Impacts of Hypoxia on Osteoclast Formation and Activity: Systematic Review
Abstract
1. Introduction
2. Results
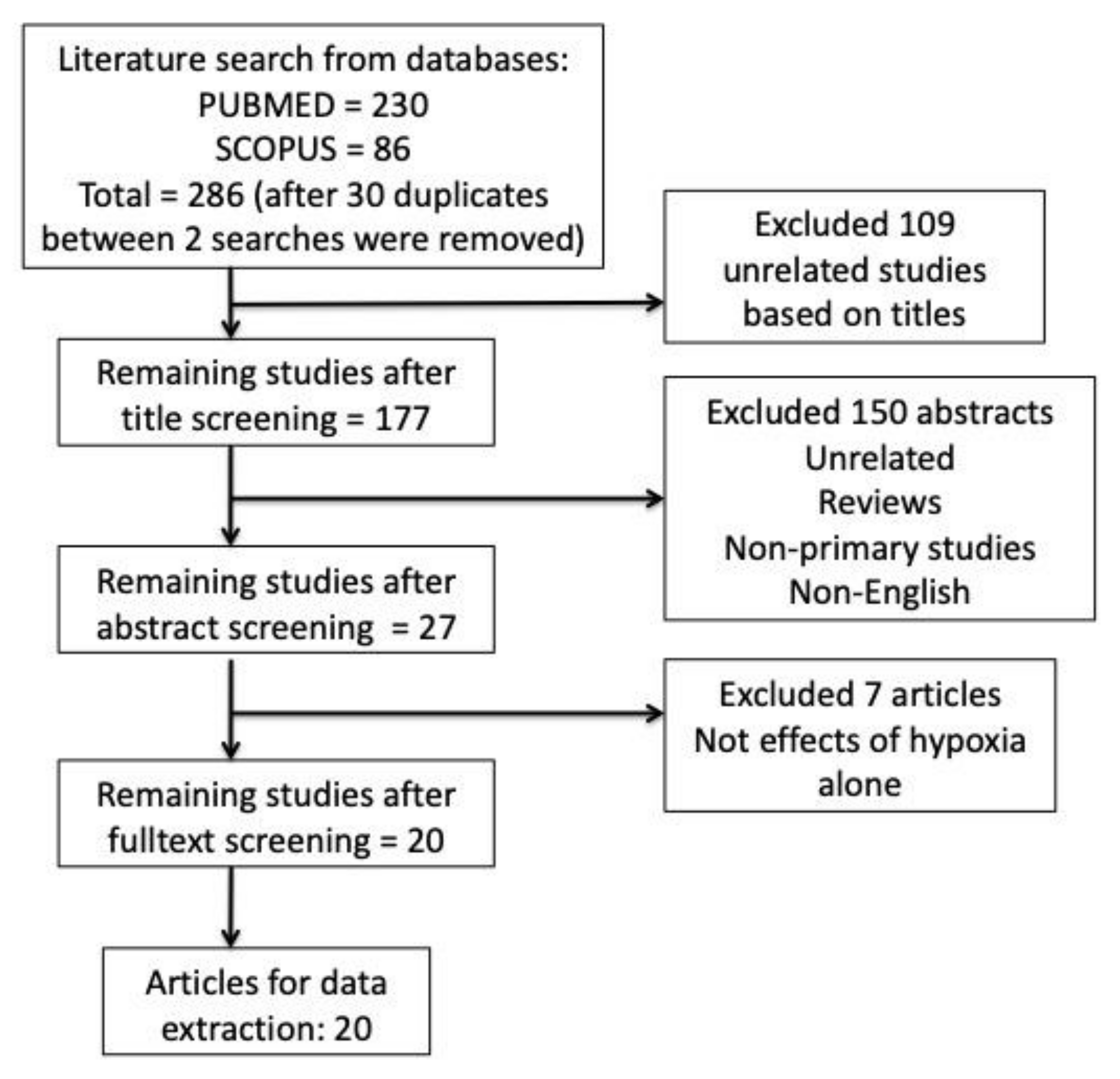
2.1. Search Results
2.2. Study Characteristics
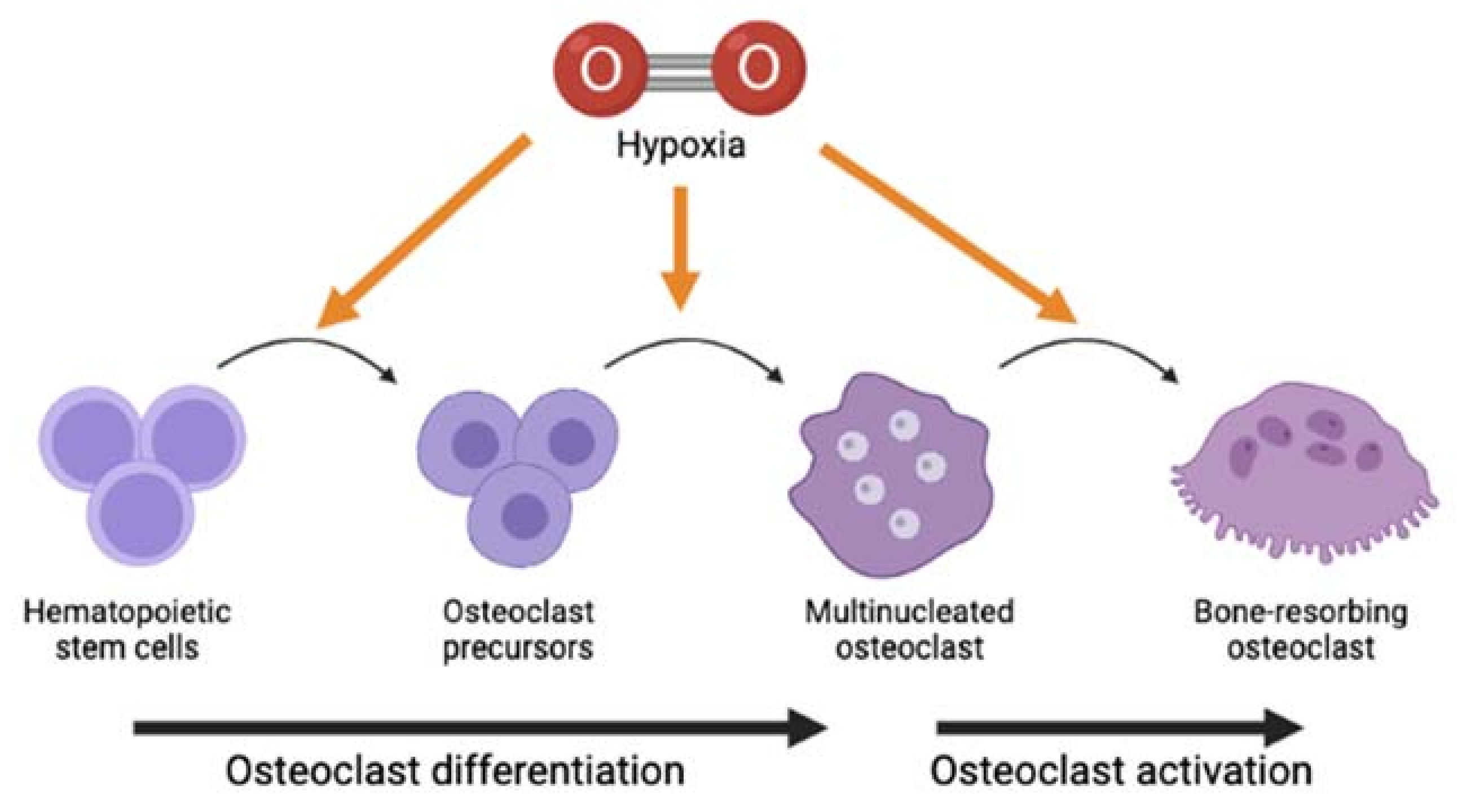
2.3. Effects of Hypoxia on Formation and Activity of Osteoclasts In Vitro
2.4. Effects of Hypoxia on Formation and Activity of Osteoclasts In Vivo
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crotti, T.N.; Dharmapatni, A.A.; Alias, E.; Haynes, D.R. Osteoimmunology: Major and Costimulatory Pathway Expression Associated with Chronic Inflammatory Induced Bone Loss. J. Immunol. Res. 2015, 2015, 281287. [Google Scholar] [CrossRef]
- Crotti, T.N.; Dharmapatni, A.A.; Alias, E.; Zannettino, A.C.; Smith, M.D.; Haynes, D.R. The immunoreceptor tyrosine-based activation motif (ITAM)-related factors are increased in synovial tissue and vasculature of rheumatoid arthritic joints. Arthritis Res. Ther. 2012, 14, R245. [Google Scholar] [CrossRef]
- Radzi, N.F.M.; Ismail, N.A.S.; Alias, E. Tocotrienols Regulate Bone Loss through Suppression on Osteoclast Differentiation and Activity: A Systematic Review. Curr. Drug Targets 2018, 19, 1095–1107. [Google Scholar] [CrossRef]
- Gorissen, B.; de Bruin, A.; Miranda-Bedate, A.; Korthagen, N.; Wolschrijn, C.; de Vries, T.J.; van Weeren, R.; Tryfonidou, M.A. Hypoxia negatively affects senescence in osteoclasts and delays osteoclastogenesis. J. Cell. Physiol. 2018, 234, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Fang, C.; Terao, C.; Giannopoulou, E.G.; Lee, Y.J.; Lee, M.J.; Mun, S.H.; Bae, S.; Qiao, Y.; Yuan, R.; et al. Hypoxia-Sensitive COMMD1 Integrates Signaling and Cellular Metabolism in Human Macrophages and Suppresses Osteoclastogenesis. Immunity 2017, 47, 66–79. [Google Scholar] [CrossRef]
- Hulley, P.A.; Bishop, T.; Vernet, A.; Schneider, J.E.; Edwards, J.R.; Athanasou, N.A.; Knowles, H.J. Hypoxia-inducible factor 1-alpha does not regulate osteoclastogenesis but enhances bone resorption activity via prolyl-4-hydroxylase 2. J. Pathol. 2017, 242, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Utting, J.C.; Flanagan, A.M.; Brandao-Burch, A.; Orriss, I.R.; Arnett, T.R. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem. Funct. 2010, 28, 374–380. [Google Scholar] [CrossRef]
