1. Introduction
Osteoporosis is a skeletal disorder characterized by the imbalance between bone formation and resorption, which leads to a decrease in bone mass and density and to progressive changes in bone structure [
1]. This, in turn, results in a higher risk of bone fracture, most commonly in the vertebrae, wrist, or hip [
2]. Currently, osteoporosis is a global health problem affecting an estimated 200 million people [
3]. Aging and chronic estrogen deficiency are the most important risk factors for osteoporosis [
4,
5]. Numerous therapeutic agents have been developed to treat osteoporosis. These include either bone-anabolic or antiresorptive drugs [
6]. Among these drugs, the most active compounds belong to the class of nitrogen-containing bisphosphonates (N-BPs). Because of their chemical structure, BPs efficiently accumulate in the bone tissue and reduce bone resorption through the inhibition of osteoclast activity [
7]. In most commercial BPs, a hydroxyl group that is covalently bound to the carbon atom of the bisphosphonic group (P-C-P) as the R
1 substituent determines the high affinity of BPs to bone mineral surface, while the antiresorptive activity is dependent basically on the chemical structure of the R
2 chain [
8,
9]. The acidic microenvironment generated by resorbing osteoclasts facilitates a release of BPs from bone tissue in osteoclast lacunae, which allows their internalization into osteoclasts by endocytosis [
10,
11]. The molecular mechanism of action of N-BPs involves the prevention of farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) through the inhibition of the mevalonate pathway [
12].
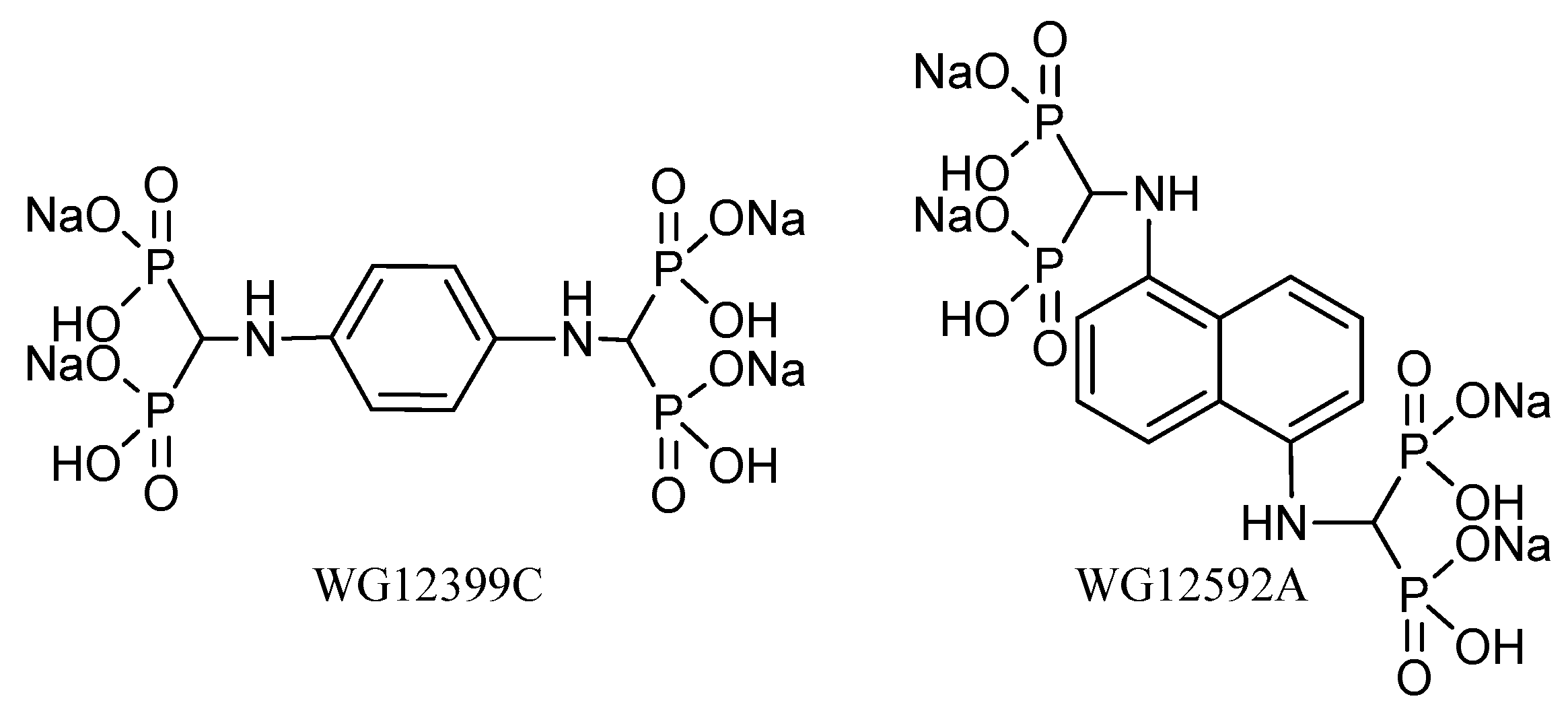
In our previous studies, we obtained two new compounds belonging to the group of aminomethylidenebisphosphonates: Benzene-1,4-bis[aminomethylidene(bisphosphonic)] acid and naphthalene-1,5-bis[aminomethylidene(bisphosphonic)] acid [
13]. These BPs have two bisphosphonic groups in their structures and aromatic ring (phenyl and naphthyl, respectively) bonded directly to the nitrogen atom of the aminomethylidenebisphosphonic group. Both BPs showed a significant antiproliferative activity toward murine J774E macrophages, a model of osteoclast precursors in vitro, through changes in cell cycle progression. Moreover, these compounds enhanced the cytotoxic activity of doxorubicin and cisplatin, especially when applied prior to cytostatics, and showed proapoptotic activity in osteoclast precursors, which was manifested by an increase in caspase-3 activity and percentage of apoptotic cells [
14]. The results obtained prompted us to perform an extended research study on the biological activity of these BPs. Because of the low solubility of the free acids, both compounds were used in the present study as their tetrasodium salt (
Figure 1).
