Connections between the Cell Cycle and the DNA Damage Response in Plants
Abstract
1. Introduction
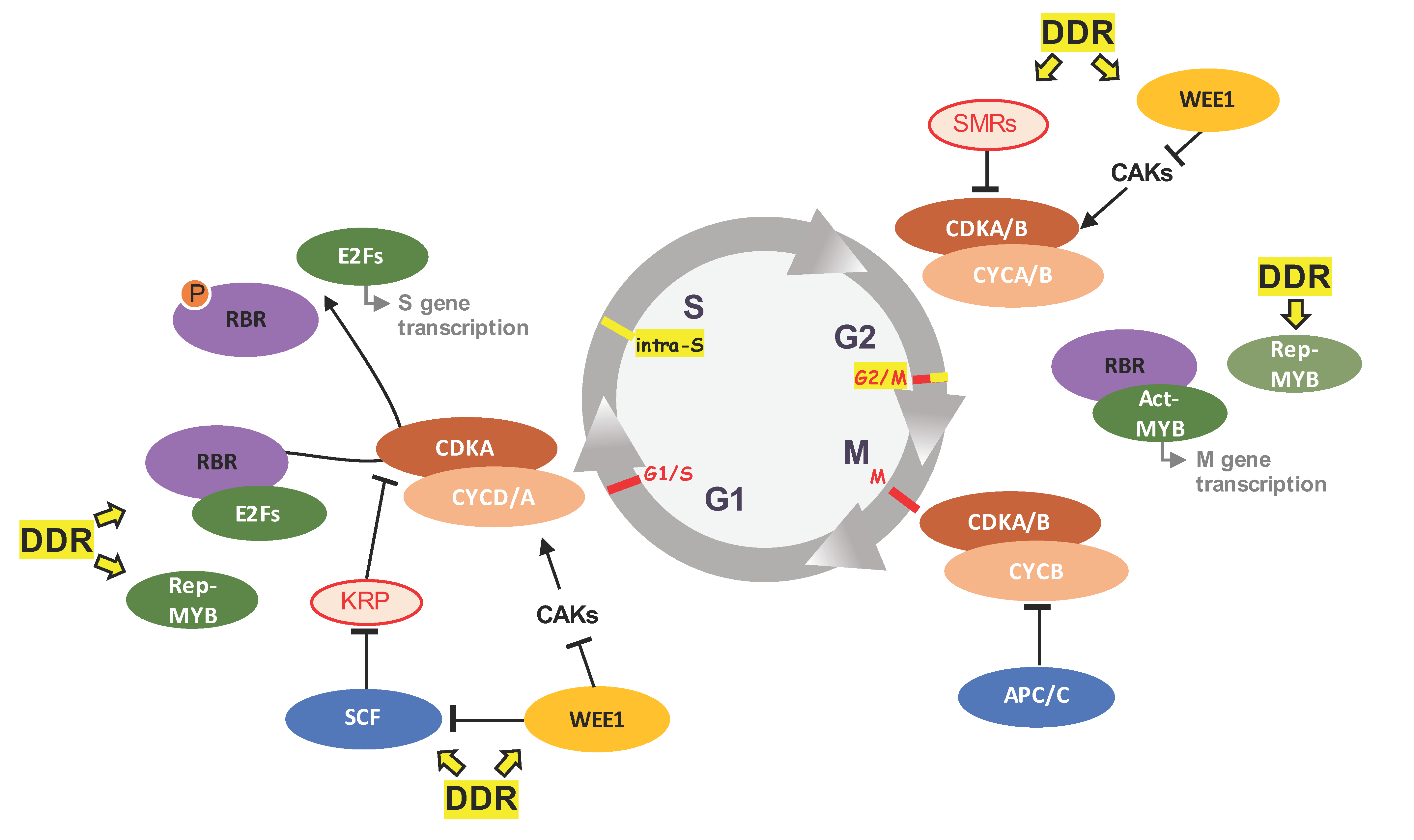
2. Plant Cell Cycle Progression: Canonical Functions of Some Key Players
3. The Plant DDR Machinery
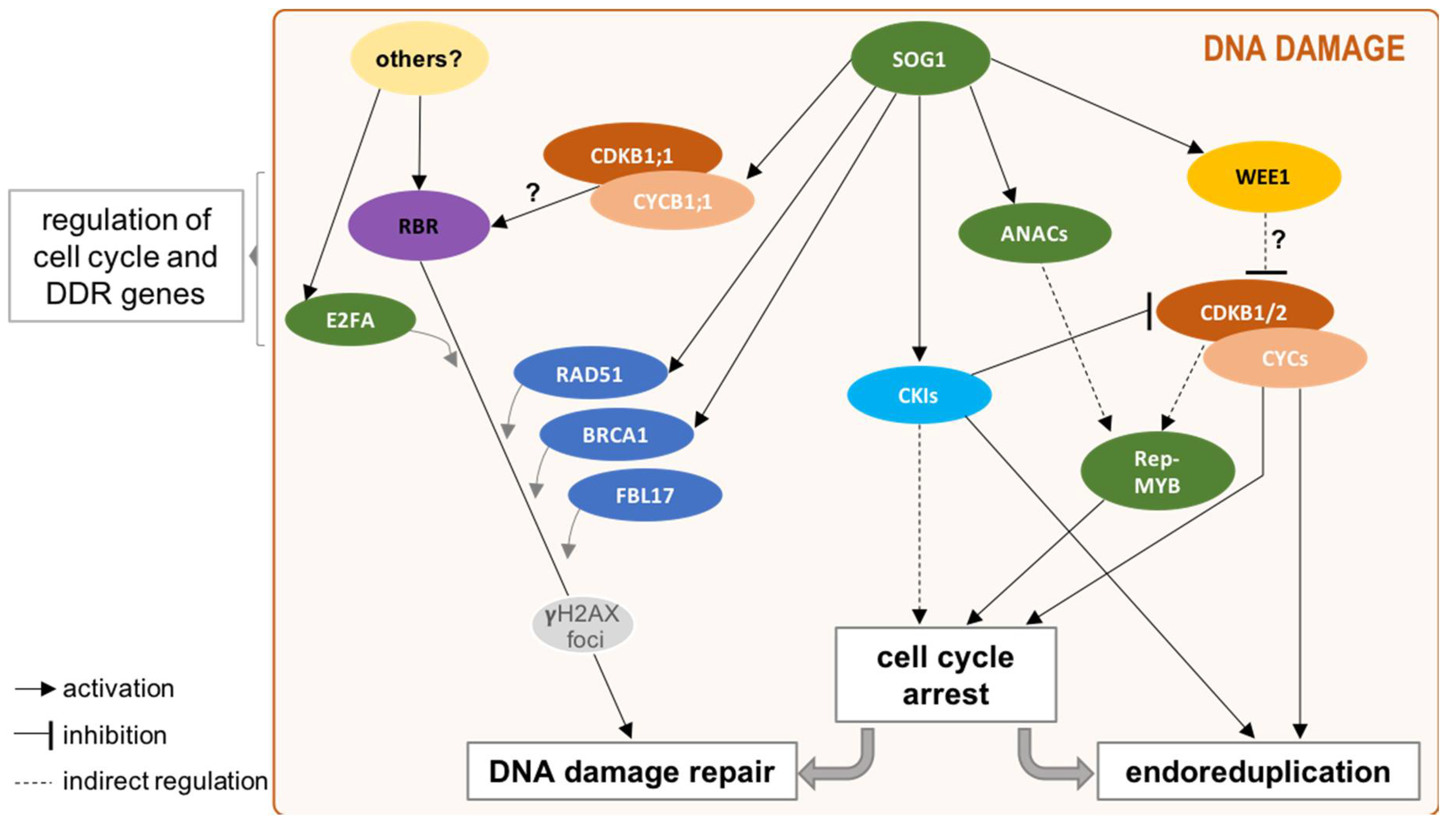
4. Beyond Cell Cycle Regulation: Cell Cycle Players in DDR
4.1. Functional Specialization of CDK/CYC Complex in DDR
4.2. DREAMs upon DNA Damage
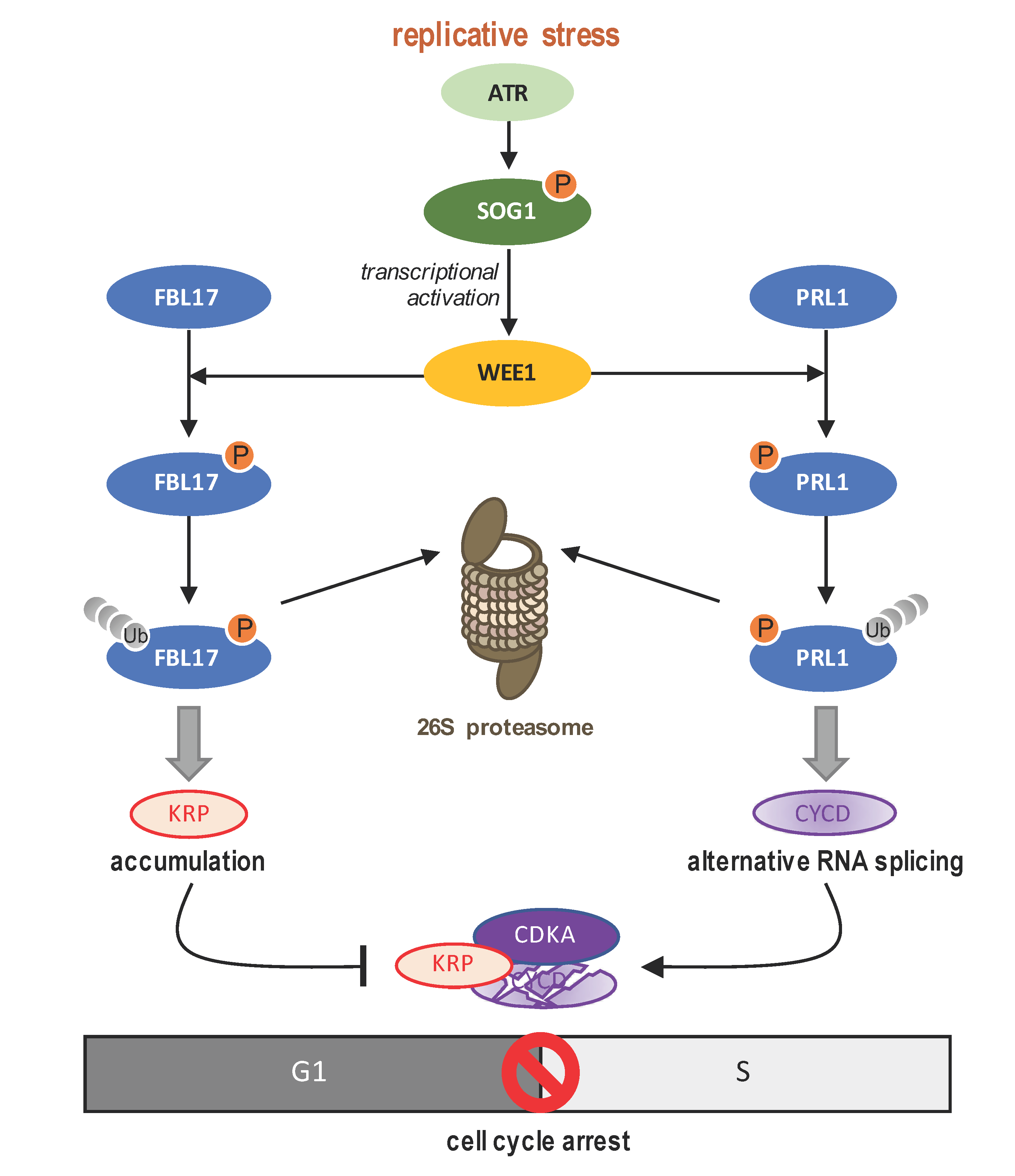
4.3. WEE1 A Key Intra-S Checkpoint Protein
4.4. Switch towards Endoreduplication: A Main Strategy in Plant DDR
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Tuteja, N.; Singh, M.B.; Misra, M.K.; Bhalla, P.L.; Tuteja, R. Molecular mechanisms of DNA damage and repair: Progress in plants. Crit. Rev. Biochem. Mol. Biol. 2001, 36, 337–397. [Google Scholar] [CrossRef]
- Hoeijmakers, J.H.J. DNA Damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Carusillo, A.; Mussolino, C. DNA damage: From threat to treatment. Cells 2020, 9, 1665. [Google Scholar] [CrossRef]
- de Veylder, L.; Larkin, J.C.; Schnittger, A. Molecular control and function of endoreplication in development and physiology. Trends Plant Sci. 2011, 16, 624–634. [Google Scholar] [CrossRef]
- Breuer, C.; Braidwood, L.; Sugimoto, K. Endocycling in the path of plant development. Curr. Opin. Plant Biol. 2014, 17, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C. Coupling cell proliferation and development in plants. Nat. Cell Biol. 2005, 7, 535–541. [Google Scholar] [CrossRef] [PubMed]
- De Veylder, L.; Beeckman, T.; Inzé, D. The ins and outs of the plant cell cycle. Nat. Rev. Mol. Cell Biol. 2007, 8, 655–665. [Google Scholar] [CrossRef]
- Inagaki, S.; Umeda, M. Cell-cycle control and plant development. Int. Rev. Cell Mol. Biol. 2011, 291, 227–261. [Google Scholar]
- Vandepoele, K.; Raes, J.; De Veylder, L.; Rouzé, P.; Rombauts, S.; Inzé, D. Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 2002, 14, 903–916. [Google Scholar] [CrossRef]
- Dissmeyer, N.; Weimer, A.K.; de Veylder, L.; Novak, B.; Schnittger, A. The regulatory network of cell cycle progression is fundamentally different in plants versus yeast or metazoans. Plant Signal. Behav. 2010, 5, 1613. [Google Scholar] [CrossRef]
- Komaki, S.; Sugimoto, K. Control of the plant cell cycle by developmental and environmental cues. Plant Cell Physiol. 2012, 53, 953–964. [Google Scholar] [CrossRef]
- Harashima, H.; Dissmeyer, N.; Schnittger, A. Cell cycle control across the eukaryotic kingdom. Trends Cell Biol. 2013, 23, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Menges, M.; De Jager, S.M.; Gruissem, W.; Murray, J.A.H. Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J. 2005, 41, 546–566. [Google Scholar] [CrossRef]
- Ferreira, P.C.G.; Hemerly, A.S.; Villarroel, R.; Van Montagu, M.; Inzé, D. The Arabidopsis functional homolog of the p34cdc2 protein kinase. Plant Cell 1991, 3, 531–540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Menges, M.; Murray, J.A.H. Synchronous Arabidopsis suspension cultures for analysis of cell-cycle gene activity. Plant J. 2002, 30, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Nowack, M.K.; Harashima, H.; Dissmeyer, N.; Zhao, X.; Bouyer, D.; Weimer, A.K.; De Winter, F.; Yang, F.; Schnittger, A. Genetic framework of cyclin-dependent kinase function in Arabidopsis. Dev. Cell 2012, 22, 1030–1040. [Google Scholar] [CrossRef]
- Stals, H.; Bauwens, S.; Traas, J.; Van Montagu, M.; Engler, G.; Inzé, D. Plant CDC2 is not only targeted to the pre-prophase band, but also co-localizes with the spindle, phragmoplast, and chromosomes. FEBS Lett. 1997, 418, 229–234. [Google Scholar] [CrossRef]
- Weingartner, M.; Binarova, P.; Drykova, D.; Schweighofer, A.; David, J.P.; Heberle-Bors, E.; Doonan, J.; Bögre, L. Dynamic recruitment of Cdc2 to specific microtubule structures during mitosis. Plant Cell 2001, 13, 1929–1943. [Google Scholar] [CrossRef]
- Porceddu, A.; Stals, H.; Reichheld, J.P.; Segers, G.; De Veylder, L.; De Pinho Barrôco, R.; Casteels, P.; Van Montagu, M.; Inzé, D.; Mironov, V. A Plant-specific cyclin-dependent kinase is involved in the control of G2/M progression in plants. J. Biol. Chem. 2001, 276, 36354–36360. [Google Scholar] [CrossRef]
