Long-term Interactions of Circulating Neutrophils with Titanium Implants, the Role of Platelets in Regulation of Leukocyte Function
Abstract
:1. Introduction
2. Results
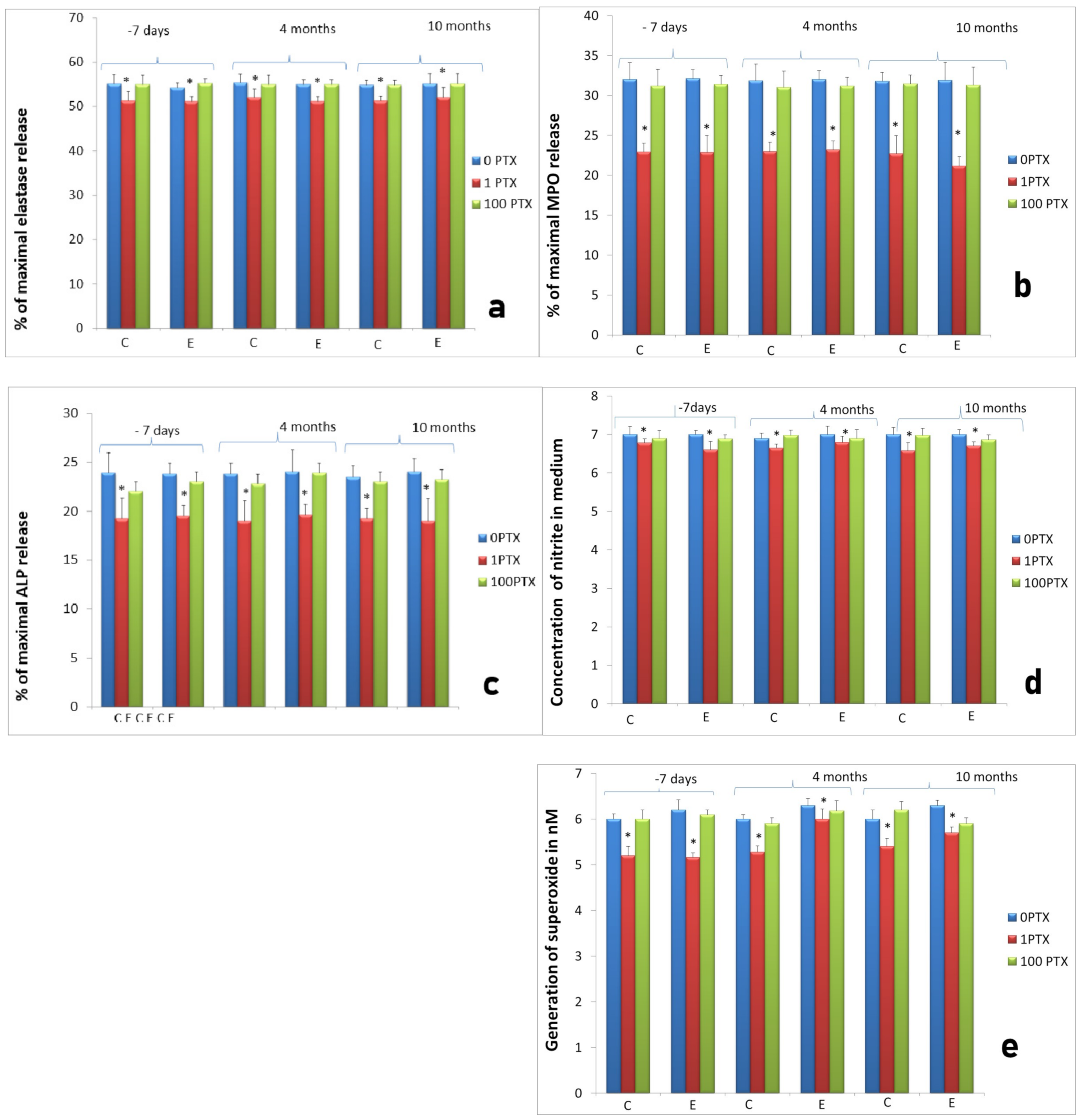
2.1. Clinical Results
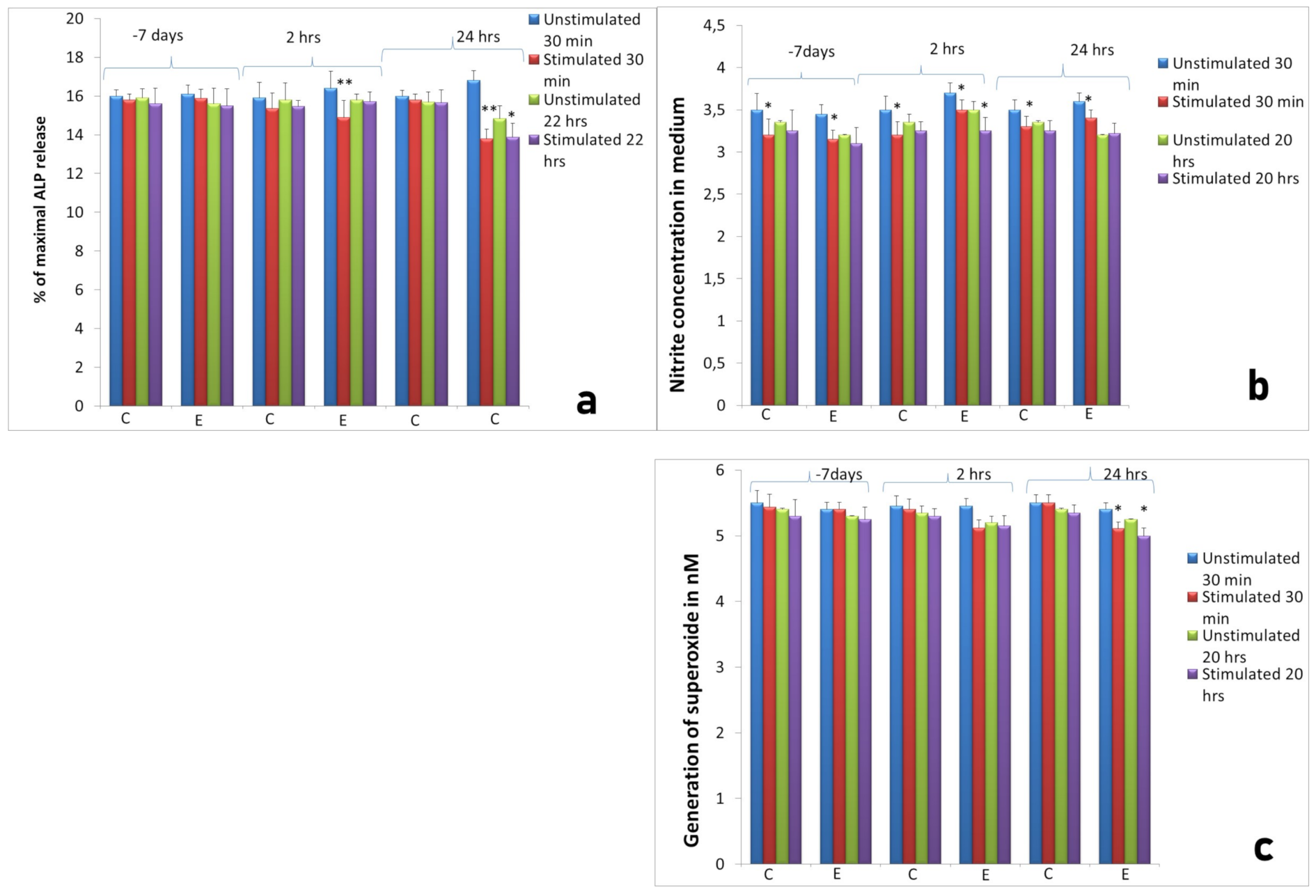
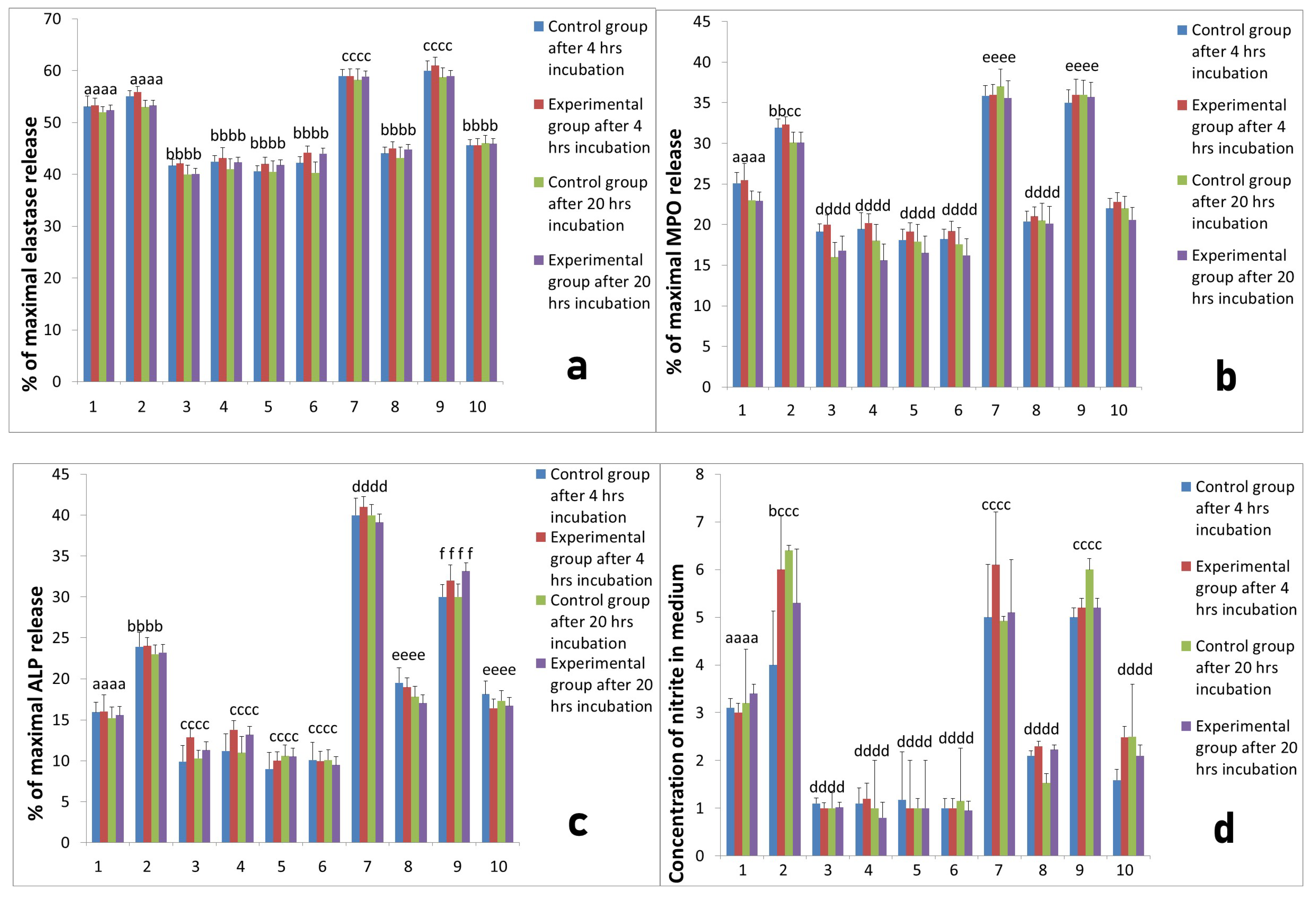
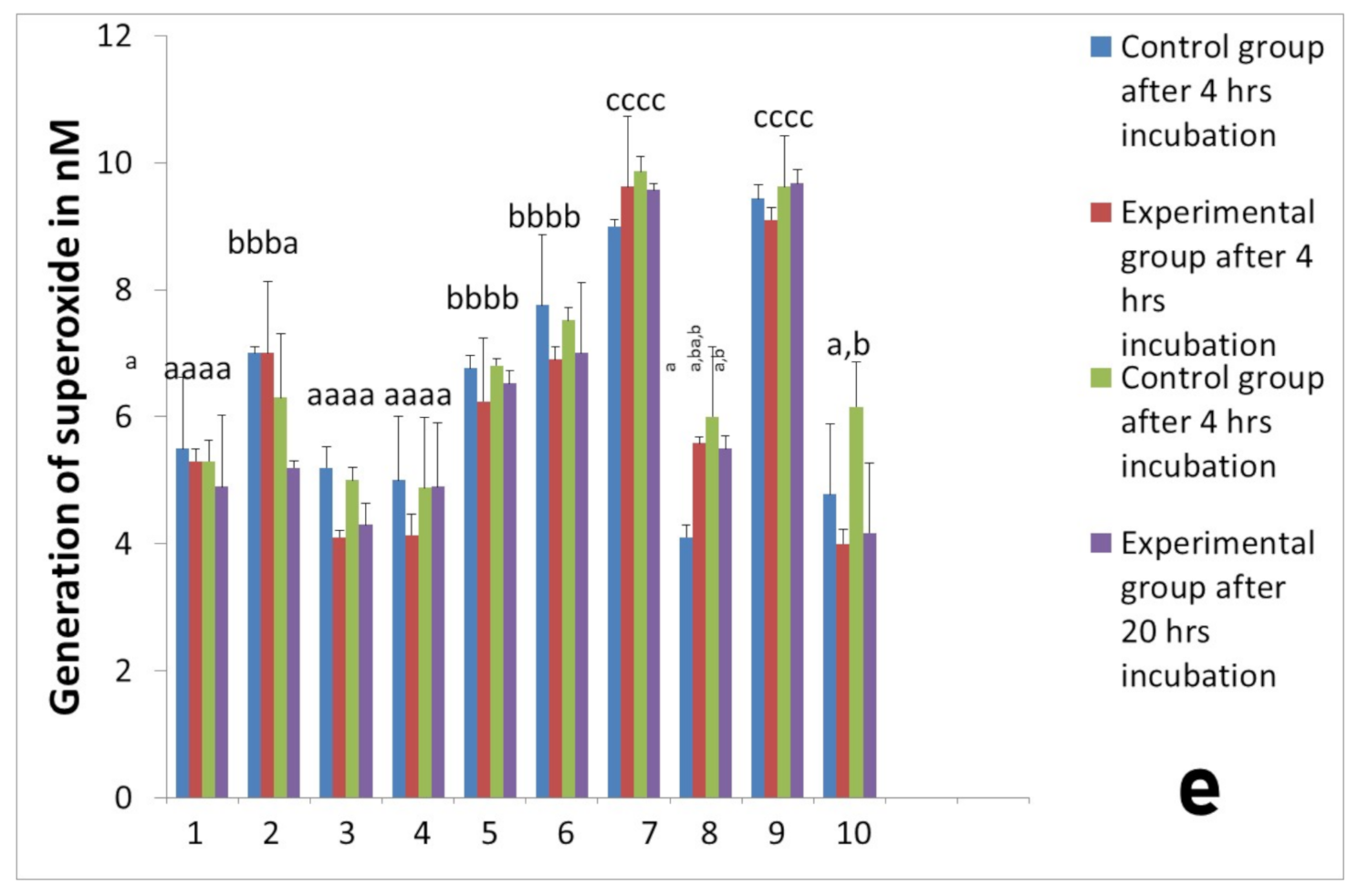
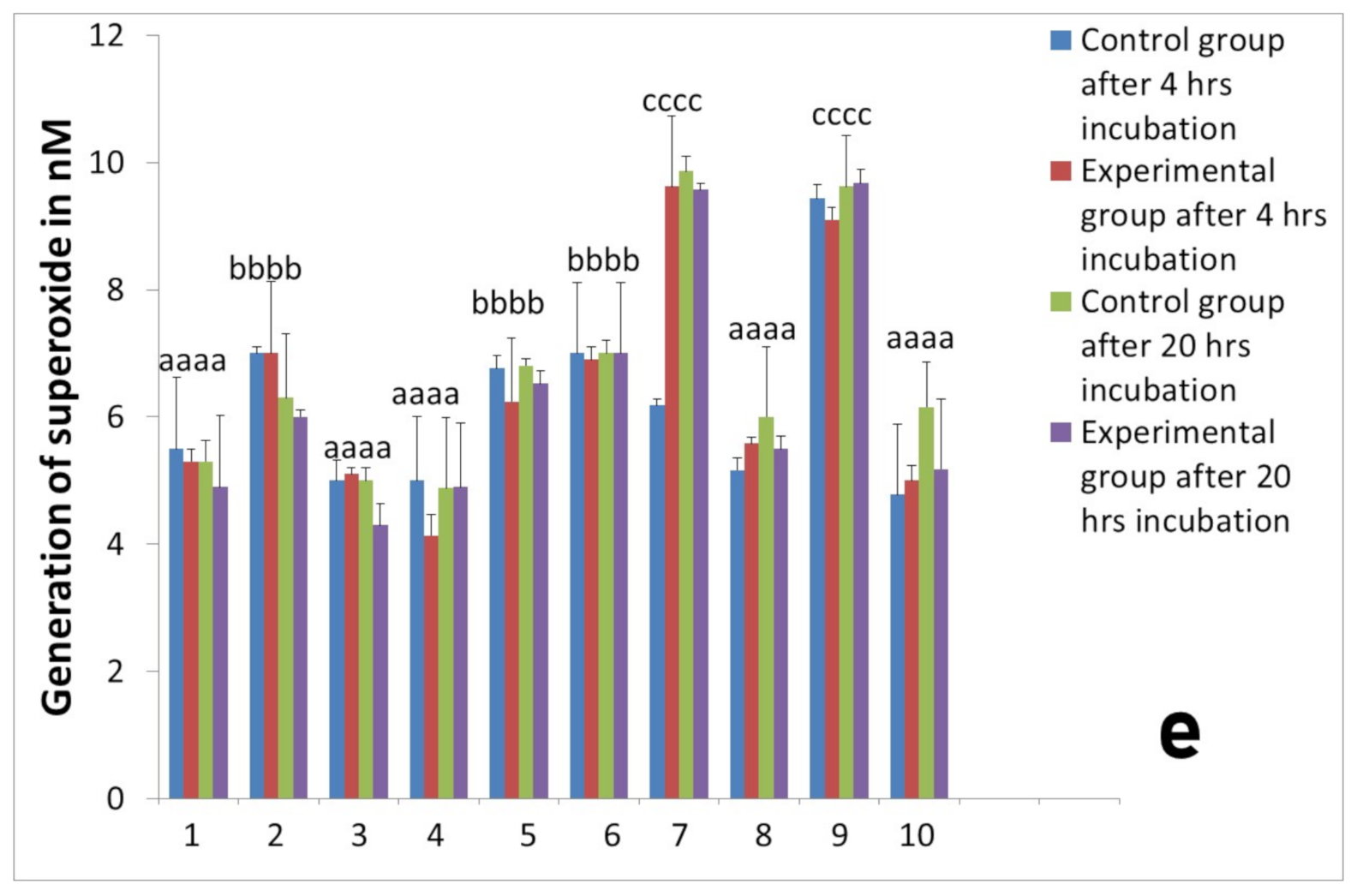
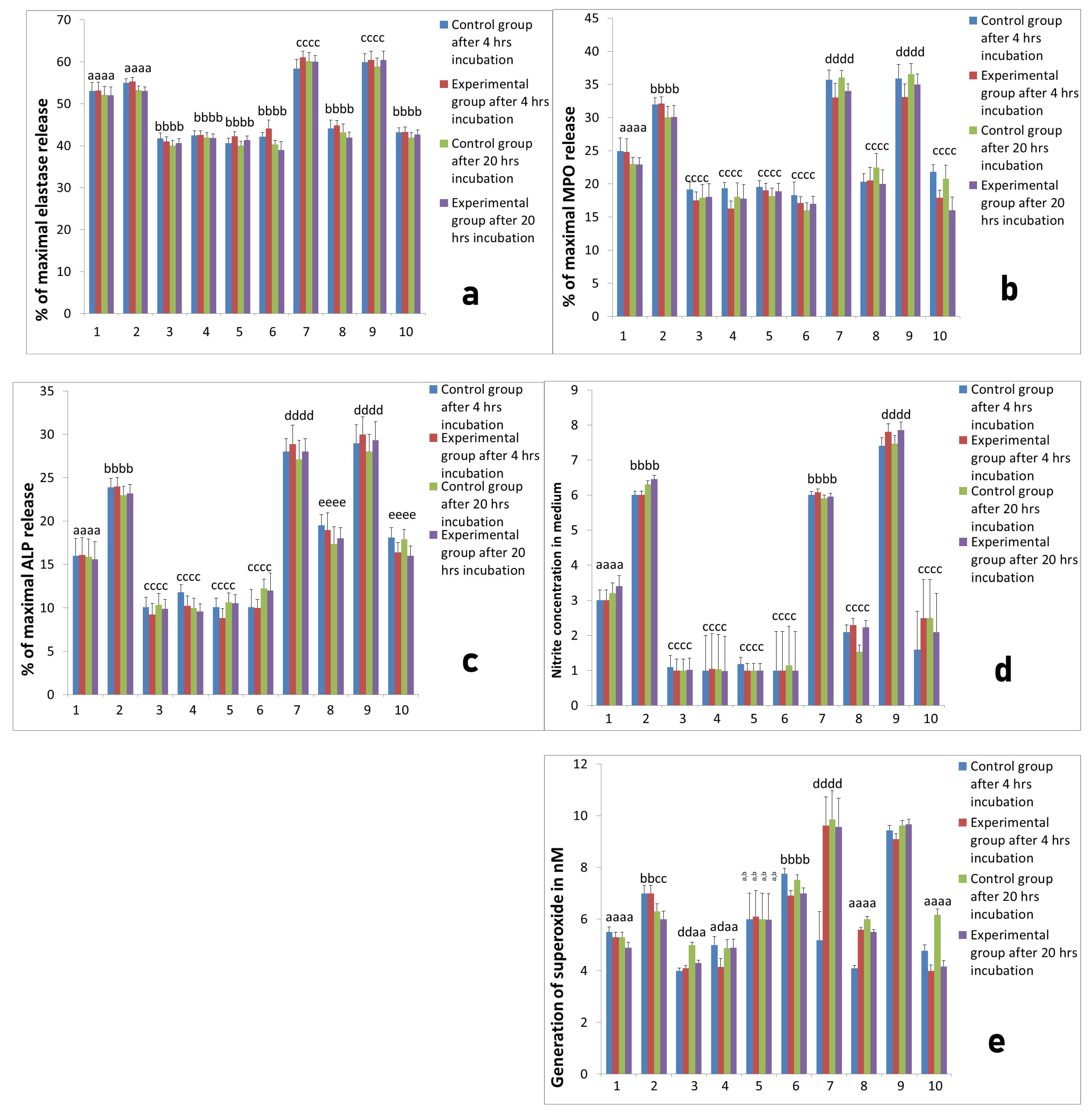
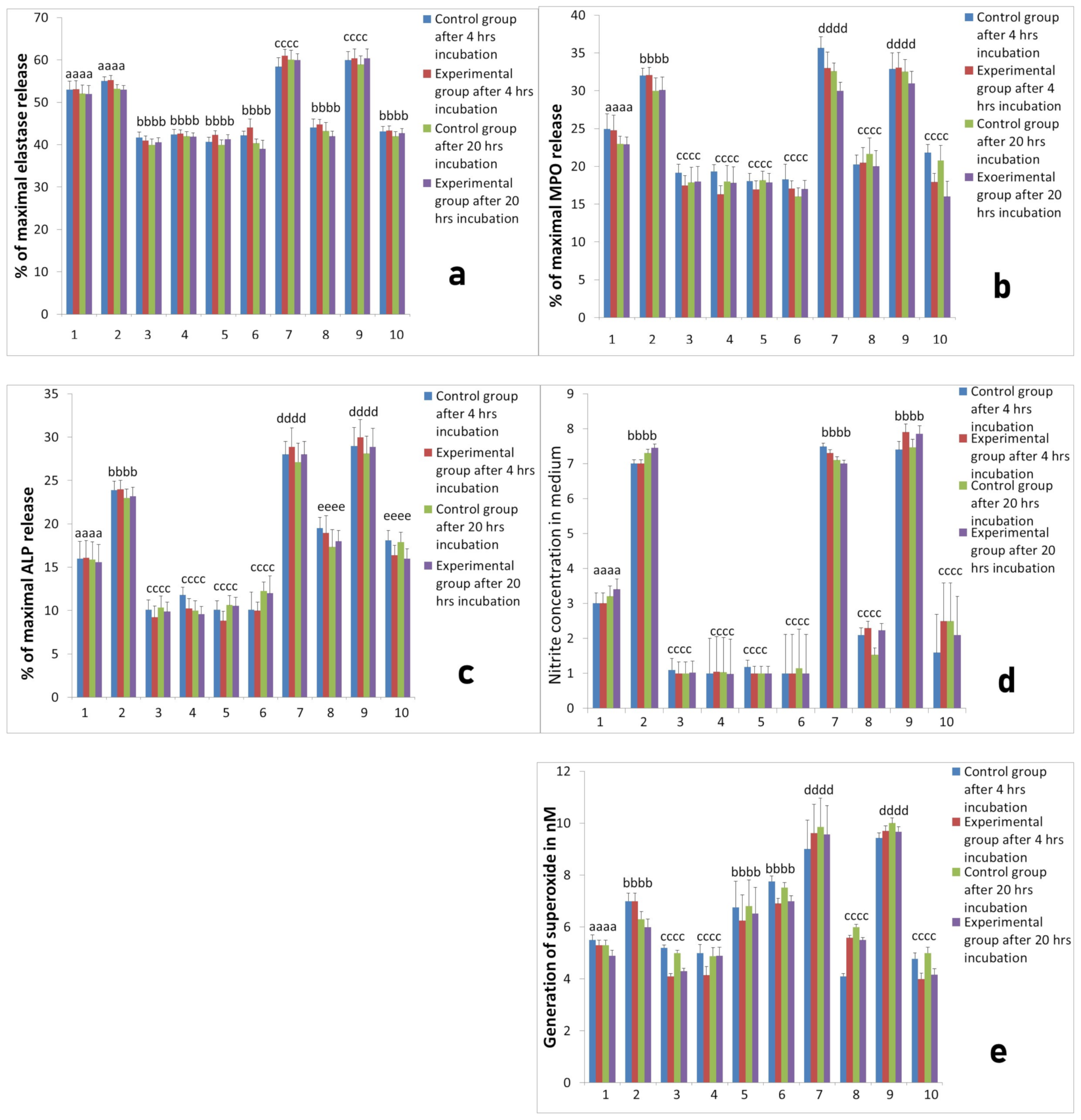
2.2. Activity of Neutrophils Isolated during the Inflammatory Phase of Repair after Implantation
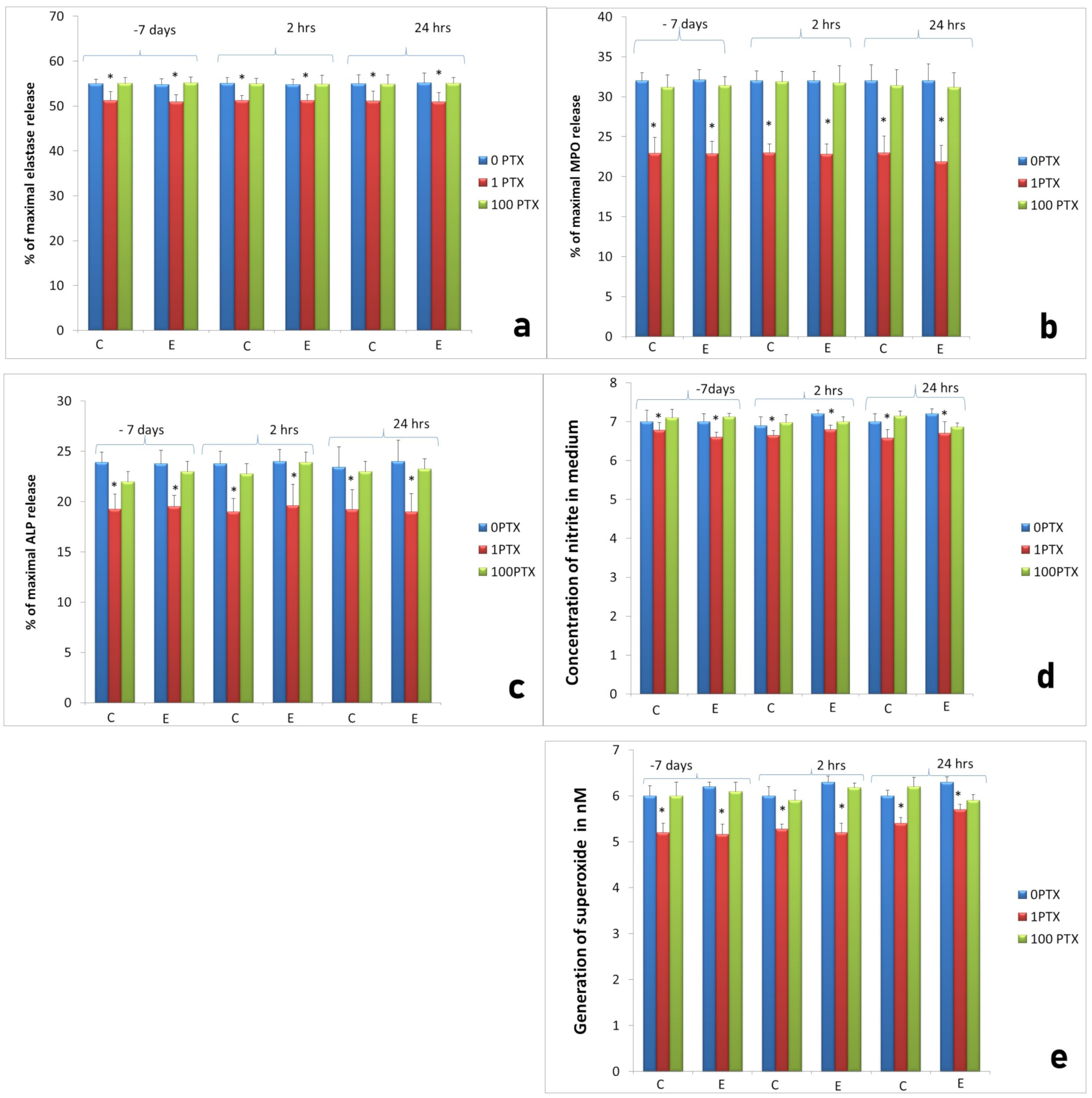
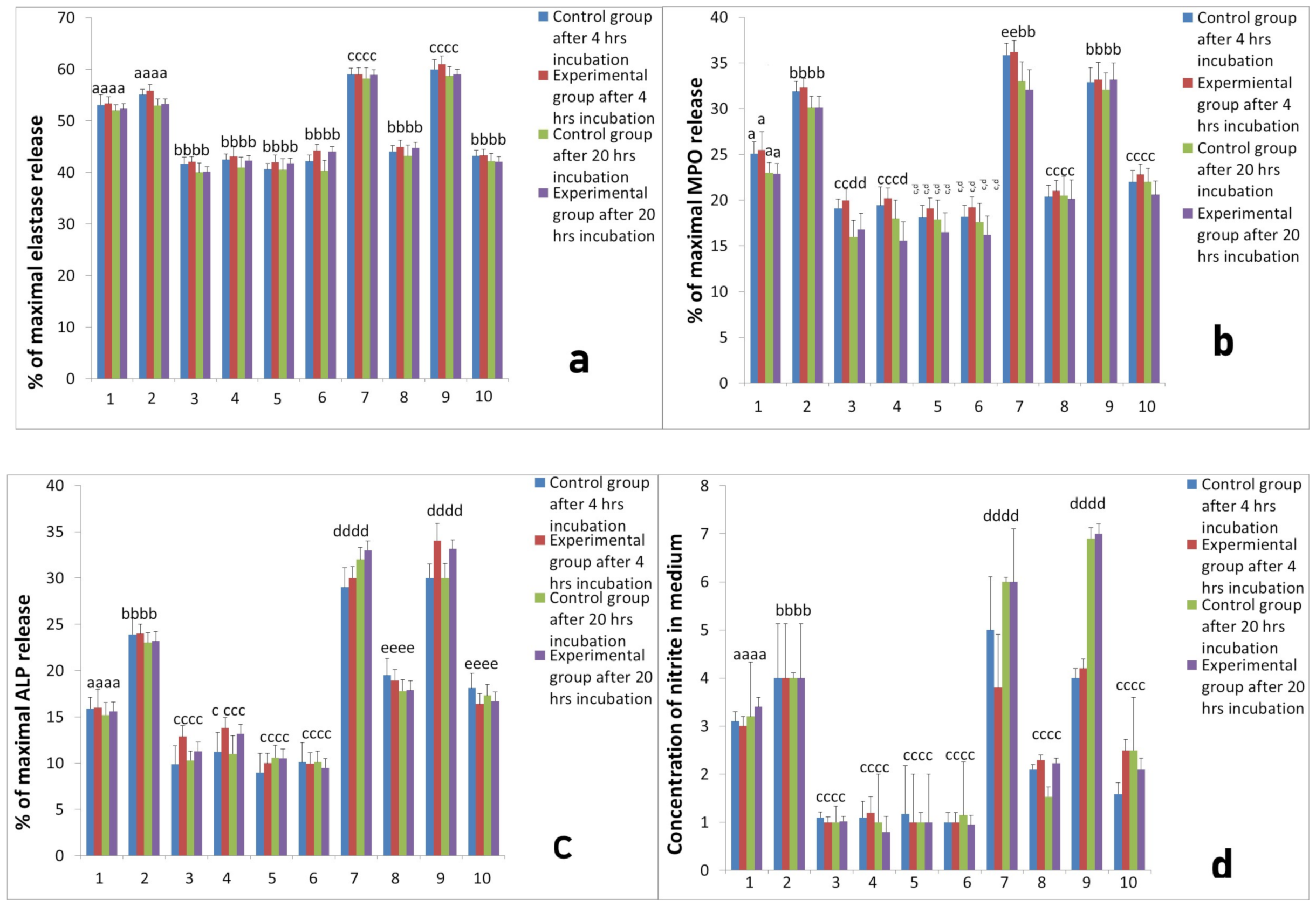
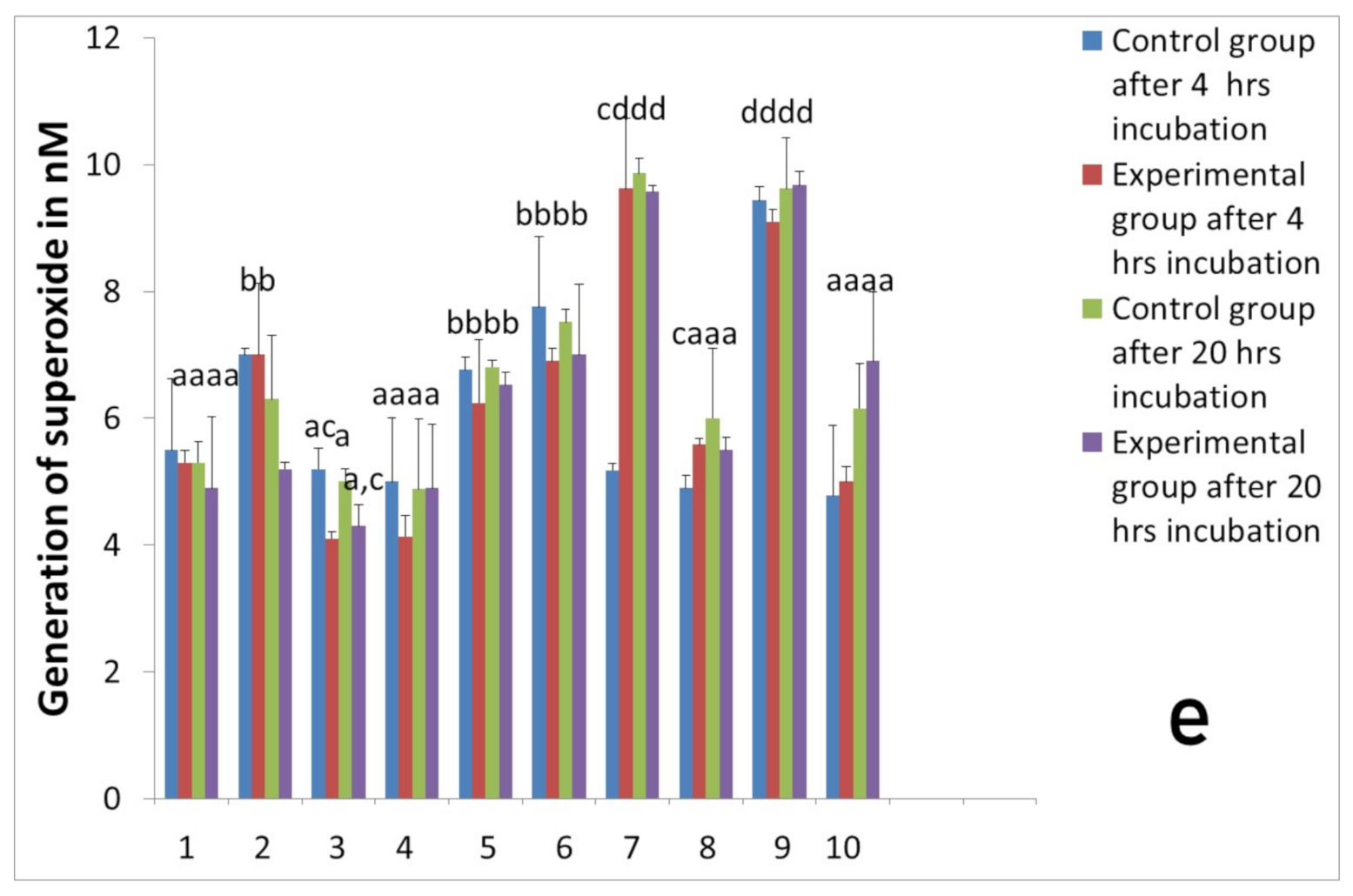
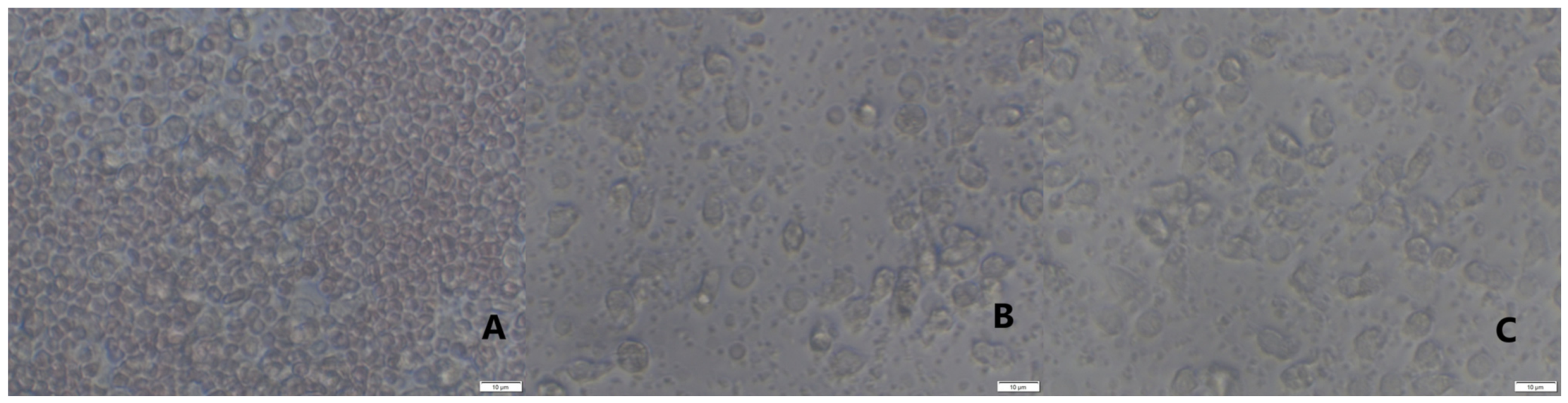
2.3. Assessment of Long-Term Interactions between Circulating Neutrophils and Ti Implants
3. Discussion
4. Materials and Methods
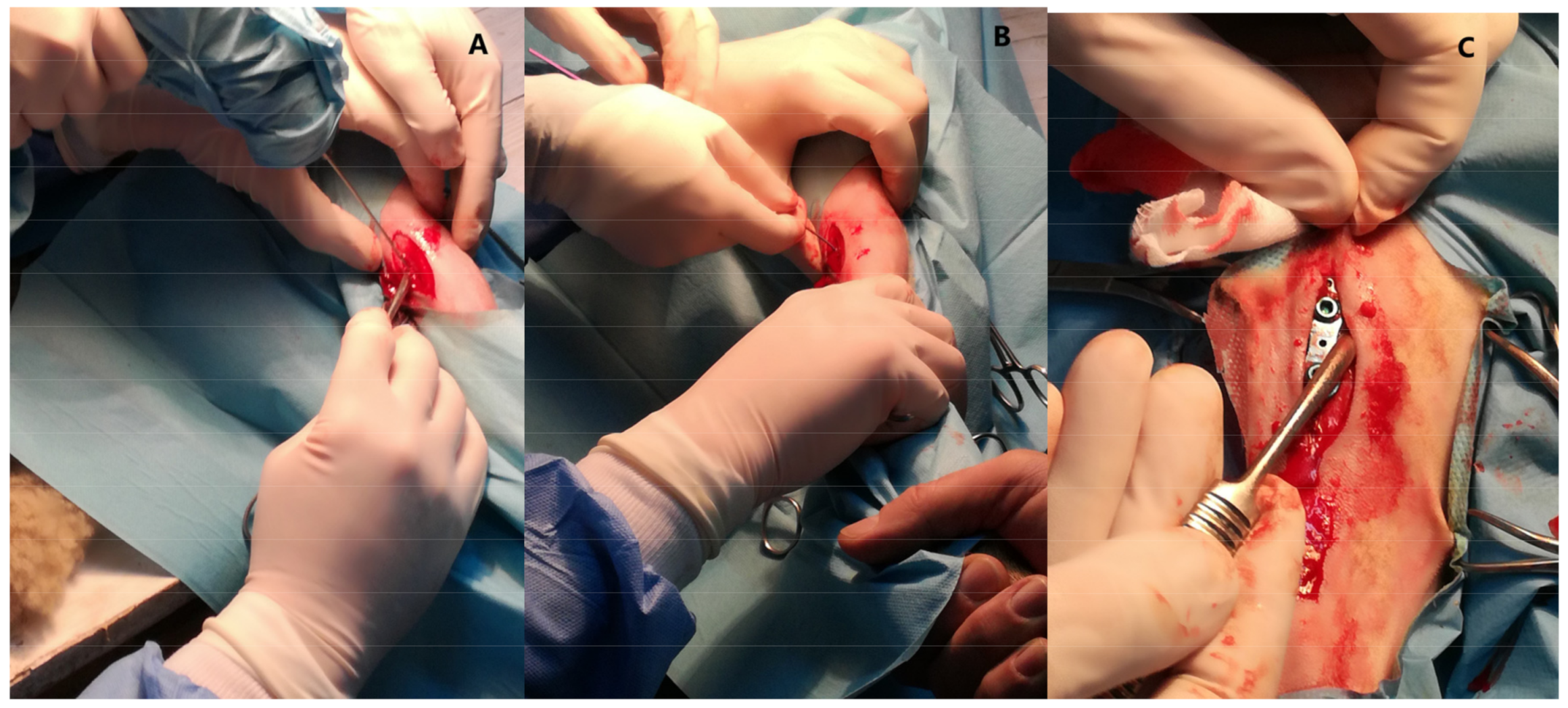
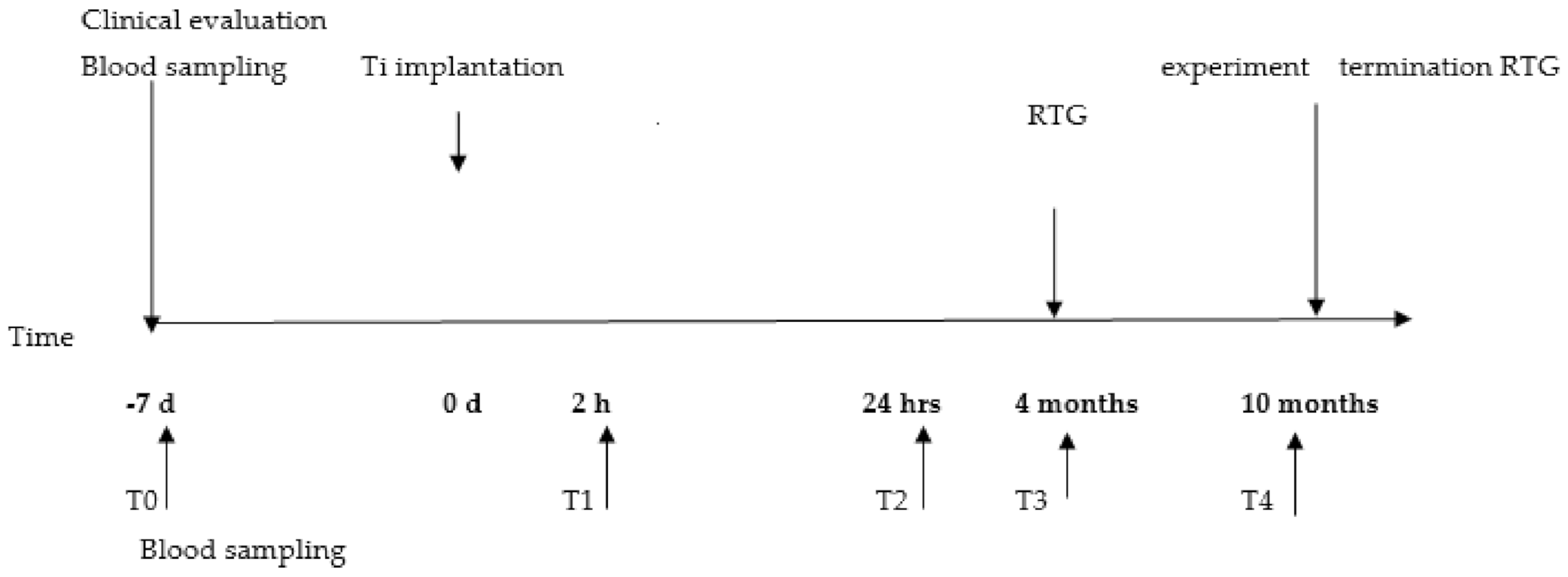
4.1. Animal Model and Surgical Procedure
4.2. Neutrophils Isolation, Culture and Stimulation
4.3. Platelet-Rich and Platelet-Poor Products
Neutrophil/Platelets Coculture
4.4. Evaluation of Cells and their Function
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mariani, E.; Lisignoli, G.; Borzi, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [Green Version]
