The Role of Melanoma Cell-Derived Exosomes (MTEX) and Photodynamic Therapy (PDT) within a Tumor Microenvironment
Abstract
:1. Introduction
1.1. Melanoma Background
1.2. Current Melanoma Treatment Modalities
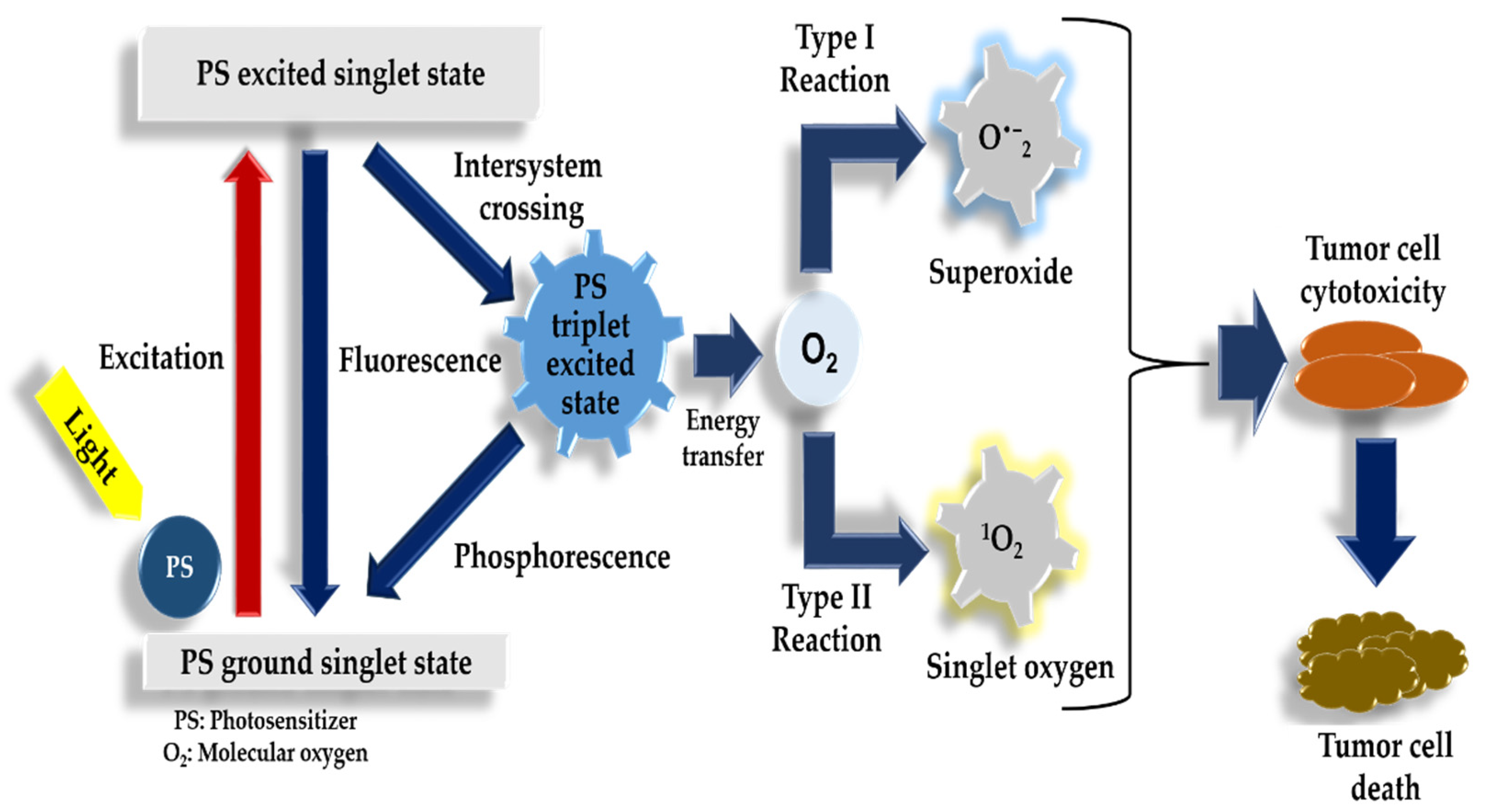
1.3. Photodynamic Therapy Mechanism of Action
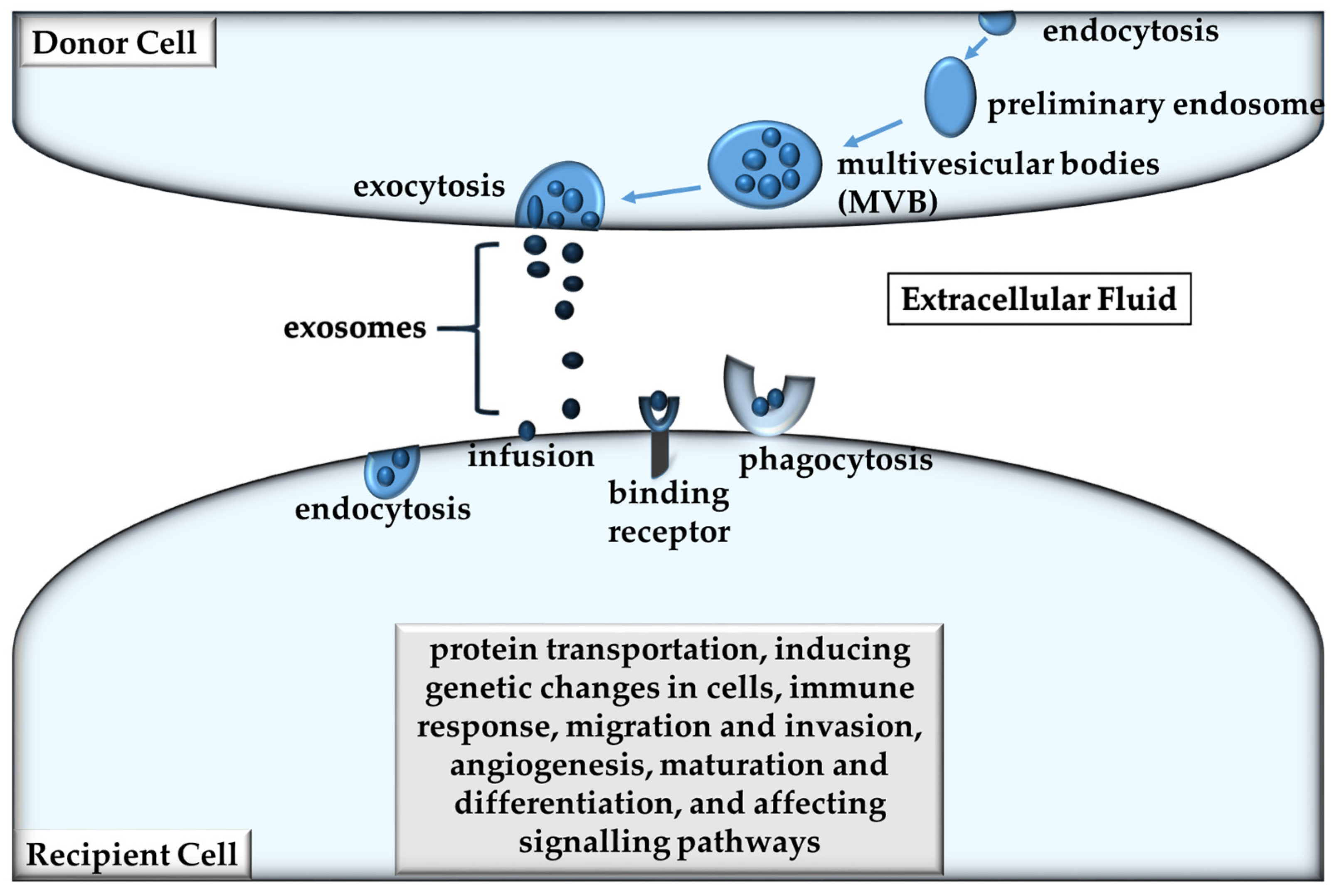
1.4. Exosomes and Extracellular Vesicles
2. The Role of Exosomes in Immune Responses
3. Role of Exosomes in Metastasis
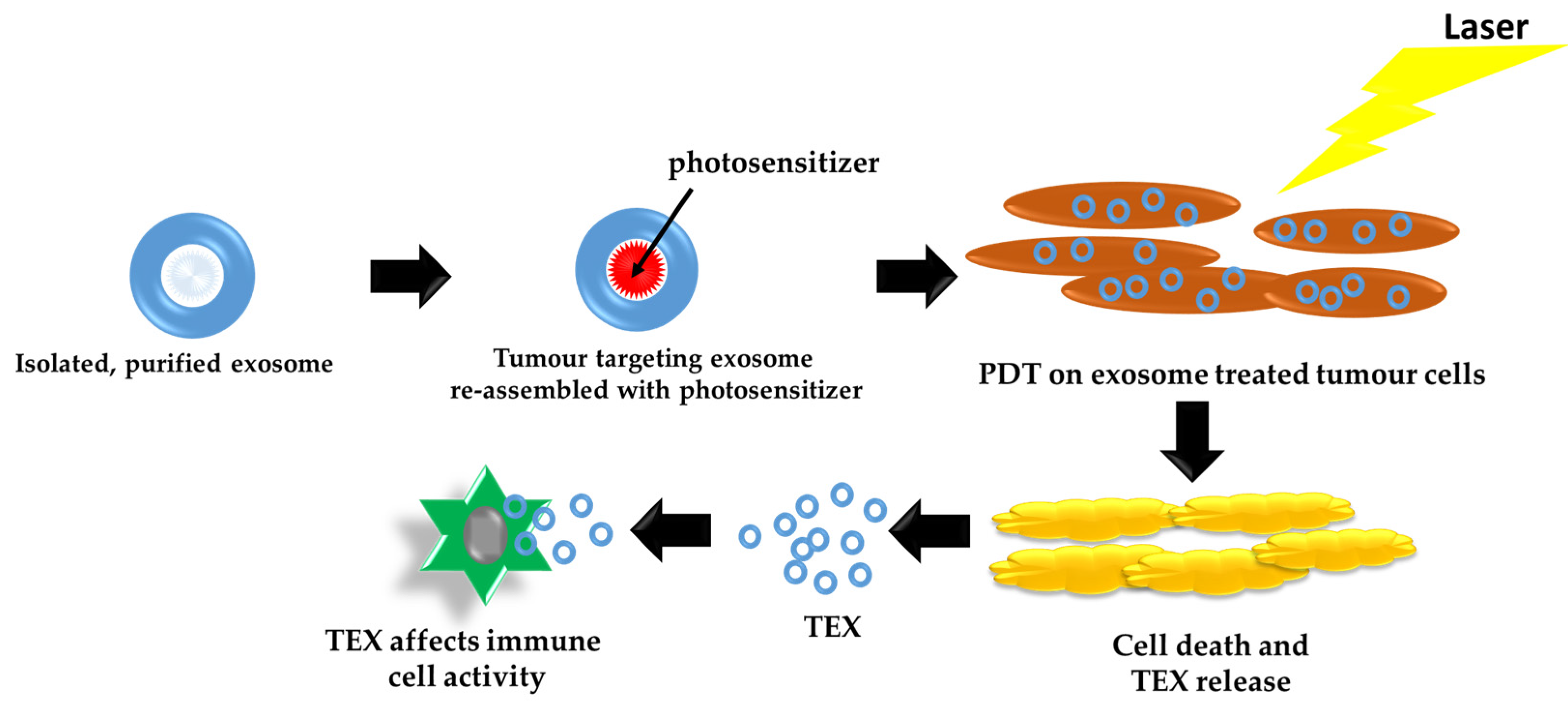
4. Exosomes as Carriers and Delivery Systems for PDT
5. Effects of Tumor Cell-Derived Exosomes (TEX) Induced by PDT
6. Clinical Trials Using Exosomes and Photodynamic Therapy in Melanoma Therapy
7. Pros and Cons of Photodynamic Therapy and Exosomes in Cancer Therapy
8. Toxicological Issues in Photodynamic Therapy
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/anual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf (accessed on 14 March 2021).
- Domingues, B.; Lopes, J.M.; Soares, P.; Pόpulo, H. Melanoma treatment in review. Immunotargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. The of tumour-derived exosomes on immune regulation and cancer immunotherapy. Future Oncol. 2017, 13, 2583–2592. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular Vesicles for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Melanoma of the Skin-Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 14 March 2021).
- National Cancer Registry, South Africa. Ekurhuleni Population-Based Cancer Registry Annual 2018 Report. Available online: https://www.nicd.ac.za/wp-content/uploads/2020/04/EPBCR-2018-report-Final-report.pdf (accessed on 14 March 2021).
- Steen, S.; Nemunaitis, J.; Fisher, T.; Kuhn, J. Circulating Tumor Cells in Melanoma: A Review of the Literature and Description of a Novel Technique. Bayl. Univ. Med. Cent. Proc. 2008, 21, 127–132. [Google Scholar] [CrossRef] [Green Version]