- Inzé, D.; De Veylder, L. Cell cycle regulation in plant development. Annu. Rev. Genet. 2006, 40, 77–105. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.U.; Buechel, S.; Zhao, Z.; Ljung, K.; Novák, O.; Busch, W.; Schuster, C.; Lohmanna, J.U. Requirement of B2-type cyclin-dependent kinases for meristem integrity in Arabidopsis thaliana. Plant Cell 2008, 20, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Boudolf, V.; Vlieghe, K.; Beemster, G.T.S.; Magyar, Z.; Torres Acosta, J.A.; Maes, S.; Van Der Schueren, E.; Inzé, D.; De Veylder, L. The plant-specific cyclin-dependent kinase CDKB1;1 and transcription factor E2Fa-DPa control the balance of mitotically dividing and endoreduplicating cells in Arabidopsis. Plant Cell 2004, 16, 2683–2692. [Google Scholar] [CrossRef]
- Boudolf, V.; Lammens, T.; Boruc, J.; van Leene, J.; van den Daele, H.; Maes, S.; van Isterdael, G.; Russinova, E.; Kondorosi, E.; Witters, E.; et al. CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. Plant Physiol. 2009, 150, 1482–1493. [Google Scholar] [CrossRef]
- Weimer, A.K.; Nowack, M.K.; Bouyer, D.; Zhao, X.; Harashima, H.; Naseer, S.; De Winter, F.; Dissmeyer, N.; Geldner, N.; Schnittger, A. RETINOBLASTOMA RELATED1 regulates asymmetric cell divisions in Arabidopsis. Plant Cell 2012, 24, 4083–4095. [Google Scholar] [CrossRef]
- Capron, A.; Serralbo, O.; Fülöp, K.; Frugier, F.; Parmentier, Y.; Dong, A.; Lecureuil, A.; Guerche, P.; Kondorosi, E.; Scheres, B.; et al. The Arabidopsis anaphase-promoting complex or cyclosome: Molecular and genetic characterization of the APC2 subunit. Plant Cell 2003, 15, 2370–2382. [Google Scholar] [CrossRef] [PubMed]
- Boruc, J.; van den Daele, H.; Hollunder, J.; Rombauts, S.; Mylle, E.; Hilson, P.; Inzé, D.; de Veylder, L.; Russinova, E. Functional modules in the Arabidopsis core cell cycle binary protein-protein interaction network. Plant Cell 2010, 22, 1264–1280. [Google Scholar] [CrossRef]
- Van Leene, J.; Hollunder, J.; Eeckhout, D.; Persiau, G.; Van De Slijke, E.; Stals, H.; Van Isterdael, G.; Verkest, A.; Neirynck, S.; Buffel, Y.; et al. Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol. Syst. Biol. 2010, 6, 397. [Google Scholar] [CrossRef]
- Dewitte, W.; Scofield, S.; Alcasabas, A.A.; Maughan, S.C.; Menges, M.; Braun, N.; Collins, C.; Nieuwland, J.; Prinsen, E.; Sundaresan, V.; et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc. Natl. Acad. Sci. USA 2007, 104, 14537–14542. [Google Scholar] [CrossRef]
- Sozzani, R.; Cui, H.; Moreno-Risueno, M.A.; Busch, W.; Van Norman, J.M.; Vernoux, T.; Brady, S.M.; Dewitte, W.; Murray, J.A.H.; Benfey, P.N. Spatiotemporal regulation of cell-cycle genes by SHORTROOT links patterning and growth. Nature 2010, 466, 128–132. [Google Scholar] [CrossRef]
- Vanneste, S.; Coppens, F.; Lee, E.; Donner, T.J.; Xie, Z.; Van Isterdael, G.; Dhondt, S.; De Winter, F.; De Rybel, B.; Vuylsteke, M.; et al. Developmental regulation of CYCA2s contributes to tissue-specific proliferation in Arabidopsis. EMBO J. 2011, 30, 3430–3441. [Google Scholar] [CrossRef]
- Loog, M.; Morgan, D.O. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 2005, 434, 104–108. [Google Scholar] [CrossRef]
- Kõivomägi, M.; Valk, E.; Venta, R.; Iofik, A.; Lepiku, M.; Morgan, D.O.; Loog, M. Dynamics of Cdk1 substrate specificity during the cell cycle. Mol. Cell 2011, 42, 610–623. [Google Scholar] [CrossRef]
- Umeda, M.; Shimotohno, A.; Yamaguchi, M. Control of cell division and transcription by cyclin-dependent kinase-activating kinases in plants. Plant Cell Physiol. 2005, 46, 1437–1442. [Google Scholar] [CrossRef]
- Dissmeyer, N.; Nowack, M.K.; Pusch, S.; Stals, H.; Inze, D.; Grini, P.E.; Schnittger, A. T-Loop phosphorylation of Arabidopsis CDKA;1 is required for its function and can be partially substituted by an aspartate residue. Plant Cell Online 2007, 19, 972–985. [Google Scholar] [CrossRef]
- Harashima, H.; Shinmyo, A.; Sekine, M. Phosphorylation of threonine 161 in plant cyclin-dependent kinase a is required for cell division by activation of its associated kinase. Plant J. 2007, 52, 435–448. [Google Scholar] [CrossRef]
- Takatsuka, H.; Ohno, R.; Umeda, M. The Arabidopsis cyclin-dependent kinase-activating kinase CDKF;1 is a major regulator of cell proliferation and cell expansion but is dispensable for CDKA activation. Plant J. 2009, 59, 475–487. [Google Scholar] [CrossRef]
- Takatsuka, H.; Umeda-Hara, C.; Umeda, M. Cyclin-dependent kinase-activating kinases CDKD;1 and CDKD;3 are essential for preserving mitotic activity in Arabidopsis thaliana. Plant J. 2015, 82, 1004–1017. [Google Scholar] [CrossRef]
