High-Throughput Gene and Protein Analysis Revealed the Response of Disc Cells to Vitamin D, Depending on the VDR FokI Variants
Abstract
:1. Introduction
2. Results
2.1. Genes Modulated by Vitamin D Treatment in Homozygous FF and Heterozygous Ff Disc Cells Cultured in Basal Condition
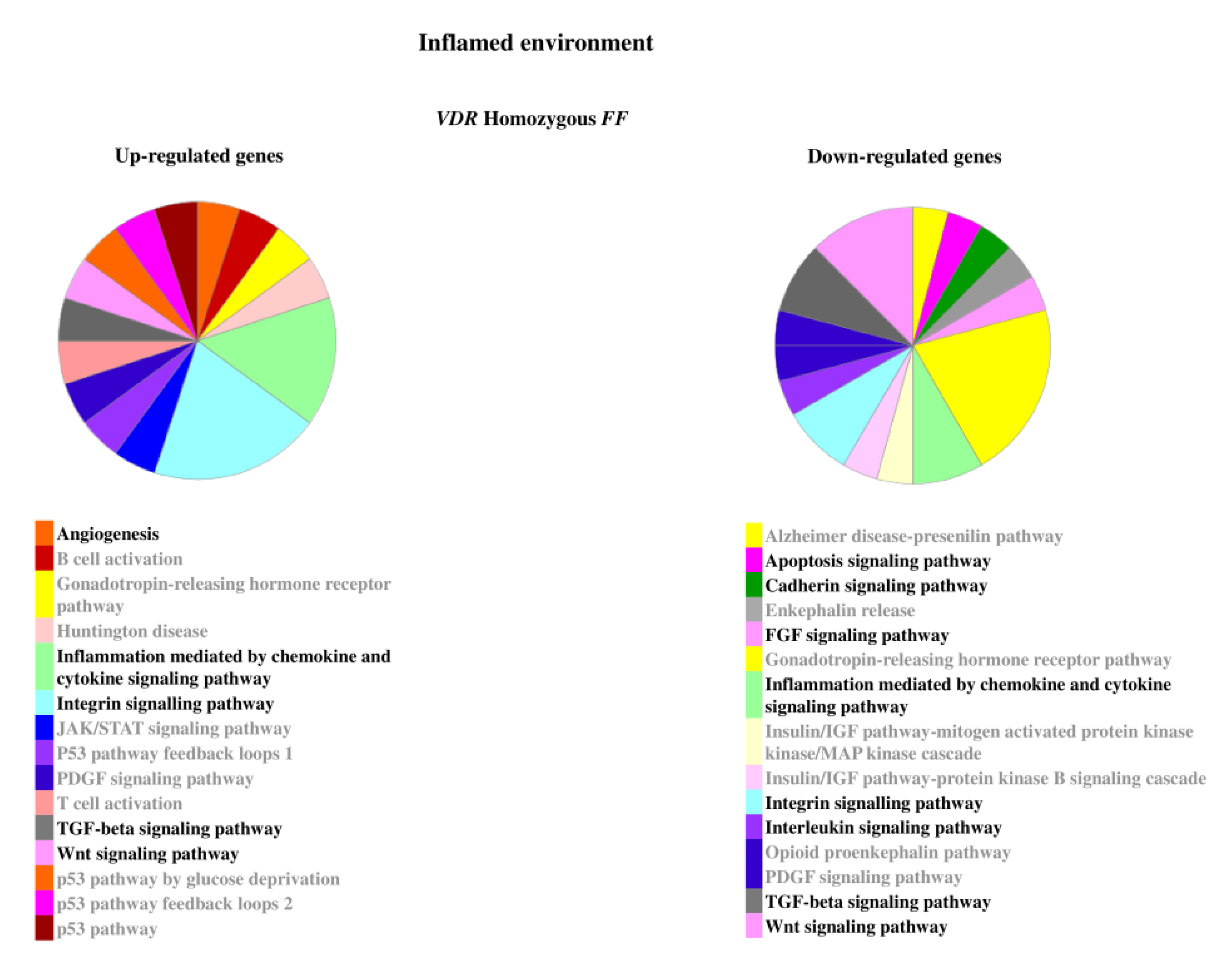
2.2. Genes Modulated by Vitamin D Treatment in Homozygous FF and Heterozygous Ff Disc Cells Cultured in Inflamed Condition
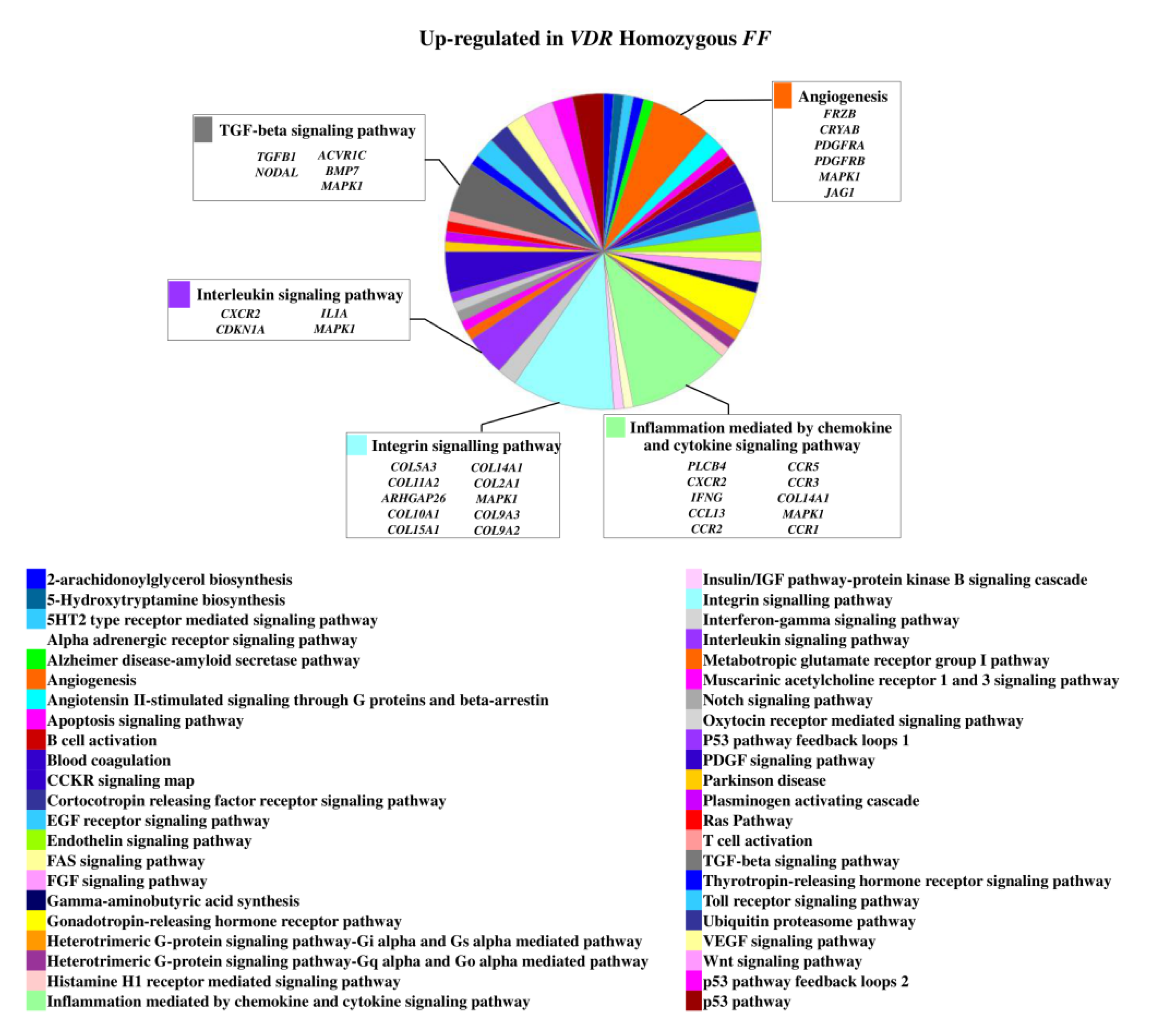
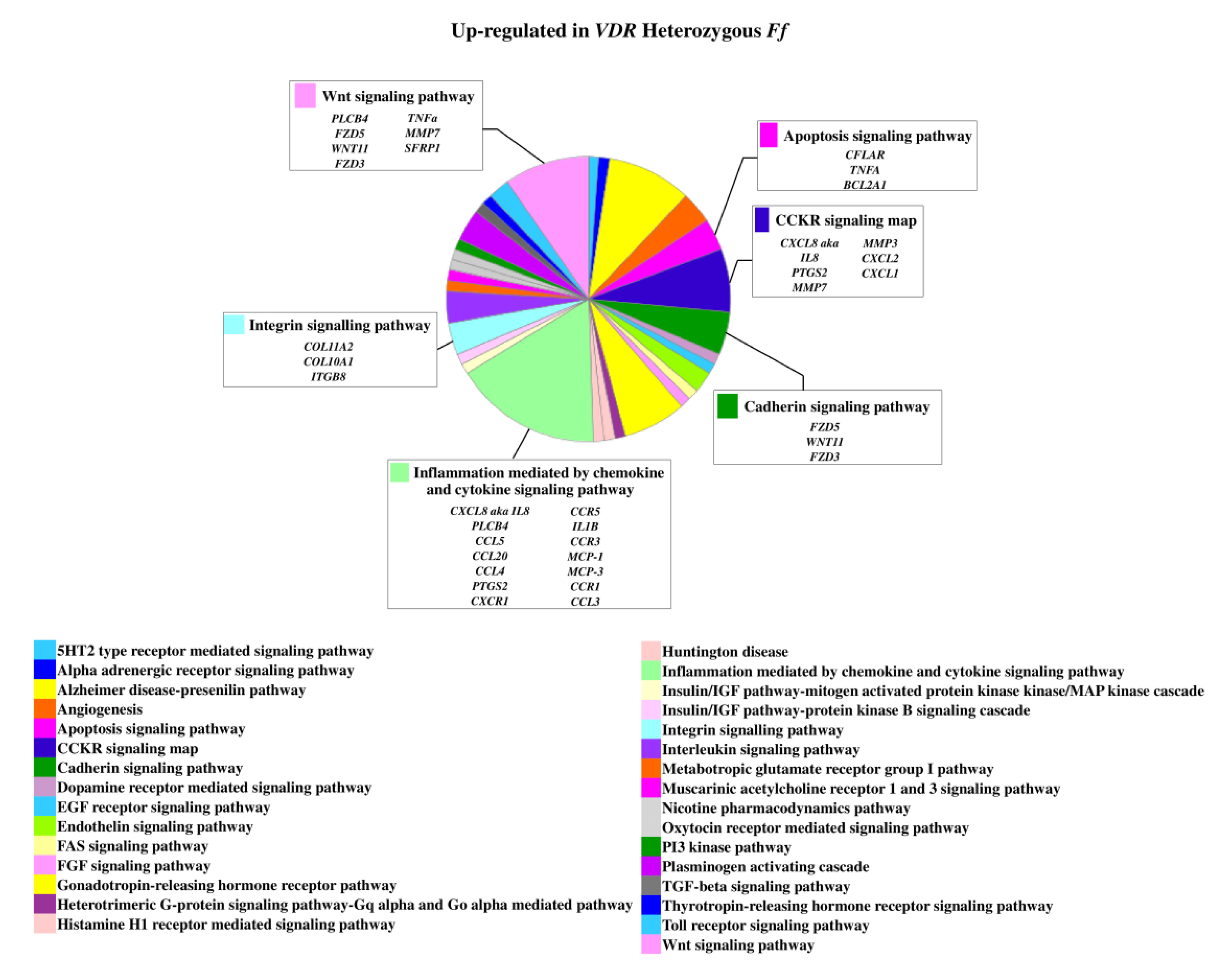
2.3. Relevant Pathways Affected by Vitamin D Depending on VDR FokI Genotypes
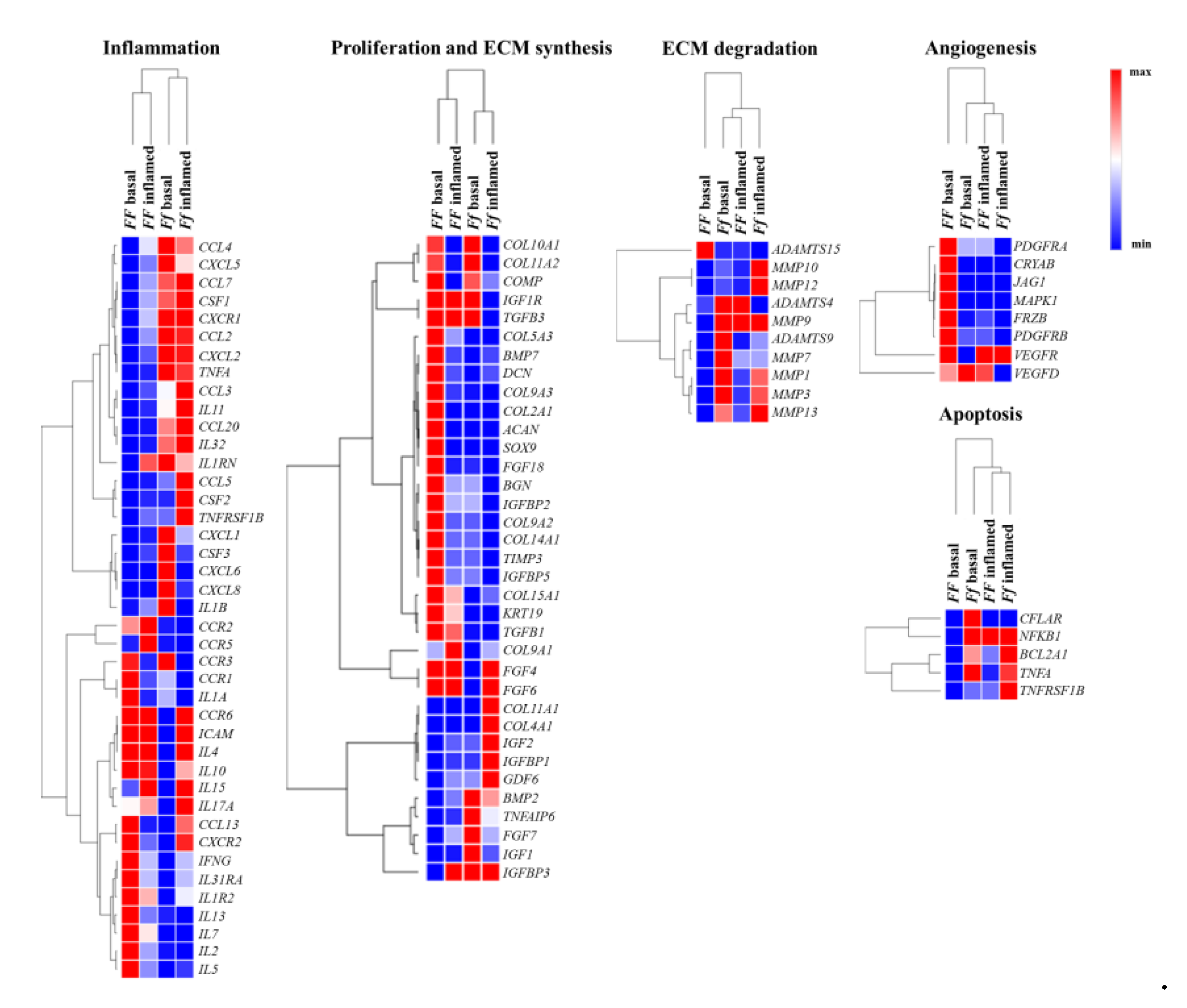
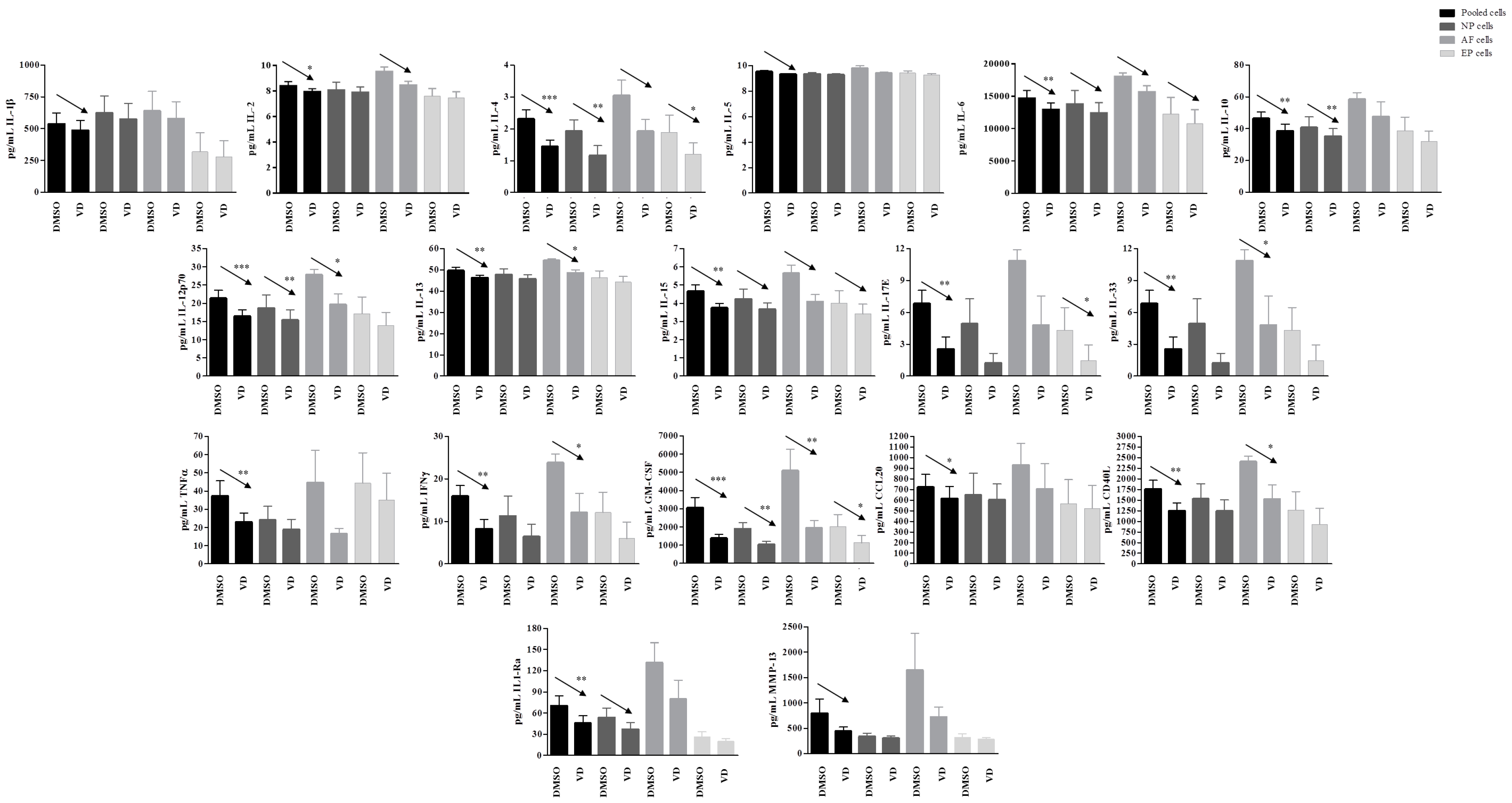
2.4. Higher Inflammatory Protein Modulation after Vitamin D Treatment Was Observed in Inflammatory Condition and Particularly in Ff Cells
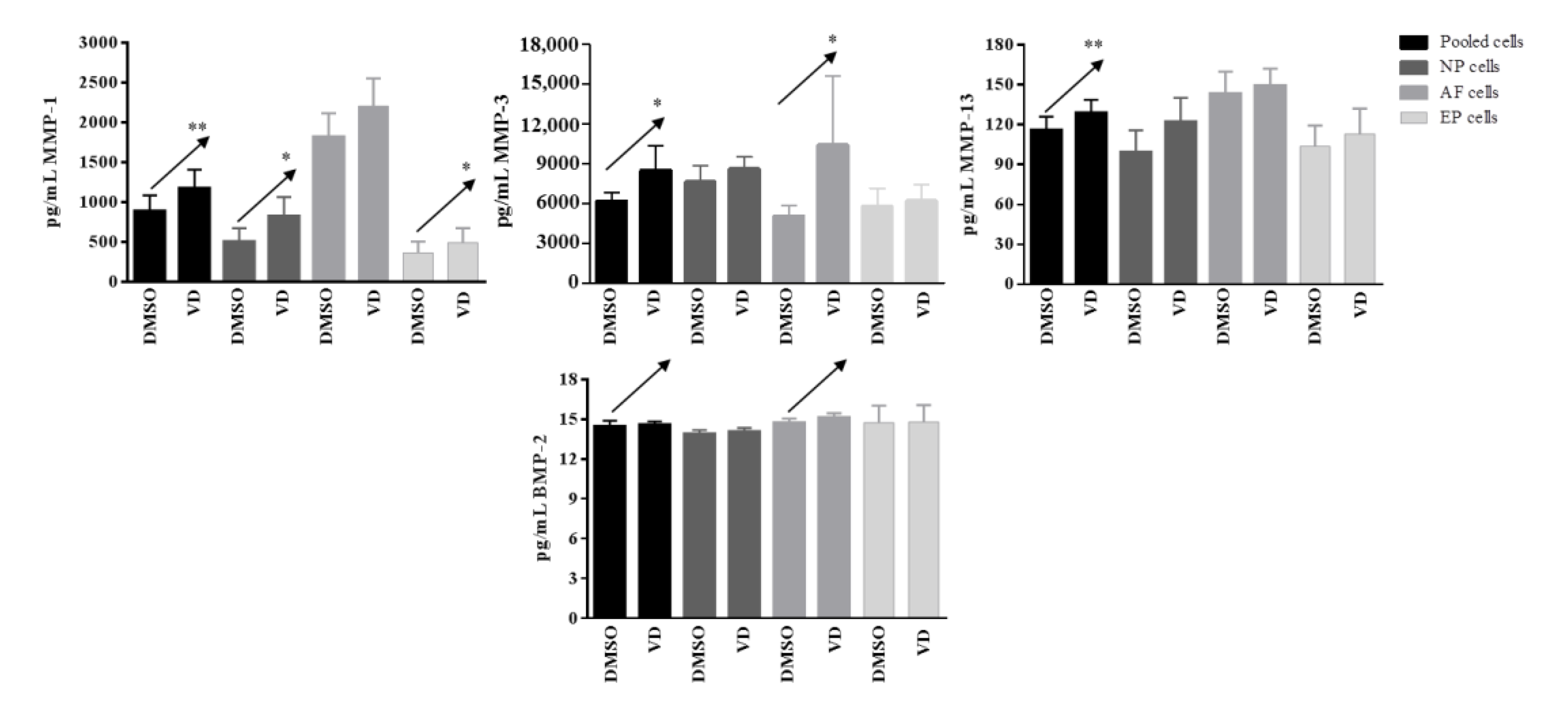
2.5. Validation of the Response to Vitamin D Observed in the Nucleus Pulposus (NP), Annulus Fibrosus (AF), and Cartilaginous Endplate (EP) Highest Responsive Ff Cells
3. Discussion
4. Materials and Methods
4.1. Tissue Sample Collection
4.2. Isolation and Expansion of Disc and Endplate Cells
4.3. Determination of FokI VDR Genotypes
4.4. 1,25(OH)2D3 and IL-1β Treatment Protocols
4.5. RNA Extraction and Quality Assessment
4.6. Gene Expression Microarray
4.7. Protein Array
4.8. Validation of the Release of Selected Proteins by Luminex® Assays
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reid, I.R.; Bolland, M.J. Skeletal and nonskeletal effects of vitamin D: Is vitamin D a tonic for bone and other tissues? Osteoporos. Int. 2014, 25, 2347–2357. [Google Scholar] [CrossRef]
- Gruber, H.E.; Hoelscher, G.; Ingram, J.A.; Chow, Y.; Loeffler, B.; Hanley, E.N., Jr. 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine 2008, 33, 755–765. [Google Scholar] [CrossRef] [Green Version]