- Tanning and Melanoma Risk. Available online: https://www.canceredinstitute.org/tanning-and-melanoma-risk.html (accessed on 14 March 2021).
- Hendi, A.; Martinez, J.C. Melanoma. In Atlas of Skin Cancers; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–89. [Google Scholar]
- Gore, M.E.; Larkin, J.M.G. Challenges and Opportunities for Converting Renal Cell Carcinoma into a Chronic Disease with Targeted Therapies. Br. J. Cancer 2011, 104, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, L.A., Jr.; Williams, R.T.; Wu, J.; Kinde, I.; Hecht, J.R.; Berlin, J.; Allen, B.; Bozic, I.; Reiter, J.G.; Nowak, M.A.; et al. The Molecular Evolution of Acquired Resistance to Targeted EGFR Blockade in Colorectal Cancers. Nature 2012, 486, 537–540. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.; Couts, K.L.; Luo, Y.; Fujita, M. Understanding Melanoma Stem Cells. Melanoma Manag. 2015, 2, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818791795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravanat, J.-L.; Douki, T.; Cadet, J. Direct and Indirect Effects of UV Radiation on DNA and Its Components. J. Photochem. Photobiol. B 2001, 63, 88–102. [Google Scholar] [CrossRef]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of Singlet Oxygen as the Cytotoxic Agent in Photoinactivation of a Murine Tumor. Cancer Res. 1976, 36, 2326–2329. [Google Scholar] [PubMed]
- Foote, C.; Doiron, D.; Gomer, C. Mechanisms of photo-oxygenation. In Porphyrin Localization and Treatment of Tumors; Alan R. Liss: New York, NY, USA, 1984; pp. 3–18. [Google Scholar]
