Isolation of Persister Cells of Bacillus subtilis and Determination of Their Susceptibility to Antimicrobial Peptides
Abstract
:1. Introduction
2. Results
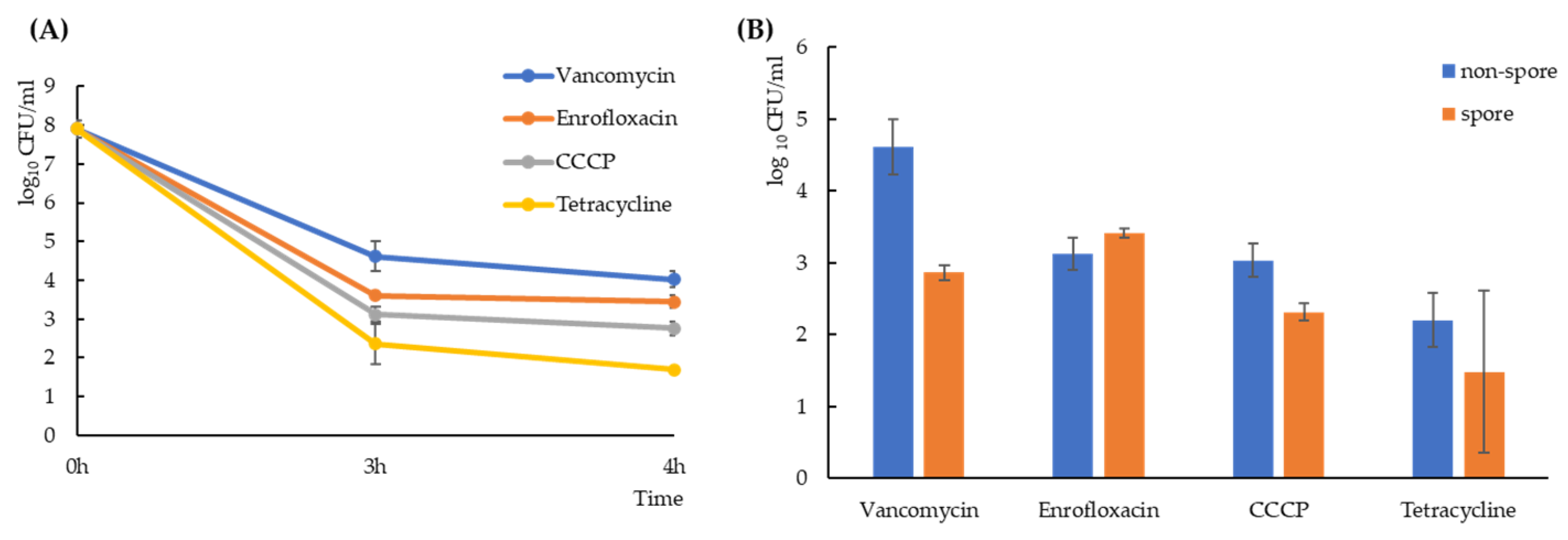
2.1. Exposure to Antimicrobial Compounds Generates B. subtilis Spores and Non-Spore Cells
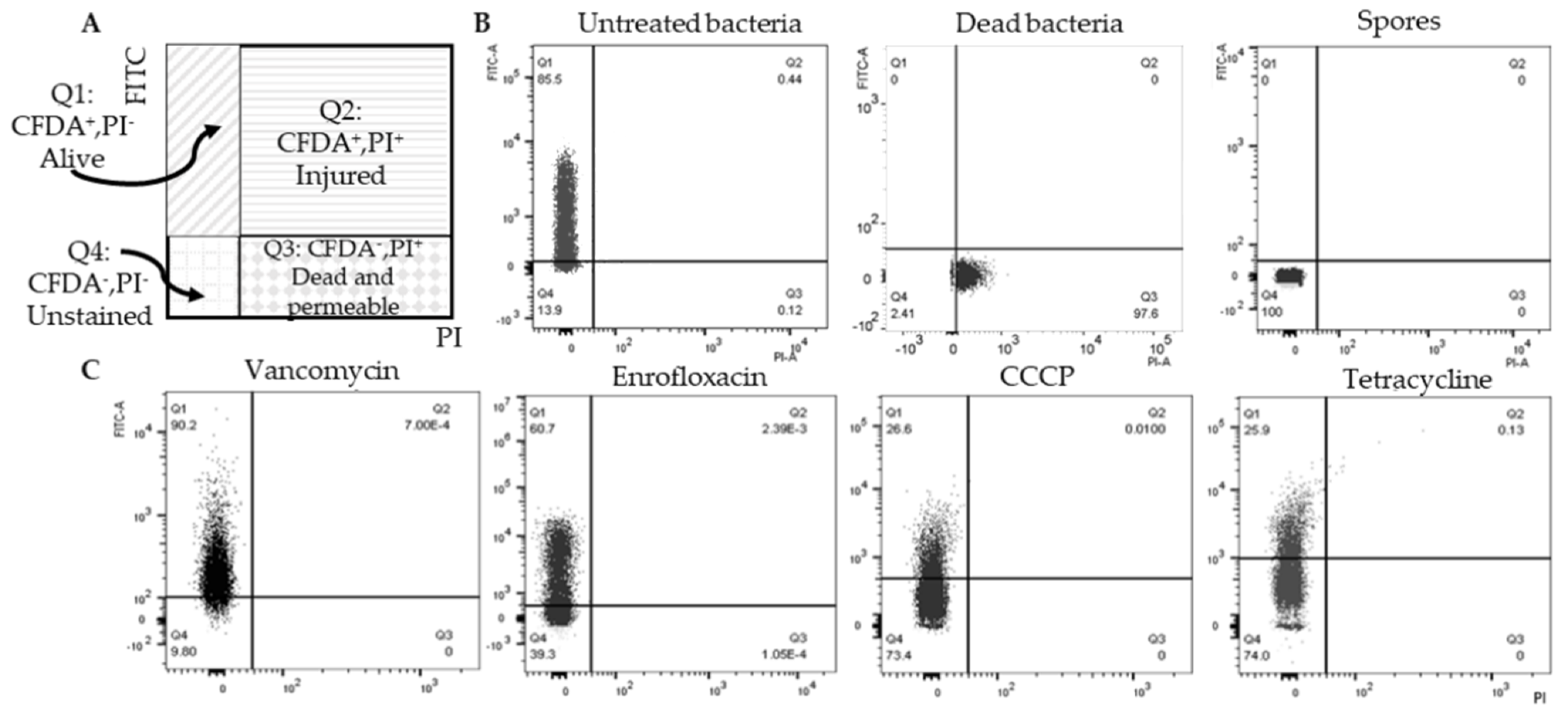
2.2. Double Staining to Indicate Surviving Non-Spore Cells after Antimicrobial Exposure
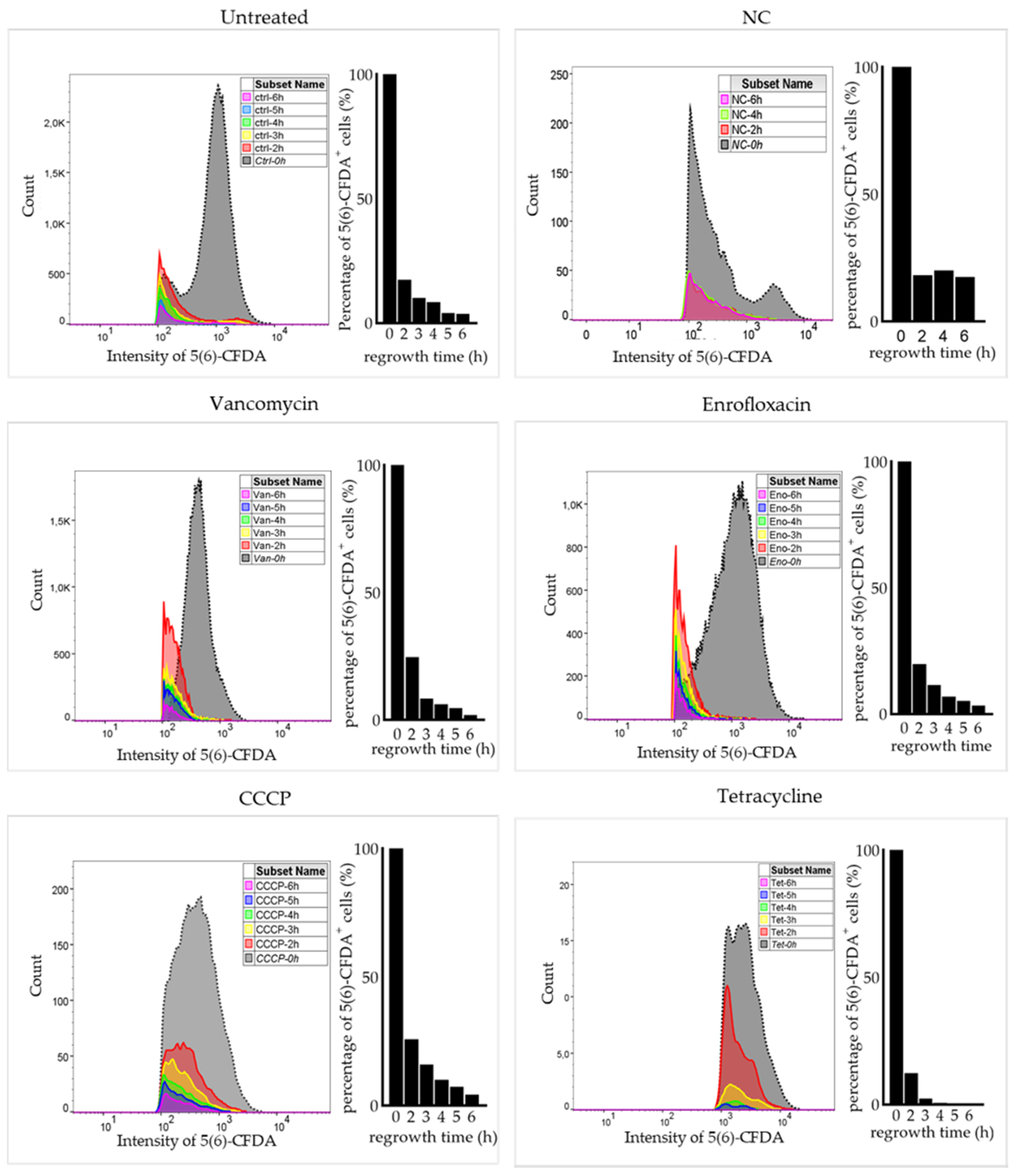
2.3. Surviving Non-Spore Cells Identified as Persisters
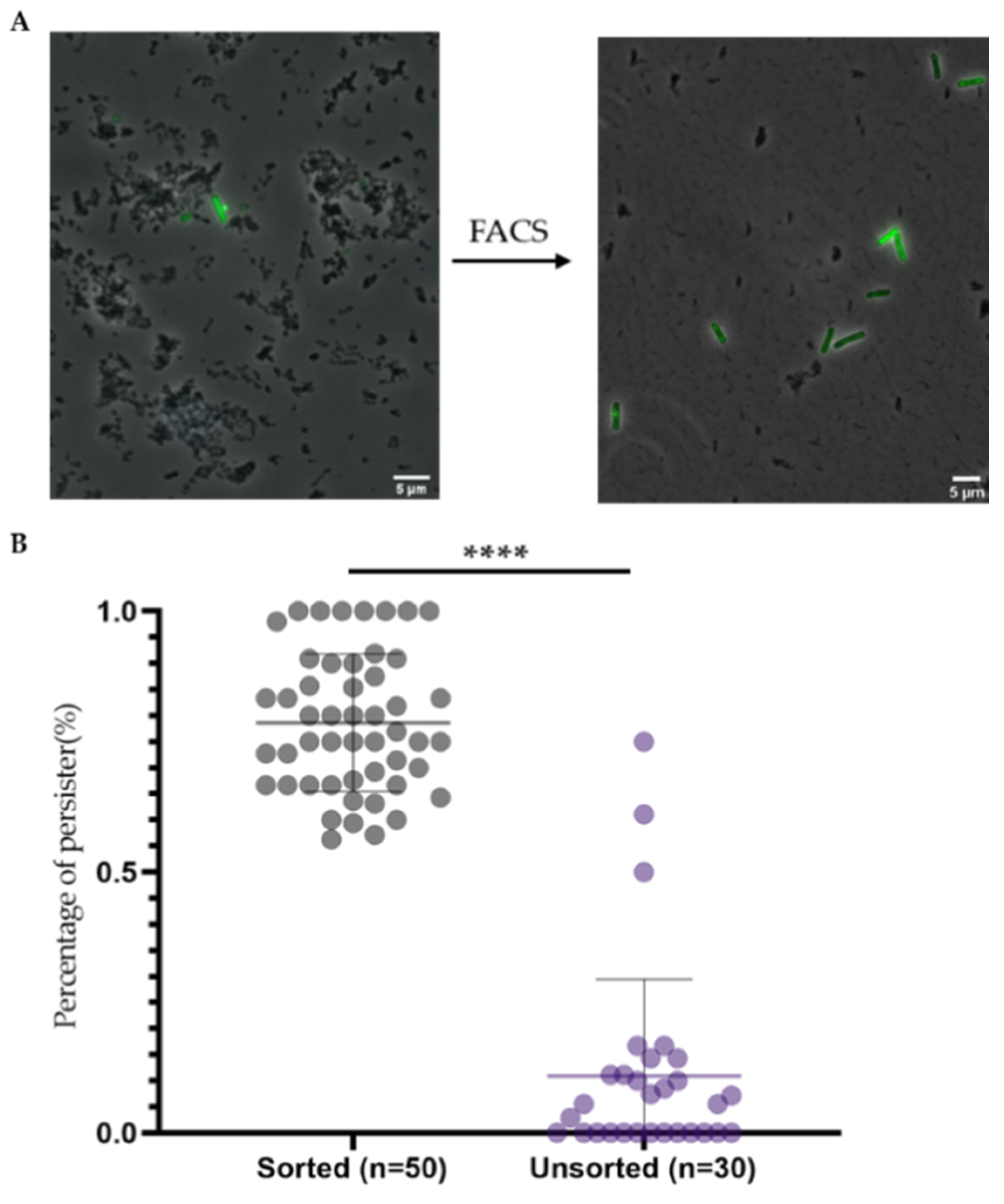
2.4. Double Staining and Subsequent Cell Sorting Is an Efficient Method to Isolate Persisters
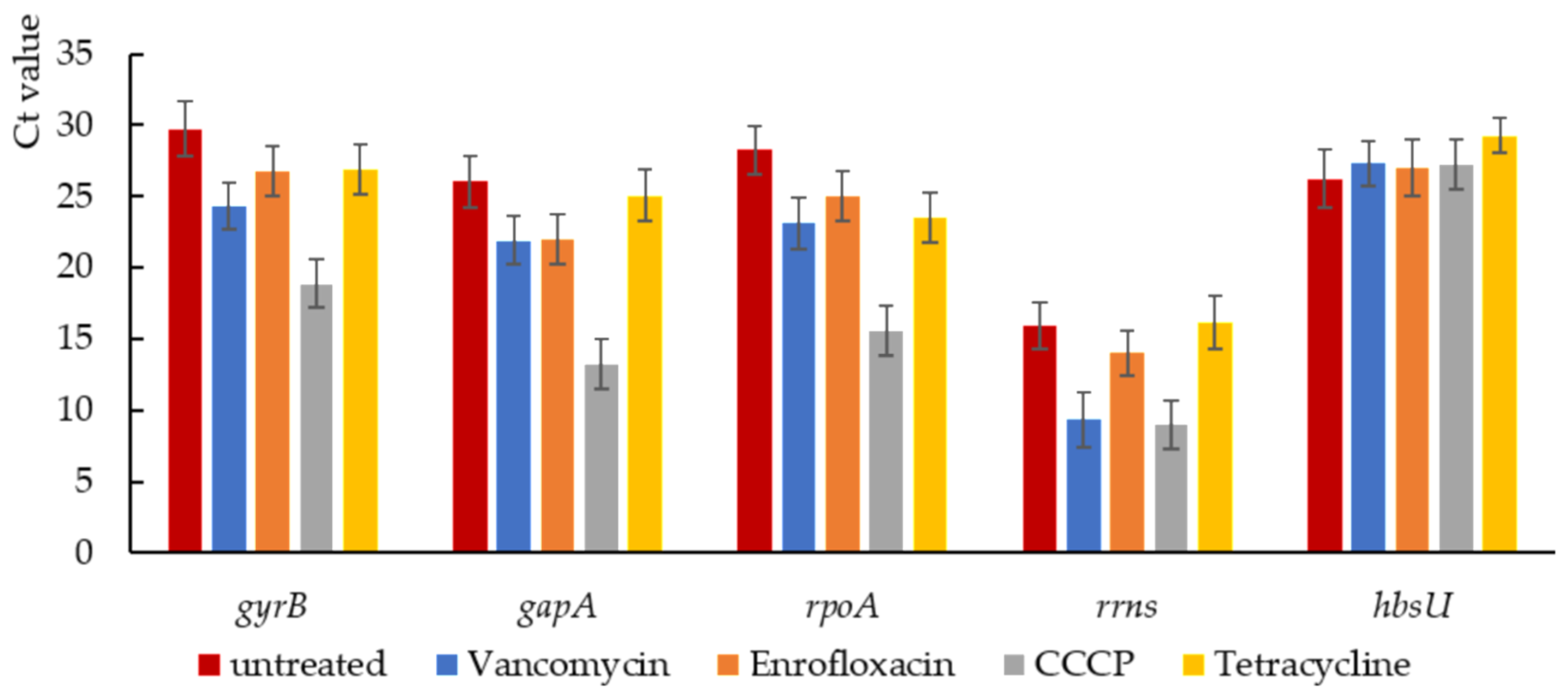
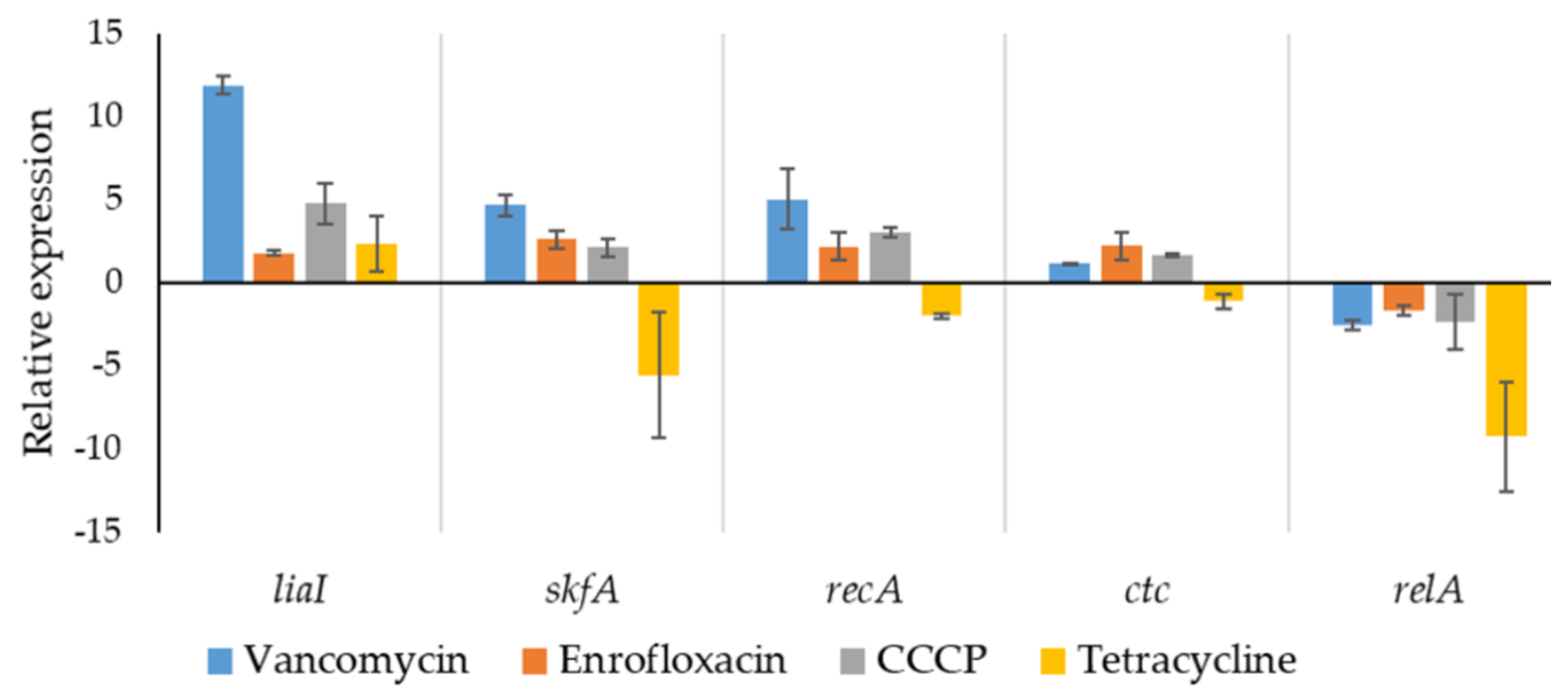
2.5. Stress-Related Gene Expression in Isolated Persisters
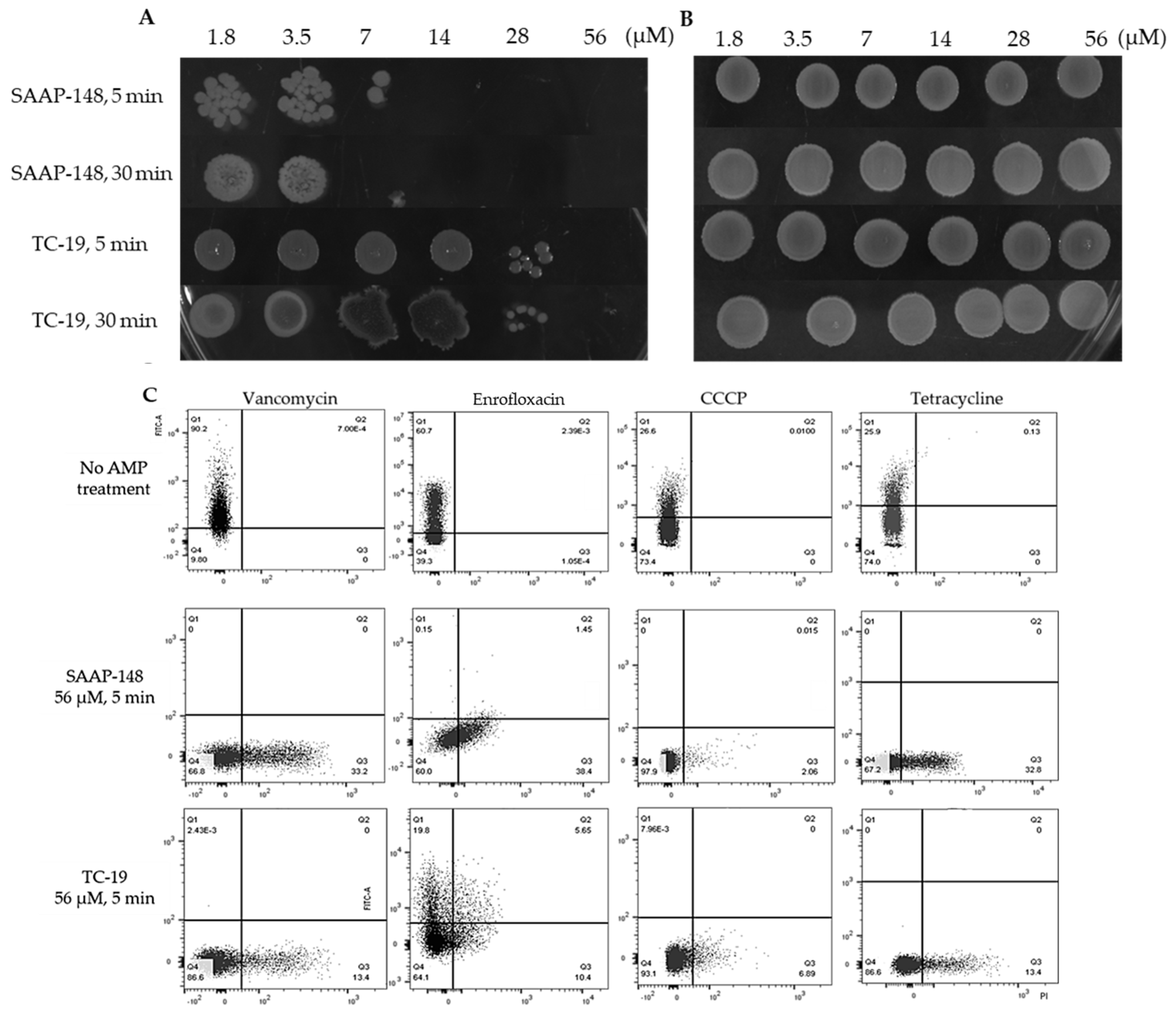
2.6. Cationic AMPs Effectively Kill B. subtilis Vegetative Cells and Persisters but Not Spores
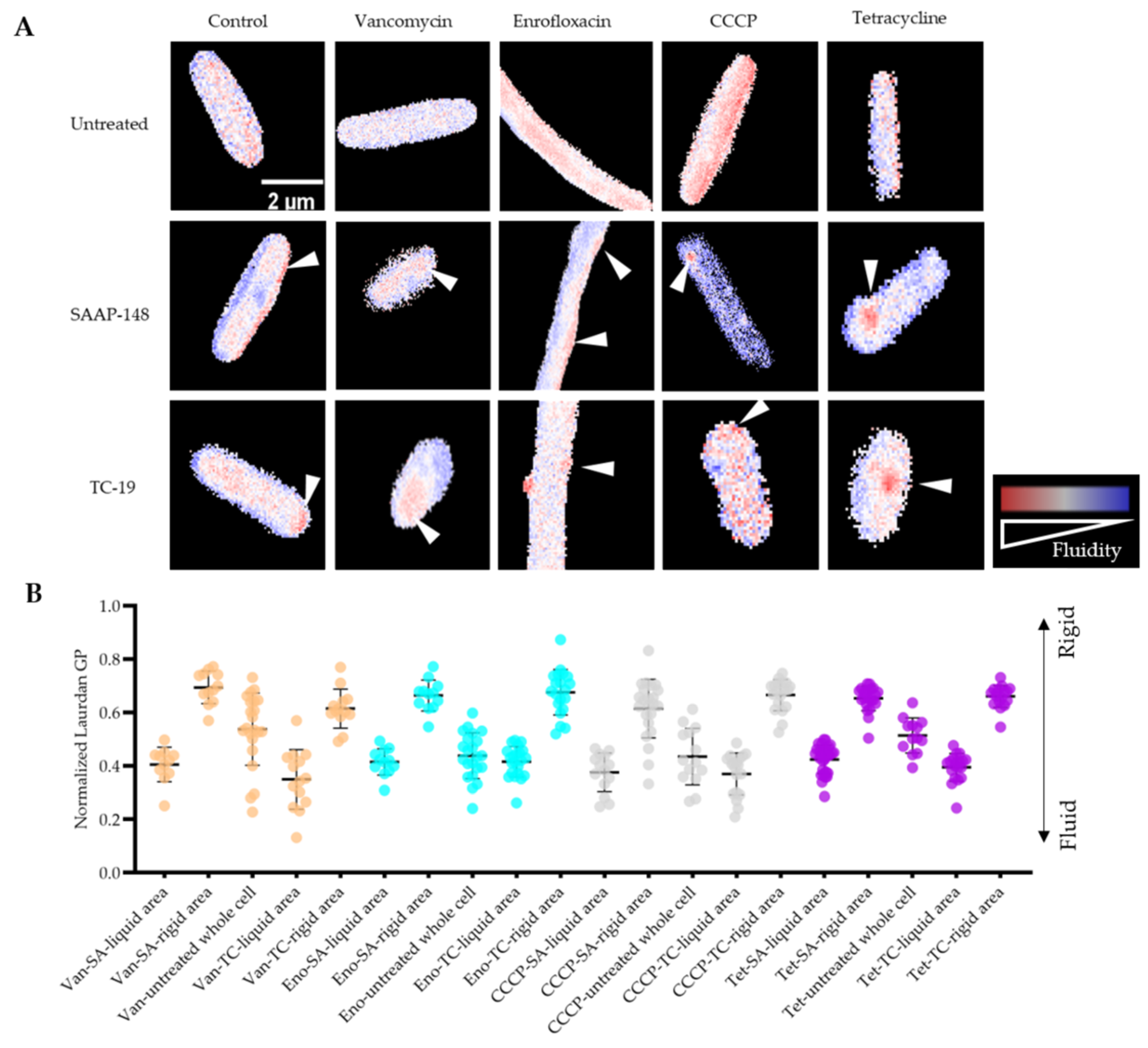
2.7. Membrane Fluidity Change during Antimicrobial Exposure and Subsequent AMPs Treatments
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Antimicrobial Compounds Information
4.2. Minimal Inhibitory Concentration (MIC) Measurement
4.3. Time Kill Assay and the Quantification of Spores and Non-Spore Cells
4.4. Flow Cytometry, FACS and Microscopy
4.5. Double Staining
4.6. Regrowth of Non-Spore Cells
4.7. qPCR Analysis with Isolated Persisters
4.8. Antimicrobial Peptides against B. subtilis Vegetative Cells, Spores and Persister Cells
4.9. Laurdan Staining to Measure the Membrane Fluidity Alteration during Antimicrobial Exposure and Subsequently AMPs Treatment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VBNC | Viable-but-nonculturable cell |
| AMP | Antimicrobial peptide |
| CCCP | Carbonyl cyanide m-chlorophenyl hydrazone |
| 5(6)-CFDA | 5-(and-6)-Carboxyfluorescein diacetate |
| PI | Propidium iodide |
| FACS | Fluorescence-activated cell sorting |
| GP | Generalized polarization |
| Van | Vancomycin |
| Eno | Enrofloxacin |
| Tet | Tetracycline |
References
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial Persistence as a Phenotypic Switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef] [Green Version]
- Hobby, L. Observations on the Mechanism of Action of Penicillin. Proc. Soc. Exp. Biol. Med. 1942, 50, 281–285. [Google Scholar] [CrossRef]
