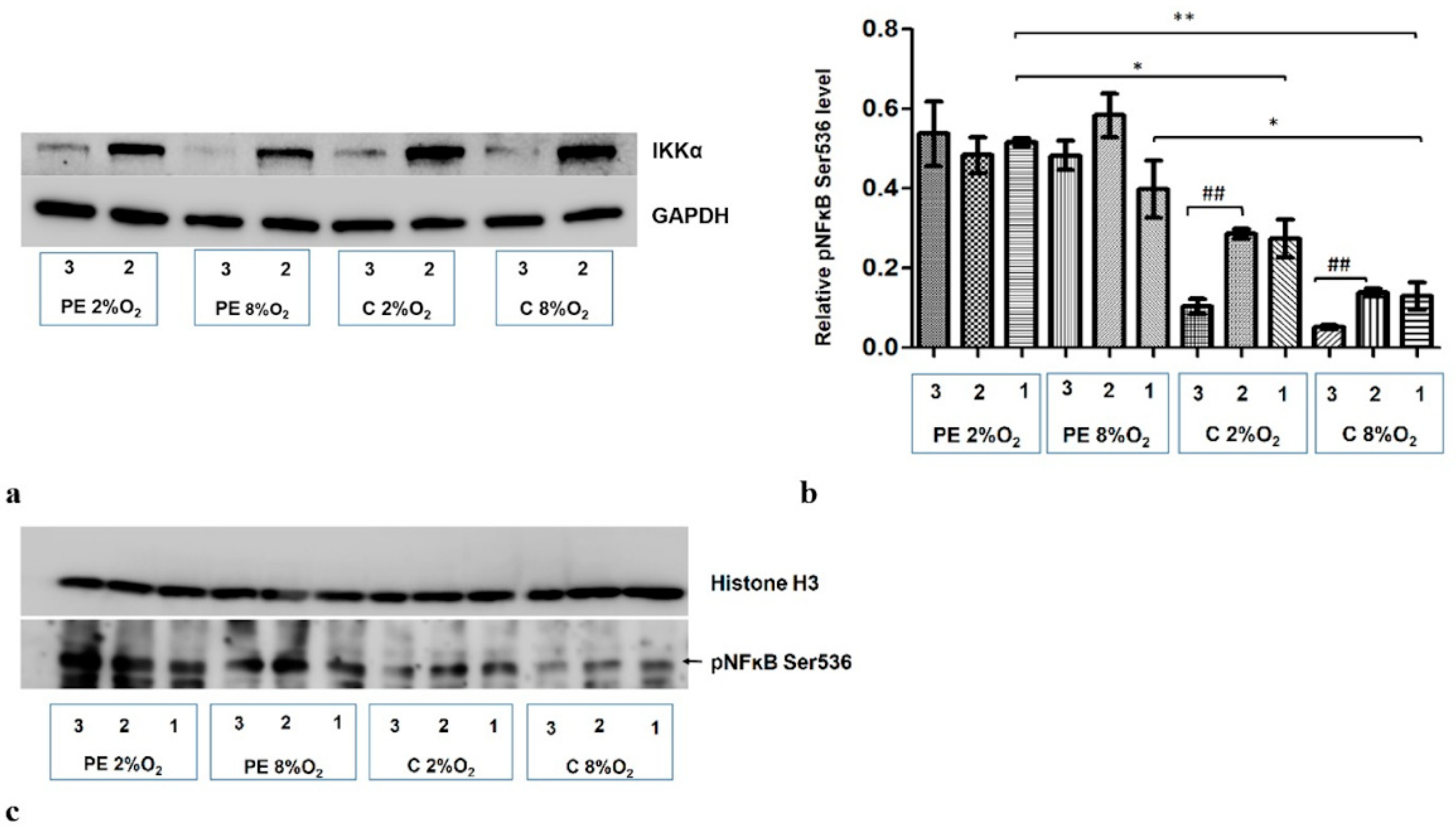
2.4. The Depletion of IKKα by Targeted siRNA Downregulates the Phosphorylated Fraction of NFĸB at Serine 536 (pNFκB) in the Nucleus of Cells Stimulated by Serum from Normotensive Women
2.5. Treatment with MG-132, an Inhibitor of Proteasome Activity, Does Not Influence the Accumulation of Phosphorylated IĸBα Protein in the Cytoplasmic Fraction of Cells Incubated in Hypoxia with Serum from Preeclamptic Mothers
2.6. The p53 Protein Is Implicated in NFκB Activation in HTR8/SVneo Cells Incubated in Hypoxic Conditions with Medium Containing 1% of Serum from Preeclamptic Mothers
3. Discussion
4. Materials and Methods
4.1. Collection of Blood Samples
4.2. Cell Culture
4.3. Total Protein Extraction
4.4. Cytoplasm and Nuclear Fraction Isolation
4.5. Small Interfering RNA (siRNA) and MG-132 Transfection
4.6. Western Blot Assays
4.7. Electrophoretic Mobility Shift Assay (EMSA)
4.8. Duolink II PLA Technology Assay
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.-Y.; Pang, Z.-J.; Yu, Y.-H. Regulation of trophoblast invasion: The role of matrix metalloproteinases. Rev. Obstet. Gynecol. 2012, 5, e137–e143. [Google Scholar] [CrossRef]
- Hernandez, I.; Fournier, T.; Chissey, A.; Therond, P.; Slama, A.; Beaudeux, J.L.; Zerrad-Saadi, A. NADPH oxidase is the major source of placental superoxide in early pregnancy: Association with MAPK pathway activation. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Tuuli, M.G.; Longtine, M.S.; Nelson, D.M. Review: Oxygen and trophoblast biology—A source of controversy. Placenta 2011, 32, S109–S118. [Google Scholar] [CrossRef] [PubMed]
- Soleymanlou, N.; Jurisica, I.; Nevo, O.; Ietta, F.; Zhang, X.; Zamudio, S.; Post, M.; Caniggia, I. Molecular evidence of placental hypoxia in preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4299–4308. [Google Scholar] [CrossRef] [PubMed]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Xu, P.; Yin, L.; Si, Y.; Zhang, C.; Meng, Y.; Feng, W.; Pan, Z.; Gao, Z.; et al. Elevated microRNA-125b inhibits cytotrophoblast invasion and impairs endothelial cell function in preeclampsia. Cell Death Discov. 2020, 6. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, 237–260. [CrossRef]
- He, C.; Shan, N.; Xu, P.; Ge, H.; Yuan, Y.; Liu, Y.; Zhang, P.; Wen, L.; Zhang, F.; Xiong, L.; et al. Hypoxia-induced Downregulation of SRC-3 Suppresses Trophoblastic Invasion and Migration Through Inhibition of the AKT/mTOR Pathway: Implications for the Pathogenesis of Preeclampsia. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Redman, C.W.G.; Sargent, I.L. Placental Debris, Oxidative Stress and Pre-eclampsia. Placenta 2000, 21, 597–602. [Google Scholar] [CrossRef]
- Raijmakers, M.T.M.; Dechend, R.; Poston, L. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension 2004, 44, 374–380. [Google Scholar] [CrossRef]