- Knowles, H.J.; Athanasou, N.A. Acute hypoxia and osteoclast activity: A balance between enhanced resorption and increased apoptosis. J. Pathol. 2009, 218, 256–264. [Google Scholar] [CrossRef]
- Muzylak, M.; Price, J.S.; Horton, M.A. Hypoxia induces giant osteoclast formation and extensive bone resorption in the cat. Calcif. Tissue Int. 2006, 79, 301–309. [Google Scholar] [CrossRef]
- Tang, Y.; Zhu, J.; Huang, D.; Hu, X.; Cai, Y.; Song, X.; Song, Z.; Hong, C.; Feng, Z.; Kang, F. Mandibular osteotomy-induced hypoxia enhances osteoclast activation and acid secretion by increasing glycolysis. J. Cell. Physiol. 2019, 234, 11165–11175. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.T.; Chen, M.Y.; Tu, M.G.; Wang, I.K.; Chang, S.S.; Li, C.Y. MicroRNA-20a regulates autophagy related protein-ATG16L1 in hypoxia-induced osteoclast differentiation. Bone 2015, 73, 145–153. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Zhang, W.; Xu, N.; Zhu, J.Y.; Jia, J.; Sun, Z.J.; Wang, Y.N.; Zhao, Y.F. Autophagy regulates hypoxia-induced osteoclastogenesis through the HIF-1alpha/BNIP3 signaling pathway. J. Cell. Physiol. 2012, 227, 639–648. [Google Scholar] [CrossRef]
- Leger, A.J.; Altobelli, A.; Mosquea, L.M.; Belanger, A.J.; Song, A.; Cheng, S.H.; Jiang, C.; Yew, N.S. Inhibition of osteoclastogenesis by prolyl hydroxylase inhibitor dimethyloxallyl glycine. J. Bone Miner. Metab. 2010, 28, 510–519. [Google Scholar] [CrossRef]
- Srinivasan, S.; Avadhani, N.G. Hypoxia-mediated mitochondrial stress in RAW264.7 cells induces osteoclast-like TRAP-positive cells. Ann. N.Y. Acad. Sci. 2007, 1117, 51–61. [Google Scholar] [CrossRef]
- Nomura, T.; Aoyama, M.; Waguri-Nagaya, Y.; Goto, Y.; Suzuki, M.; Miyazawa, K.; Asai, K.; Goto, S. Tumor necrosis factor stimulates osteoclastogenesis from human bone marrow cells under hypoxic conditions. Exp. Cell Res. 2014, 321, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, H.; Cheng, G.; Shang, W.; Zhang, S. High levels of HIF-1ɑ in hypoxic dental pulps associated with teeth with severe periodontitis. J. Mol. Histol. 2020, 51, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yu, R.; Zhao, J.; Sun, L.; Jian, L.; Li, C.; Liu, X. Constant hypoxia inhibits osteoclast differentiation and bone resorption by regulating phosphorylation of JNK and IκBα. Inflamm. Res. 2019, 68, 157–166. [Google Scholar] [CrossRef]