- Bigger, J.W. The bactericidal action of penicillin on Staphylococcus pyogenes. Irish J. Med. Sci. 1944, 19, 585–595. [Google Scholar] [CrossRef]
- Wilmaerts, D.; Windels, E.M.; Verstraeten, N.; Michiels, J. General Mechanisms Leading to Persister Formation and Awakening. Trends Genet. 2019, 35, 401–411. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Gerdes, K. Molecular Mechanisms Underlying Bacterial Persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef]
- Bakkeren, E.; Huisman, J.S.; Fattinger, S.A.; Hausmann, A.; Furter, M.; Egli, A.; Slack, E.; Sellin, M.E.; Bonhoeffer, S.; Regoes, R.R.; et al. Salmonella persisters promote the spread of antibiotic resistance plasmids in the gut. Nature 2019, 573, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Brul, S.; Zaat, S.A.J. Bacterial persister-cells and spores in the food chain: Their potential inactivation by antimicrobial peptides (amps). Int. J. Mol. Sci. 2020, 21, 8967. [Google Scholar] [CrossRef] [PubMed]
- Hingley-Wilson, S.M.; Ma, N.; Hu, Y.; Casey, R.; Bramming, A.; Curry, R.J.; Tang, H.L.; Wu, H.; Butler, R.E.; Jacobs, W.R.; et al. Loss of phenotypic inheritance associated with ydcI mutation leads to increased frequency of small, slow persisters in Escherichia coli. Proc. Natl. Acad. Sci. USA 2020, 117, 4152–4157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, S.; Fazen, C.; Henry, T.; Mok, W.; Orman, M.; Sandvik, E.; Volzing, K.; Brynildsen, M. The role of metabolism in bacterial persistence. Front. Microbiol. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Wood, T.K. Are we really studying persister cells? Environ. Microbiol. Rep. 2021, 13, 3–7. [Google Scholar] [CrossRef]
- Moyed, H.S.; Bertrand, K.P. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J. Bacteriol. 1983, 155, 768–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, aaf4268-1–aaf4628-9. [Google Scholar] [CrossRef]
- Van den Bergh, B.; Fauvart, M.; Michiels, J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev. 2017, 41, 219–251. [Google Scholar] [CrossRef]
- Iris, K.; Devang, S.; Amy, S.; Niilo, K.; Kim, L. Specialized Persister Cells and the Mechanism of Multidrug Tolerance in Escherichia coli. J. Bacteriol. 2004, 186, 8172–8180. [Google Scholar]
