Neuroprotective Effects of Cannabidiol but Not Δ9-Tetrahydrocannabinol in Rat Hippocampal Slices Exposed to Oxygen-Glucose Deprivation: Studies with Cannabis Extracts and Selected Cannabinoids
Abstract
:1. Introduction
2. Results
2.1. Effects of Cannabis Extracts and Selected Cannabinoids against OGD Neurotoxicity in Organotypic Hippocampal Slices
2.2. CBD Rescues Neuronal Damage in OGD-Treated Slices
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Materials
4.3. Preparation of Extracts from Bedrocan and FM2
4.4. Oxygen and Glucose Deprivation (OGD) in Rat Organotypic Hippocampal Slices
4.5. Immunohistochemistry
4.6. Microscopy Techniques, Qualitative and Quantitative Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, S.E.; Williams, C.M.; Iversen, L.; Whalley, B.J. Molecular Pharmacology of Phytocannabinoids. Prog. Chem. Org. Nat. Prod. 2017, 103, 61–101. [Google Scholar]
- Mechoulam, R.; Hanuš, L.O.; Pertwee, R.; Howlett, A.C. Early Phytocannabinoid Chemistry to Endocannabinoids and beyond. Nat. Rev. Neurosci. 2014, 15, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Gazzetta Ufficiale. Available online: https://www.gazzettaufficiale.it/eli/id/2015/11/30/15A08888/sg (accessed on 18 June 2021).
- Melas, P.A.; Scherma, M.; Fratta, W.; Cifani, C.; Fadda, P. Cannabidiol as a Potential Treatment for Anxiety and Mood Disorders: Molecular Targets and Epigenetic Insights from Preclinical Research. Int. J. Mol. Sci. 2021, 22, 1863. [Google Scholar] [CrossRef]
- Tomari, S.; Tanaka, T.; Ihara, M.; Matsuki, T.; Fukuma, K.; Matsubara, S.; Nagatsuka, K.; Toyoda, K. Risk Factors for Post-Stroke Seizure Recurrence after the First Episode. Seizure 2017, 52, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, J.T.; Cohan, C.H.; Dave, K.R.; Wright, C.B.; Perez-Pinzon, M.A. Global Cerebral Ischemia: Synaptic and Cognitive Dysfunction. Curr. Drug Targets 2013, 14, 20–35. [Google Scholar] [CrossRef]
- Pellegrini-Giampietro, D.E.; Mannaioni, G.; Bagetta, G. Post-Ischemic Brain Damage: The Endocannabinoid System in the Mechanisms of Neuronal Death. FEBS J. 2009, 276, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Nagayama, T.; Sinor, A.D.; Simon, R.P.; Chen, J.; Graham, S.H.; Jin, K.; Greenberg, D.A. Cannabinoids and Neuroprotection in Global and Focal Cerebral Ischemia and in Neuronal Cultures. J. Neurosci. 1999, 19, 2987–2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braida, D.; Pozzi, M.; Sala, M. CP 55,940 Protects against Ischemia-Induced Electroencephalographic Flattening and Hyperlocomotionin Mongolian Gerbils. Neurosci. Lett. 2000, 296, 69–72. [Google Scholar] [CrossRef]
- Landucci, E.; Boscia, F.; Gerace, E.; Scartabelli, T.; Cozzi, A.; Moroni, F.; Mannaioni, G.; Pellegrini-Giampietro, D.E. Chapter 23 Involvement of Endocannabinoid Signaling in the Neuroprotective Effects of Subtype 1 Metabotropic Glutamate Receptor Antagonists in Models of Cerebral Ischemia. Int. Rev. Neurobiol. 2009, 85, 337–350. [Google Scholar] [PubMed]
- Pegorini, S.; Zani, A.; Braida, D.; Guerini-Rocco, C.; Sala, M. Vanilloid VR 1 Receptor Is Involved in Rimonabant-Induced Neuroprotection. Br. J. Pharmacol. 2006, 147, 552–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Martin, B.R.; Adler, M.W.; Razdan, R.K.; Ganea, D.; Tuma, R.F. Modulation of the Balance between Cannabinoid CB1 and CB2 Receptor Activation during Cerebral Ischemic/Reperfusion Injury. Neuroscience 2008, 152, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landucci, E.; Scartabelli, T.; Gerace, E.; Moroni, F.; Pellegrini-Giampietro, D.E. CB1 Receptors and Post-Ischemic Brain Damage: Studies on the Toxic and Neuroprotective Effects of Cannabinoids in Rat Organotypic Hippocampal Slices. Neuropharmacology 2011, 60, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Muthian, S.; Rademacher, D.J.; Roelke, C.T.; Gross, G.J.; Hillard, C.J. Anandamide Content Is Increased and CB 1 Cannabinoid Receptor Blockade Is Protective during Transient, Focal Cerebral Ischemia. Neuroscience 2004, 129, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Schmid, P.C.; Schabitz, W.R.; Wolf, M.; Schwab, S.; Schmidt, H.H.O. Massive Accumulation of N-Acylethanolamines after Stroke. Cell Signalling in Acute Cerebral Ischemia? J. Neurochem. 2004, 88, 1159–1167. [Google Scholar] [CrossRef]