- Fukuoka, H.; Aoyama, M.; Miyazawa, K.; Asai, K.; Goto, S. Hypoxic stress enhances osteoclast differentiation via increasing IGF2 production by non-osteoclastic cells. Biochem. Biophys. Res. Commun. 2005, 328, 885–894. [Google Scholar] [CrossRef]
- Arnett, T.R.; Gibbons, D.C.; Utting, J.C.; Orriss, I.R.; Hoebertz, A.; Rosendaal, M.; Meghji, S. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J. Cell. Physiol. 2003, 196, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, J.; Sun, D.; Xin, J.; Wang, L.; Huang, D.; Wu, W.; Xian, C.J. Short-Term Hypoxia Accelerates Bone Loss in Ovariectomized Rats by Suppressing Osteoblastogenesis but Enhancing Osteoclastogenesis. Med. Sci. Monit. 2016, 22, 2962–2971. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Yan, H.C.; Zhang, J.; Qu, H.D.; Qiu, X.S.; Chen, L.; Li, S.J.; Cao, X.; Bean, J.C.; Chen, L.H.; et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J. Neurosci. 2010, 30, 12653–12663. [Google Scholar] [CrossRef]
- Durand, M.; Collombet, J.M.; Frasca, S.; Sarilar, V.; Lataillade, J.J.; Le Bousse-Kerdilès, M.C.; Holy, X. Separate and combined effects of hypobaric hypoxia and hindlimb suspension on skeletal homeostasis and hematopoiesis in mice. Hypoxia 2019, 7, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, L.; Matte, A.; Valenti, M.T.; Siciliano, A.; Mori, A.; Schweiger, V.; Zampieri, G.; Perbellini, L.; De Franceschi, L. Hypoxia-reperfusion affects osteogenic lineage and promotes sickle cell bone disease. Blood 2015, 126, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Takemori, T.; Kawamoto, T.; Ueha, T.; Toda, M.; Morishita, M.; Kamata, E.; Fukase, N.; Hara, H.; Fujiwara, S.; Niikura, T.; et al. Transcutaneous carbon dioxide application suppresses bone destruction caused by breast cancer metastasis. Oncol. Rep. 2018, 40, 2079–2087. [Google Scholar] [CrossRef]
- Alias, E.; Dharmapatni, A.S.; Holding, A.C.; Atkins, G.J.; Findlay, D.M.; Howie, D.W.; Crotti, T.N.; Haynes, D.R. Polyethylene particles stimulate expression of ITAM-related molecules in peri-implant tissues and when stimulating osteoclastogenesis in vitro. Acta Biomater. 2012, 8, 3104–3112. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast Activity and Subtypes as a Function of Physiology and Pathology—Implications for Future Treatments of Osteoporosis. Endocr. Rev. 2011, 32, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Kuschel, A.; Simon, P.; Tug, S. Functional regulation of HIF-1alpha under normoxia--is there more than post-translational regulation? J. Cell. Physiol. 2012, 227, 514–524. [Google Scholar] [CrossRef]