- Masuda, Y.; Sakamoto, E.; Honjoh, K.I.; Miyamoto, T. Role of toxin-antitoxin-regulated persister population and indole in bacterial heat tolerance. Appl. Environ. Microbiol. 2020, 86, e00935-20. [Google Scholar] [CrossRef]
- Keren, I.; Minami, S.; Rubin, E.; Lewis, K. Characterization and Transcriptome Analysis of Mycobacterium tuberculosis Persisters. MBio 2011, 2, e00100-11. [Google Scholar] [CrossRef] [Green Version]
- Ayrapetyan, M.; Williams, T.C.; Baxter, R.; Oliver, J.D. Viable but Nonculturable and Persister Cells Coexist Stochastically and Are Induced by Human Serum. Infect. Immun. 2015, 83, 4194–4203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; El Meouche, I.; Dunlop, M.J. Bacterial persistence induced by salicylate via reactive oxygen species. Sci. Rep. 2017, 7, 43839. [Google Scholar] [CrossRef]
- Cañas-Duarte, S.J.; Restrepo, S.; Pedraza, J.M. Novel Protocol for Persister Cells Isolation. PLoS ONE 2014, 9, e88660. [Google Scholar]
- Ayrapetyan, M.; Williams, T.; Oliver, J.D. Relationship between the Viable but Nonculturable State and Antibiotic Persister Cells. J. Bacteriol. 2021, 200, e00249-18. [Google Scholar] [CrossRef] [Green Version]
- Mohiuddin, S.G.; Kavousi, P.; Orman, M.A. Flow-cytometry analysis reveals persister resuscitation characteristics. BMC Microbiol. 2020, 20, 202. [Google Scholar] [CrossRef]
- Orman, M.A.; Brynildsen, M.P. Establishment of a Method to Rapidly Assay Bacterial Persister Metabolism. Antimicrob. Agents Chemother. 2013, 57, 4398–4409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, D.; Zhang, Z.; Khodursky, A.B.; Kaldalu, N.; Kurg, K.; Lewis, K. Persisters: A distinct physiological state of E. coli. BMC Microbiol. 2006, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Windels, E.M.; Meriem, Z.B.; Zahir, T.; Verstrepen, K.J.; Hersen, P.; Van den Bergh, B.; Michiels, J. Isolation of persisters enabled by ß-lactam-induced filamentation reveals their single-cell awakening characteristics. bioRxiv 2019, 600700. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; De Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; Van Der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef] [Green Version]
- Zaat, S.A.J.; Kwakman, P.H.S.; Drijfhout, J.W. Thrombocidin-Derived Antimicrobial Peptides. WO Patent No. WO2015099535A1, 2 July 2015. [Google Scholar]
