The Importance of Food for Endotoxemia and an Inflammatory Response
Abstract
:1. Introduction
2. Aim
3. Nutrients and Endotoxemia
3.1. The Superantigen LPS
3.2. Endotoxemia and Feeding
3.3. Endotoxemia after a Standard Breakfast
3.4. Endotoxemia after a Breakfast Containing Fibers
3.5. The Mechanism of Postprandial Uptake of LPS
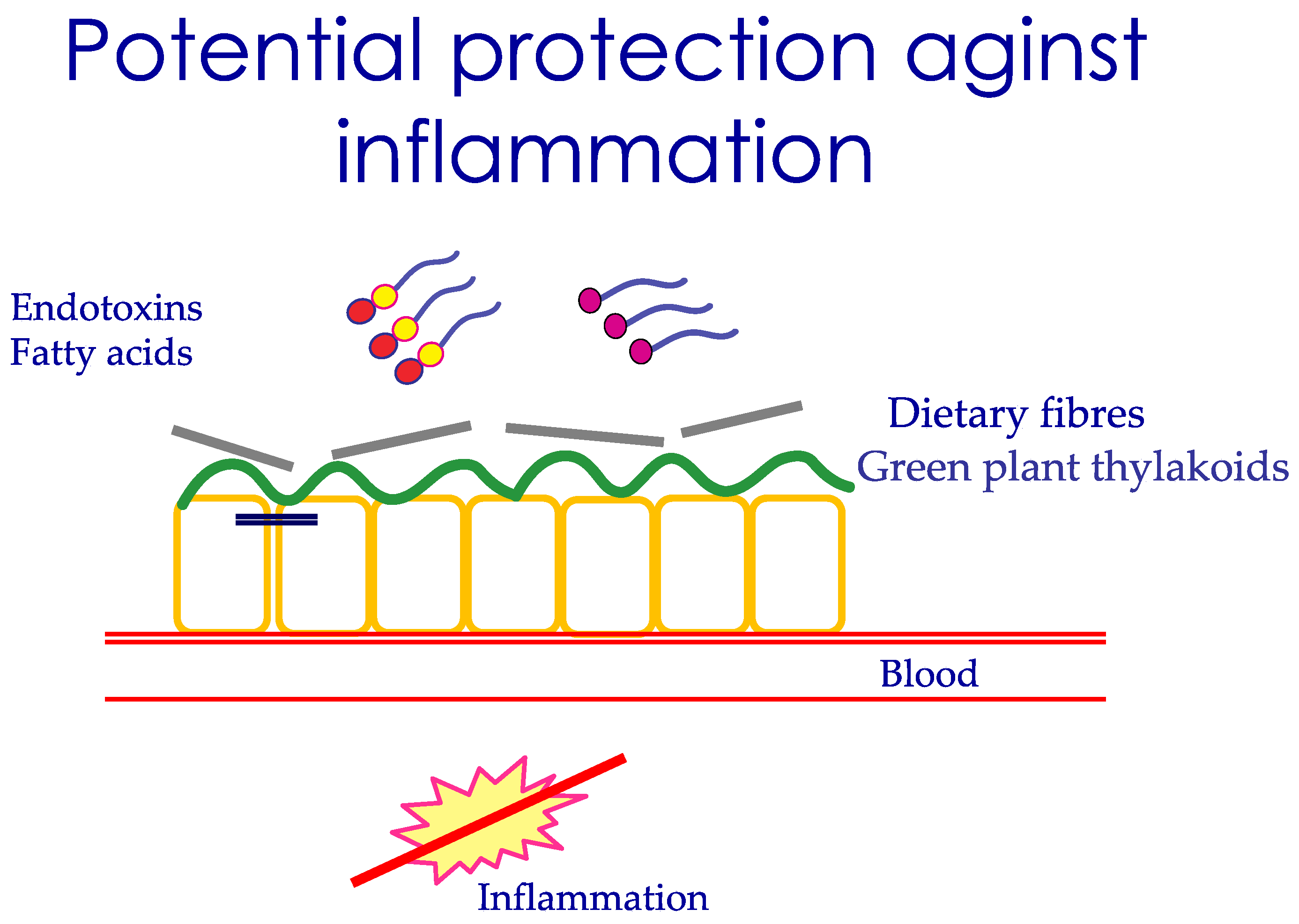
3.6. Nutrients That Protect the Uptake of LPS
3.7. Endotoxemia Postprandially during Obesity
3.8. Endotoxemia and Postprandial Inflammation, a Tipping Point in the Obese State?
3.9. Long-Chain Fatty Acids May Be the Trigger for Inflammation
3.10. Nutrients, Intestinal Permeability, Microbiome, and LPS
4. Conclusions
Funding
Conflicts of Interest
References
- Sirivongrangson, P.; Kulvichit, W.; Payungporn, S.; Pisitkun, T.; Chindamporn, A.; Peerapornratana, S.; Pisitkun, P.; Chitcharoen, S.; Sawaswong, V.; Worasilchai, N.; et al. Endotoxemia and circulating bacteriome in severe COVID-19 patients. Intensiv. Care Med. Exp. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Saad, M.J.A.; dos Santos, A.; Prada, P.D.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 1–13. [Google Scholar] [CrossRef]
- Gardiner, K.R.; Halliday, M.I.; Barclay, G.R.; Milne, L.; Brown, D.; Stephens, S.; Maxwell, R.J.; Rowlands, B.J. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut 1995, 36, 897–901. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.; Sanz, Y.; Maes, M. The Role of Microbiota and Intestinal Permeability in the Pathophysiology of Autoimmune and Neuroimmune Processes with an Emphasis on Inflammatory Bowel Disease Type 1 Diabetes and Chronic Fatigue Syndrome. Curr. Pharm. Des. 2016, 22, 6058–6075. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Geagea, A.G.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2016, 160, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2020, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.D.C.G.; Alfenas, R.D.C.G. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.; Fava, F.; Tuohy, K.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petruk, G.; Puthia, M.; Petrlova, J.; Samsudin, F.; Stromdahl, A.C.; Cerps, S.; Uller, L.; Bond, P.J.; Kjellstrom, S.; Schmidtchen, A. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J. Mol. Cell Biol. 2020, 12, 916–932. [Google Scholar] [CrossRef]
- Laugerette, F.; Vors, C.; Géloën, A.; Chauvin, M.-A.; Soulage, C.; Lambert-Porcheron, S.; Peretti, N.; Alligier, M.; Burcelin, R.; Laville, M.; et al. Emulsified lipids increase endotoxemia: Possible role in early postprandial low-grade inflammation. J. Nutr. Biochem. 