- Shimotohno, A.; Umeda-Hara, C.; Bisova, K.; Uchimiya, H.; Umeda, M. The plant-specific kinase CDKF;1 is involved in activating phosphorylation of cyclin-dependent kinase-activating kinases in arabidopsis. Plant Cell 2004, 16, 2954–2966. [Google Scholar] [CrossRef]
- Shimotohno, A.; Ohno, R.; Bisova, K.; Sakaguchi, N.; Huang, J.; Koncz, C.; Uchimiya, H.; Umeda, M. Diverse phosphoregulatory mechanisms controlling cyclin-dependent kinase-activating kinases in Arabidopsis. Plant J. 2006, 47, 701–710x. [Google Scholar] [CrossRef]
- Elbæk, C.R.; Petrosius, V.; Sørensen, C.S. WEE1 kinase limits CDK activities to safeguard DNA replication and mitotic entry. Mutat. Res.–Fundam. Mol. Mech. Mutagen. 2020, 819, 111694. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, D.A.; Marchbank, A.; McMahon, K.; Dickinson, J.R.; Rogers, H.J.; Francis, D. A WEE1 homologue from Arabidopsis thaliana. Planta 2002, 215, 518–522. [Google Scholar] [CrossRef]
- Landrieu, I.; Da Costa, M.; De Veylder, L.; Dewitte, F.; Vandepoele, K.; Hassan, S.; Wieruszeski, J.M.; Faure, J.D.; Van Montagu, M.; Inzé, D.; et al. A small CDC25 dual-specificity tyrosine-phosphatase isoform in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2004, 101, 13380–13385. [Google Scholar] [CrossRef]
- De Schutter, K.; Joubes, J.; Cools, T.; Verkest, A.; Corellou, F.; Babiychuk, E.; Van Der Schueren, E.; Beeckman, T.; Kushnir, S.; Inze, D.; et al. Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell Online 2007, 19, 211–225. [Google Scholar] [CrossRef]
- Dissmeyer, N.; Weimer, A.K.; Pusch, S.; de Schutter, K.; Kamei, C.L.A.; Nowack, M.K.; Novak, B.; Duan, G.L.; Zhu, Y.G.; de Veylder, L.; et al. Control of cell proliferation, organ growth, and DNA damage response operate independently of dephosphorylation of the arabidopsis Cdk1 Homolog CDKA;1. Plant Cell 2009, 21, 3641–3654. [Google Scholar] [CrossRef] [PubMed]
- Boudolf, V.; Inzé, D.; De Veylder, L. What if higher plants lack a CDC25 phosphatase? Trends Plant Sci. 2006, 11, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Verkest, A.; Weinl, C.; Inzé, D.; De Veylder, L.; Schnittger, A. Switching the cell cycle. Kip-related proteins in plant cell cycle control. Plant Physiol. 2005, 139, 1099–1106. [Google Scholar] [CrossRef]
- Acosta, J.A.T.; Fowke, L.C.; Wang, H. Analyses of phylogeny, evolution, conserved sequences and genome-wide expression of the ICK/KRP family of plant CDK inhibitors. Ann. Bot. 2011, 107, 1141–1157. [Google Scholar] [CrossRef]
- Peres, A.; Churchman, M.L.; Hariharan, S.; Himanen, K.; Verkest, A.; Vandepoele, K.; Magyar, Z.; Hatzfeld, Y.; Van Der Schueren, E.; Beemster, G.T.S.; et al. Novel plant-specific cyclin-dependent kinase inhibitors induced by biotic and abiotic stresses. J. Biol. Chem. 2007, 282, 25588–25596. [Google Scholar] [CrossRef]
- Kumar, N.; Harashima, H.; Kalve, S.; Bramsiepe, J.; Wang, K.; Sizani, B.L.; Bertrand, L.L.; Johnson, M.C.; Faulk, C.; Dale, R.; et al. Functional Conservation in the SIAMESE-RELATED family of cyclin-dependent kinase inhibitors in land plants. Plant Cell 2015, 27, 3065–3080. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Gilmer, S.; Whitwill, S.; Fowke, L.C. Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. Plant J. 2000, 24, 613–623. [Google Scholar] [CrossRef]
- Schnittger, A.; Weinl, C.; Bouyer, D.; Schöbinger, U.; Hülskamp, M. Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. Plant Cell 2003, 15, 303–315. [Google Scholar] [CrossRef]
- Verkest, A.; Manes, C.-L.d.O.; Vercruysse, S.; Maes, S.; Van Der Schueren, E.; Beeckman, T.; Genschik, P.; Kuiper, M.; Inzé, D.; De Veylder, L. The cyclin-dependent kinase inhibitor KRP2 controls the onset of the endoreduplication cycle during Arabidopsis leaf development through inhibition of mitotic CDKA;1 kinase complexes. Plant Cell 2005, 17, 1723–1736. [Google Scholar] [CrossRef]
- Kumar, N.; Larkin, J.C. Why do plants need so many cyclin-dependent kinase inhibitors? Why do plants need so many cyclin-dependent kinase inhibitors? Plant Signal. Behav. 2017, 12, e1282021. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Selden, K.; Bediée, A.; Rolland, G.; Baumberger, N.; Noir, S.; Bach, L.; Lamy, G.; Granier, C.; Genschik, P. SIAMESE-RELATED1 is regulated posttranslationally and participates in repression of leaf growth under moderate drought. Plant Physiol. 2018, 176, 2834–2850. [Google Scholar] [CrossRef] [PubMed]