The specific aim of this study was to evaluate the in vivo antiresorptive activity of WG12399C and WG12592A aminobisphosphonates. Ovariectomized (OVX) Balb/c mice were used in this study as a model of estrogen deficiency-related osteoporosis. Female OVX mice and rats that mimic the postmenopausal hormonal status of women are the most widely used animal model of osteoporosis for antiosteoporotic drug screening [
15,
16]. The influence of WG12399C and WG12592A administration on bone microstructure, bone strength, and biochemical parameters of bone turnover was studied. The ex vivo antiresorptive activity of aminobisphosphonates in osteoclasts was also evaluated.
3. Discussion
Bisphosphonates are the potent inhibitors of osteoclast-mediated bone resorption and have been widely used as effective therapeutic agents in bone disorders. Most of the clinically applied BPs are hydroxybisphosphonates. In these compounds the carbon atom of the methylenebisphosphonic group is attached directly to the hydroxyl group [
18]. In current study, we evaluated the skeletal effects of two BPs which contain the methylenebisphosphonic group bounded directly to the nitrogen atom.
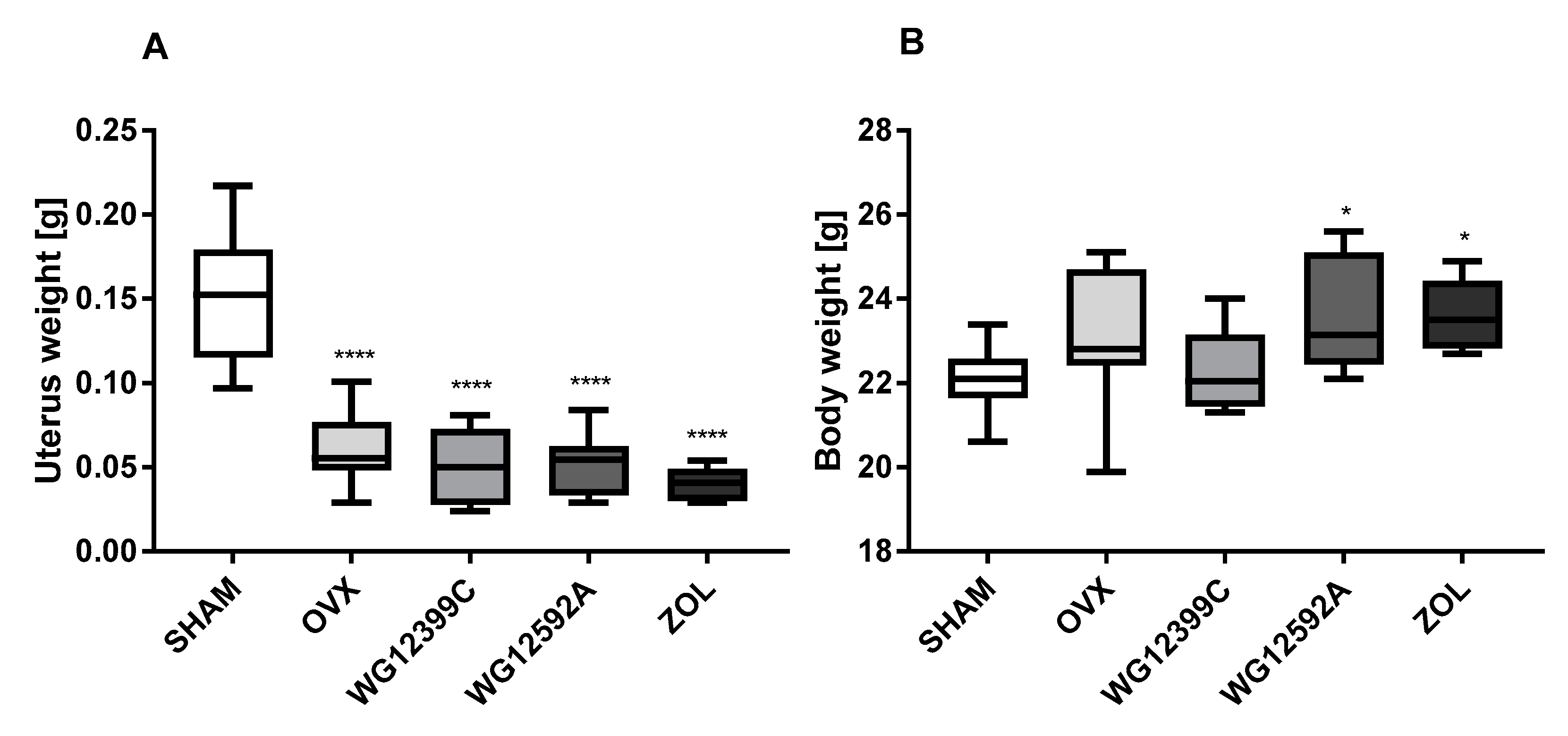
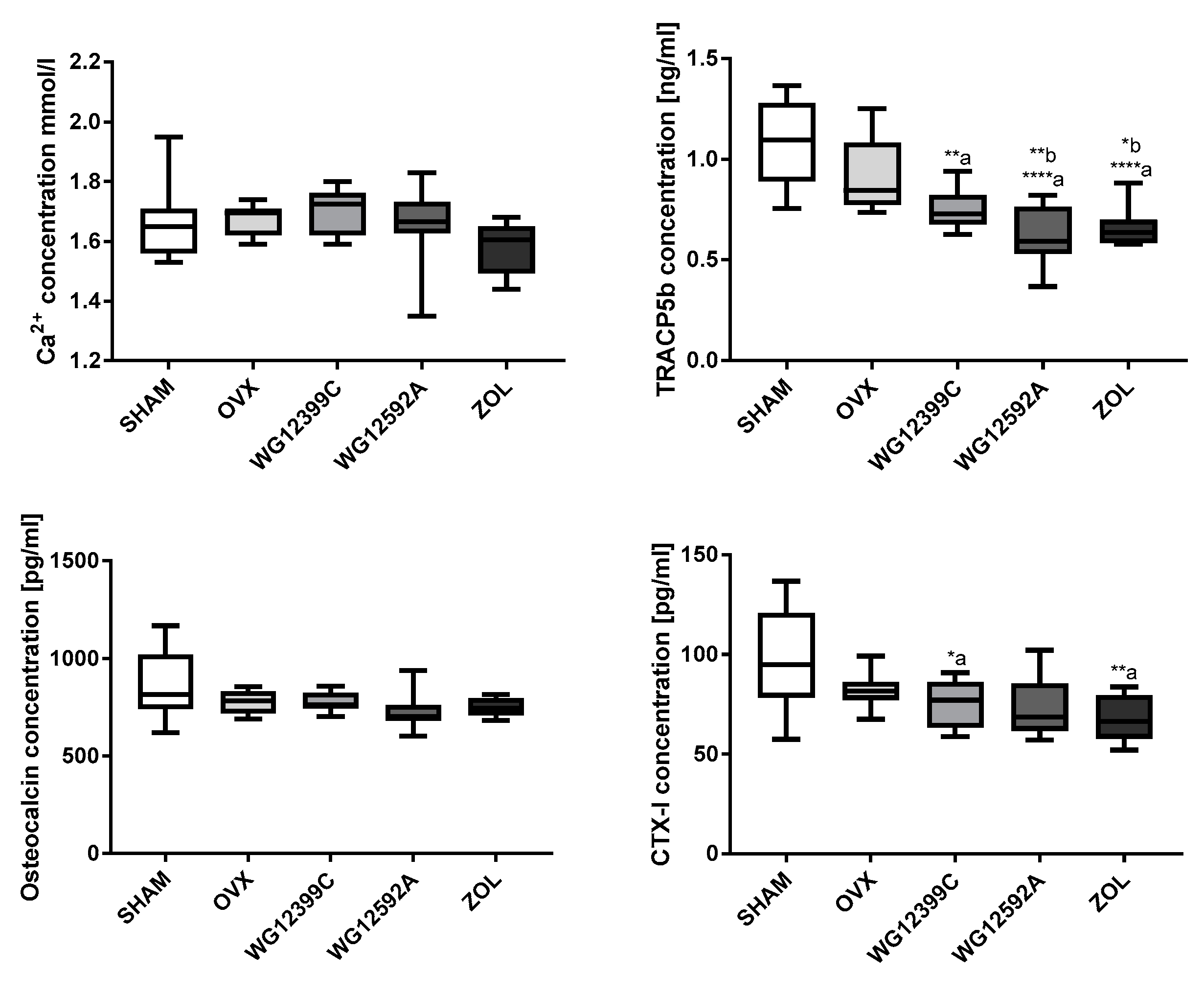
Because osteoporosis occurs most frequently in postmenopausal women due to severe estrogen deficiency, an ovariectomized (OVX) mouse model was used in the study. Our results confirmed that bilateral ovariectomy causes a significant depletion of estrogen manifested by uterus involution in all OVX mice. In addition, significant changes in trabecular bone microarchitecture were also observed in mice subjected to OVX. In the analysis of 3D structure of proximal tibial metaphysis, a decrease in BMD and an increase in Tb.Sp values induced by