Peptides Derived from Angiogenin Regulate Cellular Copper Uptake
Abstract
:1. Introduction
2. Results
2.1. ESR Study on Cu(II)-Ang1-17 System (M/L 1:1)
2.2. Potentiometric and ESR Studies on Cu(II)-Ang1-17 System (M/L 2:1)
2.3. ESR Study on Cu(II)-AcAng1-17 System (M/L 1:1)
2.4. Potentiometric and ESR Studies on Cu(II)-AcAng1-17 System (M/L 2:1)
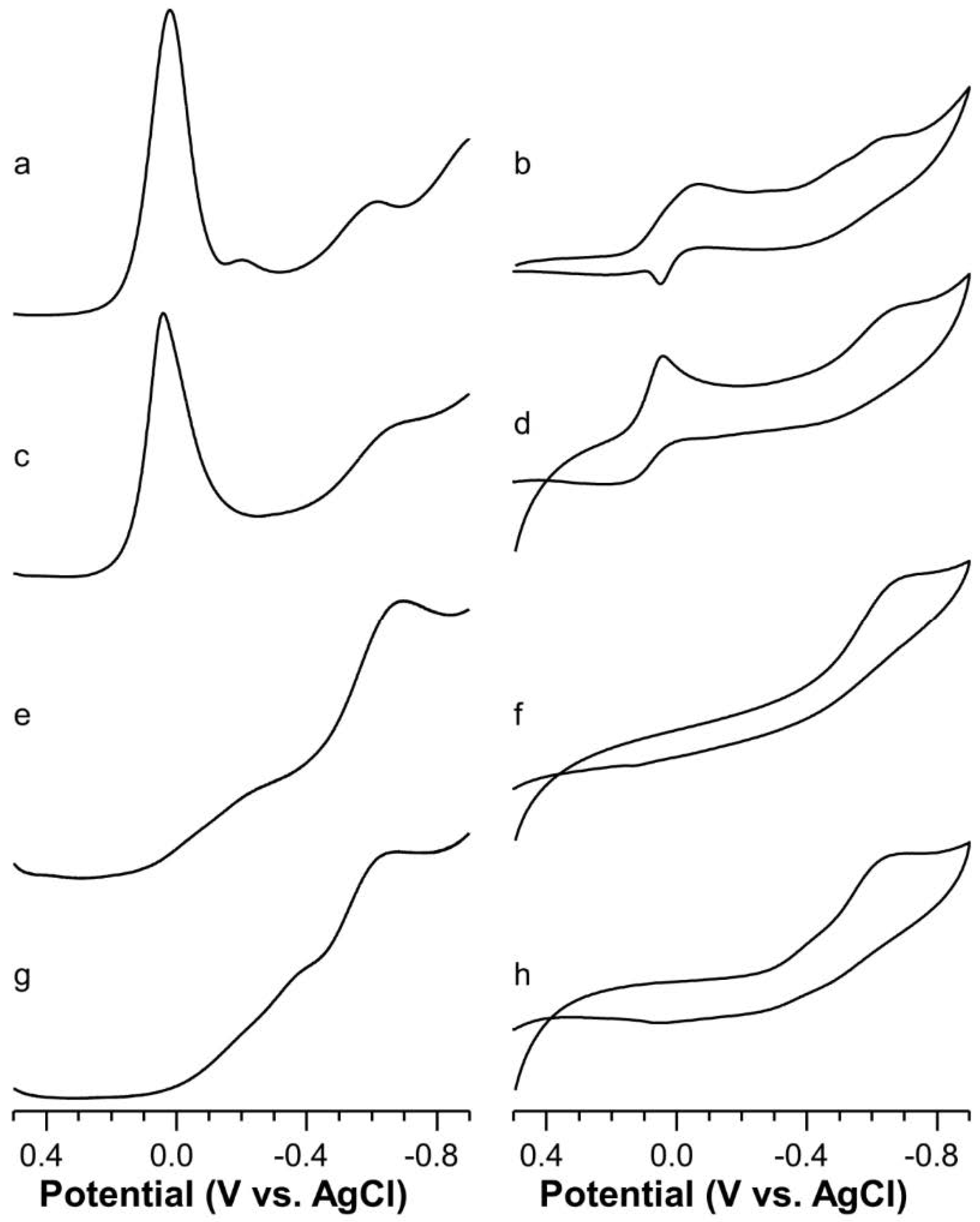
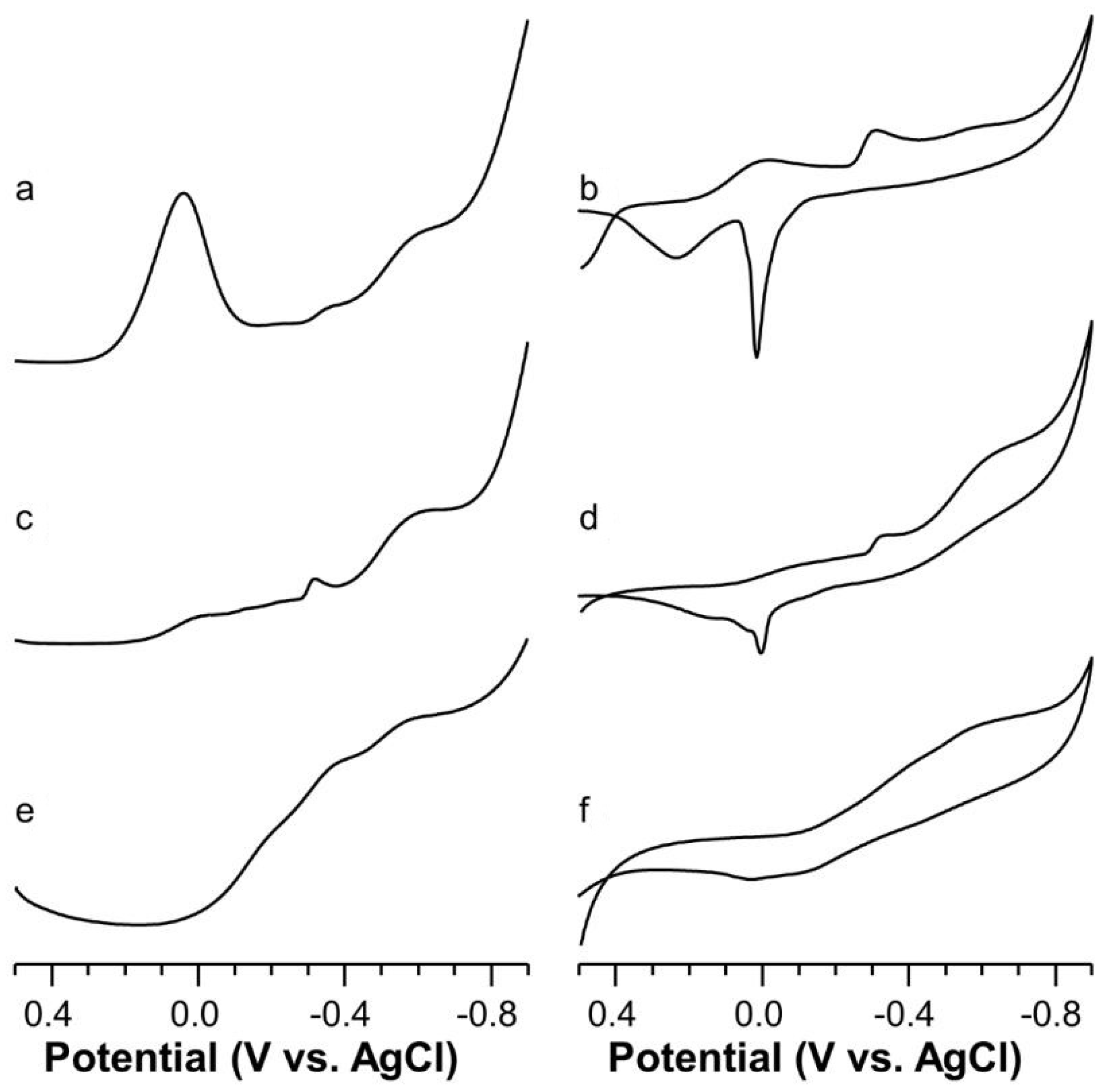