- Breyne, P.; Dreesen, R.; Vandepoele, K.; De Veylder, L.; Van Breusegem, F.; Callewaert, L.; Rombauts, S.; Raes, J.; Cannoot, B.; Engler, G.; et al. Transcriptome analysis during cell division in plants. Proc. Natl. Acad. Sci. USA 2002, 99, 14825–14830. [Google Scholar] [CrossRef]
- Menges, M.; Hennig, L.; Gruissem, W.; Murray, J.A.H. Genome-wide gene expression in an Arabidopsis cell suspension. Plant Mol. Biol. 2003, 53, 423–442. [Google Scholar] [CrossRef]
- Berckmans, B.; De Veylder, L. Transcriptional control of the cell cycle. Curr. Opin. Plant Biol. 2009, 12, 599–605. [Google Scholar] [CrossRef]
- Magyar, Z.; Bögre, L.; Ito, M. DREAMs make plant cells to cycle or to become quiescent. Curr. Opin. Plant Biol. 2016, 34, 100–106. [Google Scholar] [CrossRef]
- Desvoyes, B.; Gutierrez, C. Roles of plant retinoblastoma protein: Cell cycle and beyond. EMBO J. 2020, 39, e105802. [Google Scholar] [CrossRef]
- Fischer, M.; Müller, G.A. Cell cycle transcription control: DREAM/MuvB and RB-E2F complexes. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 638–662. [Google Scholar] [CrossRef]
- Bouyer, D.; Heese, M.; Chen, P.; Harashima, H.; Roudier, F.; Grüttner, C.; Schnittger, A. Genome-wide identification of RETINOBLASTOMA RELATED 1 binding sites in Arabidopsis reveals novel DNA damage regulators. PLoS Genet. 2018, 14, e1007797. [Google Scholar] [CrossRef] [PubMed]
- Henriques, R.; Magyar, Z.; Monardes, A.; Khan, S.; Zalejski, C.; Orellana, J.; Szabados, L.; De La Torre, C.; Koncz, C.; Bögre, L. Arabidopsis S6 kinase mutants display chromosome instability and altered RBR1-E2F pathway activity. EMBO J. 2010, 29, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Harashima, H.; Sugimoto, K. Integration of developmental and environmental signals into cell proliferation and differentiation through RETINOBLASTOMA-RELATED 1. Curr. Opin. Plant Biol. 2016, 29, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Suzuki, T.; Iwata, E.; Magyar, Z.; Bögre, L.; Ito, M. MYB3Rs, plant homologs of Myb oncoproteins, control cell cycle-regulated transcription and form DREAM-like complexes. Transcription 2015, 6, 106–111. [Google Scholar] [CrossRef]
- Genschik, P.; Marrocco, K.; Bach, L.; Noir, S.; Criqui, M.C. Selective protein degradation: A rheostat to modulate cell-cycle phase transitions. J. Exp. Bot. 2014, 65, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Santner, A.; Del Pozo, J.C.; Murray, J.A.H.; Estelle, M. Degradation of the cyclin-dependent kinase inhibitor KRP1 is regulated by two different ubiquitin E3 ligases. Plant J. 2008, 53, 705–716. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, S.A.; Brownfield, L.; Hong, S.H.; Ryu, H.; Hwang, I.; Twell, D.; Nam, H.G. Control of plant germline proliferation by SCFFBL17 degradation of cell cycle inhibitors. Nature 2008, 455, 1134–1137. [Google Scholar] [CrossRef]
- Gusti, A.; Baumberger, N.; Nowack, M.; Pusch, S.; Eisler, H.; Potuschak, T.; De Veylder, L.; Schnittger, A.; Genschik, P. The Arabidopsis thaliana F-box protein FBL17 is essential for progression through the second mitosis during pollen development. PLoS ONE 2009, 4, e4780. [Google Scholar] [CrossRef]
- Noir, S.; Marrocco, K.; Masoud, K.; Thomann, A.; Gusti, A.; Bitrian, M.; Schnittger, A.; Genschik, P. The control of Arabidopsis thaliana growth by cell proliferation and endoreplication requires the F-Box protein FBL17. Plant Cell 2015, 27, 1461–1476. [Google Scholar] [CrossRef]
- Heyman, J.; De Veylder, L. The anaphase-promoting complex/cyclosome in control of plant development. Mol. Plant 2012, 5, 1182–1194. [Google Scholar] [CrossRef]
- Lammens, T.; Boudolf, V.; Kheibarshekan, L.; Zalmas, L.P.; Gaamouche, T.; Maes, S.; Vanstraelen, M.; Kondorosi, E.; La Thangue, N.B.; Govaerts, W.; et al. Atypical E2F activity restrains APC/CCCS52A2 function obligatory for endocycle onset. Proc. Natl. Acad. Sci. USA 2008, 105, 14721–14726. [Google Scholar] [CrossRef] [PubMed]
- Ciccia, A.; Elledge, S.J. The DNA Damage Response: Making It Safe to Play with Knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, K.O.; Sakaguchi, K.; Kimura, S. DNA damage response in plants: Conserved and variable response compared to animals. Biology 2013, 2, 1338–1356. [Google Scholar] [CrossRef]