- Colombini, A.; Lanteri, P.; Lombardi, G.; Grasso, D.; Recordati, C.; Lovi, A.; Banfi, G.; Bassani, R.; Brayda-Bruno, M. Metabolic effects of vitamin D active metabolites in monolayer and micromass cultures of nucleus pulposus and annulus fibrosus cells isolated from human intervertebral disc. Int. J. Biochem. Cell Biol. 2012, 44, 1019–1030. [Google Scholar] [CrossRef]
- Tong, T.; Liu, Z.; Zhang, H.; Sun, J.; Zhang, D.; Wang, F.; Miao, D.; Shen, Y. Age-dependent expression of the vitamin D receptor and the protective effect of vitamin D receptor activation on H2O2-induced apoptosis in rat intervertebral disc cells. J. Steroid Biochem. Mol. Biol. 2019, 190, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cheng, S.; Zheng, T.; Ye, Y.; Ye, A.; Zhu, S.; Lin, X. Vitamin D retards intervertebral disc degeneration through inactivation of the NF-kappaB pathway in mice. Am. J. Transl. Res. 2019, 11, 2496–2506. [Google Scholar] [PubMed]
- Colombini, A.; Cauci, S.; Lombardi, G.; Lanteri, P.; Croiset, S.; Brayda-Bruno, M.; Banfi, G. Relationship between vitamin D receptor gene (VDR) polymorphisms, vitamin D status, osteoarthritis and intervertebral disc degeneration. J. Steroid Biochem. Mol. Biol. 2013, 138, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Miyamoto, K.; Taketani, Y.; Yamamoto, H.; Iemori, Y.; Morita, K.; Tonai, T.; Nishisho, T.; Mori, S.; Takeda, E. A vitamin D receptor gene polymorphism in the translation initiation codon: Effect on protein activity and relation to bone mineral density in Japanese women. J. Bone Miner. Res. 1997, 12, 915–921. [Google Scholar] [CrossRef]
- Videman, T.; Leppavuori, J.; Kaprio, J.; Battie, M.C.; Gibbons, L.E.; Peltonen, L.; Koskenvuo, M. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine 1998, 23, 2477–2485. [Google Scholar] [CrossRef]