- England, T.J.; Hind, W.H.; Rasid, N.A.; O’Sullivan, S.E. Cannabinoids in Experimental Stroke: A Systematic Review and Meta-Analysis. J. Perinatol. 2015, 35, 348–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, F.J.; Lafuente, H.; Rey-Santano, M.C.; Mielgo, V.E.; Gastiasoro, E.; Rueda, M.; Pertwee, R.G.; Castillo, A.I.; Romero, J.; Martínez-Orgado, J. Neuroprotective Effects of the Nonpsychoactive Cannabinoid Cannabidiol in Hypoxic-Ischemic Newborn Piglets. Pediatr. Res. 2008, 64, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Hind, W.H.; England, T.J.; O’Sullivan, S.E. Cannabidiol Protects an in Vitro Model of the Blood-Brain Barrier from Oxygen-Glucose Deprivation via PPARγ and 5-HT1A Receptors. Br. J. Pharmacol. 2016, 173, 815–825. [Google Scholar] [CrossRef] [Green Version]
- Maas, A.I.R.; Murray, G.; Henney, H.; Kassem, N.; Legrand, V.; Mangelus, M.; Muizelaar, J.P.; Stocchetti, N.; Knoller, N. Efficacy and Safety of Dexanabinol in Severe Traumatic Brain Injury: Results of a Phase III Randomised, Placebo-Controlled, Clinical Trial. Lancet Neurol. 2006, 5, 38–45. [Google Scholar] [CrossRef]
- Smajlović, D. Strokes in Young Adults: Epidemiology and Prevention. Vasc. Health Risk Manag. 2015, 11, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Jouanjus, E.; Raymond, V.; Lapeyre-Mestre, M.; Wolff, V. What Is the Current Knowledge About the Cardiovascular Risk for Users of Cannabis-Based Products? A Systematic Review. Curr. Atheroscler. Rep. 2017, 19, 26. [Google Scholar] [CrossRef]
- Kalla, A.; Krishnamoorthy, P.M.; Gopalakrishnan, A.; Figueredo, V.M. Cannabis use predicts risks of heart failure and cerebrovascular accidents: Results from the National Inpatient Sample. J. Cardiovasc. Med. 2018, 19, 480–484. [Google Scholar] [CrossRef]
- Rumalla, K.; Reddy, A.Y.; Mittal, M.K. Recreational marijuana use and acute ischemic stroke: A population-based analysis of hospitalized patients in the United States. J. Neurol. Sci. 2016, 15, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Fusco, I.; Ugolini, F.; Lana, D.; Coppi, E.; Dettori, I.; Gaviano, L.; Nosi, D.; Cherchi, F.; Pedata, F.; Giovannini, M.G.; et al. The Selective Antagonism of Adenosine A2B Receptors Reduces the Synaptic Failure and Neuronal Death Induced by Oxygen and Glucose Deprivation in Rat CA1 Hippocampus in Vitro. Front. Pharmacol. 2018, 9, 2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerace, E.; Landucci, E.; Scartabelli, T.; Moroni, F.; Pellegrini-Giampietro, D.E. Rat Hippocampal Slice Culture Models for the Evaluation of Neuroprotective Agents. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 846, ISBN 9781-617-795-350. [Google Scholar]
- Landucci, E.; Filippi, L.; Gerace, E.; Catarzi, S.; Guerrini, R.; Pellegrini-Giampietro, D.E. Neuroprotective Effects of Topiramate and Memantine in Combination with Hypothermia in Hypoxic-Ischemic Brain Injury In Vitro and In Vivo. Neurosci. Lett. 2018, 668, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.J.; Clark, P.; Brown, M.B. Potency of Δ9-THC and Other Cannabinoids in Cannabis in England in 2005: Implications for Psychoactivity and Pharmacology. J. Forensic Sci. 2008, 53, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascini, F.; Aiello, C.; Di Tanna, G. Increasing Delta-9-Tetrahydrocannabinol (δ -9-THC) Content in Herbal Cannabis over Time: Systematic Review and Meta-Analysis. Curr. Drug Abuse Rev. 2012, 5, 32–40. [Google Scholar] [CrossRef]
- Wolff, V.; Jouanjus, E. Strokes Are Possible Complications of Cannabinoids Use. Epilepsy Behav. 2017, 70, 355–363. [Google Scholar] [CrossRef]