- Kim, Y.W.; West, X.Z.; Byzova, T.V. Inflammation and oxidative stress in angiogenesis and vascular disease. J. Mol. Med. 2013, 91, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Hempstock, J.; Greenwold, N.; Burton, G.J. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am. J. Pathol. 2003, 162, 115–125. [Google Scholar] [CrossRef]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Christian, F.; Smith, E.; Carmody, R. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Glushkova, O.V.; Parfenyuk, S.B.; Novoselova, T.V.; Khrenov, M.O.; Lunin, S.M.; Novoselova, E.G. The Role of p38 and CK2 Protein Kinases in the Response of RAW 264.7 Macrophages to Lipopolysaccharide. Biochemistry (Moscow) 2018, 83, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Gang, X.; Wang, Y.; Wang, Y.; Zhao, Y.; Ding, L.; Zhao, J.; Sun, L.; Wang, G. Suppression of casein kinase 2 sensitizes tumor cells to antitumor TRAIL therapy by regulating the phosphorylation and localization of p65 in prostate cancer. Oncol. Rep. 2015, 34, 1599–1604. [Google Scholar] [CrossRef][Green Version]
- Sakowicz, A.; Bralewska, M.; Pietrucha, T.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W.; Gach, A.; Rybak-Krzyszkowska, M.; Witas, P.J.; Huras, H.; Grzesiak, M.; et al. Canonical, non-canonical and atypical pathways of nuclear factor кB activation in preeclampsia. Int. J. Mol. Sci. 2020, 21, 5574. [Google Scholar] [CrossRef]
- Bohuslav, J.; Chen, L.F.; Kwon, H.; Mu, Y.; Greene, W.C. p53 induces NF-κB activation by an IκB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J. Biol. Chem. 2004, 279, 26115–26125. [Google Scholar] [CrossRef]
- Treissman, J.; Yuan, V.; Baltayeva, J.; Le, H.T.; Castellana, B.; Robinson, W.P.; Beristain, A.G. Low oxygen enhances trophoblast column growth by potentiating differentiation of the extravillous lineage and promoting LOX activity. Development 2020, 147. [Google Scholar] [CrossRef]
- Fitzgerald, J.S.; Poehlmann, T.G.; Schleussner, E.; Markert, U.R. Trophoblast invasion: The role of intracellular cytokine signalling via signal transducer and activator of transcription 3 (STAT3). Hum. Reprod. Update 2008, 14, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Naruse, K.; Innes, B.A.; Bulmer, J.N.; Robson, S.C.; Searle, R.F.; Lash, G.E. Secretion of cytokines by villous cytotrophoblast and extravillous trophoblast in the first trimester of human pregnancy. J. Reprod. Immunol. 2010, 86, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Knofler, M. Human tumour necrosis factor: Physiological and pathological roles in placenta and endometrium. Placenta 2009, 30, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Armistead, B.; Kadam, L.; Drewlo, S.; Kohan-Ghadr, H.R. The role of NFκB in healthy and preeclamptic placenta: Trophoblasts in the spotlight. Int. J. Mol. Sci. 2020, 21, 1775. [Google Scholar] [CrossRef] [PubMed]
