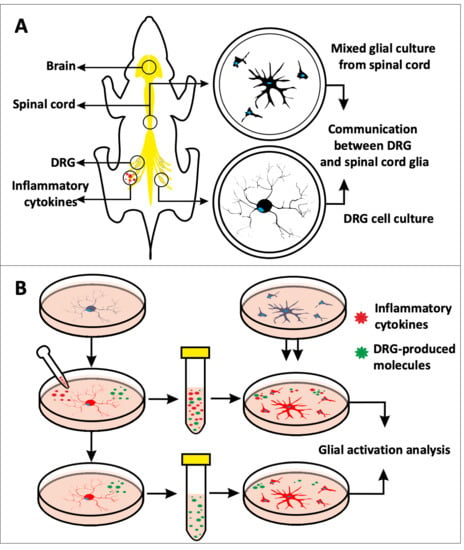
In Vitro Model to Investigate Communication between Dorsal Root Ganglion and Spinal Cord Glia
Abstract
:1. Introduction
2. Results
2.1. Inflammatory Cytokines Enhanced Spontaneous Calcium Response in DRG Neurons
2.2. Inflammatory Cytokines Did Not Influence CGRP Expression in Neonatal Rat DRG Cells
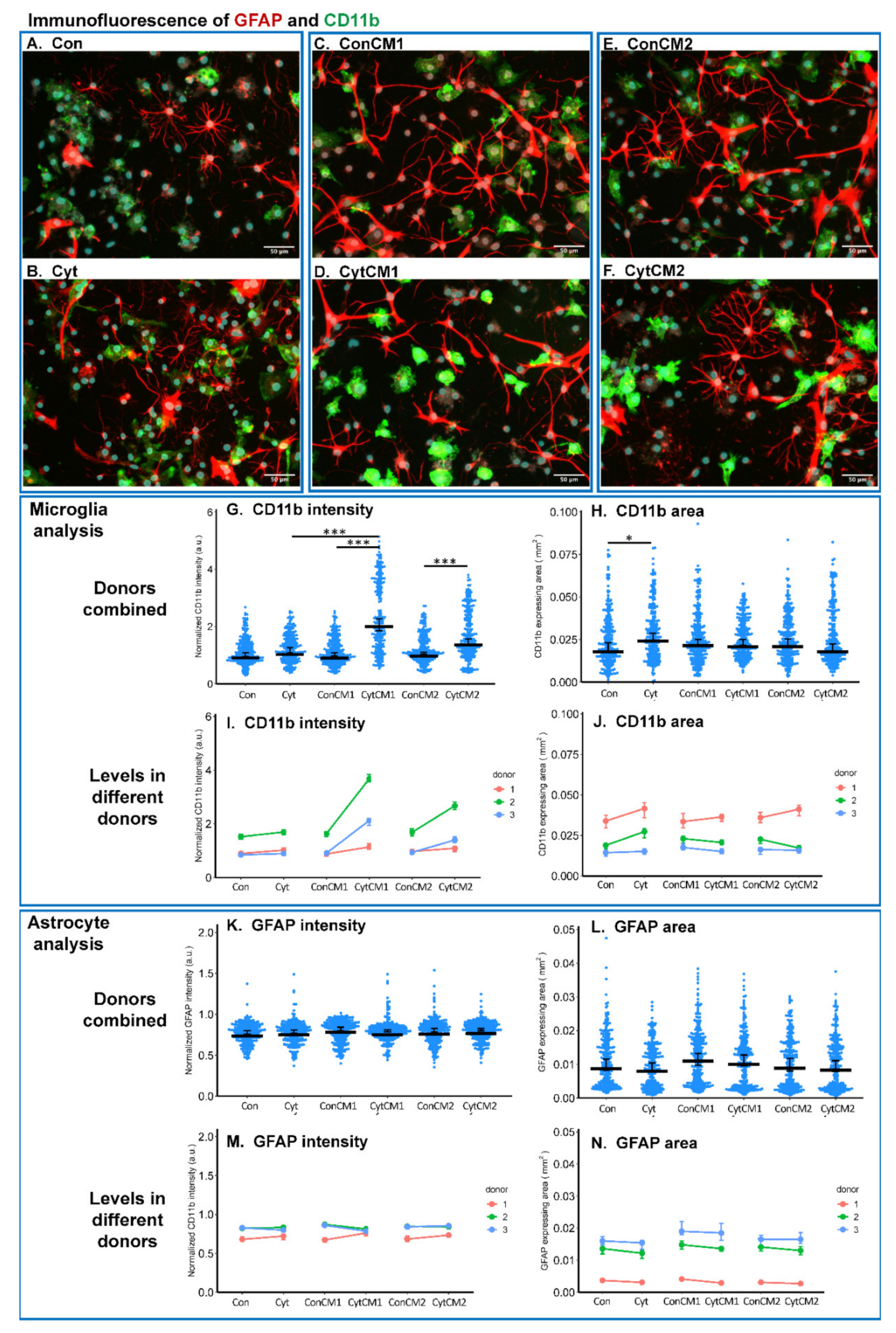
2.3. Inflammatory Cytokines Induced a DRG-Mediated Microgliosis in Mixed Glial Cuture of Neonatal Rat Spinal Cord
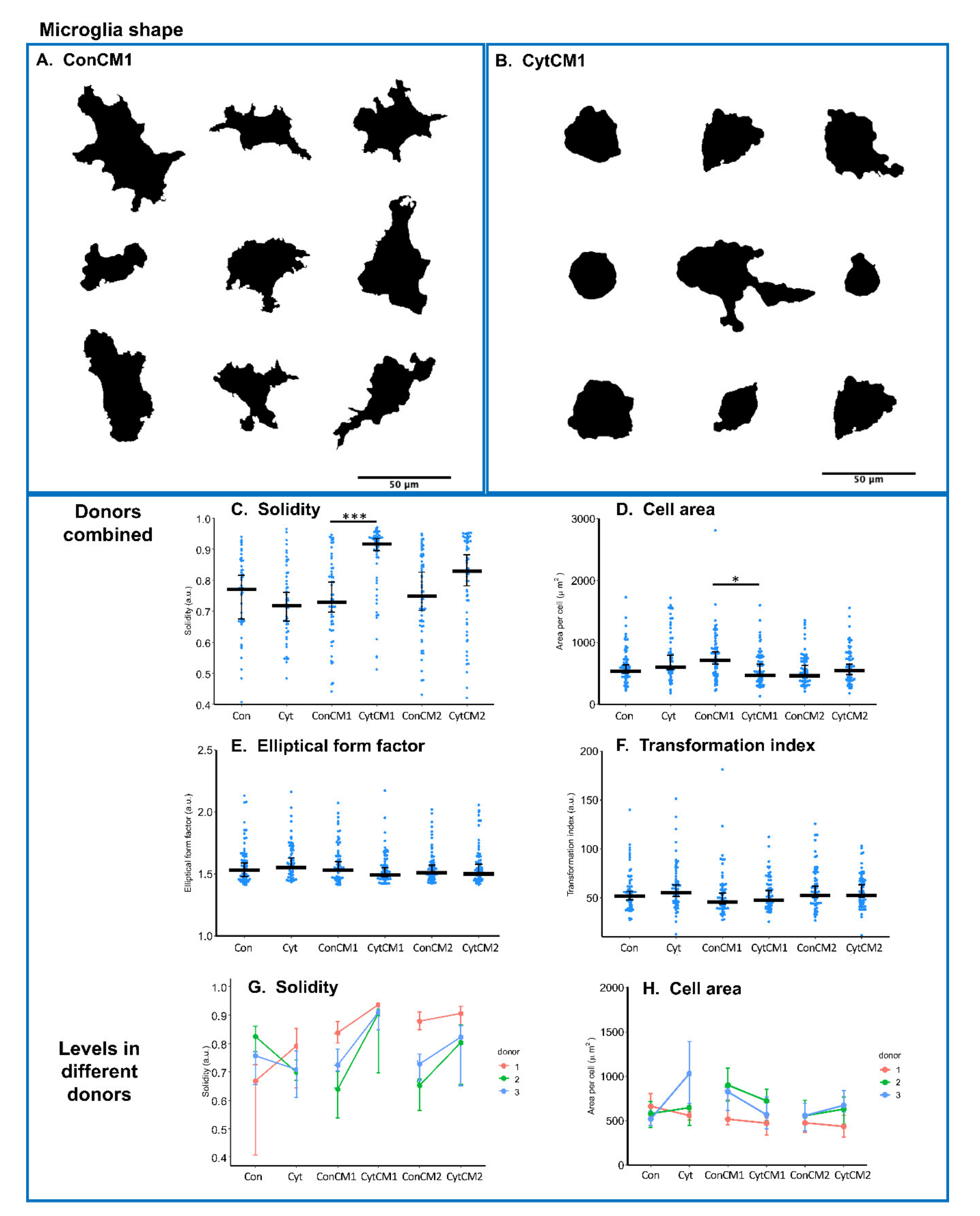
2.4. Inflammatory Cytokines Induced a DRG-Mediated Microglial Shape Change in Mixed Glial Cuture of Neonatal Rat Spinal Cord
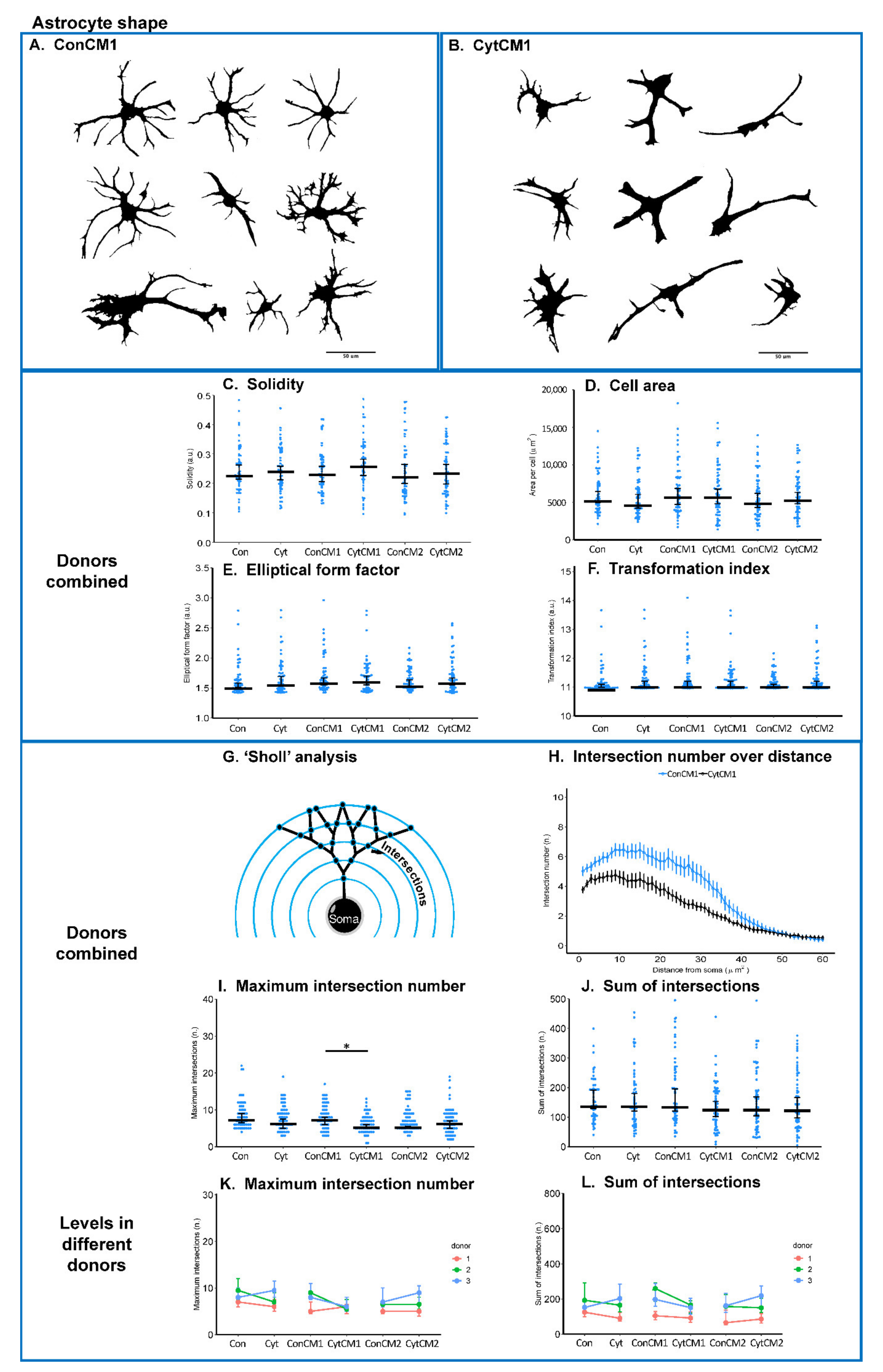
2.5. Inflammatory Cytokines Induced a DRG-Mediated Astrocyte Arbor Branching Reduction in Mixed Glial Cuture of Neonatal Rat Spinal Cord
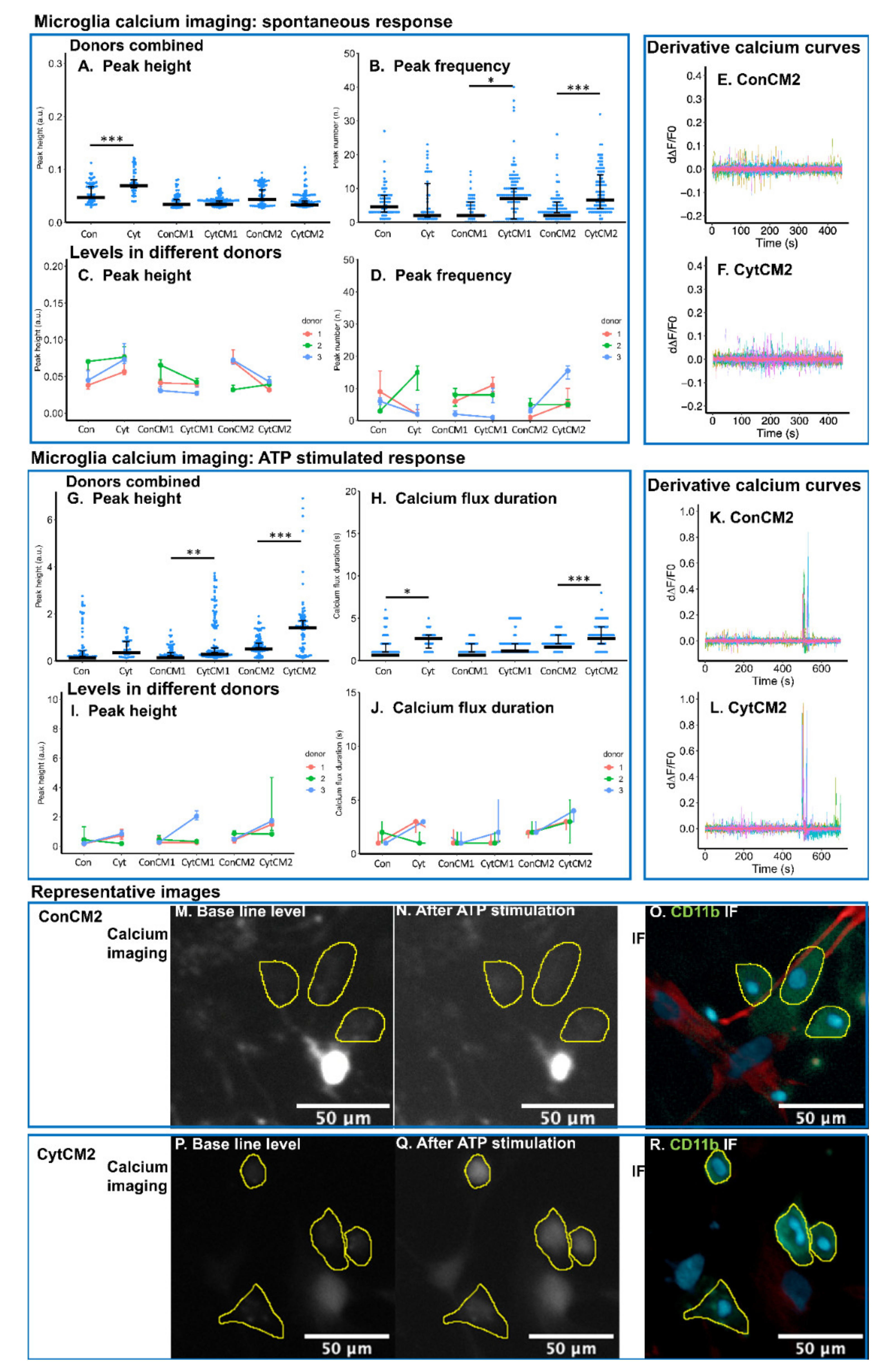
2.6. Inflammatory Cytokines Induced a DRG-Mediated Increase in Spontaneous and ATP-Stimulated Calcium Response in Neonatal Rat Microglia
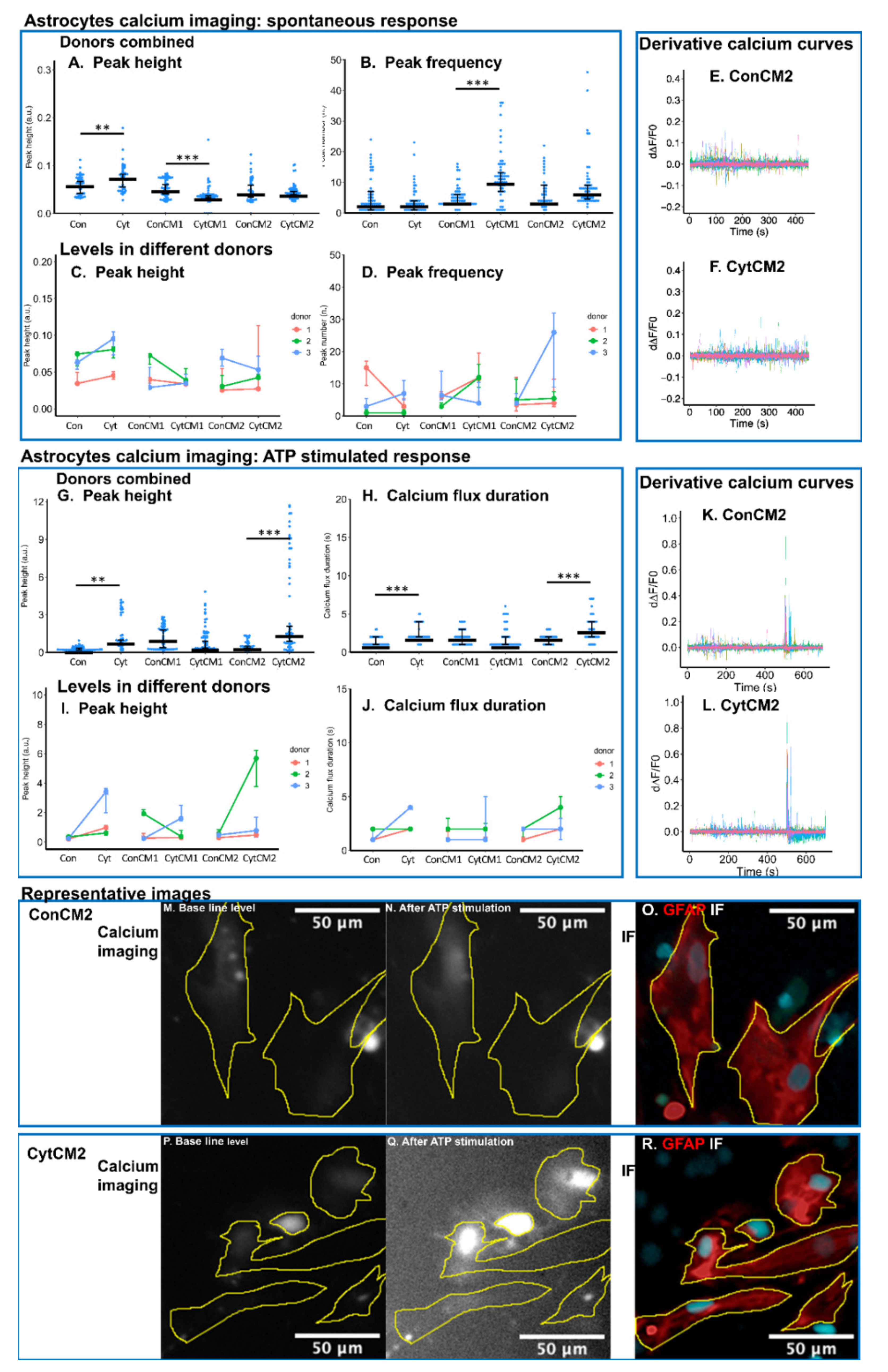
2.7. Inflammatory Cytokines Induced a DRG-Mediated Increase in ATP-Stimulated Calcium Response in Neonatal Rat Astrocytes
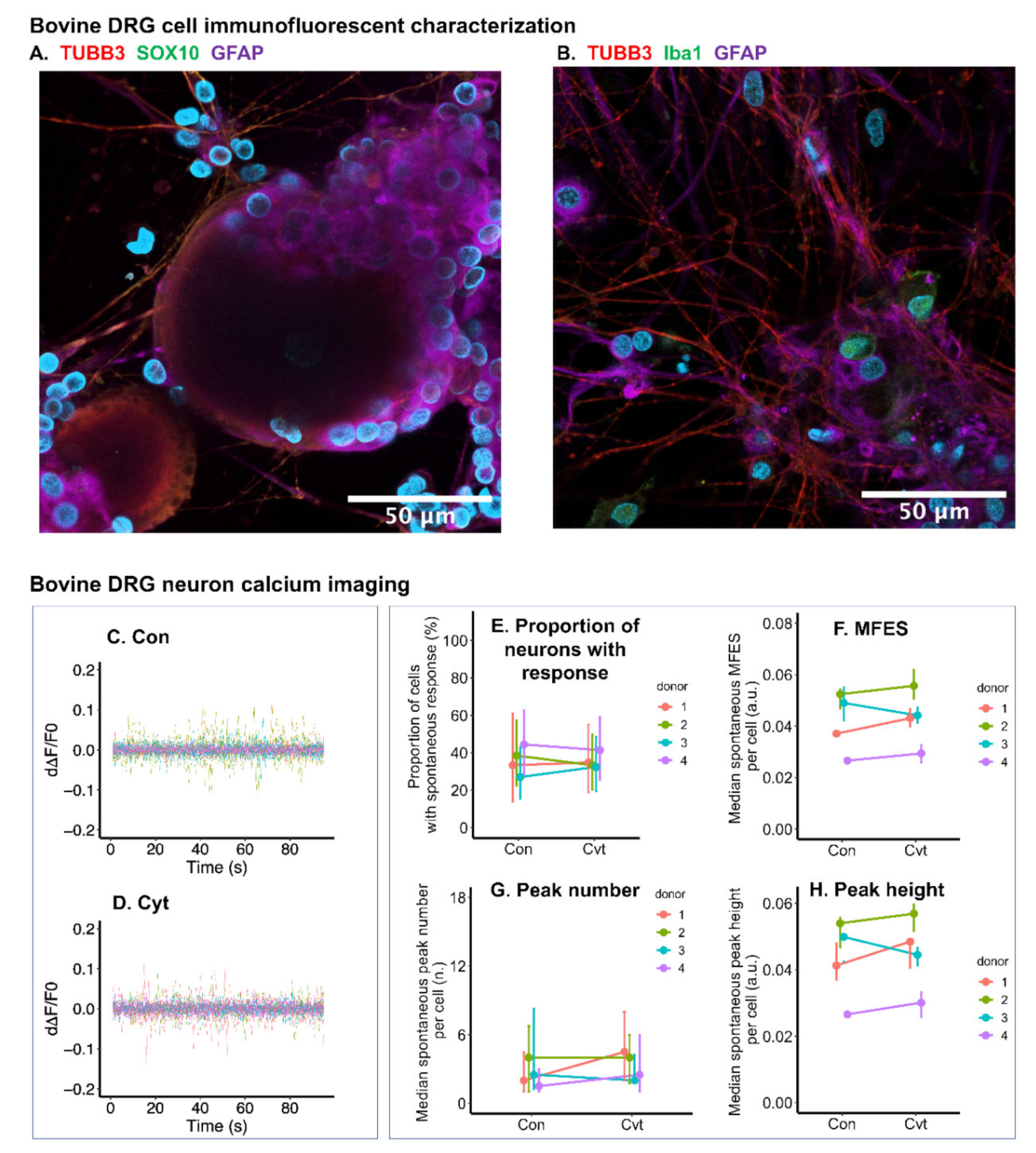
2.8. Inflammatory Cytokines Had Limited Effect on Calcium Signal of Bovine DRG Neurons
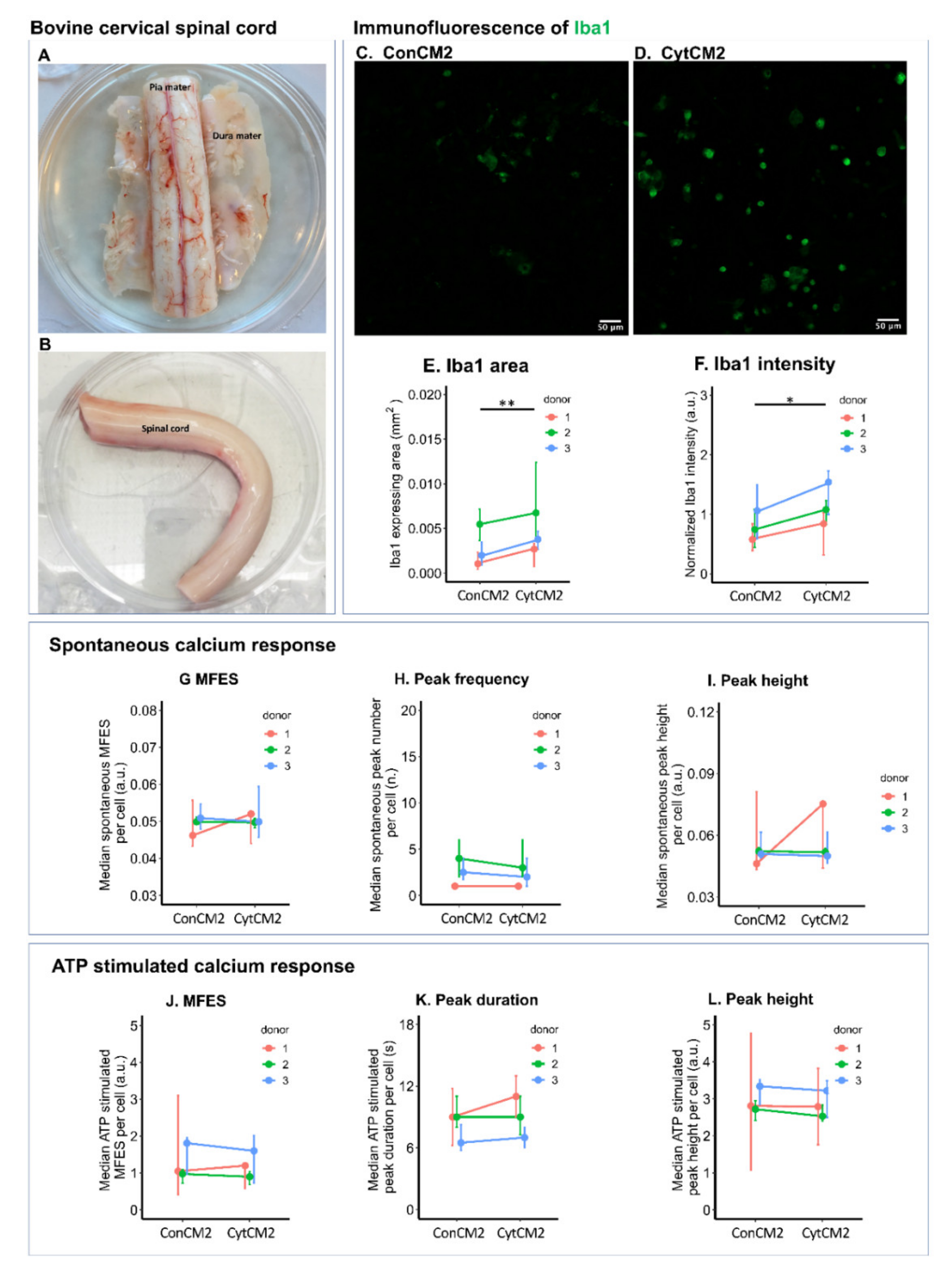
2.9. Iba1 Expression of Bovine Microglia Was Upregulated by Cytokine-Treated Bovine DRG Cells
3. Discussion
4. Materials and Methods
4.1. Cell Source and Study Design
4.2. Isolation and Culture of Primary DRG Cells Dissociated from Neonatal Rats
4.3. Pro-Inflammatory Cytokine Treatment
4.4. Calcium Imaging of Primary Neonatal Rat DRG Cells
4.5. Neonatal Rat DRG Cell-Conditioned Medium Preparation for MGC Stimulation
4.6. CGRP Immunofluorescence of the Neonatal Rat DRG Cells
4.7. Isolation and Culture of Primary MGC from Neonatal Rat Spinal Cords
4.8. Stimulation of Neonatal Rat MGC by DRG-Conditioned Medium
4.9. Immunofluorescence of Neonatal Rat MGC
4.10. Glia Shape Analysis
4.11. Calcium Imaging of Neonatal Rat MGC
4.12. Bovine Primary DRG Cell Culture
4.13. Bovine DRG Cell Cytokine Treatmemt and Calcium Imaging
4.14. Bovine DRG Cell Type Characterization Using Immunofluorescence
4.15. Bovine Spinal Cord Microglia Culture