- Deidda, R.; Avohou, H.T.; Baronti, R.; Davolio, P.L.; Pasquini, B.; Del Bubba, M.; Hubert, C.; Hubert, P.; Orlandini, S.; Furlanetto, S. Analytical quality by design: Development and control strategy for a LC method to evaluate the cannabinoids content in cannabis olive oil extracts. J. Pharm. Biomed. Anal. 2019, 166, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Irie, K.; Sano, K.; Watanabe, T.; Higuchi, S.; Enoki, M.; Nakano, T.; Harada, K.; Ishikane, S.; Ikeda, T.; et al. Therapeutic Time Window of Cannabidiol Treatment on Delayed Ischemic Damage via High-Mobility Group Box1-Inhibiting Mechanism. Biol. Pharm. Bull. 2009, 32, 1538–1544. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Mishima, K.; Fujiwara, M. Therapeutic Potential of Non-Psychotropic Cannabidiol in Ischemic Stroke. Pharmaceuticals 2010, 3, 2197–2212. [Google Scholar] [CrossRef]
- Sultan, S.R.; Millar, S.A.; England, T.J.; O’Sullivan, S.E. A Systematic Review and Meta-Analysis of the Haemodynamic Effects of Cannabidiol. Front. Pharmacol. 2017, 8, 2882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishima, K.; Hayakawa, K.; Abe, K.; Ikeda, T.; Egashira, N.; Iwasaki, K.; Fujiwara, M. Cannabidiol Prevents Cerebral Infarction via a Serotonergic 5-Hydroxytryptamine1A Receptor-Dependent Mechanism. Stroke 2005, 36, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Echeverry, C.; Prunell, G.; Narbondo, C.; de Medina, V.S.; Nadal, X.; Reyes-Parada, M.; Scorza, C. A Comparative In Vitro Study of the Neuroprotective Effect Induced by Cannabidiol, Cannabigerol, and Their Respective Acid Forms: Relevance of the 5-HT1A Receptors. Neurotox. Res. 2021, 39, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, J.Y.; Seo, J.; Choi, I.S. Neuroprotective Effect of Cannabidiol Against Hydrogen Peroxide in Hippocampal Neuron Culture. Cannabis Cannabinoid Res. 2021, 12, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, K.J.; Bi, G.H.; He, Y.; Galaj, E.; Gardner, E.L.; Xi, Z.X. Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. Br. J. Pharmacol. 2019, 176, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.L.; Murphy, A.J.; England, T.J.; O’Sullivan, S.E. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br. J. Pharmacol. 2020, 177, 4330–4352. [Google Scholar] [CrossRef] [PubMed]
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212. [Google Scholar] [CrossRef]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol Induces Rapid-Acting Antidepressant-like Effects and Enhances Cortical 5-HT/Glutamate Neurotransmission: Role of 5-HT1A Receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Scarante, F.F.; Ribeiro, M.A.; Almeida-Santos, A.F.; Guimarães, F.S.; Campos, A.C. Glial Cells and Their Contribution to the Mechanisms of Action of Cannabidiol in Neuropsychiatric Disorders. Front. Pharmacol. 2021, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Sonego, A.B.; Gomes, F.V.; Del Bel, E.A.; Guimaraes, F.S. Cannabidiol Attenuates Haloperidol-Induced Catalepsy and c-Fos Protein Expression in the Dorsolateral Striatum via 5-HT1A Receptors in Mice. Behav. Brain Res. 2016, 309, 22–28. [Google Scholar] [CrossRef]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like Effects of Cannabidiol in Mice: Possible Involvement of 5-HT 1A Receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Resstel, L.B.M.; Tavares, R.F.; Lisboa, S.F.S.; Joca, S.R.L.; Corrêa, F.M.A.; Guimarães, F.S. 5-HT 1A Receptors Are Involved in the Cannabidiol-Induced Attenuation of Behavioural and Cardiovascular Responses to Acute Restraint Stress in Rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef]
- Rock, E.M.; Bolognini, D.; Limebeer, C.L.; Cascio, M.G.; Anavi-Goffer, S.; Fletcher, P.J.; Mechoulam, R.; Pertwee, R.G.; Parker, L.A. Cannabidiol, a Nonpsychotropic Component of Cannabis, Attenuates Vomiting and Nausea-like Behaviour via Indirect Agonism of 5-HT 1A Somatodendritic Autoreceptors in the Dorsal Raphe Nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.A.; Meyer, E.; da Silva, F.F.; Milani, H.; Guimarães, F.S.; Oliveira, R.M.W. Differential contribution of CB1, CB2, 5-HT1A, and PPAR-γ receptors to cannabidiol effects on ischemia-induced emotional and cognitive impairments. Eur. J. Neurosci. 2021, 53, 1738–1751. [Google Scholar] [CrossRef] [PubMed]