OVX were evident. Unfortunately, these changes were not reflected in the plasma levels of TRAC5B and CTX-I. Based on the results obtained in this study we cannot explain this phenomenum. We postulate that this may be related to the ongoing processes of bone remodeling or simply to aging of animals, since both factors may influence the serum level of bone markers [
19,
20].
Many studies have shown that nitrogen-containing BPs may inhibit bone loss and preserve bone volume in both animal models and humans; however, their individual effect on bone resorption is variable [
21,
22,
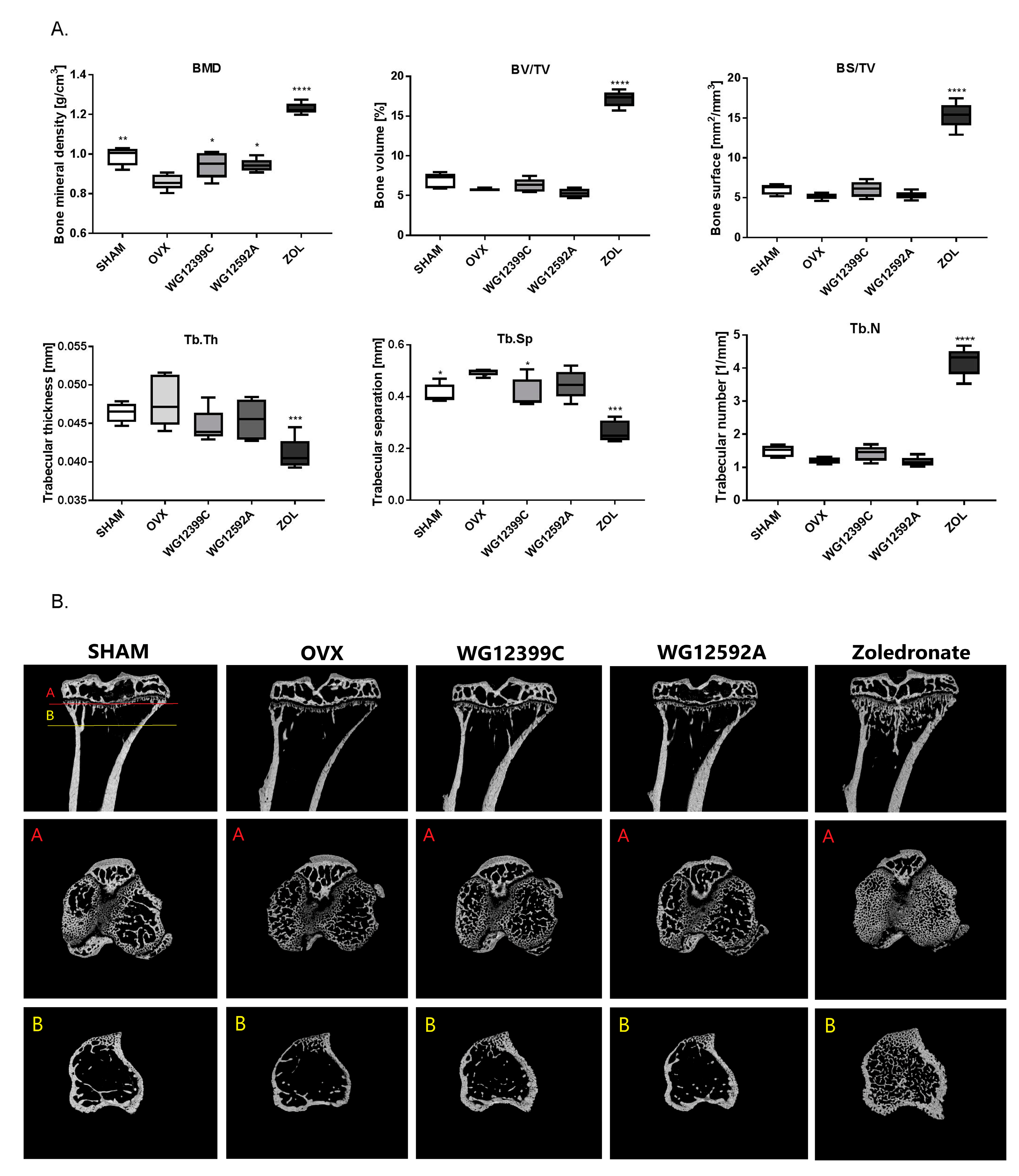
23]. Here we report that all BPs applied in the study suppressed OVX-induced bone loss. The µCT analysis revealed an improvement of important structural indices in BP-treated mice as compared to those in the untreated control group (
Figure 3 and
Figure S8). Intravenous injections with 60 mg/kg WG12399C or 6 mg/kg WG12592A almost completely prevented BMD decrease, and these indices remained at the level comparable to that in the sham group. Trabecular number and trabecular separation were also restored in mice receiving WG12399C and, to a lower degree, in the WG12592A group. A parallel analysis showed that treatment with WG12399C, WG12592A, or zoledronate also significantly improved the quality of bone structure in distal femoral metaphysis (
Figure S8). In this experiment single doses of WG12399C and WG12592A were applied, which is the main limitation of the study. However, since zoledronate chosen as the reference compound is the most active bisphosphonate applied in the clinic, we decided to use the maximum well-tolerated doses of WG12399C and WG12592A estimated in toxicity studies and evaluate their biological activity in comparison to zoledronate.