- Zhong, H.; Cao, C.; Yang, J.; Huang, Q. Research on relationship of HIF-1 Signaling pathway and postmenstrual osteoporosis. J. Sichuan Univ. China Med Sci. Ed. 2017, 48, 862–868. [Google Scholar]
- Utting, J.C.; Robins, S.P.; Brandao-Burch, A.; Orriss, I.R.; Behar, J.; Arnett, T.R. Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp. Cell Res. 2006, 312, 1693–1702. [Google Scholar] [CrossRef]
- Li, B.; Lee, W.C.; Song, C.; Ye, L.; Abel, E.D.; Long, F. Both aerobic glycolysis and mitochondrial respiration are required for osteoclast differentiation. FASEB J. 2020, 34, 11058–11067. [Google Scholar] [CrossRef]
- Lemma, S.; Sboarina, M.; Porporato, P.E.; Zini, N.; Sonveaux, P.; Di Pompo, G.; Baldini, N.; Avnet, S. Energy metabolism in osteoclast formation and activity. Int. J. Biochem. Cell Biol. 2016, 79, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Roiniotis, J.; Dinh, H.; Masendycz, P.; Turner, A.; Elsegood, C.L.; Scholz, G.M.; Hamilton, J.A. Hypoxia prolongs monocyte/macrophage survival and enhanced glycolysis is associated with their maturation under aerobic conditions. J. Immunol. 2009, 182, 7974–7981. [Google Scholar] [CrossRef]
- Diepenhorst, N.A.; Nowell, C.J.; Rueda, P.; Henriksen, K.; Pierce, T.; Cook, A.E.; Pastoureau, P.; Sabatini, M.; Charman, W.N.; Christopoulos, A.; et al. High throughput, quantitative analysis of human osteoclast differentiation and activity. Anal. Biochem. 2017, 519, 51–56. [Google Scholar] [CrossRef]
- Rissanen, J.P.; Suominen, M.I.; Peng, Z.; Halleen, J.M. Secreted tartrate-resistant acid phosphatase 5b is a Marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif. Tissue Int. 2008, 82, 108–115. [Google Scholar] [CrossRef]
- Sun, X.; Liang, J.; Wang, C.; Cao, S.; Hu, Y.; Xu, X. Transient Effect of 17beta-estradiol on Osteoporosis in Ovariectomized Rats Accompanied with Unilateral Disuse in the Early Phase. Int. J. Med Sci. 2015, 12, 423–431. [Google Scholar] [CrossRef][Green Version]