- Cassetta, M.; Perrotti, V.; Calasso, S.; Piattelli, A.; Sinjari, B.; Iezzi, G. Bone formation in sinus augmentation procedures using autologous bone, porcine bone, and a 50:50 mixture: A human clinical and histological evaluation at 2 months. Clin. Oral Implant Res. 2015, 26, 1180–1184. [Google Scholar] [CrossRef]
- Athanasou, N.A. The pathobiology and pathology of aseptic implant failure. Bone Jt. Res. 2016, 5, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Pettersson, J.; Johansson, A.; Thorén, M.M. Titanium release in peri-implantitis. J. Oral Rehabil. 2019, 46, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amer, Y.; Darwech, I.; Clohisy, J.C. Aseptic loosening of total joint replacements: Mechanisms underlying osteolysis and potential therapies. Arthritis Res. Ther. 2007, 9, S6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumazawa, R.; Watari, F.; Takashi, N.; Tanimura, Y.; Uo, M.; Totsuka, Y. Effects of Ti ions and particles on neutrophil function and morphology. Biomaterials 2002, 23, 3757–3764. [Google Scholar] [CrossRef]
- Perobelli, S.M.; Silva, T.G.; Bonomo, A. Neutrophils Plasticity: The Regulatory Interface in Various Pathological Conditions; INTECH: London, UK, 2017; Chapter 7; pp. 145–169. [Google Scholar] [CrossRef] [Green Version]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef] [PubMed]