- Webber, J.; Steadman, R.; Mason, M.D.; Tabi, Z.; Clayton, A. Cancer Exosomes Trigger Fibroblast to Myofibroblast Differentiation. Cancer Res. 2010, 70, 9621–9630. [Google Scholar] [CrossRef] [Green Version]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [Green Version]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome Mediated Communication within the Tumor Microenvironment. J. Control. Release 2015, 219, 278–294. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Kalluri, R. The Biology and Function of Exosomes in Cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like Vesicles Are Present in Human Blood Plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, B.; Wu, L.; Hvam, M.L.; Aslan, H.; Dong, M.; Dyrskjøt, L.; Ostenfeld, M.S.; Moghimi, S.M.; Howard, K.A. Tumour Exosomes Display Differential Mechanical and Complement Activation Properties Dependent on Malignant State: Implications in Endothelial Leakiness. J. Extracell. Vesicles 2015, 4. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- Hugel, B.; Martínez, M.C.; Kunzelmann, C.; Freyssinet, J.-M. Membrane Microparticles: Two Sides of the Coin. Physiology 2005, 20, 22–27. [Google Scholar] [CrossRef]
- Saleem, S.; Abdel-Mageed, A. Tumor-Derived Exosomes in Oncogenic Reprogramming and Cancer Progression. Cell. Mol. Life Sci. 2014, 72. [Google Scholar] [CrossRef] [Green Version]
- Villarroya-Beltri, C.; Baixauli, F.; Gutiérrez-Vázquez, C.; Sánchez-Madrid, F.; Mittelbrunn, M. Sorting It out: Regulation of Exosome Loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Namee, N.M.; O’Driscoll, L. Extracellular Vesicles and Anti-Cancer Drug Resistance. Biochim. Biophys. Acta Rev. Cancer 2018, 1870, 123–136. [Google Scholar] [CrossRef]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W.E. Proteomic Profiling of Exosomes: Current Perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Mannavola, F.; D’Oronzo, S.; Cives, M.; Stucci, L.S.; Ranieri, G.; Silvestris, F.; Tucci, M. Extracellular Vesicles and Epigenetic Modifications Are Hallmarks of Melanoma Progression. Int. J. Mol. Sci. 2020, 21, 52. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Wu, N.; Yang, J.-M.; Gould, S.; Shen, B.; Wu, N.; Yang, J.M.; Gould, S.J. Protein Targeting to Exosomes/Microvesicles by Plasma Membrane Anchors. J. Biol. Chem. 2011, 286, 14383–14395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappa, G.; Mercapide, J.; Anzanello, F.; Pope, R.M.; Loricio, A. Biochemical and Biological Characterization of Exosomes Containing Prominin-1/CD133. Mol. Cancer 2013, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.; Ohlendorf, J.; Chen, Y.; Taylor, D.D.; Rai, S.N.; Waigel, S.; Zacharias, W.; Hao, H.; McMasters, K.M. Identifying MRNA, MicroRNA and Protein Profiles of Melanoma Exosomes. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dressel, R.; Johnson, J.P.; Günther, E. Heterogeneous Patterns of Constitutive and Heat Shock Induced Expression of HLA-Linked HSP70–1 and HSP70–2 Heat Shock Genes in Human Melanoma Cell Lines. Melanoma Res. 1998, 8, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic Analysis of Melanoma-Derived Exosomes by Two-Dimensional Polyacrylamide Gel Electrophoresis and Mass Spectrometry. Proteomics 2004, 4, 4019–4031. [Google Scholar] [CrossRef] [PubMed]
- Wolfers, J.; Lozier, A.; Raposo, G.; Regnault, A.; Théry, C.; Masurier, C.; Flament, C.; Pouzieux, S.; Faure, F.; Tursz, T.; et al. Tumor-Derived Exosomes Are a Source of Shared Tumor Rejection Antigens for CTL Cross-Priming. Nat. Med. 2001, 7, 297–303. [Google Scholar] [CrossRef] [PubMed]
- ExoCarta Exosome Protein, RNA and Lipid Database. Available online: http://www.exocarta.org/ (accessed on 18 March 2021).