2011, 22, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deopurkar, R.; Ghanim, H.; Friedman, J.; Abuaysheh, S.; Sia, C.L.; Mohanty, P.; Viswanathan, P.; Chaudhuri, A.; Dandona, P. Differential Effects of Cream, Glucose, and Orange Juice on Inflammation, Endotoxin, and the Expression of Toll-Like Receptor-4 and Suppressor of Cytokine Signaling-3. Diabetes Care 2010, 33, 991–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erridge, C.; Attina, T.; Spickett, C.M.; Webb, D.J. A high-fat meal induces low-grade endotoxemia: Evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 2007, 86, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Abuaysheh, S.; Sia, C.L.; Korzeniewski, K.; Chaudhuri, A.; Fernández-Real, J.M.; Dandona, P. Increase in Plasma Endotoxin Concentrations and the Expression of Toll-Like Receptors and Suppressor of Cytokine Signaling-3 in Mononuclear Cells After a High-Fat, High-Carbohydrate Meal: Implications for insulin resistance. Diabetes Care 2009, 32, 2281–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vors, C.; Pineau, G.; Drai, J.; Meugnier, E.; Pesenti, S.; Laville, M.; Laugerette, F.; Malpuech-Brugère, C.; Vidal, H.; Michalski, M.-C. Postprandial Endotoxemia Linked With Chylomicrons and Lipopolysaccharides Handling in Obese Versus Lean Men: A Lipid Dose-Effect Trial. J. Clin. Endocrinol. Metab. 2015, 100, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Guerville, M.; Boudry, G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G1–G15. [Google Scholar] [CrossRef] [Green Version]
- Akiba, Y.; Maruta, K.; Takajo, T.; Narimatsu, K.; Said, H.; Kato, I.; Kuwahara, A.; Kaunitz, J.D. Lipopolysaccharides transport during fat absorption in rodent small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G1070–G1087. [Google Scholar] [CrossRef] [PubMed]
- Kvietys, P.R.; Specian, R.D.; Grisham, M.B.; Tso, P. Jejunal mucosal injury and restitution: Role of hydrolytic products of food digestion. Am. J. Physiol. Liver Physiol. 1991, 261, G384–G391. [Google Scholar] [CrossRef]
- Lairon, D.; Lafont, H.; Vigne, J.L.; Nalbone, G.; Léonardi, J.; Hauton, J.C. Effects of dietary fibers and cholestyramine on the activity of pancreatic lipase in vitro. Am. J. Clin. Nutr. 1985, 42, 629–638. [Google Scholar] [CrossRef] [Green Version]
- Montelius, C.; Gustafsson, K.; Weström, B.; Albertsson, P.; Emek, S.C.; Rayner, M.; Erlanson-Albertsson, C. Chloroplast thylakoids reduce glucose uptake and decrease intestinal macromolecular permeability. Br. J. Nutr. 2011, 106, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Köhnke, R.; Lindbo, A.; Larsson, T.; Lindqvist, A.; Rayner, M.; Emek, S.C.; Albertsson, P.; Rehfeld, J.F.; Landin-Olsson, M.; Erlanson-Albertsson, C. Thylakoids promote release of the satiety hormone cholecystokinin while reducing insulin in healthy humans. Scand. J. Gastroenterol. 2009, 44, 712–719. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Ortuño, M.I.Q.; Murri, M.; Boto-Ordoñez, M.; Martínez, P.P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J. Lipid Res. 2012, 53, 973–978. [Google Scholar] [CrossRef] [Green Version]
- Reilly, S.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Stenkula, K.; Erlanson-Albertsson, C. Adipose cell size: Importance in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R284–R295. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.A. The role of TLR4 in endotoxin responsiveness in humans. J. Endotoxin. Res. 2001, 7, 389–393. [Google Scholar] [CrossRef]