- Nisa, M.U.; Huang, Y.; Benhamed, M.; Raynaud, C. The plant DNA damage response: Signaling pathways leading to growth inhibition and putative role in response to stress conditions. Front. Plant Sci. 2019, 10, 653. [Google Scholar] [CrossRef]
- Nikitaki, Z.; Holá, M.; Donà, M.; Pavlopoulou, A.; Michalopoulos, I.; Angelis, K.J.; Georgakilas, A.G.; Macovei, A.; Balestrazzi, A. Integrating plant and animal biology for the search of novel DNA damage biomarkers. Mutat. Res.–Rev. Mutat. Res. 2018, 775, 21–38. [Google Scholar] [CrossRef]
- Oakley, G.G.; Patrick, S.M. Replication protein A: Directing traffic at the intersection of replication and repair. Front. Biosci. 2010, 15, 883–900. [Google Scholar] [CrossRef]
- Yang, X.H.; Zou, L. Recruitment of ATR-ATRIP, Rad17, and 9-1-1 Complexes to DNA Damage. Methods Enzymol. 2006, 409, 118–131. [Google Scholar]
- Fischer, M. Census and evaluation of p53 target genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef]
- Preuss, S.B.; Britt, A.B. A DNA-damage-induced cell cycle checkpoint in arabidopsis. Genetics 2003, 164, 323–334. [Google Scholar] [CrossRef]
- Yoshiyama, K.; Conklin, P.A.; Huefner, N.D.; Britt, A.B. Suppressor of gamma response 1 (SOG1) encodes a putative transcription factor governing multiple responses to DNA damage. Proc. Natl. Acad. Sci. USA 2009, 106, 12843–12848. [Google Scholar] [CrossRef]
- Yoshiyama, K.O.; Kobayashi, J.; Ogita, N.; Ueda, M.; Kimura, S.; Maki, H.; Umeda, M. ATM-mediated phosphorylation of SOG1 is essential for the DNA damage response in Arabidopsis. EMBO Rep. 2013, 14, 817–822. [Google Scholar] [CrossRef]
- Yoshiyama, K.O.; Kaminoyama, K.; Sakamoto, T.; Kimura, S. Increased phosphorylation of ser-gln sites on SUPPRESSOR OF GAMMA RESPONSE1 strengthens the DNA damage response in Arabidopsis thaliana. Plant Cell 2017, 29, 3255–3268. [Google Scholar] [CrossRef]
- Sjogren, C.A.; Bolaris, S.C.; Larsen, P.B. Aluminum-dependent terminal differentiation of the arabidopsis root tip is mediated through an ATR-, ALT2-, and SOG1-regulated transcriptional response. Plant Cell 2015, 27, 2501–2515. [Google Scholar] [CrossRef]
- Ogita, N.; Okushima, Y.; Tokizawa, M.; Yamamoto, Y.Y.; Tanaka, M.; Seki, M.; Makita, Y.; Matsui, M.; Okamoto-Yoshiyama, K.; Sakamoto, T.; et al. Identifying the target genes of SUPPRESSOR OF GAMMA RESPONSE 1, a master transcription factor controlling DNA damage response in Arabidopsis. Plant J. 2018, 94, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Bourbousse, C.; Vegesna, N.; Law, J.A. SOG1 activator and MYB3R repressors regulate a complex DNA damage network in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E12453–E12462. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cools, T.; De Veylder, L. Mechanisms used by plants to cope with DNA damage. Annu. Rev. Plant Biol. 2016, 67, 439–462. [Google Scholar] [CrossRef]
- Spampinato, C.P. Protecting DNA from errors and damage: An overview of DNA repair mechanisms in plants compared to mammals. Cell. Mol. Life Sci. 2017, 74, 1693–1709. [Google Scholar] [CrossRef]
- Berens, P.J.T.; Molinier, J. Formation and recognition of uv-induced dna damage within genome complexity. Int. J. Mol. Sci. 2020, 21, 1–23. [Google Scholar]
- Cannan, W.J.; Pederson, D.S. Mechanisms and consequences of double-strand DNA break formation in chromatin. J. Cell. Physiol. 2016, 231, 3–14. [Google Scholar] [CrossRef]
- Friesner, J.D.; Liu, B.; Culligan, K.; Britt, A.B. Ionizing radiation-dependent γ-H2AX focus formation requires ataxia telangiectasia mutated and ataxia telangiectasia mutated and Rad3-related. Mol. Biol. Cell 2005, 16, 2566–2576. [Google Scholar] [CrossRef]
- Amiard, S.; Charbonnel, C.; Allain, E.; Depeiges, A.; White, C.I.; Gallego, M.E. Distinct roles of the ATR kinase and the Mre11-Rad50-Nbs1 complex in the maintenance of chromosomal stability in Arabidopsis. Plant Cell 2010, 22, 3020–3033. [Google Scholar] [CrossRef]
- Dickey, J.S.; Redon, C.E.; Nakamura, A.J.; Baird, B.J.; Sedelnikova, O.A.; Bonner, W.M. H2AX: Functional roles and potential applications. Chromosoma 2009, 118, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Lorković, Z.J.; Berger, F. Heterochromatin and DNA damage repair: Use different histone variants and relax. Nucleus 2017, 8, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, A.A.; Jeggo, P.; Lobrich, M. The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax. DNA Repair 2010, 9, 1273–1282. [Google Scholar] [CrossRef]