- Eser, B.; Cora, T.; Eser, O.; Kalkan, E.; Haktanir, A.; Erdogan, M.O.; Solak, M. Association of the polymorphisms of vitamin D receptor and aggrecan genes with degenerative disc disease. Genet. Test. Mol. Biomark. 2010, 14, 313–317. [Google Scholar] [CrossRef]
- Omair, A.; Lie, B.A.; Reikeras, O.; Brox, J.I. An association study of interleukin 18 receptor genes (IL18R1 and IL18RAP) in lumbar disc degeneration. Open Orthop. J. 2012, 6, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Noponen-Hietala, N.; Kyllonen, E.; Mannikko, M.; Ilkko, E.; Karppinen, J.; Ott, J.; Ala-Kokko, L. Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann. Rheum. Dis. 2003, 62, 1208–1214. [Google Scholar] [CrossRef]
- Cauci, S.; Vigano, M.; de Girolamo, L.; De Luca, P.; Perucca Orfei, C.; Banfi, G.; Lombardi, G.; Brayda-Bruno, M.; Colombini, A. High levels of circulating type II collagen degradation marker (CTx-II) are associated with specific VDR polymorphisms in patients with adult vertebral osteochondrosis. Int. J. Mol. Sci. 2017, 18, 2073. [Google Scholar] [CrossRef] [Green Version]
- Colombini, A.; Brayda-Bruno, M.; Lombardi, G.; Croiset, S.J.; Vrech, V.; Maione, V.; Banfi, G.; Cauci, S. FokI polymorphism in the vitamin D receptor gene (VDR) and its association with lumbar spine pathologies in the Italian population: A case-control study. PLoS ONE 2014, 9, e97027. [Google Scholar] [CrossRef]
- Pekala, P.A.; Henry, B.M.; Taterra, D.; Piwowar, M.; Vikse, J.; Tubbs, R.S.; Tomaszewski, K.A. FokI as a genetic factor of intervertebral disc degeneration: A PRISMA-compliant systematic review of overlapping meta-analyses. J. Clin. Neurosci. 2019, 60, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Luo, Z.J. Osteoporosis therapies might lead to intervertebral disc degeneration via affecting cartilage endplate. Med. Hypotheses 2019, 125, 5–7. [Google Scholar] [CrossRef]