Interestingly, a remarkable improvement of microstructure was observed in zoledronate-treated mice as compared to that in healthy sham-operated animals. This phenomenon has been previously reported in other studies. In tumor-bearing mice, zoledronate caused a significant increase in bone volume in both OVX and sham-operated animals compared to that in the respective controls; this demonstrated that bone resorption is reduced to the same extent in the models of pre- and postmenopausal bone [
24]. Similarly, Kim et al. showed that treatment with zoledronate spectacularly improved BMD as well as bone volume and thickness in a murine model of irradiation-induced bone loss [
25]. It is also worth noting that although the overall bone microstructure in zoledronate-treated mice was improved as compared to that in the other experimental groups, the trabecular thickness was reduced in both tibial (
Figure 3A) and femoral metaphyses (
Figure S8). This was compensated by a significant increase in the number of probably newly formed bone trabeculae, which resulted in a decrease in Tb.Sp values.
Bone mass is a major determinant of bone strength, and BMD is considered to be the standard parameter to evaluate bone strength and the risk of bone fractures. However, other factors related to bone microstructure also contribute to bone integrity and its resistance to mechanical stress. These factors include trabecular thickness and separation, porosity, and collagen content [
26]. Thicker plate-like trabeculae provide better bone quality and strength than thinner rod-like trabeculae [
27]. Thus, the bone quality defined as the sum of structural and material properties rather than BMD alone seems to be a better predictor of bone strength.
In these studies, mechanical tests were performed using the four-point bending test, the main advantage of which is the constant bending moment in the area of the examined bone (middle region) [
28]. Unexpectedly, a significant improvement in the quality of bone microstructure in tibial and femoral metaphysis did not correlate with an improvement of parallel indices in femoral shaft (
Figure S9) and with an increase in bone strength in our study. This may be related to the fact the long bone is very diversified along its entire length, both in terms of shape and size of the cross-section, which is determined precisely by the thickness of the cortex layer and the cross-section diameter. A bone fracture always occurs in the weakest place, often below the distal femoral epiphysis, where a cortical layer of fairly large diameter but a small thickness is observed. Moreover, because of their chemical structure, BPs show high affinity to bone tissue and binds readily to resorbing surfaces. Therefore, they accumulate much more profusely in trabecular bone than in cortical bone [
29]. This may be one of the reasons why a significant improvement in trabecular structure in the area of tibial and femoral metaphyses is not correlated with a remarkable increase of parallel parameters in bone shafts (
Figure S9) as well as in bone strength in the bending test.
Bone sustains its homeostatic state by maintaining the balance between two opposite processes: Bone formation by osteoblasts and bone resorption mediated by osteoclasts. Osteoporosis is caused by an excessive osteoclast-related osteolysis. Estrogen deficiency affects bone remodeling through direct and indirect mechanisms [
30]. These mechanisms include the influence on osteoclast lifespan and apoptosis [
31], RANKL-induced osteoclast differentiation [
32], and the effects on the production of RANKL [
33] and osteoprotegrin [
34]. Previous studies have shown that staining for TRAP activity reflects the increase in the activity and size of osteoclasts in OVX mice [
35,
36], and the inhibition of osteoclast activity is correlated with the improvement in bone microarchitecture [
37,
38]. Thus far, the influence of BPs on the morphology and activity of osteoclasts has been shown in many animal model studies and in bone biopsies from BPs-treated patients [
39,
40]. However, the exact nature of the effect of BPs on osteoclast number, activity, and life-span remains inconclusive. In our present study, the staining for TRAP activity showed an elevated number of active osteoclasts in distal tibial metaphysis in OVX mice as compared to that in sham-operated animals. Treatment with WG12399C or WG12592A significantly reduced the number of large multinucleated TRAP-positive cells when compared with that in OVX mice. These changes were at comparable level to those observed for the reference zoledronic acid.
The reduction in the TRAP activity reflects the decreased number of and suppressed resorptive activity of osteoclasts and correlates with the overall condition of bone structure. However, the exact mechanism involved in this phenomenon remains elusive.
Thus far, it has been shown that zoledronic acid decreased the number of TRAP-positive multinuclear cells derived from mouse osteoclast precursors through the inhibition of differentiation and multinucleation [
41]. In turn, Hirayama et al. postulated that in rheumatoid arthritis, an increased osteoclast functional activity rather than osteoclast formation is more likely to play a role in generalized bone loss [
42]. Thus, we posed the question of whether the changes in osteoclast number and activity in BP-treated mice result from the diminished maturation of osteoclast precursors or from their reduced resorptive activity.