- Gibson, H.E.; Edwards, J.G.; Page, R.S.; Van Hook, M.J.; Kauer, J.A. TRPV1 Channels Mediate Long-Term Depression at Synapses on Hippocampal Interneurons. Neuron 2008, 57, 746–759. [Google Scholar] [CrossRef] [Green Version]
- Saffarzadeh, F.; Eslamizade, M.J.; Mousavi, S.M.; Abraki, S.B.; Hadjighassem, M.R.; Gorji, A. TRPV1 receptors augment basal synaptic transmission in CA1 and CA3 pyramidal neurons in epilepsy. Neuroscience. 2016, 9, 170–178. [Google Scholar] [CrossRef]
- Balleza-Tapia, H.; Crux, S.; Andrade-Talavera, Y.; Eslamizade, M.J.; Olivieri, A.; Santoni, M. TrpV1 receptor activation rescues neuronal function and network gamma oscillations from Aβ-induced impairment in mouse hippocampus in vitro. eLife 2018, 7, e37703. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Neeper, M.P.; Liu, Y.; Hutchinson, T.L.; Lubin, M.L.; Flores, C.M. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 2008, 28, 6231–6238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelli, M.B.; Offidani, M.; Alesiani, F.; Discepoli, G.; Liberati, S.; Olivieri, A.; Santoni, M.; Santoni, G.; Leoni, P.; Nabissi, M. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int. J. Cancer 2014, 1, 2534–2546. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Samanta, A.; Liu, Y.; Hughes, T.E.; Zhao, S.; Yudin, Y.; Rohacs, T.; Han, S.; Moiseenkova-Bell, V.Y. Molecular mechanism of TRPV2 channel modulation by cannabidiol. eLife 2019, 30, e48792. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic Plant Cannabinoids, Cannabidivarin (CBDV) and Cannabidiol (CBD), Activate and Desensitize Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in Vitro: Potential for the Treatment of Neuronal Hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dettori, I.; Gaviano, L.; Ugolini, F.; Lana, D.; Bulli, I.; Magni, G.; Rossi, F.; Giovannini, M.G.; Pedata, F. Protective Effect of Adenosine A2B Receptor Agonist, BAY60-6583, Against Transient Focal Brain Ischemia in Rat. Front. Pharmacol. 2021, 11, 588757. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, Z.; Li, F.; Sun, L. Morphological characteristics of eosinophilic neuronal death after transient unilateral forebrain ischemia in Mongolian gerbils. Neuropathology 2016, 36, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Fu, W.; Li, Y.; Witke, W.; Kwiatkowski, D.J.; Mattson, M.P. The Actin-Severing Protein Gelsolin Modulates Calcium Channel and NMDA Receptor Activities and Vulnerability to Excitotoxicity in Hippocampal Neurons. J. Neurosci. 1997, 17, 8178–8186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.L.; Strickland, S. Neuronal Death in the Hippocampus Is Promoted by Plasmin-Catalyzed Degradation of Laminin. Cell 1997, 91, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Melrose, J.; Hayes, A.J.; Bix, G. The CNS/PNS Extracellular Matrix Provides Instructive Guidance Cues to Neural Cells and Neuroregulatory Proteins in Neural Development and Repair. Int. J. Mol. Sci. 2021, 22, 5583. [Google Scholar] [CrossRef]
- Reinhard, J.; Renner, M.; Wiemann, S.; Shakoor, D.A.; Stute, G.; Dick, H.B.; Faissner, A.; Joachim, S.C. Ischemic Injury Leads to Extracellular Matrix Alterations in Retina and Optic Nerve. Sci. Rep. 2017, 7, 43470. [Google Scholar] [CrossRef]
- Khelif, Y.; Toutain, J.; Quittet, M.S.; Chantepie, S.; Laffray, X.; Valable, S.; Divoux, D.; Sineriz, F.; Pascolo-Rebouillat, E.; Papy-Garcia, D.; et al. A Heparan Sulfate-Based Matrix Therapy Reduces Brain Damage and Enhances Functional Recovery Following Stroke. Theranostics 2018, 8, 5814–5827. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; Van Antwerp, J.; Parcher, J.F.; Elsohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Estratto Oleoso di Infiorescenze Femminili di Cannabis. Available online: https://www.sifap.org/procedure/estrazione-oleosa-di-infiorescenze-femminili-di-cannabi (accessed on 18 June 2021).