- Pishgahi, A.; Dolatkhah, N.; Shakouri, S.K.; Hashemian, M.; Amiri, A.; Delkhosh Reihany, M.; Jahanjou, F. Lower serum 25-hydroxyvitamin D3 concentration is associated with higher pain and disability in subjects with low back pain: A case-control study. BMC Res. Notes 2019, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Gokcek, E.; Kaydu, A. Assessment of relationship between vitamin d deficiency and pain severity in patients with low back pain: A retrospective, observational study. Anesth. Essays Res. 2018, 12, 680–684. [Google Scholar] [CrossRef]
- Panwar, A.; Valupadas, C.; Veeramalla, M.; Vishwas, H.N. Prevalence of vitamin D deficiency in chronic and subacute low back pain patients in India: A triple-arm controlled study. Clin. Rheumatol. 2018, 37, 1367–1374. [Google Scholar] [CrossRef]
- Zadro, J.R.; Shirley, D.; Ferreira, M.; Carvalho Silva, A.P.; Lamb, S.E.; Cooper, C.; Ferreira, P.H. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician 2018, 21, 121–145. [Google Scholar] [CrossRef]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Cutolo, M.; Paolino, S.; Sulli, A.; Smith, V.; Pizzorni, C.; Seriolo, B. Vitamin D, steroid hormones, and autoimmunity. Ann. N. Y. Acad. Sci. 2014, 1317, 39–46. [Google Scholar] [CrossRef]
- Colombini, A.; Lombardi, G.; Corsi, M.M.; Banfi, G. Pathophysiology of the human intervertebral disc. Int. J. Biochem. Cell Biol. 2008, 40, 837–842. [Google Scholar] [CrossRef]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Goncalves, R.M.; Barbosa, M.A. Inflammation in intervertebral disc degeneration and regeneration. J. R. Soc. Interface 2015, 12, 20150429. [Google Scholar] [CrossRef]