4.16. Immunofluorescence and Calcium Imaging of Bovine Microglia Stimulated by the Second DRG-Conditioned Medium
4.17. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LBP | low back pain |
| IVD | intervertebral disc |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| DRG | dorsal root ganglion |
| AP | action potentials |
| PGE2 | prostaglandin E2 |
| CD11b | cluster of differentiation molecule 11-b |
| GFAP | glial fibrillary acidic protein |
| MGC | mixed glial culture |
| MFES | maximum fluorescent elevation speed |
| CGRP | calcitonin gene-related peptide |
| TUBB3 | tubulin β-III |
| ATP | adenosine triphosphate |
| ROI | region of interest |
| PBS | phosphate buffered saline |
| NGF | nerve growth factor |
References
- Middendorp, M.; Vogl, T.J.; Kollias, K.; Kafchitsas, K.; Khan, M.F.; Maataoui, A. Association between intervertebral disc degeneration and the Oswestry Disability Index. J. Back Musculoskelet. Rehabil. 2017, 30, 819–823. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Kepler, C.K.; Markova, D.Z.; Dibra, F.; Yadla, S.; Vaccaro, A.R.; Risbud, M.V.; Albert, T.J.; Anderson, D.G. Expression and Relationship of Proinflammatory Chemokine RANTES/CCL5 and Cytokine IL-1 beta in Painful Human Intervertebral Discs. Spine 2013, 38, 873–880. [Google Scholar] [CrossRef]
- Wang, H.; Schiltenwof, M.; Buchner, M. The role of TNF-alpha in patients with chronic low back pain—A prospective comparative longitudinal study. Clin. J. Pain 2008, 24, 273–278. [Google Scholar] [CrossRef]
- Teodorczyk-Injeyan, J.A.; Triano, J.J.; Injeyan, H.S. Nonspecific Low Back Pain: Inflammatory Profiles of Patients with Acute and Chronic Pain. Clin. J. Pain 2019, 35, 818–825. [Google Scholar] [CrossRef]
- Weber, K.T.; Alipui, D.O.; Sison, C.P.; Bloom, O.; Quraishi, S.; Overby, M.C.; Levine, M.; Chahine, N.O. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis. Res. Ther. 2016, 18, 3. [Google Scholar] [CrossRef] [Green Version]
- Lai, A.; Moon, A.; Purmessur, D.; Skovrlj, B.; Laudier, D.M.; Winkelstein, B.A.; Cho, S.K.; Hecht, A.C.; Iatridis, J.C. Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. Spine J. 2016, 16, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Evashwick-Rogler, T.W.; Lai, A.; Watanabe, H.; Salandra, J.M.; Winkelstein, B.A.; Cho, S.K.; Hecht, A.C.; Iatridis, J.C. Inhibiting tumor necrosis factor-alpha at time of induced intervertebral disc injury limits long-term pain and degeneration in a rat model. JOR Spine 2018, 1, e1014. [Google Scholar] [CrossRef] [PubMed]