- Deriano, L.; Roth, D.B. Modernizing the nonhomologous end-joining repertoire: Alternative and classical NHEJ share the stage. Annu. Rev. Genet. 2013, 47, 433–455. [Google Scholar] [CrossRef]
- Cejka, P. DNA end resection: Nucleases team up with the right partners to initiate homologous recombination. J. Biol. Chem. 2015, 290, 22931–22938. [Google Scholar] [CrossRef]
- Puchta, H. The repair of double-strand breaks in plants: Mechanisms and consequences for genome evolution. J. Exp. Bot. 2005, 56, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Weimer, A.K.; Biedermann, S.; Harashima, H.; Roodbarkelari, F.; Takahashi, N.; Foreman, J.; Guan, Y.; Pochon, G.; Heese, M.; Van Damme, D.; et al. The plant-specific CDKB1-CYCB1 complex mediates homologous recombination repair in Arabidopsis. EMBO J. 2016, 35, 2068–2086. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, N.; Sablowski, R. Hypersensitivity to DNA damage in plant stem cell niches. Proc. Natl. Acad. Sci. USA 2009, 106, 20984–20988. [Google Scholar] [CrossRef]
- Furukawa, T.; Curtis, M.J.; Tominey, C.M.; Duong, Y.H.; Wilcox, B.W.L.; Aggoune, D.; Hays, J.B.; Britt, A.B. A shared DNA-damage-response pathway for induction of stem-cell death by UVB and by gamma irradiation. DNA Repair 2010, 9, 940–948. [Google Scholar] [CrossRef]
- Adachi, S.; Minamisawa, K.; Okushima, Y.; Inagaki, S.; Yoshiyama, K.; Kondou, Y.; Kaminuma, E.; Kawashima, M.; Toyoda, T.; Matsui, M.; et al. Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 10004–10009. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Schnittger, A. Endoreplication—A means to an end in cell growth and stress response. Curr. Opin. Plant Biol. 2020, 54, 85–92. [Google Scholar] [CrossRef]
- Trovesi, C.; Manfrini, N.; Falcettoni, M.; Longhese, M.P. Regulation of the DNA damage response by cyclin-dependent kinases. J. Mol. Biol. 2013, 425, 4756–4766. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, A.; De Veylder, L. The Dual Face of Cyclin B1. Trends Plant Sci. 2018, 23, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Haga, N.; Kato, K.; Murase, M.; Araki, S.; Kubo, M.; Demura, T.; Suzuki, K.; Müller, I.; Voß, U.; Jürgens, G.; et al. R1R2R3-Myb proteins positively regulate cytokinesis through activation of KNOLLE transcription in Arabidopsis thaliana. Development 2007, 134, 1101–1110. [Google Scholar] [CrossRef]
- Haga, N.; Kobayashi, K.; Suzuki, T.; Maeo, K.; Kubo, M.; Ohtani, M.; Mitsuda, N.; Demura, T.; Nakamura, K.; Jurgens, G.; et al. Mutations in MYB3R1 and MYB3R4 cause pleiotropic developmental defects and preferential down-regulation of multiple G2/M-specific genes in Arabidopsis. Plant Physiol. 2011, 157, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ndathe, R.W.; Kumar, N.; Zeringue, E.A.; Kato, N.; Larkin, J.C. The CDK inhibitor SIAMESE targets both CDKA;1 and CDKB1 complexes to establish endoreplication in trichomes. Plant Physiol. 2020, 184, 165–175. [Google Scholar] [CrossRef]
- Chen, P.; Takatsuka, H.; Takahashi, N.; Kurata, R.; Fukao, Y.; Kobayashi, K.; Ito, M.; Umeda, M. Arabidopsis R1R2R3-Myb proteins are essential for inhibiting cell division in response to DNA damage. Nat. Commun. 2017, 8, 635. [Google Scholar] [CrossRef]
- Horvath, B.M.; Kourova, H.; Nagy, S.; Nemeth, E.; Magyar, Z.; Papdi, C.; Ahmad, Z.; Sanchez-Perez, G.F.; Perilli, S.; Blilou, I.; et al. Arabidopsis RETINOBLASTOMA RELATED directly regulates DNA damage responses through functions beyond cell cycle control. EMBO J. 2017, 36, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, S.; Harashima, H.; Chen, P.; Heese, M.; Bouyer, D.; Sofroni, K.; Schnittger, A. The retinoblastoma homolog RBR1 mediates localization of the repair protein RAD51 to DNA lesions in Arabidopsis. EMBO J. 2017, 36, 1279–1297. [Google Scholar] [CrossRef]
- Gentric, N.; Masoud, K.; Journot, R.P.; Cognat, V.; Chabouté, M.-E.; Noir, S.; Genschik, P. The F-box-like protein FBL17 is a regulator of DNA-damage response and co-localizes with RETINOBLASTOMA RELATED 1 at DNA lesion sites. Plant Physiol. 2020, 183, 1295–1305. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle arrest through indirect transcriptional repression by p53: I have a DREAM. Cell Death Differ. 2018, 25, 114–132. [Google Scholar] [CrossRef]