- Olejarz, W.; Dominiak, A.; Żołnierzak, A.; Kubiak-Tomaszewska, G.; Lorenc, T. Tumor-Derived Exosomes in Immunosuppression and Immunotherapy. J. Immunol. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Iglesias, T.; del Toro-Arreola, A.; Albarran-Somoza, B.; del Toro-Arreola, S.; Sanchez-Hernandez, P.E.; Ramirez-Dueñas, M.G.; Balderas-Peña, L. Ma. A.; Bravo-Cuellar, A.; Ortiz-Lazareno, P.C.; Daneri-Navarro, A. Low NKp30, NKp46 and NKG2D Expression and Reduced Cytotoxic Activity on NK Cells in Cervical Cancer and Precursor Lesions. BMC Cancer 2009, 9, 186. [Google Scholar] [CrossRef] [Green Version]
- Szczepanski, M.J.; Szajnik, M.; Welsh, A.; Whiteside, T.L.; Boyiadzis, M. Blast-Derived Microvesicles in Sera from Patients with Acute Myeloid Leukemia Suppress Natural Killer Cell Function via Membrane-Associated Transforming Growth Factor-Beta1. Haematologica 2011, 96, 1302–1309. [Google Scholar] [CrossRef]
- Kunigelis, K.; Graner, M. The Dichotomy of Tumor Exosomes (TEX) in Cancer Immunity: Is It All in the ConTEXt? Vaccines 2015, 3, 1019–1051. [Google Scholar] [CrossRef]
- Janco, J.M.T.; Lamichhane, P.; Karyampudi, L.; Knutson, K.L. Tumor-Infiltrating Dendritic Cells in Cancer Pathogenesis. J.Immunol. 2015, 194, 2985–2991. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.; Shen, K.; Wu, Q.; Sun, X.; Bai, Y.; Xie, Y.; Pan, J.; Qi, C. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol. Lett. 2018, 199, 36–43. [Google Scholar] [CrossRef]
- Tian, X.; Shen, H.; Li, Z.; Wang, T.; Wang, S. Tumor-Derived Exosomes, Myeloid-Derived Suppressor Cells, and Tumor Microenvironment. J. Hematol. Oncol 2019, 12, 84. [Google Scholar] [CrossRef] [Green Version]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J.; Whiteside, T.L. Tumor-Derived Microvesicles Promote Regulatory T Cell Expansion and Induce Apoptosis in Tumor-Reactive Activated CD8+ T Lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef] [Green Version]
- Wieckowski, E.; Whiteside, T. Human Tumor-Derived vs. Dendritic Cell-Derived Exosomes Have Distinct Biologic Roles and Molecular Profiles. Immunol. Res. 2006, 36, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Mannavola, F.; Passarelli, A.; Stucci, L.; Cives, M.; Silvestris, F. Exosomes in Melanoma: A Role in Tumor Progression, Metastasis and Impaired Immune System Activity. Oncotarget 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Viaud, S.; Théry, C.; Ploix, S.; Tursz, T.; Lapierre, V.; Lantz, O.; Zitvogel, L.; Chaput, N. Dendritic Cell-Derived Exosomes for Cancer Immunotherapy: What’s Next? Cancer Res. 2010, 70, 1281–1285. [Google Scholar] [CrossRef] [Green Version]
- Valenti, R.; Huber, V.; Iero, M.; Filipazzi, P.; Parmiani, G.; Rivoltini, L. Tumor-Released Microvesicles as Vehicles of Immunosuppression. Cancer Res. 2007, 67, 2912–2915. [Google Scholar] [CrossRef] [Green Version]
- Rivoltini, L.; Chiodoni, C.; Squarcina, P.; Tortoreto, M.; Villa, A.; Vergani, B.; Bürdek, M.; Botti, L.; Arioli, I.; Cova, A.; et al. TNF-Related Apoptosis-Inducing Ligand (TRAIL)–Armed Exosomes Deliver Proapoptotic Signals to Tumor Site. Clin. Cancer Res. 2016, 22, 3499–3512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune System and Melanoma Biology: A Balance between Immunosurveillance and Immune Escape. Oncotarget 2017, 8, 106132–106142. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Meng, Y.; Blank, C.; Brown, I.; Kacha, A.; Kline, J.; Harlin, H. Immune Resistance Orchestrated by the Tumor Microenvironment. Immunol. Rev. 2006, 213, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.-N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1+ Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. Exosomes Carrying Immunoinhibitory Proteins and Their Role in Cancer. Clin. Exp. Immunol. 2017, 189, 259–267. [Google Scholar] [CrossRef] [Green Version]