- Omardien, S.; Drijfhout, J.W.; Vaz, F.M.; Wenzel, M.; Hamoen, L.W.; Zaat, S.A.J.; Brul, S. Bactericidal activity of amphipathic cationic antimicrobial peptides involves altering the membrane fluidity when interacting with the phospholipid bilayer. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2404–2415. [Google Scholar] [CrossRef]
- Fang, C.; Stiegeler, E.; Cook, G.M.; Mascher, T.; Gebhard, S. Bacillus subtilis as a platform for molecular characterisation of regulatory mechanisms of Enterococcus faecalis resistance against cell wall antibiotics. PLoS ONE 2014, 9, e93169. [Google Scholar] [CrossRef] [Green Version]
- Prazdnova, E.V.; Mazanko, M.S.; Bren, A.B.; Chistyakov, V.A.; Weeks, R.; Chikindas, M.L. SOS Response Inhibitory Properties by Potential Probiotic Formulations of Bacillus amyloliquefaciens B-1895 and Bacillus subtilis KATMIRA1933 Obtained by Solid-State Fermentation. Curr. Microbiol. 2019, 76, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Strahl, H.; Hamoen, L.W. Membrane potential is important for bacterial cell division. Proc. Natl. Acad. Sci. USA 2010, 107, 12281–12286. [Google Scholar] [CrossRef] [Green Version]
- Ian, C.; Marilyn, R. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar]
- Wenzel, M.; Dekker, M.P.; Wang, B.; Burggraaf, M.J.; Bitter, W.; van Weering, J.R.T.; Hamoen, L.W. A flat embedding method for transmission electron microscopy reveals an unknown mechanism of tetracycline. Commun. Biol. 2021, 4, 306. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448. [Google Scholar] [CrossRef] [Green Version]
- Hoefel, D.; Grooby, W.L.; Monis, P.T.; Andrews, S.; Saint, C.P. A comparative study of carboxyfluorescein diacetate and carboxyfluorescein diacetate succinimidyl ester as indicators of bacterial activity. J. Microbiol. Methods 2003, 52, 379–388. [Google Scholar] [CrossRef]
- Basiji, D.A.; Ortyn, W.E.; Liang, L.; Venkatachalam, V.; Morrissey, P. Cellular image analysis and imaging by flow cytometry. Clin. Lab. Med. 2007, 27, 653–670. [Google Scholar] [CrossRef] [Green Version]
- Muratori, M.; Forti, G.; Baldi, E. Comparing flow cytometry and fluorescence microscopy for analyzing human sperm DNA fragmentation by TUNEL labeling. Cytom. Part A 2008, 73, 785–787. [Google Scholar] [CrossRef]