- Hally, K.E.; Bird, G.K.; La Flamme, A.C.; Harding, S.A.; Larsen, P.D. Platelets modulate multiple markers of neutrophil function in response to in vitro Toll-like receptor stimulation. PLoS ONE 2019, 14, e0223444. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.; Rehders, M.; Yu, P.-L. Antimicrobial fragments of the pro-region of cathelicidins and other immune peptides. Biotechnol. Lett. 2008, 30, 813–818. [Google Scholar] [CrossRef]
- Zdziennicka, J.; Szponder, T.; Wessely-Szponder, J. Application of Natural Neutrophil Products for Stimulation of Monocyte-Derived Macrophages Obtained before and after Osteochondral or Bone Injury. Microorganisms 2021, 9, 124. [Google Scholar] [CrossRef]
- Wessely-Szponder, J.; Michalska, J.; Szponder, T.; Żylińska, B.; Tarczyńska, M.; Szubstarski, M. The Role of Antimicrobial Neutrophil Extract in Modification of the Inflammatory Response During Osteochondral Autograft and Allograft Transplantation in Rabbits. J. Comp. Pathol. 2020, 175, 49–63. [Google Scholar] [CrossRef]
- Cohn, J.; Bone, R.C. New strategies in nonantibiotic treatment of gram-negative sepsis. Clevel. Clin. J. Med. 1992, 59, 608–615. [Google Scholar] [CrossRef]
- Atalay, Y.; Gunes, N.; Guner, M.D.; Akpolat, V.; Celik, M.S.; Guner, R. Pentoxifylline and electromagnetic field improved bone fracture healing in rats. Drug Design Develop. Ther. 2015, 9, 5195–5201. [Google Scholar] [CrossRef] [Green Version]