- .Uxa, S.; Bernhart, S.H.; Mages, C.F.S.; Fischer, M.; Kohler, R.; Hoffmann, S.; Stadler, P.F.; Engeland, K.; Müller, G.A. DREAM and RB cooperate to induce gene repression and cell-cycle arrest in response to p53 activation. Nucleic Acids Res. 2019, 47, 9087–9103. [Google Scholar] [CrossRef]
- Gómez, M.S.; Falcone Ferreyra, M.L.; Sheridan, M.L.; Casati, P. Arabidopsis E2Fc is required for the DNA damage response under UV-B radiation epistatically over the microRNA396 and independently of E2Fe. Plant J. 2019, 97, 749–764. [Google Scholar] [CrossRef]
- Manickavinayaham, S.; Dennehey, B.K.; Johnson, D.G. Direct regulation of dna repair by e2f and rb in mammals and plants: Core function or convergent evolution? Cancers 2021, 13, 934. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Wang, C.; Hu, Z.; Yan, S. Negative regulator of E2F transcription factors links cell cycle checkpoint and DNA damage repair. Proc. Natl. Acad. Sci. USA 2018, E3837–E3845. [Google Scholar] [CrossRef]
- Culligan, K.; Tissier, A.; Britt, A. ATR Regulates a G2-Phase Cell-Cycle Checkpoint in Arabidopsis thaliana. Plant Cell 2004, 16, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Roitinger, E.; Hofer, M.; Köcher, T.; Pichler, P.; Novatchkova, M.; Yang, J.; Schlögelhofer, P.; Mechtler, K. Quantitative phosphoproteomics of the Ataxia Telangiectasia-Mutated (ATM) and Ataxia Telangiectasia-Mutated and Rad3-related (ATR) dependent DNA damage response in Arabidopsis thaliana. Mol. Cell. Proteom. 2015, 14, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Cools, T.; Iantcheva, A.; Weimer, A.K.; Boens, S.; Takahashi, N.; Maes, S.; Van den Daele, H.; Van Isterdael, G.; Schnittger, A.; De Veylder, L. The Arabidopsis thaliana checkpoint kinase WEE1 protects against premature vascular differentiation during replication stress. Plant Cell 2011, 23, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, H.; Song, J.; Cao, X.; Rogers, H.J.; Francis, D.; Jia, C.; Sun, L.; Hou, M.; Yang, Y.; et al. Cell cycle arrest mediated by Cd-induced DNA damage in Arabidopsis root tips. Ecotoxicol. Environ. Saf. 2017, 145, 569–574. [Google Scholar] [CrossRef]
- Cabral, D.; Banora, M.Y.; Antonino, J.D.; Rodiuc, N.; Vieira, P.; Coelho, R.R.; Chevalier, C.; Eekhout, T.; Engler, G.; De Veylder, L.; et al. The plant WEE1 kinase is involved in checkpoint control activation in nematode-induced galls. New Phytol. 2020, 225, 430–447. [Google Scholar] [CrossRef]
- Pan, T.; Qin, Q.; Nong, C.; Gao, S.; Wang, L.; Cai, B.; Zhang, M.; Wu, C.; Chen, H.; Li, T.; et al. A novel WEE1 pathway for replication stress responses. Nat. Plants 2021, 7, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhan, L.; Zhao, Y.; Huang, Y.; Wu, C.; Pan, T.; Qin, Q.; Xu, Y.; Deng, Z.; Li, J.; et al. The ATR-WEE1 kinase module inhibits the MAC complex to regulate replication stress response. Nucleic Acids Res. 2021, 49, 1411–1425. [Google Scholar] [CrossRef]
- Zhao, X.; Harashima, H.; Dissmeyer, N.; Pusch, S.; Weimer, A.K.; Bramsiepe, J.; Bouyer, D.; Rademacher, S.; Nowack, M.K.; Novak, B.; et al. A General G1/S-Phase cell-cycle control module in the flowering plant Arabidopsis thaliana. PLoS Genet. 2012, 8, e1002847. [Google Scholar] [CrossRef]
- D’Ario, M.; Tavares, R.; Schiessl, K.; Desvoyes, B.; Gutierrez, C.; Howard, M.; Sablowski, R. Cell size controlled in plants using DNA content as an internal scale. Science 2021, 372, 1176–1181. [Google Scholar] [CrossRef]
- Yu, Z.K.; Gervais, J.L.M.; Zhang, H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 11324–11329. [Google Scholar] [CrossRef] [PubMed]
- Sutterlüty, H.; Chatelain, E.; Marti, A.; Wirbelauer, C.; Senften, M.; Müller, U.; Krek, W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1999, 1, 207–214. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, B.; You, C.; Zhang, Y.; Zeng, L.; Li, S.; Johnson, K.C.M.; Yu, B.; Li, X.; Chen, X. The arabidopsis MOS4-associated complex promotes microRNA biogenesis and precursor messenger RNA splicing. Plant Cell 2017, 29, 2626–2643. [Google Scholar] [CrossRef] [PubMed]
- Lenzken, S.C.; Loffreda, A.; Barabino, S.M.L. RNA splicing: A new player in the DNA damage response. Int. J. Cell Biol. 2013, 2013, 153634. [Google Scholar] [CrossRef] [PubMed]
- Nimeth, B.A.; Riegler, S.; Kalyna, M. Alternative splicing and DNA damage response in plants. Front. Plant Sci. 2020, 11, 91. [Google Scholar] [CrossRef]