- Gonzalez-Quintela, A.; Alonso, M.; Campos-Franco, J.; Vizcaino, L.; Loidi, L.; Gude, F. Determinants of Serum Concentrations of Lipopolysaccharide-Binding Protein (LBP) in the Adult Population: The Role of Obesity. PLoS ONE 2013, 8, e54600. [Google Scholar] [CrossRef]
- Bahr, I.; Spielmann, J.; Quandt, D.; Kielstein, H. Obesity-Associated Alterations of Natural Killer Cells and Immunosurveillance of Cancer. Front. Immunol. 2020, 11, 245. [Google Scholar] [CrossRef] [Green Version]
- Mo, Z.; Huang, S.; Burnett, D.J.; Rutledge, J.C.; Hwang, D.H. Endotoxin May Not Be the Major Cause of Postprandial Inflammation in Adults Who Consume a Single High-Fat or Moderately High-Fat Meal. J. Nutr. 2020, 150, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Gitau, R.; Griffin, B.A.; Gibson, G.R.; Tuohy, K.M.; Lovegrove, J.A. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int. J. Obes. 2012, 37, 216–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakayama, J.; Yamamoto, A.; Palermo-Conde, L.A.; Higashi, K.; Sonomoto, K.; Tan, J.; Lee, Y.-K. Impact of Westernized Diet on Gut Microbiota in Children on Leyte Island. Front. Microbiol. 2017, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Bellido, C.; Lopez-Miranda, J.; Blanco-Colio, L.M.; Perez-Martinez, P.; Muriana, F.J.; Martin-Ventura, J.L.; Marin, C.; Fuentes, F.; Gomez, P.; Egido, J.; et al. Butter and walnuts, but not olive oil, elicit postprandial activation of nuclear transcription factor kappaB in peripheral blood mononuclear cells from healthy men. Am. J. Clin. Nutr. 2004, 80, 1487–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2019, 11, 77–91. [Google Scholar] [CrossRef] [Green Version]
- Precup, G.; Vodnar, D.-C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019, 122, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rebholz, C.M.; Hegde, S.; LaFiura, C.; Raghavan, M.; Lloyd, J.F.; Cheng, C.; Seidelmann, S.B. Plant-based diets, pescatarian diets and COVID-19 severity: A population-based case-control study in six countries. BMJ Nutr. Prev. Health 2021, 4, 257–266. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erlanson-Albertsson, C.; Stenkula, K.G. The Importance of Food for Endotoxemia and an Inflammatory Response. Int. J. Mol. Sci. 2021, 22, 9562. https://doi.org/10.3390/ijms22179562
Erlanson-Albertsson C, Stenkula KG. The Importance of Food for Endotoxemia and an Inflammatory Response. International Journal of Molecular Sciences. 2021; 22(17):9562. https://doi.org/10.3390/ijms22179562
Chicago/Turabian StyleErlanson-Albertsson, Charlotte, and Karin G. Stenkula. 2021. "The Importance of Food for Endotoxemia and an Inflammatory Response" International Journal of Molecular Sciences 22, no. 17: 9562. https://doi.org/10.3390/ijms22179562
APA StyleErlanson-Albertsson, C., & Stenkula, K. G. (2021). The Importance of Food for Endotoxemia and an Inflammatory Response. International Journal of Molecular Sciences, 22(17), 9562. https://doi.org/10.3390/ijms22179562