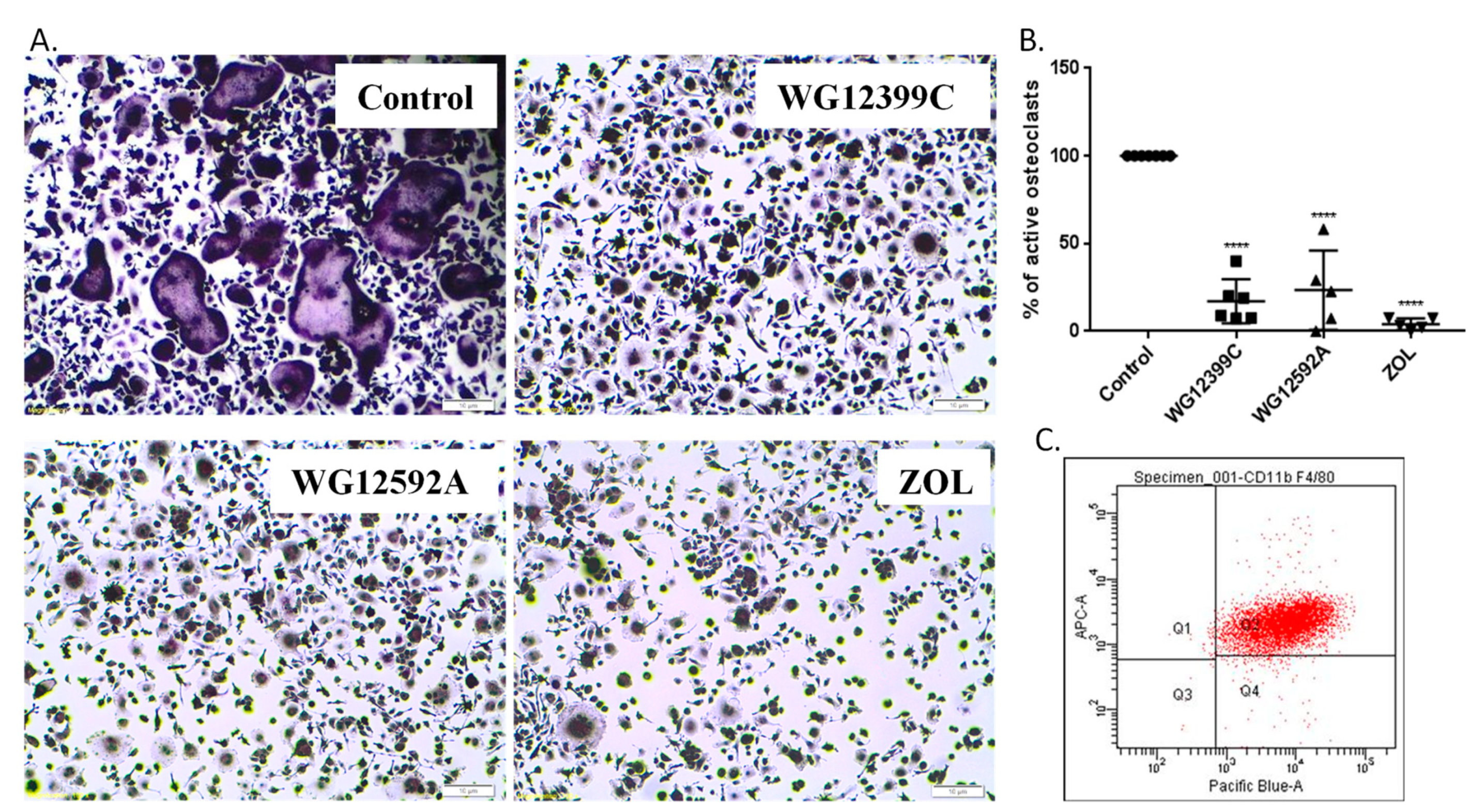
In previous studies, we have shown that WG12399C and WG12592A significantly restrain the proliferation rate of J774E cells, which are a convenient model of osteoclast precursors for in vitro studies [
13]. The antiproliferative effect of new BPs was related to the changes in cell cycle progression and proapoptotic activity [
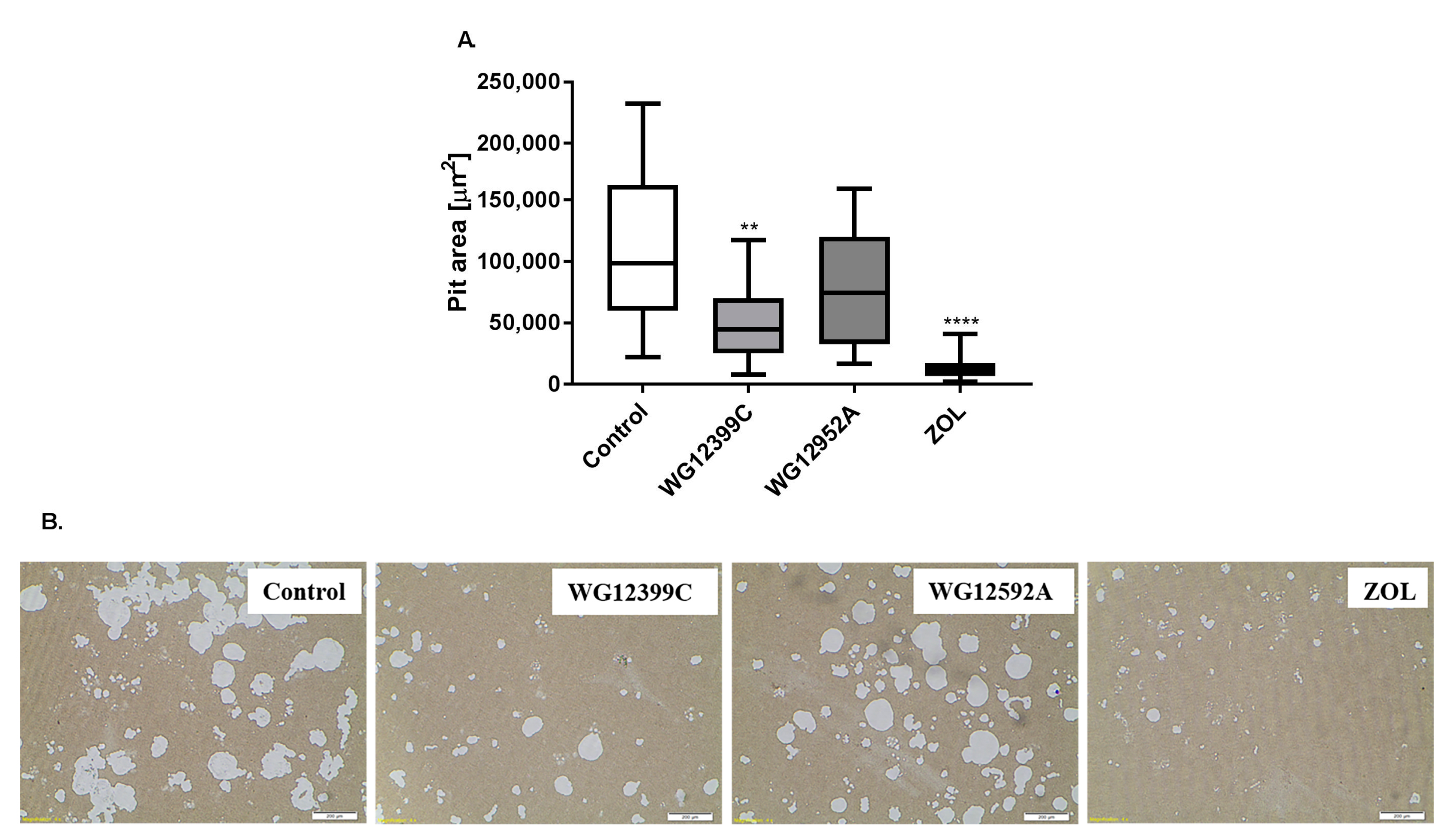
14]. However, these model cells do not readily differentiate into mature osteoclasts under standard culturing conditions. Thus, primary bone marrow cells were isolated from murine marrow and differentiated to macrophages. Then, BMDMs were cultured in the presence of RANKL and M-CSF and either WG12399C, WG12592A, or reference zoledronate. Both the studied BPs remarkably inhibited osteoclastogenesis, resulting in the reduction in the number of active, TRAP-positive osteoclasts by 83% and 77%, respectively, for WG12399C and WG12592A. The influence of these BPs on osteoclast differentiation was similar to the effects observed for the reference zoledronic acid. Then, WG12399C and WG12592A compounds were tested for their antiresorptive properties. Fully differentiated macrophages cultured on osteo-microplates in the presence of RANKL and M-CSF develop into active osteoclasts and efficiently resorb the mineral coating of the plates. In these studies, the treatment of cells with WG12399C or WG12592A resulted in partial inhibition of their resorptive activity; however, these effects were not as pronounced as for the reference zoledronate. Nevertheless, both aminomethylidenebisphosphonates significantly affected the maturation and antiresorptive activity of osteoclasts.
4. Materials and Methods
4.1. Compounds
Tetrasodium salts of benzene-1,4-bis[aminomethylidene(bisphosphonic)] acid (WG12399C) and naphthalene-1,5-bis[aminomethylidene(bisphosphonic)] acid (WG12592A) were prepared from free acids using the procedure described below. The preparation of the starting free bisphosphonic acids was carried out in a large scale using the procedure developed in our previous studies [
13] and was described in detail in the
Supplementary Materials.
1H and
31P NMR spectra were recorded on a 600 MHz Bruker Avance spectrometer in D