- Whiteside, T.L. Exosomes in Cancer: Another Mechanism of Tumor-Induced Immune Suppression. In Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy; Advances in Experimental Medicine and Biology; Kalinski, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 81–89. [Google Scholar] [CrossRef]
- Pitt, J.; Charrier, M.; Viaud, S.; Andre, F.; Besse, B.; Chaput, N.; Zitvogel, L. Dendritic Cell-Derived Exosomes as Immunotherapies in the Fight against Cancer. J. Immunol. 2014, 193, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Näslund, T.I.; Gehrmann, U.; Qazi, K.R.; Karlsson, M.C.I.; Gabrielsson, S. Dendritic Cell-Derived Exosomes Need to Activate Both T and B Cells to Induce Antitumor Immunity. J. Immunol. 2013, 190, 2712–2719. [Google Scholar] [CrossRef]
- Bardi, G.T.; Smith, M.A.; Hood, J.L. Melanoma Exosomes Promote Mixed M1 and M2 Macrophage Polarization. Cytokine 2018, 105, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Bland, C.L.; Byrne-Hoffman, C.N.; Fernandez, A.; Rellick, S.L.; Deng, W.; Klinke, D.J. Exosomes Derived from B16F0 Melanoma Cells Alter the Transcriptome of Cytotoxic T Cells That Impacts Mitochondrial Respiration. FEBS J. 2018, 285, 1033–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Yang, Y.; Wang, W.; Zhang, Y.; Chen, Z.; Hao, C.; Zhang, J. Melanoma-Released Exosomes Directly Activate the Mitochondrial Apoptotic Pathway of CD4+ T Cells through Their MicroRNA Cargo. Exp. Cell Res. 2018, 371, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.L.; Chapoval, A.I.; Nelson, J.; Orhue, V.; Zhang, X.; Schulze, D.H.; Strome, S.E.; Gastman, B.R. Tumor-Induced Senescent T Cells with Suppressor Function: A Potential Form of Tumor Immune Evasion. Cancer Res. 2008, 68, 870–879. [Google Scholar] [CrossRef] [Green Version]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes Secreted under Hypoxia Enhance Invasiveness and Stemness of Prostate Cancer Cells by Targeting Adherens Junction Molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harmati, M.; Gyukity-Sebestyen, E.; Dobra, G.; Janovak, L.; Dekany, I.; Saydam, O.; Hunyadi-Gulyas, E.; Nagy, I.; Farkas, A.; Pankotai, T.; et al. Small Extracellular Vesicles Convey the Stress-Induced Adaptive Responses of Melanoma Cells. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Hood, J.L.; Pan, H.; Lanza, G.M.; Wickline, S.A. Paracrine Induction of Endothelium by Tumor Exosomes. Lab. Investig. 2009, 89, 1317–1328. [Google Scholar] [CrossRef] [Green Version]
- Ekström, E.J.; Bergenfelz, C.; von Bülow, V.; Serifler, F.; Carlemalm, E.; Jönsson, G.; Andersson, T.; Leandersson, K. WNT5A Induces Release of Exosomes Containing Pro-Angiogenic and Immunosuppressive Factors from Malignant Melanoma Cells. Mol. Cancer 2014, 13, 88. [Google Scholar] [CrossRef] [Green Version]
- Katoh, M. Therapeutics Targeting Angiogenesis: Genetics and Epigenetics, Extracellular MiRNAs and Signaling Networks (Review). Int. J. Mol. Med. 2013, 32, 763–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajos-Michniewicz, A.; Duechler, M.; Czyz, M. MiRNA in Melanoma-Derived Exosomes. Cancer Lett. 2014, 347, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Vences-Catalán, F.; Levy, S. Immune Targeting of Tetraspanins Involved in Cell Invasion and Metastasis. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes Released by Melanoma Cells Prepare Sentinel Lymph Nodes for Tumor Metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A Comprehensive Overview of Exosomes as Drug Delivery Vehicles—Endogenous Nanocarriers for Targeted Cancer Therapy. Biochim. Biophys. Acta Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef]
- Zhu, M.; Tian, X.; Song, X.; Li, Y.; Tian, Y.; Zhao, Y.; Nie, G. Nanoparticle-Induced Exosomes Target Antigen-Presenting Cells to Initiate Th1-Type Immune Activation. Small 2012, 8, 2841–2848. [Google Scholar] [CrossRef]
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Murakami, Y.; Rikitake, Y. Mechanism of Recipient Cell-Dependent Differences in Exosome Uptake. BMC Cancer 2018, 18. [Google Scholar] [CrossRef] [Green Version]