- Bozzini, C.; Champin, G.M.; Alippi, R.M.; Bozzini, C.E. Static biomechanics in bone from growing rats exposed chronically to simulated high altitudes. High Alt. Med. Biol. 2013, 14, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Rankin, E.B.; Castellini, L.; Alcudia, J.F.; LaGory, E.L.; Andersen, R.; Rhodes, S.D.; Wilson, T.L.; Mohammad, K.S.; Castillo, A.B.; et al. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev. 2015, 29, 817–831. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Wu, Q.; Ji, Y.C.; Feng, Z.F.; Kang, F.W. HIF-1α facilitates osteocyte-mediated osteoclastogenesis by activating JAK2/STAT3 pathway in vitro. J. Cell. Physiol. 2019, 234, 21182–21192. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Bakiri, L.; Hoebertz, A.; Eferl, R.; Schilling, A.F.; Komnenovic, V.; Scheuch, H.; Priemel, M.; Stewart, C.L.; Amling, M.; et al. Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature 2008, 454, 221–225. [Google Scholar] [CrossRef]
- Jolly, J.J.; Chin, K.Y.; Farhana, M.F.N.; Alias, E.; Chua, K.H.; Hasan, W.N.W.; Ima-Nirwana, S. Optimization of the Static Human Osteoblast/Osteoclast Co-culture System. Iran. J. Med Sci. 2018, 43, 208–213. [Google Scholar]
- Al Hadi, H.; Smerdon, G.R.; Fox, S.W. Hyperbaric oxygen therapy suppresses osteoclast formation and bone resorption. J. Orthop. Res. 2013, 31, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.H.; Aly, F.A.; Gabr, S.A. Effect of Moderate Aerobic Training on Bone Metabolism Indices among Adult Humans. Pak. J. Med Sci. 2014, 30, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Knowles, H.J. Hypoxic regulation of osteoclast differentiation and bone resorption activity. Hypoxia 2015, 3, 73–82. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, J.K.; Mohamad Hazir, N.S.; Alias, E. Impacts of Hypoxia on Osteoclast Formation and Activity: Systematic Review. Int. J. Mol. Sci. 2021, 22, 10146. https://doi.org/10.3390/ijms221810146
Tan JK, Mohamad Hazir NS, Alias E. Impacts of Hypoxia on Osteoclast Formation and Activity: Systematic Review. International Journal of Molecular Sciences. 2021; 22(18):10146. https://doi.org/10.3390/ijms221810146
Chicago/Turabian StyleTan, Jen Kit, Nur Shukriyah Mohamad Hazir, and Ekram Alias. 2021. "Impacts of Hypoxia on Osteoclast Formation and Activity: Systematic Review" International Journal of Molecular Sciences 22, no. 18: 10146. https://doi.org/10.3390/ijms221810146
APA StyleTan, J. K., Mohamad Hazir, N. S., & Alias, E. (2021). Impacts of Hypoxia on Osteoclast Formation and Activity: Systematic Review. International Journal of Molecular Sciences, 22(18), 10146. https://doi.org/10.3390/ijms221810146