- Kunsch, C.; Rosen, C.A. NF-κB Subunit-Specific Regulation of the Interleukin-8 Promoter. Mol. Cell. Biol. 1993, 13, 6137–6146. [Google Scholar] [PubMed]
- Libermann, T.A.; Baltimore, D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef]
- Furmento, V.A.; Marino, J.; Blank, V.C.; Roguin, L.P. The granulocyte colony-stimulating factor (G-CSF) upregulates metalloproteinase-2 and VEGF through PI3K/Akt and Erk1/2 activation in human trophoblast Swan 71 cells. Placenta 2014, 35, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J.; Marois, J.; Garoufalis, J.; D’Addario, M.; Roulston, A.; Kwan, I.; Pepin, N.; Lacoste, J.; Nguyen, H.; Bensi, G. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: Evidence for a positive autoregulatory loop. Mol. Cell. Biol. 1993, 13, 6231–6240. [Google Scholar] [CrossRef]
- Liu, H.; Sidiropoulos, P.; Song, G.; Pagliari, L.J.; Birrer, M.J.; Stein, B.; Anrather, J.; Pope, R.M. TNF-α Gene Expression in Macrophages: Regulation by NF-κB Is Independent of c-Jun or C/EBPβ. J. Immunol. 2000, 164, 4277–4285. [Google Scholar] [CrossRef]
- Collart, M.A.; Baeuerle, P.; Vassalli, P. Regulation of tumor necrosis factor alpha transcription in macrophages: Involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol. Cell. Biol. 1990, 10, 1498–1506. [Google Scholar] [CrossRef]
- Nakamura, H.; Kimura, T.; Ogita, K.; Koyama, S.; Tsujie, T.; Tsutsui, T.; Shimoya, K.; Koyama, M.; Kaneda, Y.; Murata, Y. Alteration of the timing of implantation by in vivo gene transfer: Delay of implantation by suppression of nuclear factor κB activity and partial rescue by leukemia inhibitory factor. Biochem. Biophys. Res. Commun. 2004, 321, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Ghosh, S.; Baltimore, D. Embryonic lethality and liver degeneration in mice lacking RelA component of NF-κB. Nature 1995, 376, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Gauster, M.; Orendi, K.; König, J.; Moser, G. Oxygen as modulator of trophoblast invasion. J. Anat. 2009, 215, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Zhang, L. Hypoxia and mitochondrial dysfunction in pregnancy complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz, A. The role of NFκB in the three stages of pregnancy—Implantation, maintenance, and labour: A review article. BJOG Int. J. Obstet. Gynaecol. 2018. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Aban, M.; Cinel, L.; Arslan, M.; Dilek, U.; Kaplanoglu, M.; Arpaci, R.; Dilek, S. Expression of nuclear factor-kappa B and placental apoptosis in pregnancies complicated with intrauterine growth restriction and preeclampsia: An immunohistochemical study. Tohoku J. Exp. Med. 2004, 204, 195–202. [Google Scholar] [CrossRef]
- Vaughan, J.E.; Walsh, S.W. Activation of NF-κB in Placentas of Women with Preeclampsia. Hypertens Pregnancy 2012, 31, 243–251. [Google Scholar] [CrossRef]