- Pištěková, H.; Jančová, P.; Buňková, L.; Šopík, T.; Maršálková, K.; Berčíková, L.; Buňka, F. Detection and relative quantification of amine oxidase gene (yobN) in Bacillus subtilis: Application of real-time quantitative PCR. J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Liu, S.; Qian, K.; Xu, M.; Yang, T.; Xu, J.; Rao, Z. Improving the Production of Salt-Tolerant Glutaminase by Integrating Multiple Copies of Mglu into the Protease and 16S rDNA Genes of Bacillus subtilis 168. Molecules 2019, 24, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wen, J.; Jia, X. Engineering Bacillus subtilis for isobutanol production by heterologous Ehrlich pathway construction and the biosynthetic 2-ketoisovalerate precursor pathway overexpression. Appl. Microbiol. Biotechnol. 2011, 91, 577–589. [Google Scholar] [CrossRef]
- Prajapati, R.K.; Sur, R.; Mukhopadhyay, J. A novel function of δ factor from Bacillus subtilis as a transcriptional repressor. J. Biol. Chem. 2016, 291, 24029–24035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Chen, T.; Liu, Y.; Lv, X.; Li, J.; Du, G.; Ledesma-Amaro, R.; Liu, L. CRISPRi allows optimal temporal control of N-acetylglucosamine bioproduction by a dynamic coordination of glucose and xylose metabolism in Bacillus subtilis. Metab. Eng. 2018, 49, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Escobar, J.; Wolf, D.; Fritz, G.; Höfler, C.; Wedlich-Söldner, R.; Mascher, T. Subcellular localization, interactions and dynamics of the phage-shock protein-like Lia response in Bacillus subtilis. Mol. Microbiol. 2014, 92, 716–732. [Google Scholar] [CrossRef] [PubMed]
- López, D.; Kolter, R. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol. Rev. 2010, 34, 134–149. [Google Scholar] [CrossRef] [Green Version]
- Kreuzer, K.N. DNA Damage Responses in Prokaryotes: Regulating Gene Expression, Modulating Growth Patterns, and Manipulating Replication Forks. Cold Spring Harb. Perspect. Biol. 2013, 5, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, M.; Cogliati, S.; Vileta, D.; Bauman, C.; Rateni, L.; Leñini, C.; Argañaraz, F.; Francisco, M.; Villalba, J.M.; Steil, L.; et al. Regulation of biofilm aging and dispersal in Bacillus subtilis by the alternative sigma factor SigB. J. Bacteriol. 2019, 201, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauryliuk, V.; Atkinson, G.C.; Murakami, K.S.; Tenson, T.; Gerdes, K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat. Rev. Microbiol. 