- Gerace, E.; Landucci, E.; Scartabelli, T.; Moroni, F.; Chiarugi, A.; Pellegrini-Giampietro, D.E. Interplay between Histone Acetylation/Deacetylation and Poly(ADP-Ribosyl)Ation in the Development of Ischemic Tolerance in Vitro. Neuropharmacology 2015, 92, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, D.; Landucci, E.; Gerace, E.; Lana, D.; Ugolini, F.; Henley, J.M.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuroprotective Effects of MGluR5 Activation through the PI3K/Akt Pathway and the Molecular Switch of AMPA Receptors. Neuropharmacology 2020, 162, 107810. [Google Scholar] [CrossRef] [PubMed]
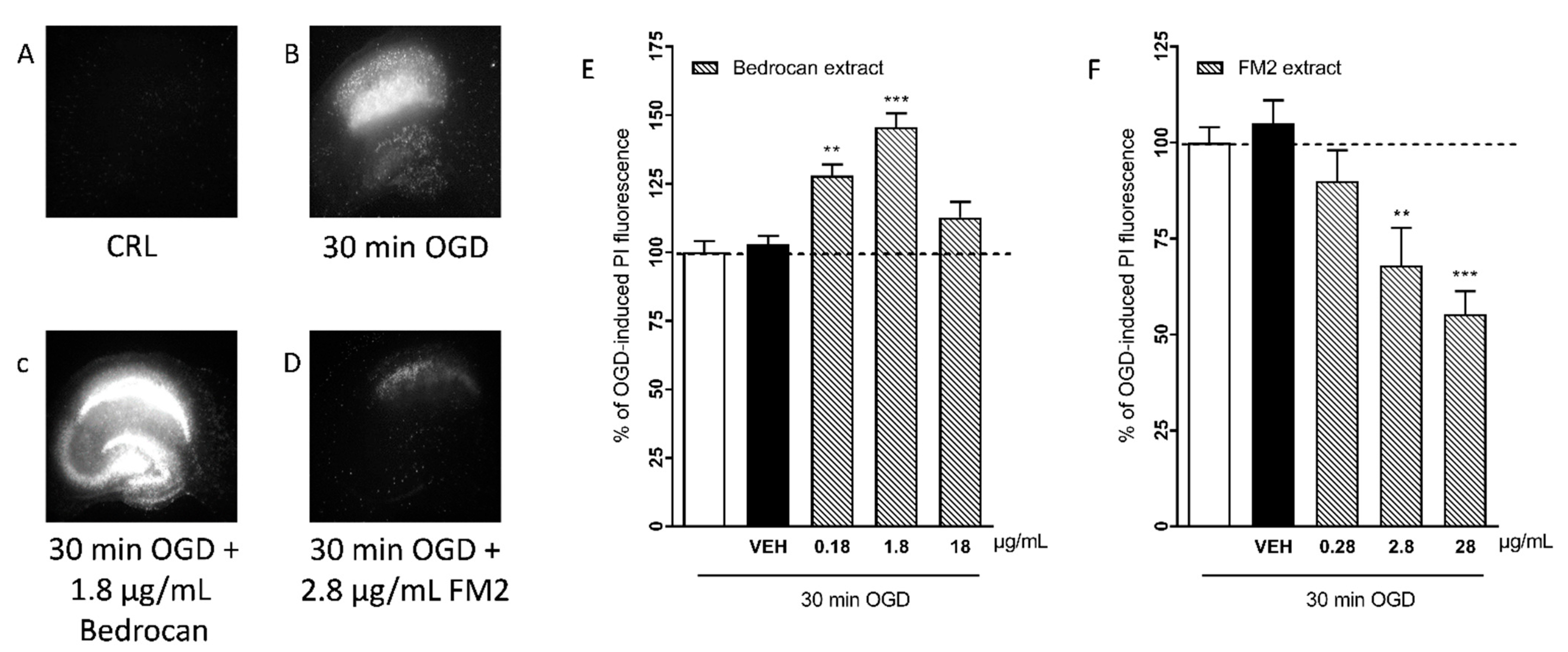