- De Luca, P.; de Girolamo, L.; Perucca Orfei, C.; Vigano, M.; Cecchinato, R.; Brayda-Bruno, M.; Colombini, A. Vitamin D’s effect on the proliferation and inflammation of human intervertebral disc cells in relation to the functional vitamin D receptor gene foki polymorphism. Int. J. Mol. Sci. 2018, 19, 2002. [Google Scholar] [CrossRef] [Green Version]
- Pockert, A.J.; Richardson, S.M.; Le Maitre, C.L.; Lyon, M.; Deakin, J.A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 2009, 60, 482–491. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, M.; Wang, X.; Xu, X.; Ni, M.; Wang, Y. Negative effects of ADAMTS-7 and ADAMTS-12 on endplate cartilage differentiation. J. Orthop. Res. 2012, 30, 1238–1243. [Google Scholar] [CrossRef]
- Glant, T.T.; Kamath, R.V.; Bardos, T.; Gal, I.; Szanto, S.; Murad, Y.M.; Sandy, J.D.; Mort, J.S.; Roughley, P.J.; Mikecz, K. Cartilage-specific constitutive expression of TSG-6 protein (product of tumor necrosis factor alpha-stimulated gene 6) provides a chondroprotective, but not antiinflammatory, effect in antigen-induced arthritis. Arthritis Rheum. 2002, 46, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, H.G.; Vilcek, J. TSG-6: An IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997, 8, 143–156. [Google Scholar] [CrossRef]
- Miclea, R.L.; van der Horst, G.; Robanus-Maandag, E.C.; Lowik, C.W.; Oostdijk, W.; Wit, J.M.; Karperien, M. Apc bridges Wnt/beta-catenin and BMP signaling during osteoblast differentiation of KS483 cells. Exp. Cell Res. 2011, 317, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003, 278, 41227–41236. [Google Scholar] [CrossRef] [Green Version]
- Fischer, L.; Boland, G.; Tuan, R.S. Wnt signaling during BMP-2 stimulation of mesenchymal chondrogenesis. J. Cell. Biochem. 2002, 84, 816–831. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; de Girolamo, L.; Kouroupis, D.; Castagnetta, M.; Perucca Orfei, C.; Coviello, D.; Coco, S.; Correa, D.; Brayda-Bruno, M.; Colombini, A. Intervertebral disc and endplate cells response to IL-1beta inflammatory cell priming and identification of molecular targets of tissue degeneration. Eur. Cells Mater. 