- Hally, K.E.; La Flamme, A.C.; Harding, S.A.; Larsen, P.D. Platelets regulate leucocyte responses to Toll-like receptor stimulation. Clin. Transl. Immunol. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Camargo Garbin, L.; Lopez, C.; Carmona, J.U. A Critical Overview of the Use of Platelet-Rich Plasma in Equine Medicine Over the Last Decade. Front. Vet. Sci. 2021, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.; Macedo, A.; Ingaro, I.L.; Huber, S.C.; Santos, G.S.; Santana, M.H.A. Leukocyte-rich PRP for knee osteoarthritis: Current concepts. J. Clin. Orthop. Trauma 2019, 10, 179–182. [Google Scholar] [CrossRef]
- Gomes de Melo, B.A.; Martins Shimojo, A.A.M.; Gomez Marcelino Perez, A.; Santos Duarte Lana, J.F.; Andrade Santana, M.H. Distribution, recovery and concentration of platelets and leukocytes in L-PRP prepared by centrifugation. Colloids Surf. B Biointerfaces 2018, 161, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Zhang, N.; Li, T.; Zhou, X.; Jia, J.; Liang, Y.; Sun, X.; Chen, H. The Effects of Platelet-Rich and Platelet-Poor Plasma on Biological Characteristics of BM-MSCs In Vitro. Anal. Cell. Pathol. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Rexhepi, I.; Paolantonio, M.; Romano, L.; Serroni, M.; Santamaria, P.; Secondi, L.; Paolantonio, G.; Sinjari, B.; De Ninis, P.; Femminella, B. Efficacy of inorganic bovine bone combined with leukocyte and platelet-rich fibrin or collagen membranes for treating unfavorable periodontal infrabony defects: Randomized non-inferiority trial. J. Periodontol. 2021, 6, 1–12. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.H.-C. PRP Treatment Efficacy for Tendinopathy: A Review of Basic Science Studies. BioMed Res. Int. 2016, 2016, 9103792. [Google Scholar] [CrossRef] [Green Version]