- Hackel, D.; Brack, A.; Rittner, H.L. Chapter 19–Leukocytes as Mediators of Pain and Analgesia. In NeuroImmune Biology; Arnason, B.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 9, pp. 237–250. [Google Scholar]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Vanderwall, A.G.; Milligan, E.D. Cytokines in Pain: Harnessing Endogenous Anti-Inflammatory Signaling for Improved Pain Management. Front. Immunol. 2019, 10, 3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Pang, R.P.; Shen, K.F.; Zimmermann, M.; Xin, W.J.; Li, Y.Y.; Liu, X.G. TNF-alpha enhances the currents of voltage gated sodium channels in uninjured dorsal root ganglion neurons following motor nerve injury. Exp. Neurol. 2011, 227, 279–286. [Google Scholar] [CrossRef]
- Binshtok, A.M.; Wang, H.; Zimmermann, K.; Amaya, F.; Vardeh, D.; Shi, L.; Brenner, G.J.; Ji, R.R.; Bean, B.P.; Woolf, C.J.; et al. Nociceptors are interleukin-1beta sensors. J. Neurosci. 2008, 28, 14062–14073. [Google Scholar] [CrossRef] [Green Version]
- Black, B.J.; Atmaramani, R.; Kumaraju, R.; Plagens, S.; Romero-Ortega, M.; Dussor, G.; Price, T.J.; Campbell, Z.T.; Pancrazio, J.J. Adult mouse sensory neurons on microelectrode arrays exhibit increased spontaneous and stimulus-evoked activity in the presence of interleukin-6. J. Neurophysiol. 2018, 120, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. S1), S10–S28. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Dubner, R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010, 16, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a020420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farber, K.; Kettenmann, H. Functional role of calcium signals for microglial function. Glia 2006, 54, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Garrison, C.J.; Dougherty, P.M.; Kajander, K.C.; Carlton, S.M. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991, 565, 1–7. [Google Scholar] [CrossRef]