2020, 39, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Nie, L.; Wang, Y.; Wang, X.P.; Zhao, H.; Dongol, S.; Maharjan, S.; Cheng, L. CCL20 secretion from the nucleus pulposus improves the recruitment of CCR6-expressing Th17 cells to degenerated IVD tissues. PLoS ONE 2013, 8, e66286. [Google Scholar] [CrossRef] [Green Version]
- Shamji, M.F.; Setton, L.A.; Jarvis, W.; So, S.; Chen, J.; Jing, L.; Bullock, R.; Isaacs, R.E.; Brown, C.; Richardson, W.J. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010, 62, 1974–1982. [Google Scholar] [CrossRef] [Green Version]
- Colombini, A.; Lopa, S.; Ceriani, C.; Lovati, A.B.; Croiset, S.J.; Di Giancamillo, A.; Lombardi, G.; Banfi, G.; Moretti, M. In vitro characterization and in vivo behavior of human nucleus pulposus and annulus fibrosus cells in clinical-grade fibrin and collagen-enriched fibrin gels. Tissue Eng. Part A 2015, 21, 793–802. [Google Scholar] [CrossRef]
- Lopa, S.; Ceriani, C.; Cecchinato, R.; Zagra, L.; Moretti, M.; Colombini, A. Stability of housekeeping genes in human intervertebral disc, endplate and articular cartilage cells in multiple conditions for reliable transcriptional analysis. Eur. Cells Mater. 2016, 31, 395–406. [Google Scholar] [CrossRef]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- De Luca, P.; Castagnetta, M.; de Girolamo, L.; Coco, S.; Malacarne, M.; Ragni, E.; Vigano, M.; Lugano, G.; Brayda-Bruno, M.; Coviello, D.; et al. Intervertebral disc and endplate cell characterisation highlights annulus fibrosus cells as the most promising for tissue-specific disc degeneration therapy. Eur. Cells Mater. 2020, 39, 156–170. [Google Scholar] [CrossRef]