- Abaricia, J.O.; Arth, H.; Shah, A.H.; Musselman, R.M.; Olivares-Navarrete, R. Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater. Sci. 2020, 8, 2289–2299. [Google Scholar] [CrossRef] [Green Version]
- Baht, G.S.; Vi, L.; Alman, B.A. The Role of the Immune Cells in Fracture Healing. Curr. Osteoporos. Rep. 2018, 16, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhunjhunwala, S. Neutrophils at the Biological- Material Interface. ACS Biomater. 2018, 4, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Haiwen, Z.; Rui, H.; Bingxi, Z.; Qingfeng, G.; Beibei, W.; Jifeng, Z.; Xuemei, W.; Kebang, W. Cathelicidin- derived PR39 protects enterohemorrhagic Escherichia coli O157:H7 challenged mice by improving epithelial function and balancing the microbiota in the intestine. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Caputi, S.; Trubiani, O.; Sinjari, B.; Trofimova, S.; Diomede, F.; Linkova, N.; Diatlova, A.; Khavinson, V. Effect of short peptides on neuronal differentiation of stem cells. Int. J. Immunopathol. Phamacol. 2019, 33, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fantin, M.; Quintieri, L.; Kusz, E.; Kis, E.; Glavinas, H.; Floreani, M.; Padrini, R.; Duda, E.; Vizler, C. Pentoxifylline and its major oxidative metabolites exhibit different pharmacological properties. Eur. J. Pharmacol. 2006, 535, 301–309. [Google Scholar] [CrossRef]