- Raghavendra, V.; Tanga, F.Y.; DeLeo, J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004, 20, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Liu, B.; Wang, L.; Ren, K.; Qiao, J.T.; Berman, B.M.; Lao, L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain 2005, 118, 125–136. [Google Scholar] [CrossRef]
- Shi, Y.; Gelman, B.B.; Lisinicchia, J.G.; Tang, S.J. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 10833–10840. [Google Scholar] [CrossRef] [Green Version]
- Miyagi, M.; Millecamps, M.; Danco, A.T.; Ohtori, S.; Takahashi, K.; Stone, L.S. ISSLS Prize winner: Increased innervation and sensory nervous system plasticity in a mouse model of low back pain due to intervertebral disc degeneration. Spine (Phila Pa 1976) 2014, 39, 1345–1354. [Google Scholar] [CrossRef]
- Yang, G.; Chen, L.; Gao, Z.; Wang, Y. Implication of microglia activation and CSF-1/CSF-1Rpathway in lumbar disc degeneration-related back pain. Mol. Pain 2018, 14, 1744806918811238. [Google Scholar] [CrossRef] [Green Version]
- Krock, E.; Millecamps, M.; Anderson, K.M.; Srivastava, A.; Reihsen, T.E.; Hari, P.; Sun, Y.R.; Jang, S.H.; Wilcox, G.L.; Belani, K.G.; et al. Interleukin-8 as a therapeutic target for chronic low back pain: Upregulation in human cerebrospinal fluid and pre-clinical validation with chronic reparixin in the SPARC-null mouse model. EBioMedicine 2019, 43, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Handa, J.; Sekiguchi, M.; Krupkova, O.; Konno, S. The effect of serotonin-noradrenaline reuptake inhibitor duloxetine on the intervertebral disk-related radiculopathy in rats. Eur. Spine J. 2016, 25, 877–887. [Google Scholar] [CrossRef]
- Zhuang, Z.Y.; Wen, Y.R.; Zhang, D.R.; Borsello, T.; Bonny, C.; Strichartz, G.R.; Decosterd, I.; Ji, R.R. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 3551–3560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padi, S.S.; Kulkarni, S.K. Minocycline prevents the development of neuropathic pain, but not acute pain: Possible anti-inflammatory and antioxidant mechanisms. Eur. J. Pharmacol. 2008, 601, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hathway, G.J.; Vega-Avelaira, D.; Moss, A.; Ingram, R.; Fitzgerald, M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain 2009, 144, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberberger, R.V.; Barry, C.; Dominguez, N.; Matusica, D. Human Dorsal Root Ganglia. Front. Cell. Neurosci. 