- van der Meel, R.; Fens, M.H.A.M.; Vader, P.; van Solinge, W.W.; Eniola-Adefeso, O.; Schiffelers, R.M. Extracellular Vesicles as Drug Delivery Systems: Lessons from the Liposome Field. J. Control. Release 2014, 195, 72–85. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.-H.; Wilcher, S.A.; Gupta, R.C. Milk Exosomes—Natural Nanoparticles for SiRNA Delivery. Cancer Lett. 2019, 449, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Kim, H.; Yoon, S.; Lee, H.; Hwang, J.; Jung, J.; Chang, J.H.; Choi, J.; Kim, H. Exosome-Based Photoacoustic Imaging Guided Photodynamic and Immunotherapy for the Treatment of Pancreatic Cancer. J. Control. Release 2021, 330, 293–304. [Google Scholar] [CrossRef]
- Yim, N.; Ryu, S.-W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.-H.; et al. Exosome Engineering for Efficient Intracellular Delivery of Soluble Proteins Using Optically Reversible Protein-Protein Interaction Module. Nat. Commun. 2016, 7, 12277. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of SiRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, J.; Karlson, T.D.L.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma Exosomes Can Deliver Exogenous Short Interfering RNA to Monocytes and Lymphocytes. Nucleic Acids Res. 2012, 40. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Fan, J.-H.; Zhao, L.-P.; Fan, G.-L.; Zheng, R.-R.; Qiu, X.-Z.; Yu, X.-Y.; Li, S.-Y.; Zhang, X.-Z. Chimeric Peptide Engineered Exosomes for Dual-Stage Light Guided Plasma Membrane and Nucleus Targeted Photodynamic Therapy. Biomaterials 2019, 211, 14–24. [Google Scholar] [CrossRef]
- Aalberts, M.; Stout, T.A.E.; Stoorvogel, W. Prostasomes: Extracellular Vesicles from the Prostate. Reproduction 2014, 147, R1–R14. [Google Scholar] [CrossRef] [Green Version]
- Pitson, S.; Powell, J. Modification of the Tumour Microenvironment via Exosomal Shedding of Sphingosine 1-Phosphate Receptor 2 by Breast Cancer Cells. Oncotarget 2018, 9. [Google Scholar] [CrossRef]
- Bae, S.; Brumbaugh, J.; Bonavida, B. Exosomes Derived from Cancerous and Non-Cancerous Cells Regulate the Anti-Tumor Response in the Tumor Microenvironment. Genes Cancer 2018, 9, 87–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Harris, S.L.; Levine, A.J. The Regulation of Exosome Secretion: A Novel Function of the P53 Protein. Cancer Res. 2006, 66, 4795–4801. [Google Scholar] [CrossRef] [Green Version]
- Kucharzewska, P.; Belting, M. Emerging Roles of Extracellular Vesicles in the Adaptive Response of Tumour Cells to Microenvironmental Stress. J. Extracell. Vesicles 2013, 2, 20304. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane Vesicles as Conveyors of Immune Responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Aubertin, K.; Silva, A.K.A.; Luciani, N.; Espinosa, A.; Djemat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive Release of Extracellular Vesicles from Cancer Cells after Photodynamic Treatment or Chemotherapy. Sci. Rep. 2016, 6, 35376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, H.; Zeng, Q.; Wang, P.; Zhang, G.; Ji, J.; Li, M.; Shen, S.; Wang, X. Exosomes from 5-Aminolevulinic Acid Photodynamic Therapy-Treated Squamous Carcinoma Cells Promote Dendritic Cell Maturation. Photodiagnosis Photodyn. Ther. 2020, 30, 101746. [Google Scholar] [CrossRef]
- Theodoraki, M.-N.; Yerneni, S.S.; Brunner, C.; Theodorakis, J.; Hoffmann, T.K.; Whiteside, T.L. Plasma-Derived Exosomes Reverse Epithelial-to-Mesenchymal Transition after Photodynamic Therapy of Patients with Head and Neck Cancer. Oncoscience 2018, 5, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Mahmoodzadeh, H.H.; Halabian, R.; Amin, M.; Imani Fooladi, A.A. Texosome-based drug delivery system for cancer therapy: From past to present. Ann. Transl Med. 2015, 12, 150–162. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of the first phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, C.K.; Jena, B.C.; Banerjee, I.; Das, S.; Parekh, A.; Bhutia, S.K.; Mandal, M. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol. Pharm. 2019, 16, 24–40. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Soltani, F.; Parhiz, H.; Mokhtarzadeh, A.; Ramezani, M. Synthetic and biological vesicular nano-carriers designed for gene delivery. Curr. Pharm. Des. 2015, 21, 6214–6235. [Google Scholar] [CrossRef] [PubMed]