- Amini, A.; Velaei, K.; Bayat, M.; Dadpay, M.; Nourozian, M.; Jakanbakhsh Asl, E. The effects of Pentoxifylline on the Wound Healing Process in a Rat Experimental Pressure Sore Model. Anatom. Sci. J. 2013, 10, 15–24. [Google Scholar]
- Chalmeth, A. Therapeutic regimens of endotoxaemia in sheep. Bulg. J. Vet. Med. 2020, 1311–1477. [Google Scholar] [CrossRef]
- Gros, A.; Ollivier, V.; Ho-Tin-Noé, B. Platelets in inflammation: Regulation of leukocyte activities and vascular repair. Front. Immunol. 2015, 5, 678. [Google Scholar] [CrossRef]
- Lösche, W.; Dressel, M.; Krause, S.; Redlich, H.; Spangenberg, P.; Heptinstall, S. Contact-induced modulation of neutrophil elastase secretion and phagocytic activity by platelets. Blood Coagul. Fibrinolysis Int. J. Haemostatis Thromb. 1996, 7. [Google Scholar] [CrossRef]
- Międzobrodzki, J.; Panz, T.; Płonka, P.M.; Zając, K.; Dracz, J.; Pytel, K.; Mateuszuk, Ł.; Chłopicki, S. Platelets augment respiratory burst in neutrophils activated by selected species of gram-positive or gram-negative bacteria. Folia Histochem. Cytobiol. 2008, 46, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Weibrich, G.; Kleis, W.K.G.; Hafner, G.; Hitzler, W.E.; Wagner, W. Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin. Oral Implants Res. 2003, 14, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Mannava, S.; Whitney, K.; Kennedy, M.I.; King, J. Characterizing The Biological Constituents Of Platelet-poor Plasma--do The Platelets Matter? A Prospective Cohort Study. Orthop. J. Sports Med. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef]
- Szponder, T.; Wessely-Szponder, J.; Świeca, M.; Smolira, A.; Gruszecki, T. The combined use of ozone therapy and autologous platelet-rich plasma as an alternative approach to foot rot treatment for sheep. A preliminary study. Small Rumin. Res. 2017, 156, 50–56. [Google Scholar] [CrossRef]
- Wessely-Szponder, J.; Szponder, T.; Bobowiec, R. Different activation of monocyte-derived macrophages by antimicrobial peptides at a titanium tibial implantation in rabbits. Res. Vet. Sci. 2017, 115, 201–210. [Google Scholar] [CrossRef]
- Skerlavaj, B.; Beninicasa, M.; Risso, A.; Zanetti, M.; Gennaro, R. SMAP-29: A Potent Antibacterial and Antifungal Peptide From Sheep Leukocytes. FEBS Lett. 1999, 463, 58–62. [Google Scholar] [CrossRef] [Green Version]


| Parameter | T0 | T2 | T3 | T4 |
|---|---|---|---|---|
| WBC (109/L) | 5.99 ± 1.22 | 5.9 ± 1.9 | 6.13 ± 1.46 | 5.95 ± 1.34 |
| GRAN (109/L) | 3.4 ± 1.1 | 3.35 ± 0.97 | 3.67 ± 1.46 | 3.42 ± 0.98 |
| RBC (1012/L) | 9.2 ± 0.91 | 9.06 ± 0.82 | 9.1 ± 0.79 | 9.15 ± 0.9 |
| HCT (%) | 27.45 ± 3.21 | 27.12 ± 2.45 | 27.57 ± 2.32 | 27.34 ± 3.11 |
| HGB (g/L) | 113.2 ± 1.22 | 117.9 ± 1.14 | 114.8 ± 1.03 | 117.5 ± 1.06 |
| Fibrinogen (g/L) | 4.10 ± 1.00 | 4.97 ± 0.51 | 4.52 ± 0.62 | 4.15 ± 0.86 |