- Thomas, P.D.; Kejariwal, A.; Campbell, M.J.; Mi, H.; Diemer, K.; Guo, N.; Ladunga, I.; Ulitsky-Lazareva, B.; Muruganujan, A.; Rabkin, S.; et al. PANTHER: A browsable database of gene products organized by biological function, using curated protein family and subfamily classification. Nucleic Acids Res. 2003, 31, 334–341. [Google Scholar] [CrossRef]



| Basal | Inflamed | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| FF | Ff | FF | Ff | ||||||
| Gene | Protein | Gene | Protein | Gene | Protein | Gene | Protein | Gene | Protein |
| CXCL5 | ENA-78 | − | n.d. | = | n.d. | + | = | + | − |
| CXCL1 | GRO/GROα | − | n.d. | + | n.d. | = | = | + | − |
| CXCL8 | IL-8 | − | n.d. | + | + | = | = | + | − |
| CCL2 | MCP-1 | − | = | + | + | = | = | + | − |
| CCL5 | RANTES | − | n.d. | + | n.d. | = | n.d. | + | n.d. |
| CCL7 | MCP-3 | − | n.d. | + | n.d. | = | n.d. | + | n.d. |
| IL1A | IL-1α | + | n.d. | = | n.d. | − | n.d. | − | n.d. |
| IL1B | IL-1β | − | n.d. | + | n.d. | = | − | − | n.d. |
| IL2 | IL-2 | + | = | − | + | = | = | − | − |
| IL3 | IL-3 | = | n.d. | = | + | = | + | = | − |
| IL4 | IL-4 | = | n.d. | − | n.d. | = | n.d. | = | n.d. |
| IL5 | IL-5 | + | n.d. | − | n.d. | + | n.d. | = | n.d. |
| IL6 | IL-6 | = | n.d. | = | n.d. | = | = | = | − |
| IL7 | IL-7 | + | n.d. | = | n.d. | + | n.d. | = | n.d. |
| IL10 | IL-10 | + | n.d. | − | n.d. | + | n.d. | + | n.d. |
| IL13 | IL-13 | = | n.d. | − | n.d. | − | n.d. | − | n.d. |
| IL15 | IL-15 | − | n.d. | − | n.d. | = | n.d. | = | n.d. |
| IFNG | IFNγ | + | n.d. | − | − | = | n.d. | = | n.d. |
| TNFA | TNFα | − | n.d. | + | n.d. | − | + | + | n.d. |
| CSF1 | M-CSF | − | n.d. | + | n.d. | = | n.d. | + | n.d. |
| CSF2 | GM-CSF | − | n.d. | = | n.d. | = | − | + | − |
| CSF3 | G-CSF | − | n.d. | + | n.d. | = | n.d. | = | − |
| LEP | Leptin | = | = | = | − | = | = | = | + |