2015, 13, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Manganelli, R.; Gennaro, M.L. Protecting from Envelope Stress: Variations on the Phage-Shock-Protein Theme. Trends Microbiol. 2017, 25, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Ter Beek, A.; Wijman, J.G.E.; Zakrzewska, A.; Orij, R.; Smits, G.J.; Brul, S. Comparative physiological and transcriptional analysis of weak organic acid stress in Bacillus subtilis. Food Microbiol. 2015, 45, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Hachmann, A.-B.; Angert, E.R.; Helmann, J.D. Genetic analysis of factors affecting susceptibility of Bacillus subtilis to daptomycin. Antimicrob. Agents Chemother. 2009, 53, 1598–1609. [Google Scholar] [CrossRef] [Green Version]
- Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; Ambroa, A.; López, M.; Bou, G.; Cantón, R.; Garcia-Contreras, R.; Wood, T.K.; et al. (p)ppGpp and its role in bacterial persistence: New challenges. Antimicrob. Agents Chemother. 2020, 64, e01283-20. [Google Scholar] [CrossRef] [PubMed]
- Pavel, K.; Vallo, V.; Alves, O.S.R.; Jelena, B.; Teresa, D.P.S.; Ievgen, D.; Dominik, R.; Felipe, C.; Tanel, T.; Vasili, H. Subinhibitory Concentrations of Bacteriostatic Antibiotics Induce relA-Dependent and relA-Independent Tolerance to β-Lactams. Antimicrob. Agents Chemother. 2017, 61, e02173-16. [Google Scholar]
- Kwan, B.W.; Valenta, J.A.; Benedik, M.J.; Wood, T.K. Arrested protein synthesis increases persister-like cell formation. Antimicrob. Agents Chemother. 2013, 57, 1468–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paranjape, S.S.; Shashidhar, R. Inhibition of protein synthesis eradicates persister cells of V. cholerae. 3 Biotech 2019, 9, 380. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.M.; Best, K.B.; Bell, J.D. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1565, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Mulyukin, A.L.; Kozlova, A.N.; Sorokin, V.V.; Suzina, N.E.; Cherdyntseva, T.A.; Kotova, I.B.; Gaponov, A.M.; Tutel’yan, A.V.; El’-Registan, G.I. Surviving forms in antibiotic-treated Pseudomonas aeruginosa. Microbiology 2015, 84, 751–763. [Google Scholar] [CrossRef]
- Wood, T.K. Combatting bacterial persister cells. Biotechnol. Bioeng. 2016, 113, 476–483. [Google Scholar] [CrossRef]
- Fauvart, M.; de Groote, V.N.; Michiels, J. Role of persister cells in chronic infections: Clinical relevance and perspectives on anti-persister therapies. J. Med. Microbiol. 2011, 60, 699–709. [Google Scholar] [CrossRef]