2019, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; Corradini, L.; Just, S.; Arndt, K.; Doods, H. The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. Pain 2013, 154, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Saegusa, H.; Tanabe, T. N-type voltage-dependent Ca2+ channel in non-excitable microglial cells in mice is involved in the pathophysiology of neuropathic pain. Biochem. Biophys. Res. Commun. 2014, 450, 142–147. [Google Scholar] [CrossRef]
- Finzsch, M.; Schreiner, S.; Kichko, T.; Reeh, P.; Tamm, E.R.; Bösl, M.R.; Meijer, D.; Wegner, M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J. Cell Biol. 2010, 189, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Hoyland, J.A.; Le Maitre, C.; Freemont, A.J. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology 2008, 47, 809–814. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, S.; Taira, A.; Inoue, G.; Koshi, T.; Ito, T.; Yamashita, M.; Yamauchi, K.; Suzuki, M.; Takahashi, K.; Ohtori, S. TNF-alpha in nucleus pulposus induces sensory nerve growth—A study of the mechanism of discogenic low back pain using TNF-alpha-deficient mice. Spine 2008, 33, 1542–1546. [Google Scholar] [CrossRef]
- Leung, L.; Cahill, C.M. TNF-alpha and neuropathic pain—A review. J. Neuroinflammation 2010, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Chapman, C.R.; Vierck, C.J. The Transition of Acute Postoperative Pain to Chronic Pain: An Integrative Overview of Research on Mechanisms. J. Pain 2017, 18, 359.e1–359.e38. [Google Scholar] [CrossRef]
- Nguyen, C.; Upadhyay, H.; Murphy, M.; Borja, G.; Rozsahegyi, E.J.; Barnett, A.; Brookings, T.; McManus, O.B.; Werley, C.A. Simultaneous voltage and calcium imaging and optogenetic stimulation with high sensitivity and a wide field of view. Biomed. Opt. Express. 2019, 10, 789–806. [Google Scholar] [CrossRef]
- Bradesi, S. Role of spinal cord glia in the central processing of peripheral pain perception. Neurogastroenterol. Motil. 2010, 22, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Seybold, V.S.; Galeazza, M.T.; Garry, M.G.; Hargreaves, K.M. Plasticity of calcitonin gene related peptide neurotransmission in the spinal cord during peripheral inflammation. Can. J. Physiol. Pharmacol. 1995, 73, 1007–1014. [Google Scholar] [CrossRef]