| IGF1 | IGF-1 | − | n.d. | + | n.d. | − | n.d. | = | n.d. |
| PDGFB | PDGF-BB | = | + | = | n.d. | = | − | = | + |
| TGFB1 | TGFβ1 | + | n.d. | − | − | + | n.d. | − | n.d. |
| VEGF | VEGF | = | + | = | n.d. | = | − | = | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombini, A.; De Luca, P.; Cangelosi, D.; Perucca Orfei, C.; Ragni, E.; Viganò, M.; Malacarne, M.; Castagnetta, M.; Brayda-Bruno, M.; Coviello, D.; et al. High-Throughput Gene and Protein Analysis Revealed the Response of Disc Cells to Vitamin D, Depending on the VDR FokI Variants. Int. J. Mol. Sci. 2021, 22, 9603. https://doi.org/10.3390/ijms22179603
Colombini A, De Luca P, Cangelosi D, Perucca Orfei C, Ragni E, Viganò M, Malacarne M, Castagnetta M, Brayda-Bruno M, Coviello D, et al. High-Throughput Gene and Protein Analysis Revealed the Response of Disc Cells to Vitamin D, Depending on the VDR FokI Variants. International Journal of Molecular Sciences. 2021; 22(17):9603. https://doi.org/10.3390/ijms22179603
Chicago/Turabian StyleColombini, Alessandra, Paola De Luca, Davide Cangelosi, Carlotta Perucca Orfei, Enrico Ragni, Marco Viganò, Michela Malacarne, Mauro Castagnetta, Marco Brayda-Bruno, Domenico Coviello, and et al. 2021. "High-Throughput Gene and Protein Analysis Revealed the Response of Disc Cells to Vitamin D, Depending on the VDR FokI Variants" International Journal of Molecular Sciences 22, no. 17: 9603. https://doi.org/10.3390/ijms22179603
APA StyleColombini, A., De Luca, P., Cangelosi, D., Perucca Orfei, C., Ragni, E., Viganò, M., Malacarne, M., Castagnetta, M., Brayda-Bruno, M., Coviello, D., & de Girolamo, L. (2021). High-Throughput Gene and Protein Analysis Revealed the Response of Disc Cells to Vitamin D, Depending on the VDR FokI Variants. International Journal of Molecular Sciences, 22(17), 9603. https://doi.org/10.3390/ijms22179603