2O as the solvent (at 600 MHz and 243 MHz, respectively) and locked on deuterium from a solvent. Chemical shifts (δ) are expressed in parts per million (ppm). High-resolution mass spectra were recorded on a Bruker MicrOTOF-QII spectrometer (Bruker Daltonic, Bremen, Germany) equipped with an electrospray ion source. The instrument was operated in the negative-ion mode and calibrated externally with a sodium formate (10 mM). The capillary temperature was 200 °C, and N
2 was used as a nebulizing gas. Data were acquired with micrOTOFcontrol 4.0 and processed for calibration with DataAnalysis software from Daltonic GmbH (Germany). Samples of the studied compounds were infused into the mass spectrometer at a flow rate of 3 μL/min in water/isopropanol mixture (50/50,
v/
v). All reagents and solvents used in the synthesis were of commercial quality and purchased from Sigma-Aldrich (Darmstadt, Germany) and a local supplier (Avantor, Poland). Zoledronate was synthesized according to literature procedure [
43].
Procedure for the preparation of WG12399C and WG12592A: First, 10 mmol of free benzene-1,4-bis[aminomethylidene(bisphosphonic)] acid and naphthalene-1,5-bis-[aminomethylidene(bisphosphonic)] acid were dissolved in 40 mL of 1.0 M NaOH (40 mmol). The resulting solutions were added dropwise to the vigorously stirred 96% ethanol (250 mL), and the resulting suspensions aged over night at room temperature. The suspensions were centrifuged at 4700 rpm for 30 min. After centrifugation, the obtained solid was washed with 96% ethanol (4∙25 mL) and dried in vacuo, yielding the tetrasodium salts of benzene-1,4-bis[aminomethylidene(bisphosphonic)] acid (WG12399C) and naphthalene-1,5-bis[aminomethylidene(bisphosphonic)] acid (WG12592A) with 90% and 98% yield, respectively. The structures of the obtained BPs were confirmed by
1H,
31P NMR and HRMS-ESI spectroscopy: WG12399C:
1H NMR (D
2O, 600 MHz): δ 3.61 (t, 2H,
J = 19.5 Hz, CH), 6.69 (s, 4H, ArH);
31P {
1H} NMR (D
2O, 243 MHz): δ 15.47 (s); HRMS–ESI:
m/z [M-4H+3Na]
− calcd: 520.9028; found: 520.9044; WG12592A:
1H NMR (D
2O, 600 MHz): δ 4.07 (t, 2H,
J = 19.9 Hz, CH), 6.81 (d, 2H,
J = 6.5 Hz, ArH), 7.34–7.38 (m, 4H, ArH);
31P {
1H} NMR (D
2O, 243 MHz): δ 15.46 (s); HRMS–ESI: m/z [M-4H+3Na]
− calcd: 570.9185; found: 570.9193. Copies of
1H,
31P NMR and HRMS-ESI spectra are available in
Supplementary Materials (Figures S1–S6).