- Fisher, R.A.; Thurston, T.L.; Saliba, A.; Blommestein, I.; Vogel, J.; Helaine, S. Salmonella persisters undermine host immune defenses during antibiotic treatment. Science 2018, 1160, 1156–1160. [Google Scholar]
- Ayrapetyan, M.; Williams, T.C.; Oliver, J.D. Bridging the gap between viable but non-culturable and antibiotic persistent bacteria. Trends Microbiol. 2015, 23, 7–13. [Google Scholar] [CrossRef]
- Amato, S.M.; Brynildsen, M.P. Persister Heterogeneity Arising from a Single Metabolic Stress. Curr. Biol. 2015, 25, 2090–2098. [Google Scholar] [CrossRef] [Green Version]
- Schrank, C.L.; Wilt, I.K.; Monteagudo Ortiz, C.; Haney, B.A.; Wuest, W.M. Using membrane perturbing small molecules to target chronic persistent infections. RSC Med. Chem. 2021, 12, 1312–1324. [Google Scholar] [CrossRef]
- Babii, O.; Afonin, S.; Schober, T.; Komarov, I.V.; Ulrich, A.S. Flexibility vs. rigidity of amphipathic peptide conjugates when interacting with lipid bilayers. Biochim. Biophys. Acta (BBA)-Biomembr. 2017, 1859, 2505–2515. [Google Scholar] [CrossRef]
- Henderson, J.M.; Iyengar, N.S.; Lam, K.L.H.; Maldonado, E.; Suwatthee, T.; Roy, I.; Waring, A.J.; Lee, K.Y.C. Beyond electrostatics: Antimicrobial peptide selectivity and the influence of cholesterol-mediated fluidity and lipid chain length on protegrin-1 activity. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1861, 182977. [Google Scholar] [CrossRef]
- Bessa, L.J.; Manickchand, J.R.; Eaton, P.; Leite, J.R.S.A.; Brand, G.D.; Gameiro, P. Intragenic Antimicrobial Peptide Hs02 Hampers the Proliferation of Single- and Dual-Species Biofilms of P. aeruginosa and S. aureus: A Promising Agent for Mitigation of Biofilm-Associated Infections. Int. J. Mol. Sci. 2019, 20, 3604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiemstra, H.S.; Duinkerken, G.; Benckhuijsen, W.E.; Amons, R.; de Vries, R.R.P.; Roep, B.O.; Drijfhout, J.W. The identification of CD4+ T cell epitopes with dedicated synthetic peptide libraries. Proc. Natl. Acad. Sci. USA 1997, 94, 10313–10318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rüger, M.; Bensch, G.; Tüngler, R.; Reichl, U. A flow cytometric method for viability assessment of Staphylococcus aureus and Burkholderia cepacia in mixed culture. Cytom. Part A 2012, 81, 1055–1066. [Google Scholar] [CrossRef]