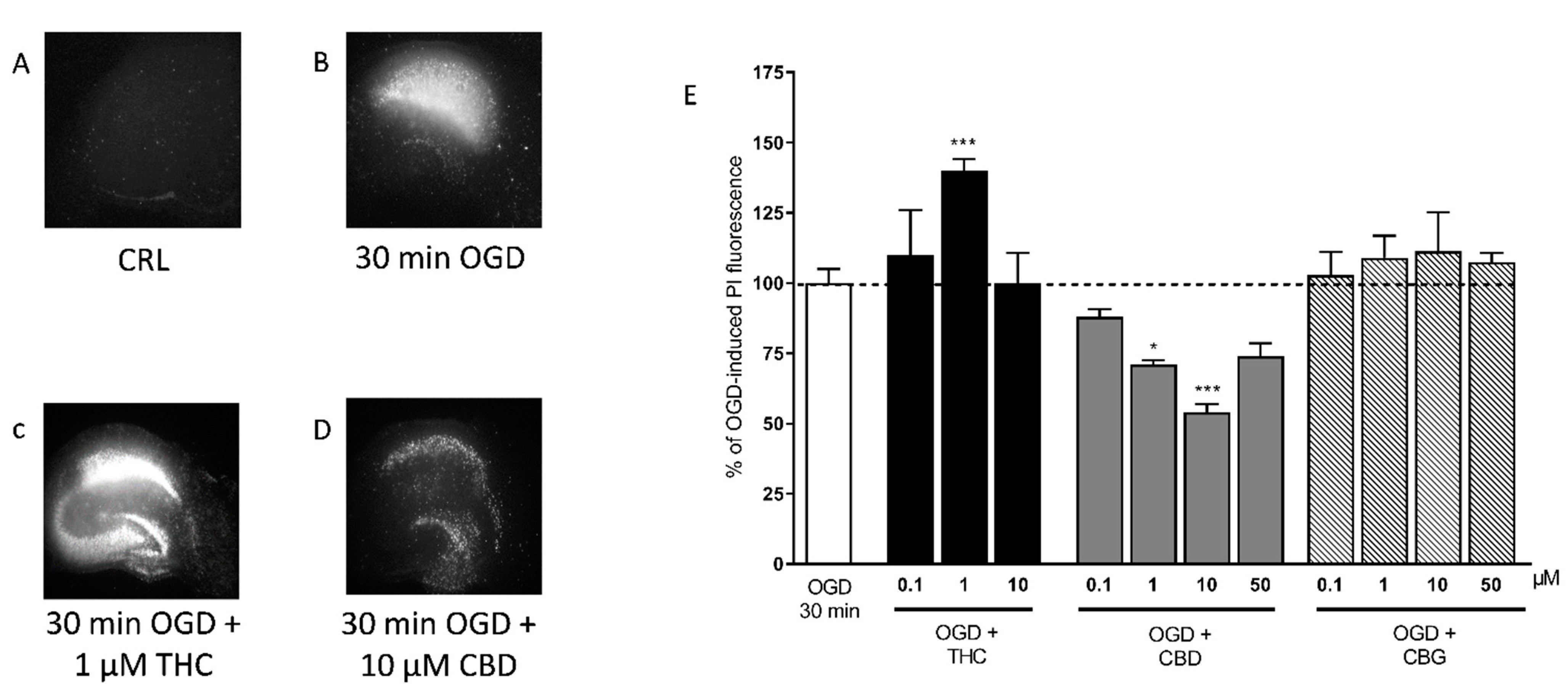

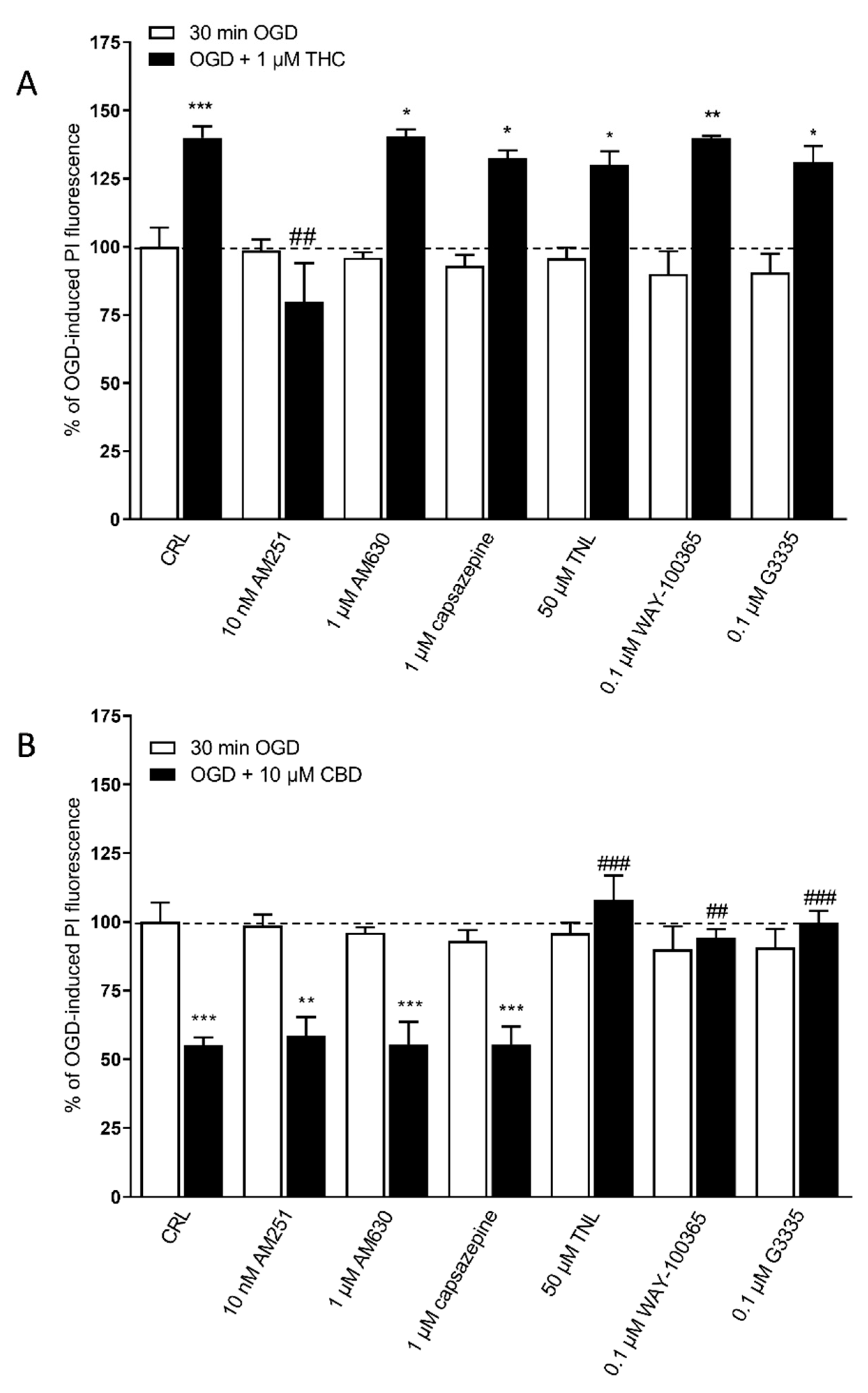
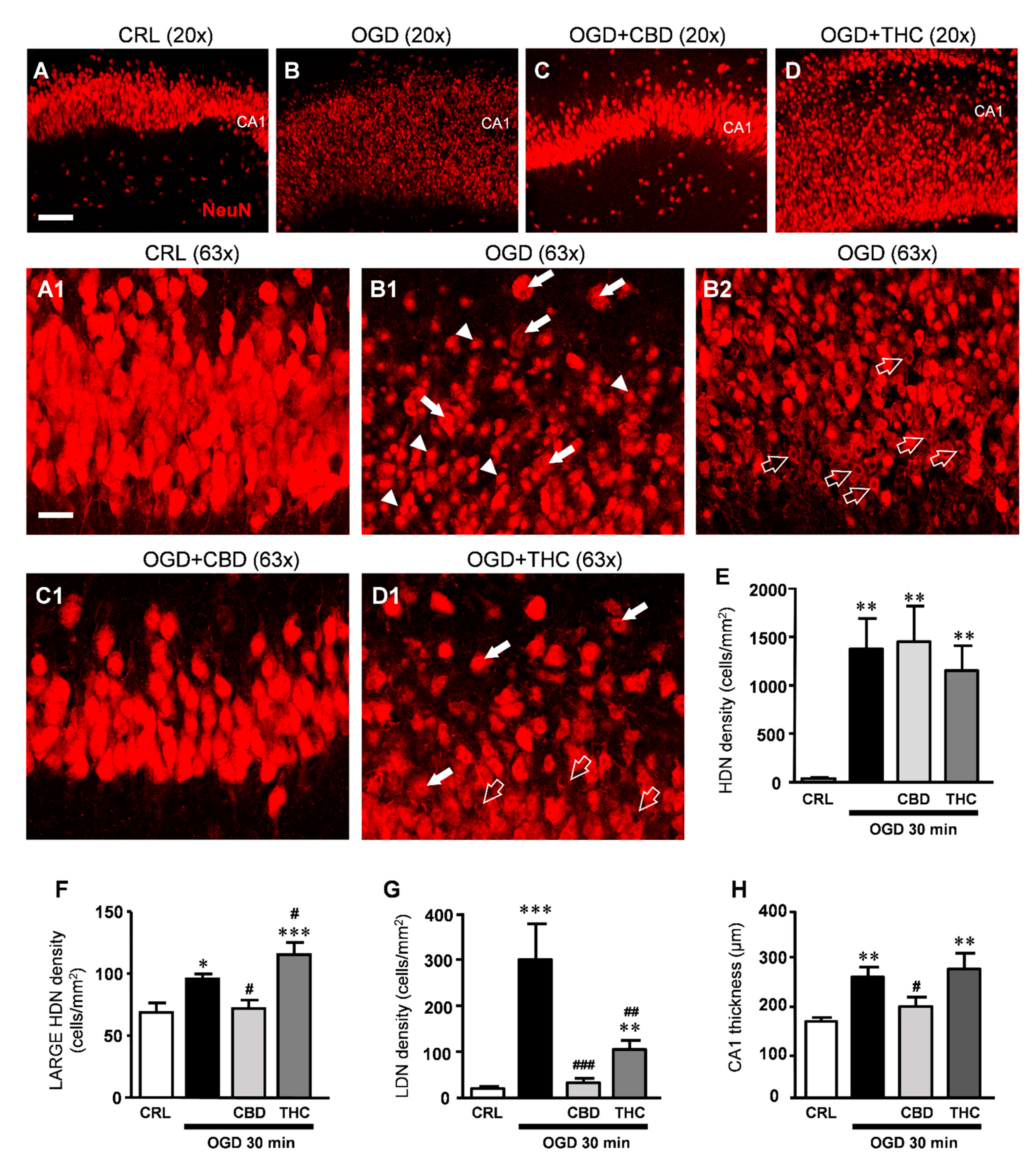
| Concentration (mg/mL) | ||
|---|---|---|
| Compounds | Bedrocan | FM2 |
| CBD-A | 0.39 | 0.2 |
| CBD | 0.66 | 8.2 |
| CBN | 0.32 | 0.26 |
| Δ-9-THC | 16.96 | 4.6 |
| THC-A | 0.61 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landucci, E.; Mazzantini, C.; Lana, D.; Davolio, P.L.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuroprotective Effects of Cannabidiol but Not Δ9-Tetrahydrocannabinol in Rat Hippocampal Slices Exposed to Oxygen-Glucose Deprivation: Studies with Cannabis Extracts and Selected Cannabinoids. Int. J. Mol. Sci. 2021, 22, 9773. https://doi.org/10.3390/ijms22189773