4.2. Animals
For toxicity studies and osteoporosis experiment, eight-week-old female BALB/c mice were purchased from the Center of Experimental Medicine of the Medical University of Bialystok (Bialystok, Poland) and maintained in specific pathogen-free conditions. For ex vivo analyses, six- to eight-week-old male Foxp3 × Balb/c male mice were obtained from the Hirszfeld Institute of Immunology and Experimental Therapy, Wroclaw. The experiments were performed according to EU Directive 2010/63/EU on the protection of animals used for scientific purposes and were approved by the first Local Committee for Experiments with the Use of Laboratory Animals, Wroclaw, Poland (Permission No.: 4/2015 and 111/2017). Mice were maintained in a temperature-controlled specific pathogen-free facility with access to mouse chow (S8435-S023, Sniff Spezialdiaten, Soest, Germany) and water ad libitum.
4.3. Toxicity Studies
The single-dose acute intravenous toxicity was evaluated in Balb/c female mice, weighing 17–22 g. Each group of five mice received, respectively, a single dose of 1.75; 5.5; 17.5; 35; 55.5; 175 mg/kg body weight of WG12399C or 1.75; 3.5; 5.5; 17.5 mg/kg body weight of WG12592A. The general behavior of mice and the signs of toxicity were observed continuously for 30 min after the treatment and then every 30 min for 4 h. Thereafter, mice were further observed and weighted once a day up to 14 days. Behavioral changes, clinical signs of toxicity and deaths were recorded. Animals showing signs of severe pain, permanent signs of severe distress or being in agony, were humanely sacrificed, and the time of death was recorded. The LD50 values were estimated from the dose-response curves.
Subacute toxicity of WG12399C and WG12592A was examined based on OECD Test Guideline No. 407 with minor modifications. Each experimental group consisted of five Balb/c female mice, weighing 15–20 g. WG12399C and WG12592A were administered intravenously with the following total doses divided into four weekly injections: WG12399C—18, 35, and 50 mg/kg body weight; WG12592A—1, 2, and 4 mg/kg body weight. The doses for the subacute toxicity test were established taking into account the LD50. The animals receiving vehicle (saline) served as control. Mice were observed every day for 28 days, and body weight changes were recorded twice a week. Observations included the condition of skin and fur, eyes, any signs of diarrhea, food and water intake, as well as changes in activity or behavior patterns. After 28 days of observation the animals were sacrificed, the major visceral organs such as the heart, liver, spleen, lungs, and kidneys were removed carefully for gross examination.
4.4. Ovariectomy-Induced Osteoporosis
The animals were either sham-operated (n = 8) or ovariectomized. Ovariectomy was performed under general anesthesia. Mice received an intraperitoneal injection of buprenorphine at the dose of 0.2 mg/kg (VET-AGRO, Lublin, Poland) and were anaesthetized with a mixture of synthetic air and isoflurane (4% for induction and 2–3% v/v for maintenance; 200 mL/min; Aerrane isoflurane, Baxter, Guayama, Puerto Ricoc, IL, USA). To induce the estrogen deficiency, ovariectomy was performed by removing both ovaries through the dorsal approach, and the wounds were closed with soluble surgical sutures. Mice that underwent the same surgical procedure without removing the ovaries (SHAM) served as a control. Twenty-four hours after the surgery, buprenorphine (0.1 mg/kg) was injected. The OVX mice were randomly divided into four groups (n = 8, each): Untreated ovariectomized mice (OVX), and mice treated with WG12399C, WG12592A, or zoledronate (ZOL). The animals were kept in gangs of four individuals. Ten days after the surgery, mice started receiving the treatment. Mice were administered intravenously with vehicle (saline), WG12399C 60 mg/kg, WG12592A 6 mg/kg, or zoledronate 120 μg/kg divided into six weekly doses. The doses applied in the study were based on previous toxicological tests and did not cause any clinical signs of toxicity. After 56 days from ovariectomy, mice were anesthetized, and blood samples were obtained for further analyses. The animals were then euthanized by cervical translocation. The femurs and tibias were collected post mortem. For micro-computed tomography (μCT) analysis, bones were immediately frozen and stored in −80 °C, while for histological studies, bones were fixed in 70% ethanol.
4.5. Micro-Computed Tomography (μCT)
μCT analysis was performed on excised femurs and tibias. Proximal tibial metaphyses, the distal femoral metaphysis and femoral shafts were scanned with an X-ray μCT system (SkyScan 1172, Bruker, Kontich, Belgium). Each sample was registered at resolution of 6 μm with lamp parameters of 51 kV/194 μA by using an additional 0.5 mm Al filter. 3D structural properties were measured using CTAn software. For each long bone (femur and tibia), measurements were taken for two areas: Spongy bone and compact bone. The method of selecting the region and the representative area of analysis (volume of interest (VOI)) was performed in accordance with the guidelines developed by Bruker for testing on small animals [
44]. The following parameters were quantitatively analyzed: Bone mineral density (BMD), bone volume (BV/TV), bone surface (BS/TV), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), and trabecular number (Tb.N). The BMD value of the examined bones was obtained by comparing them with the BMD value of external density phantoms normalized for murine bones.
4.6. Examination of Mechanical Properties of Bones
Mechanical test was conducted on mouse femurs by using the four-point bending test. To eliminate changes in the measured values associate with tissue deformation during the test, the measurement was performed using a protocol dedicated to measuring the mechanical properties of small animals [
45]. For this purpose, each bone epiphysis was fixed with Duracryl Plus
® adhesive in an aluminum sleeve with a diameter of 6 mm. The distance between the lower supports was 12 mm, and the distance between the point where the force is applied and the lower support was 6 mm. The measurement distance of the embedded femur between the aluminum sleeves was 10 mm. Tests were performed on an MTS 858 MiniBionix machine (Eden Prairie, USA), with displacement speed of 1 mm/min. Because of the shape of the femur, the bone cross-section was approximated with an ellipse to determine the mechanical parameters. For each femur, the values of elastic modulus (E), ultimate strength (σ
ult), and stiffness (k) were determined. All mechanical parameters determined in the four-point bending test were calculated using classical mechanics formulas.