- Vrouenraets, M.B.; Visser, G.W.; Snow, G.B.; van Dongen, G.A. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003, 23, 505–522. [Google Scholar] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacha, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Borgia, F.; Giuffrida, R.; Caradonna, E.; Vaccaro, M.; Guarneri, F.; Cannavò, S.P. Early and Late Onset Side Effects of Photodynamic Therapy. Biomedicines 2018, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
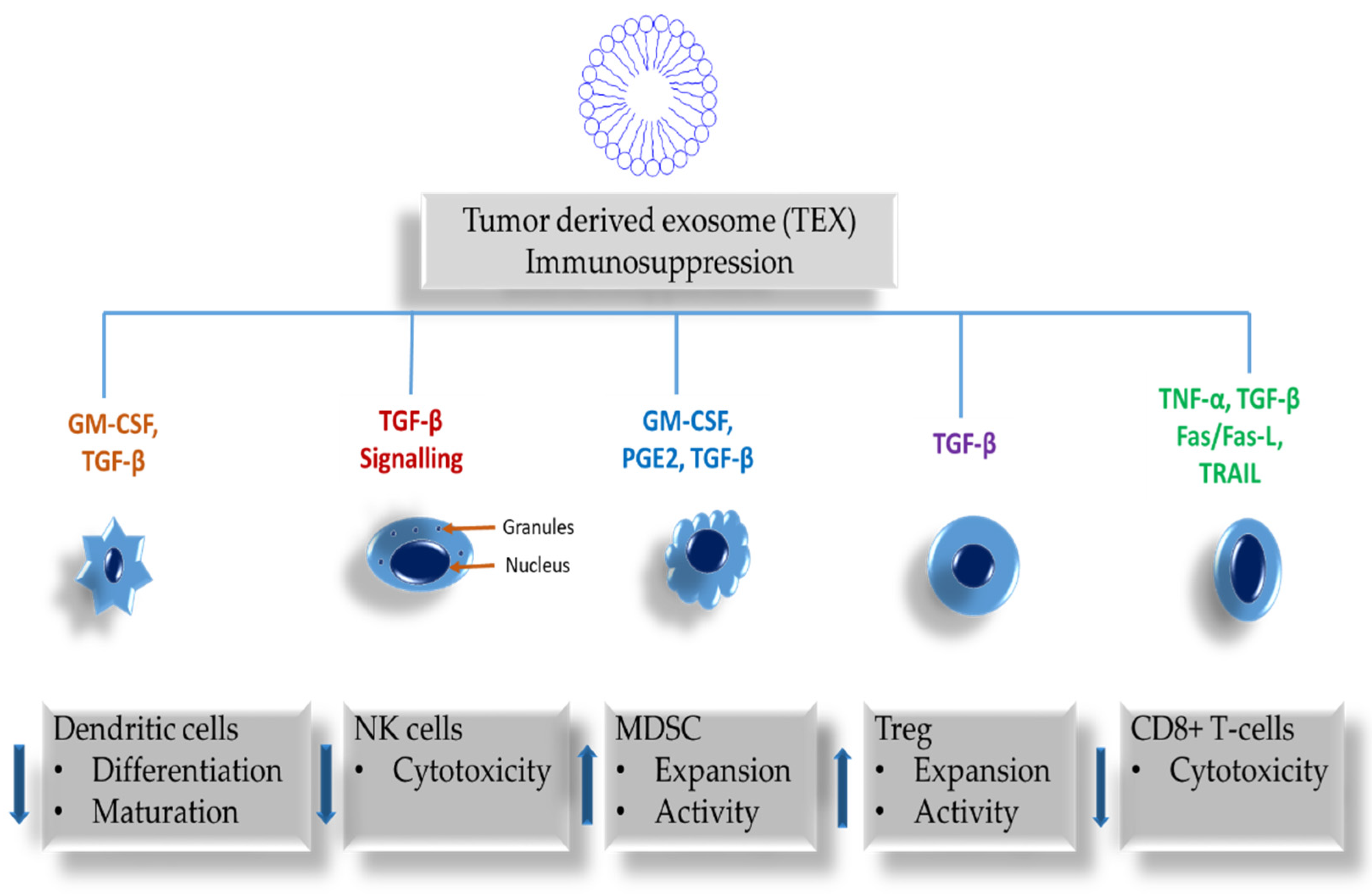
| Immune Cells | TEX Effects | Non-TEX Effects | References |
|---|---|---|---|
| Natural killer (NK) cells | Suppressed cytolytic activity. Downregulation of NKG2D and impaired cell cytotoxic function. | Activating NKG2D, NKP30, NKP46, and NKG2C receptors inducing NK cell cytotoxicity | [41,42] |
| Dendritic cells (DC) | Suppressed differentiation, maturation, and activity. Suppress migration through chemokine receptor expression inhibition. | [43,44,45] | |
| Myeloid-derived suppressor cells (MDSC) | Increase the progression of MDSCs, expediting their activation, aiding expansion, and increasing the immunosuppressive role. | [46] | |
| Regulatory T-cells (Treg) | Increased proliferation and expansion. Induced immune suppression by supporting extension of CD4(+)CD25(+)FOXP3(+)T regulatory cells and the diminishing of antitumor CD8(+) effector T-cells. | [47] | |
| CD8+T-cells | Suppressed cytotoxicity, inhibiting signaling and proliferation, increased apoptosis, and alteration of antitumor phenotype. | Promote the proliferation of all T-cells | [47,48] |
| Photosensitizer (PS) | Tumor Cell Line | Exosome Effects | References |
|---|---|---|---|
| Foscan® m-THPC (5,10,15,20-tetra(3-hydroxyphenyl)chlorin) photosensitizer | Human prostatic cancer cells (PC-3, ATCC ® CRL-1435™) | Increased EV release 1-h post photo-activation, secreted EVs may transmit extracellular membrane constituents, drugs and large intracellular objects to harmless target cells | [89] |
| (5,10,15,20-tetra(3-hydroxyphenyl)chlorin) | SCC cells (PECA, primary mice SCCs, A431) | ALA-PDT-treated SCCs trigger dendritic cell maturation and fibroblast secretion of TGF-β1, resulting in increased anti-tumor immunity. | [90] |
| Temoporfin (Foscan® Biolitec, Vienna, Austria) | PCI13, a HNSCC HPV-negative cell line and SCC-90, a HNSCC HPV+ cell line; A549 (human lung adenocarcinoma), BT549 (human breast ductal carcinoma), and MDAMB231 (human breast adenocarcinoma) | Post-PDT exosome-mediated conversion from mesenchymal to epithelial tumor phenotype mediated by exosomes. | [91] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkhobongo, B.; Chandran, R.; Abrahamse, H. The Role of Melanoma Cell-Derived Exosomes (MTEX) and Photodynamic Therapy (PDT) within a Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 9726. https://doi.org/10.3390/ijms22189726
Mkhobongo B, Chandran R, Abrahamse H. The Role of Melanoma Cell-Derived Exosomes (MTEX) and Photodynamic Therapy (PDT) within a Tumor Microenvironment. International Journal of Molecular Sciences. 2021; 22(18):9726. https://doi.org/10.3390/ijms22189726
Chicago/Turabian StyleMkhobongo, Bridgette, Rahul Chandran, and Heidi Abrahamse. 2021. "The Role of Melanoma Cell-Derived Exosomes (MTEX) and Photodynamic Therapy (PDT) within a Tumor Microenvironment" International Journal of Molecular Sciences 22, no. 18: 9726. https://doi.org/10.3390/ijms22189726
APA StyleMkhobongo, B., Chandran, R., & Abrahamse, H. (2021). The Role of Melanoma Cell-Derived Exosomes (MTEX) and Photodynamic Therapy (PDT) within a Tumor Microenvironment. International Journal of Molecular Sciences, 22(18), 9726. https://doi.org/10.3390/ijms22189726