- Wen, J.; Pasman, R.; Manders, E.M.M.; Setlow, P.; Brul, S. Visualization of germinosomes and the inner membrane in Bacillus subtilis spores. J. Vis. Exp. 2019, 2019, e59388. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Omardien, S.; Drijfhout, J.W.; van Veen, H.; Schachtschabel, S.; Riool, M.; Hamoen, L.W.; Brul, S.; Zaat, S.A.J. Synthetic antimicrobial peptides delocalize membrane bound proteins thereby inducing a cell envelope stress response. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.A.; Tricerri, M.A.; Gratton, E. Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 7314–7319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| gyrB | GGAGGAAAATTTGACGGAAG | GTTTATAGGTTTGGCGGTGA |
| rrns | AGCATTCAGTTGGGCACTCT | CAGGTCATAAGGGGCATGAT |
| rpoA | GAAGGCGTTGTGGAAGATGT | GCTGCCGTTACAGTTCCTTC |
| gapA | GCTCTTAAAGAAGCGGCTGA | ACCATGCTGCCTTCCATAAC |
| hbsU | TTCCGGCAACTGCGTCTTTA | TGGTAACTTCGAGGTGCGTG |
| liaI | ACAAGAAAACAATAGGCGGA | AACGGAAGTGAGCAGATGA |
| skfA | AGCCGGGAGGTACTTCGATT | AGGATGCGGAAGTGCACAAA |
| recA | GTTCGGCAAAGGTTCCATTA | AGCGCCACAGTTGTTTTACC |
| ctc | TGCAGTCATTACGCTTGAGG | TTCACTCCAATGGCTTCTCC |
| relA | GGCATTGACAACCTCCTTGT | TTCCTTGCGCTTTTGAACTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Brul, S.; Zaat, S.A.J. Isolation of Persister Cells of Bacillus subtilis and Determination of Their Susceptibility to Antimicrobial Peptides. Int. J. Mol. Sci. 2021, 22, 10059. https://doi.org/10.3390/ijms221810059
Liu S, Brul S, Zaat SAJ. Isolation of Persister Cells of Bacillus subtilis and Determination of Their Susceptibility to Antimicrobial Peptides. International Journal of Molecular Sciences. 2021; 22(18):10059. https://doi.org/10.3390/ijms221810059
Chicago/Turabian StyleLiu, Shiqi, Stanley Brul, and Sebastian A. J. Zaat. 2021. "Isolation of Persister Cells of Bacillus subtilis and Determination of Their Susceptibility to Antimicrobial Peptides" International Journal of Molecular Sciences 22, no. 18: 10059. https://doi.org/10.3390/ijms221810059
APA StyleLiu, S., Brul, S., & Zaat, S. A. J. (2021). Isolation of Persister Cells of Bacillus subtilis and Determination of Their Susceptibility to Antimicrobial Peptides. International Journal of Molecular Sciences, 22(18), 10059. https://doi.org/10.3390/ijms221810059