- Litang, Z.; Hong, W.; Weimin, Z.; Xiaohui, T.; Qian, S. Serum NF-κBp65, TLR4 as biomarker for diagnosis of preeclampsia. Open Med. 2017, 12, 399–402. [Google Scholar] [CrossRef]
- Herrington, F.D.; Carmody, R.J.; Goodyear, C.S. Modulation of NF-κB Signaling as a Therapeutic Target in Autoimmunity. J. Biomol. Screen. 2016, 21, 223–242. [Google Scholar] [CrossRef]
- Adli, M.; Merkhofer, E.; Cogswell, P.; Baldwin, A.S. IKKα and IKKβ each function to regulate NF-κB activation in the TNF-induced/canonical pathway. PLoS ONE 2010, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nottingham, L.K.; Yan, C.H.; Yang, X.; Si, H.; Coupar, J.; Bian, Y.; Cheng, T.F.; Allen, C.; Arun, P.; Gius, D.; et al. Aberrant IKKα and IKKβ cooperatively activate NF-κB and induce EGFR/AP1 signaling to promote survival and migration of head and neck cancer. Oncogene 2014, 33, 1135–1147. [Google Scholar] [CrossRef]
- Sakurai, H.; Chiba, H.; Miyoshi, H.; Sugita, T.; Toriumi, W. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 1999, 274, 30353–30356. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Asano, T.; Nakayama, K.; Kato, T.; Karin, M.; Kamata, H. Nuclear IKKβ Is an Adaptor Protein for IκBα Ubiquitination and Degradation in UV-Induced NF-κB Activation. Mol. Cell 2010, 39, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Qi, W.; Mu, J.; Wei, Y.; Yang, L.L.; Zhang, Q.; Wu, Q.; Tang, J.Y.; Feng, B. MG132 protects against renal dysfunction by regulating Akt-mediated inflammation in diabetic nephropathy. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Taylor, J.A.; Bren, G.D.; Pennington, K.N.; Trushin, S.A.; Asin, S.; Paya, C.V. Serine 32 and serine 36 of IκBα are directly phosphorylated by protein kinase CKII in vitro. J. Mol. Biol. 1999, 290, 839–850. [Google Scholar] [CrossRef]
- Scherer, D.C.; Brockman, J.A.; Chent, Z.; Maniatist, T.O.M.; Ballard, D.W. Signal-induced degradation of IicBa requires site- specific ubiquitination. Proc. Natl. Acad. Sci. USA 1995, 92, 11259–11263. [Google Scholar] [CrossRef]
- Canty, T.G.; Boyle, E.M.; Farr, A.; Morgan, E.N.; Verrier, E.D.; Pohlman, T.H. Oxidative stress induces NF-κB nuclear translocation without degradation of IκBα. Circulation 1999, 100, 361–365. [Google Scholar] [CrossRef]
- Ryan, K.M.; Ernst, M.K.; Rice, N.R.; Vousden, K.H. Role of NF-kB in p53-mediated programmed cell death. Nature 2000, 404, 892–897. [Google Scholar] [CrossRef]
- Catarino, C.; Santos-Silva, A.; Belo, L.; Rocha-Pereira, P.; Rocha, S.; Patrício, B.; Quintanilha, A.; Rebelo, I. Inflammatory disturbances in preeclampsia: Relationship between maternal and umbilical cord blood. J. Pregnancy 2012, 2012. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Vázquez, C.M.; Mate, A.; Sobrevia, L.; Marín, R. Oxidative stress: Normal pregnancy versus preeclampsia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, D.C. Preeclampsia: From inflammation to immunoregulation. Clin. Med. Insights Blood Disord. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Aleyasin, H.; Cregan, S.P.; Iyirhiaro, G.; O’Hare, M.J.; Callaghan, S.M.; Slack, R.S.; Park, D.S. Nuclear Factor-κB Modulates the p53 Response in Neurons Exposed to DNA Damage. J. Neurosci. 2004, 24, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Bujold, E.; Roberge, S.; Lacasse, Y.; Bureau, M.; Audibert, F.; Marcoux, S.; Forest, J.-C.; Giguère, Y. Prevention of Preeclampsia and Intrauterine Growth Restriction With Aspirin Started in Early Pregnancy: A Meta-Analysis. Obstet. Gynecol. 2010, 116, 402–414. [Google Scholar] [CrossRef]
- Wang, Y.P.; Wu, Y.; Li, L.Y.; Zheng, J.; Liu, R.G.; Zhou, J.P.; Yuan, S.Y.; Shang, Y.; Yao, S.L. Aspirin-triggered lipoxin A4attenuates LPS-induced pro-inflammatory responses by inhibiting activation of NF-κB and MAPKs in BV-2 microglial cells. J. Neuroinflammation 2011, 8, 95. [Google Scholar] [CrossRef]
- Romano, M.; Cianci, E.; Simiele, F.; Recchiuti, A. Lipoxins and aspirin-triggered lipoxins in resolution of inflammation. Eur. J. Pharmacol. 2015, 760, 49–63. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakowicz, A.; Bralewska, M.; Pietrucha, T.; Figueras, F.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W.; Gach, A.; Sakowicz, B.; Rybak-Krzyszkowska, M.; Huras, H.; et al. The Preeclamptic Environment Promotes the Activation of Transcription Factor Kappa B by P53/RSK1 Complex in a HTR8/SVneo Trophoblastic Cell Line. Int. J. Mol. Sci. 2021, 22, 10200. https://doi.org/10.3390/ijms221910200
Sakowicz A, Bralewska M, Pietrucha T, Figueras F, Habrowska-Górczyńska DE, Piastowska-Ciesielska AW, Gach A, Sakowicz B, Rybak-Krzyszkowska M, Huras H, et al. The Preeclamptic Environment Promotes the Activation of Transcription Factor Kappa B by P53/RSK1 Complex in a HTR8/SVneo Trophoblastic Cell Line. International Journal of Molecular Sciences. 2021; 22(19):10200. https://doi.org/10.3390/ijms221910200
Chicago/Turabian StyleSakowicz, Agata, Michalina Bralewska, Tadeusz Pietrucha, Francesc Figueras, Dominika E. Habrowska-Górczyńska, Agnieszka W. Piastowska-Ciesielska, Agnieszka Gach, Bartosz Sakowicz, Magda Rybak-Krzyszkowska, Hubert Huras, and et al. 2021. "The Preeclamptic Environment Promotes the Activation of Transcription Factor Kappa B by P53/RSK1 Complex in a HTR8/SVneo Trophoblastic Cell Line" International Journal of Molecular Sciences 22, no. 19: 10200. https://doi.org/10.3390/ijms221910200
APA StyleSakowicz, A., Bralewska, M., Pietrucha, T., Figueras, F., Habrowska-Górczyńska, D. E., Piastowska-Ciesielska, A. W., Gach, A., Sakowicz, B., Rybak-Krzyszkowska, M., Huras, H., Grzesiak, M., & Biesiada, L. (2021). The Preeclamptic Environment Promotes the Activation of Transcription Factor Kappa B by P53/RSK1 Complex in a HTR8/SVneo Trophoblastic Cell Line. International Journal of Molecular Sciences, 22(19), 10200. https://doi.org/10.3390/ijms221910200