| Haptoglobin (g/L) | 0.22 ± 0.20 | 0.61 ± 0.13 | 0.21 ± 0.15 | 0.28 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdziennicka, J.; Junkuszew, A.; Latalski, M.; Świeca, M.; Wessely-Szponder, J. Long-term Interactions of Circulating Neutrophils with Titanium Implants, the Role of Platelets in Regulation of Leukocyte Function. Int. J. Mol. Sci. 2021, 22, 10060. https://doi.org/10.3390/ijms221810060
Zdziennicka J, Junkuszew A, Latalski M, Świeca M, Wessely-Szponder J. Long-term Interactions of Circulating Neutrophils with Titanium Implants, the Role of Platelets in Regulation of Leukocyte Function. International Journal of Molecular Sciences. 2021; 22(18):10060. https://doi.org/10.3390/ijms221810060
Chicago/Turabian StyleZdziennicka, Joanna, Andrzej Junkuszew, Michał Latalski, Michał Świeca, and Joanna Wessely-Szponder. 2021. "Long-term Interactions of Circulating Neutrophils with Titanium Implants, the Role of Platelets in Regulation of Leukocyte Function" International Journal of Molecular Sciences 22, no. 18: 10060. https://doi.org/10.3390/ijms221810060
APA StyleZdziennicka, J., Junkuszew, A., Latalski, M., Świeca, M., & Wessely-Szponder, J. (2021). Long-term Interactions of Circulating Neutrophils with Titanium Implants, the Role of Platelets in Regulation of Leukocyte Function. International Journal of Molecular Sciences, 22(18), 10060. https://doi.org/10.3390/ijms221810060