- Romero-Sandoval, A.; Chai, N.; Nutile-McMenemy, N.; Deleo, J.A. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008, 1219, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Bardoni, R.; Ghirri, A.; Zonta, M.; Betelli, C.; Vitale, G.; Ruggieri, V.; Sandrini, M.; Carmignoto, G. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J. Physiol. 2010, 588, 831–846. [Google Scholar] [CrossRef]
- Kofuji, P.; Araque, A. G-Protein-Coupled Receptors in Astrocyte-Neuron Communication. Neuroscience 2021, 456, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Weyer, A.D.; Zappia, K.J.; Garrison, S.R.; O’Hara, C.L.; Dodge, A.K.; Stucky, C.L. Nociceptor Sensitization Depends on Age and Pain Chronicity(1,2,3). eNeuro 2016, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wangzhou, A.; McIlvried, L.A.; Paige, C.; Barragan-Iglesias, P.; Shiers, S.; Ahmad, A.; Guzman, C.A.; Dussor, G.; Ray, P.R.; Gereau, R.W.I.V.; et al. Pharmacological target-focused transcriptomic analysis of native vs cultured human and mouse dorsal root ganglia. PAIN 2020, 161, 1497–1517. [Google Scholar] [CrossRef]
- Kupari, J.; Usoskin, D.; Parisien, M.; Lou, D.; Hu, Y.; Fatt, M.; Lonnerberg, P.; Spangberg, M.; Eriksson, B.; Barkas, N.; et al. Single cell transcriptomics of primate sensory neurons identifies cell types associated with chronic pain. Nat. Commun. 2021, 12, 1510. [Google Scholar] [CrossRef]
- Fadda, A.; Bartschi, M.; Hemphill, A.; Widmer, H.R.; Zurbriggen, A.; Perona, P.; Vidondo, B.; Oevermann, A. Primary Postnatal Dorsal Root Ganglion Culture from Conventionally Slaughtered Calves. PLoS ONE 2016, 11, e0168228. [Google Scholar] [CrossRef] [Green Version]
- Rostock, C.; Schrenk-Siemens, K.; Pohle, J.; Siemens, J. Human vs. Mouse Nociceptors–Similarities and Differences. Neuroscience 2018, 387, 13–27. [Google Scholar] [CrossRef]
- Joukal, M.; Klusakova, I.; Dubovy, P. Direct communication of the spinal subarachnoid space with the rat dorsal root ganglia. Ann. Anat. 2016, 205, 9–15. [Google Scholar] [CrossRef]
- Grace, P.M.; Hutchinson, M.R.; Maier, S.F.; Watkins, L.R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 2014, 14, 217–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef]
- Milligan, E.D.; Zapata, V.; Chacur, M.; Schoeniger, D.; Biedenkapp, J.; O′Connor, K.A.; Verge, G.M.; Chapman, G.; Green, P.; Foster, A.C.; et al. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur. J. Neurosci. 2004, 20, 2294–2302. [Google Scholar] [CrossRef]