Landucci E, Mazzantini C, Lana D, Davolio PL, Giovannini MG, Pellegrini-Giampietro DE. Neuroprotective Effects of Cannabidiol but Not Δ9-Tetrahydrocannabinol in Rat Hippocampal Slices Exposed to Oxygen-Glucose Deprivation: Studies with Cannabis Extracts and Selected Cannabinoids. International Journal of Molecular Sciences. 2021; 22(18):9773. https://doi.org/10.3390/ijms22189773
Chicago/Turabian StyleLanducci, Elisa, Costanza Mazzantini, Daniele Lana, Pier Luigi Davolio, Maria Grazia Giovannini, and Domenico E. Pellegrini-Giampietro. 2021. "Neuroprotective Effects of Cannabidiol but Not Δ9-Tetrahydrocannabinol in Rat Hippocampal Slices Exposed to Oxygen-Glucose Deprivation: Studies with Cannabis Extracts and Selected Cannabinoids" International Journal of Molecular Sciences 22, no. 18: 9773. https://doi.org/10.3390/ijms22189773
APA StyleLanducci, E., Mazzantini, C., Lana, D., Davolio, P. L., Giovannini, M. G., & Pellegrini-Giampietro, D. E. (2021). Neuroprotective Effects of Cannabidiol but Not Δ9-Tetrahydrocannabinol in Rat Hippocampal Slices Exposed to Oxygen-Glucose Deprivation: Studies with Cannabis Extracts and Selected Cannabinoids. International Journal of Molecular Sciences, 22(18), 9773. https://doi.org/10.3390/ijms22189773