- Scholes, D.R.; Paige, K.N. Plasticity in ploidy: A generalized response to stress. Trends Plant Sci. 2015, 20, 165–175. [Google Scholar] [CrossRef]
- Mahapatra, K.; Roy, S. An insight into the mechanism of DNA damage response in plants-role of SUPPRESSOR OF GAMMA RESPONSE 1: An overview. Mutat. Res. Mol. Mech. Mutagen. 2020, 819, 111689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Dale, R.; Kemboi, D.; Zeringue, E.A.; Kato, N.; Larkin, J.C. Functional analysis of short linear motifs in the plant cyclin-dependent kinase inhibitor SIAMESE. Plant Physiol. 2018, 177, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Churchman, M.L.; Brown, M.L.; Kato, N.; Kirik, V.; Hulskamp, M.; Inze, D.; De Veylder, L.; Walker, J.D.; Zheng, Z.; Oppenheimer, D.G.; et al. SIAMESE, a plant-specific cell cycle regulator, controls endoreplication onset in Arabidopsis thaliana. Plant Cell Online 2006, 18, 3145–3157. [Google Scholar] [CrossRef]
- Wen, B.; Nieuwland, J.; Murray, J.A.H. The Arabidopsis CDK inhibitor ICK3/KRP5 is rate limiting for primary root growth and promotes growth through cell elongation and endoreduplication. J. Exp. Bot. 2013, 64, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, K.; Muiño, J.M.; Sablowski, R. Arabidopsis JAGGED links floral organ patterning to tissue growth by repressing Kip-related cell cycle inhibitors. Proc. Natl. Acad. Sci. USA 2014, 111, 2830–2835. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Alvim Kamei, C.L.; Cools, T.; Vanderauwera, S.; Takahashi, N.; Okushima, Y.; Eekhout, T.; Yoshiyama, K.O.; Larkin, J.; Van den Daele, H.; et al. The Arabidopsis SIAMESE-RELATED cyclin-dependent kinase inhibitors SMR5 and SMR7 regulate the DNA damage checkpoint in response to reactive oxygen species. Plant Cell 2014, 26, 296–309. [Google Scholar] [CrossRef]
- Yamasaki, S.; Shimada, E.; Kuwano, T.; Kawano, T.; Noguchi, N. Continuous UV-B irradiation induces endoreduplication and peroxidase activity in epidermal cells surrounding trichomes on cucumber cotyledons. J. Radiat. Res. 2010, 51, 187–196. [Google Scholar] [CrossRef]
- Yamasaki, S.; Murakami, Y. Continuous UV-B irradiation induces endoreduplication and trichome formation in cotyledons, and reduces epidermal cell division and expansion in the first leaves of pumpkin seedlings (Cucurbita maxima Duch. × C. moschata Duch.). Environ. Control Biol. 2014, 52, 203–209. [Google Scholar] [CrossRef][Green Version]
- Matsuda, M.; Iwata, Y.; Koizumi, N.; Mishiba, K. ichiro DNA double-strand breaks promote endoreduplication in radish cotyledon. Plant Cell Rep. 2018, 37, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Nakayama, S.; Umeda-Hara, C.; Ohtsuki, N.; Saika, H.; Umeda, M.; Toki, S. CDKB2 is involved in mitosis and DNA damage response in rice. Plant J. 2012, 69, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Garcia, J.A.; Eekhout, T.; Achon, I.; Nisa, M.-U.; Coussens, G.; Vercauteren, I.; Van den Daele, H.; Pauwels, L.; Van Lijsebettens, M.; Raynaud, C.; et al. Maize ATR safeguards genome stability during kernel development to prevent early endosperm endocycle onset and cell death. Plant Cell 2021, 8, 2662–2684. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Shikazono, N.; Tanaka, A.; Hase, Y.; Funayama, T.; Wada, S.; Inoue, M. Comparative radiation tolerance based on the induction of DNA double-strand breaks in tobacco BY-2 cells and CHO-K1 cells irradiated with gamma rays. Radiat. Res. 2005, 163, 520–525. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gentric, N.; Genschik, P.; Noir, S. Connections between the Cell Cycle and the DNA Damage Response in Plants. Int. J. Mol. Sci. 2021, 22, 9558. https://doi.org/10.3390/ijms22179558
Gentric N, Genschik P, Noir S. Connections between the Cell Cycle and the DNA Damage Response in Plants. International Journal of Molecular Sciences. 2021; 22(17):9558. https://doi.org/10.3390/ijms22179558
Chicago/Turabian StyleGentric, Naomie, Pascal Genschik, and Sandra Noir. 2021. "Connections between the Cell Cycle and the DNA Damage Response in Plants" International Journal of Molecular Sciences 22, no. 17: 9558. https://doi.org/10.3390/ijms22179558
APA StyleGentric, N., Genschik, P., & Noir, S. (2021). Connections between the Cell Cycle and the DNA Damage Response in Plants. International Journal of Molecular Sciences, 22(17), 9558. https://doi.org/10.3390/ijms22179558