- Wolf, Y.; Yona, S.; Kim, K.W.; Jung, S. Microglia, seen from the CX3CR1 angle. Front. Cell. Neurosci. 2013, 7, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Shi, X.Q.; Echeverry, S.; Mogil, J.S.; De Koninck, Y.; Rivest, S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J. Neurosci. 2007, 27, 12396–12406. [Google Scholar] [CrossRef] [PubMed]
- Delree, P.; Leprince, P.; Schoenen, J.; Moonen, G. Purification and culture of adult rat dorsal root ganglia neurons. J. Neurosci. Res. 1989, 23, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.A.; Davis, B.M.; Molliver, D.C. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat. Protoc. 2007, 2, 152–160. [Google Scholar] [CrossRef]
- Patil, V.; O’Connell, E.; Quinlan, L.R.; Fearnhead, H.; McMahon, S.; Pandit, A. A robust platform for high-throughput screening of therapeutic strategies for acute and chronic spinal cord injury. iScience 2021, 24, 102182. [Google Scholar] [CrossRef]
- Fu, M.; Sun, Z.H.; Zong, M.; He, X.P.; Zuo, H.C.; Xie, Z.P. Deoxyschisandrin modulates synchronized Ca2+ oscillations and spontaneous synaptic transmission of cultured hippocampal neurons. Acta. Pharmacol. Sin. 2008, 29, 891–898. [Google Scholar] [CrossRef]
- Ma, J.; Stefanoska, D.; Grad, S.; Alini, M.; Peroglio, M. Direct and Intervertebral Disc-Mediated Sensitization of Dorsal Root Ganglion Neurons by Hypoxia and Low pH. Neurospine 2020, 17, 42–59. [Google Scholar] [CrossRef]
- Simpson, A.W. Fluorescent measurement of [Ca(2)+]c: Basic practical considerations. Methods. Mol. Biol. 2005, 312, 3–36. [Google Scholar] [CrossRef]
- Francis, M.; Waldrup, J.; Qian, X.; Taylor, M.S. Automated Analysis of Dynamic Ca(2+) Signals in Image Sequences. J. Vis. Exp. JoVE 2014, 51560. [Google Scholar] [CrossRef] [Green Version]
- Richner, M.; Jager, S.B.; Siupka, P.; Vaegter, C.B. Hydraulic Extrusion of the Spinal Cord and Isolation of Dorsal Root Ganglia in Rodents. J. Vis. Exp. 2017, 119, 55226. [Google Scholar] [CrossRef] [Green Version]
- Flamant, Q.; Stanciuc, A.M.; Pavailler, H.; Sprecher, C.M.; Alini, M.; Peroglio, M.; Anglada, M. Roughness gradients on zirconia for rapid screening of cell-surface interactions: Fabrication, characterization and application. J. Biomed. Mater. Res. A 2016, 104, 2502–2514. [Google Scholar] [CrossRef]