4.7. Histology
Tibias were fixed in 70% ethanol for one week. The samples were then decalcified in formic acid-sodium citrate reagent (45% formic acid and 20% sodium citrate, 1:1) for three days and embedded in paraffin. Next, 3-µm-thick sections of proximal metaphyses were made and stained with a Leukocyte Acid Phosphatase (TRAP) Kit (387A-1KT; Sigma-Aldrich). According to the procedure of Sigma-Aldrich, slides were fixed by immersing in a Fixative Solution (citrate solution, acetone, 37% formaldehyde) for 30 s and rinsed thoroughly in deionized water. The slides were then incubated in the TRAP staining solution (deionized water prewarmed to 37 °C, 45 mL; Diazotized Fast Garnet GBC Solution, 1 mL; Naphthol AS-BI Phosphate Solution, 0.5 mL, Acetate Solution, 2 mL; Tartrate Solution, 1 mL) for 1 h at 37 °C protected from light, rinsed in deionized water, counterstained in hematoxylin solution for 2 min, dehydrated through graded alcohols, cleared in xylene, and mounted.
Microphotographs of all the studied tissues were subjected to a computer-assisted image analysis through a computer coupled to a BX53 optical microscope (Olympus, Tokyo, Japan). The microscopy set had the potential to record images and to analyze them digitally. The measurements were carried out using the CellA software (Olympus Soft Imaging Solution GmbH, Munich, Germany).
4.8. Measurement of Serum Levels of Bone Turnover Markers
The expression of selected proteins was detected in mouse plasma. Blood specimens were collected during mice euthanasia to heparinized tubes. Next, the blood samples were immediately centrifuged at 2000× g for 15 min at 4 °C, and plasma was transferred into fresh tubes. Calcium level was measured in each sample of plasma using the Cobas c 111 z ISE device (Roche Diagnostics Ltd., Rotkreuz, Switzerland). Tartrate-resistant acid phosphatase 5b (TRACP5b), osteocalcin (OC), and cross linked C-telopeptide of type I collagen (CTX-I) levels were estimated in plasma by CLIA kits (Elabscience, Houston, TX, USA) according to the manufacturer’s instructions.
4.9. Cell Culture
Bone marrow was isolated from six- to nine-week-old mice by flushing tibias and femurs with α-MEM medium (Thermo Scientific, Waltham, MA, USA) containing antibiotics. Cells were then centrifuged (1300 rpm, 5 min, 4 °C) and resuspended in α-MEM medium with 10% fetal bovine serum (FBS), 100 U/l penicillin, 100 μg/mL streptomycin, and 75 ng/mL M-CSF (Chiron Corp., Emeryville, CA, USA). Primary bone marrow cells were cultured for 48 h, and the floating cells were discarded; the medium was refreshed, and adherent bone marrow-derived macrophages (BMDMs) were cultured for additional 48 h. The cells were then harvested, and osteoclastogenesis or pit assays were performed.
4.10. Macrophage Purity
Macrophage culture was tested for purity by cytofluorescence. Briefly, cells were harvested with Cell Dissociation Solution (Sigma-Aldrich, Darmstadt, Germany), centrifuged, resuspended in PBS (IIET, Wroclaw, Poland) with 2% FBS (Sigma-Aldrich), and transferred to separate tubes at the density of 1 × 105/tube. The cells were stained with fluorophore-conjugated antibody against CD11b and/or F4/80 antigen (BD Biosciences, San Jose, CA, USA), a mouse-specific macrophage marker. The samples were stained on ice for 40 min and then washed with PBS and analyzed. Data analysis was performed by flow cytometry using the Diva Software program for data acquisition (BD Biosciences).
4.11. In Vitro Osteoclastogenesis Assay
A total of 2 × 10
4 differentiated BMDMs were seeded in 24-well plates in α-MEM supplemented with 30 ng/mL M-CSF and 40 ng/mL RANKL (R&D Systems, Minneapolis, USA) and treated with BPs at the concentration of 0.919 μM for WG12399C and 0.421 µM for WG12592A. The concentrations of BPs were based on parallel studies on RAW264.7 macrophages. The IC
50 values determined for RAW264.7 cells in cytotoxic test (according to the protocol used for J774E macrophages [
46]) were 9 µM for WG12399C and 4.5 µM for WG12592A, thus 10 times higher than the doses applied in osteoclastogenesis assay. Untreated cells and cells treated with 0.345 μM zoledronic acid served as controls. Media were replaced every alternate day, and cultures were maintained for eight days. Next, the cells were stained for tartrate-resistant acid phosphatase (TRAP) using an Acid Phosphatase Leukocyte Kit (Sigma-Aldrich) according to the manufacturer’s instructions and counterstained with hematoxylin solution Gill No. 3. Microscopic examination was performed and photographs were captured at 100× magnification using a bright field microscope (Olympus IX81) connected to a camera equipped with Olympus Stream Image Analysis software (Olympus Europe Holding GmBH, Hamburg, Germany). TRAP-positive cells with at least three nuclei were counted as osteoclasts. The number of active osteoclasts in the BP-treated groups was compared to the values in the control wells. Two wells were assessed per treatment in five independent experiments.
4.12. Pit Formation Assay
A total of 2 × 103 differentiated BMDMs were seeded in 24-well Corning Osteo Assay Surface Microplates (Corning Inc., Corning, NY, USA) in α-MEM supplemented with 10% FBS and treated with 0.919 μM WG12399C or 0.421 µM WG12592A in the presence of 30 ng/mL M-CSF and 40 ng/mL RANKL. Untreated cells and cells treated with 0.345 μM zoledronic acid served as controls. Media were replaced every alternate day, and cultures were maintained for 10 days. Subsequently, osteostrips were sonicated for 15 min and washed with distilled water. Microscopic examination was performed and microphotographs were captured at 100× magnification with a bright field microscope. The area of resorption pits was measured using Olympus Stream Start 1.6.1 program (Olympus). The area of resorption pits in the BP-treated groups was compared to the values in the control wells. Two wells were assessed per treatment in seven independent experiments.
4.13. Statistical Evaluation
Statistical analysis was performed using GraphPad Prism 7.01 (GraphPad Software Inc., San Diego, CA, USA). Shapiro–Wilk’s normality test and Bartlett’s test were used to confirm the assumptions for analysis of variance (ANOVA). Tests used for each data analysis are indicated in figure legends. p < 0.05 was considered to be statistically significant.