- Caldeira, C.; Oliveira, A.F.; Cunha, C.; Vaz, A.R.; Falcao, A.S.; Fernandes, A.; Brites, D. Microglia change from a reactive to an age-like phenotype with the time in culture. Front. Cell. Neurosci. 2014, 8, 152. [Google Scholar] [CrossRef]
- Smith, A.M.; Gibbons, H.M.; Oldfield, R.L.; Bergin, P.M.; Mee, E.W.; Curtis, M.A.; Faull, R.L.; Dragunow, M. M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. J. Neuroinflammation 2013, 10, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, T.A.; Blackman, A.V.; Oyrer, J.; Jayabal, S.; Chung, A.J.; Watt, A.J.; Sjöström, P.J.; van Meyel, D.J. Neuronal morphometry directly from bitmap images. Nat. Methods 2014, 11, 982–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Stefanoska, D.; Stone, L.S.; Hildebrand, M.; van Donkelaar, C.C.; Zou, X.; Basoli, V.; Grad, S.; Alini, M.; Peroglio, M. Hypoxic stress enhances extension and branching of dorsal root ganglion neuronal outgrowth. JOR Spine 2020, 3, e1090. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Patil, V.; Pandit, A.; Quinlan, L.R.; Finn, D.P.; Grad, S.; Alini, M.; Peroglio, M. In Vitro Model to Investigate Communication between Dorsal Root Ganglion and Spinal Cord Glia. Int. J. Mol. Sci. 2021, 22, 9725. https://doi.org/10.3390/ijms22189725
Ma J, Patil V, Pandit A, Quinlan LR, Finn DP, Grad S, Alini M, Peroglio M. In Vitro Model to Investigate Communication between Dorsal Root Ganglion and Spinal Cord Glia. International Journal of Molecular Sciences. 2021; 22(18):9725. https://doi.org/10.3390/ijms22189725
Chicago/Turabian StyleMa, Junxuan, Vaibhav Patil, Abhay Pandit, Leo R. Quinlan, David P. Finn, Sibylle Grad, Mauro Alini, and Marianna Peroglio. 2021. "In Vitro Model to Investigate Communication between Dorsal Root Ganglion and Spinal Cord Glia" International Journal of Molecular Sciences 22, no. 18: 9725. https://doi.org/10.3390/ijms22189725
APA StyleMa, J., Patil, V., Pandit, A., Quinlan, L. R., Finn, D. P., Grad, S., Alini, M., & Peroglio, M. (2021). In Vitro Model to Investigate Communication between Dorsal Root Ganglion and Spinal Cord Glia. International Journal of Molecular Sciences, 22(18), 9725. https://doi.org/10.3390/ijms22189725