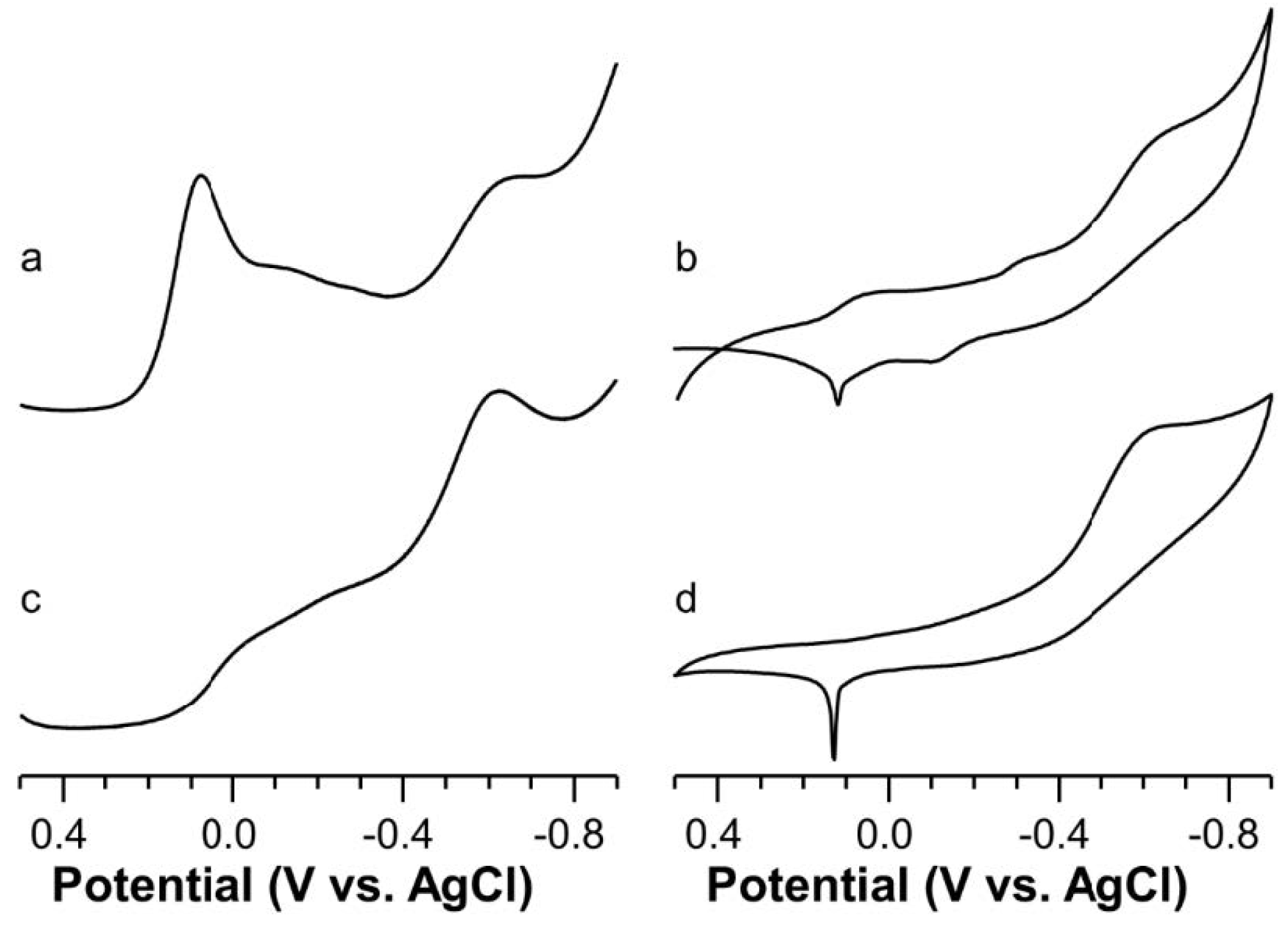
2.5. Voltammetric Study on Cu(II)-Ang1-17 System (M/L 1:1)
2.6. Voltammetric Study on Cu(II)-AcAng1-17 System (M/L 1:1)
2.7. Voltammetric Study on Cu(II) with Ang1-17 or AcAng1-17 Systems (M/L 2:1)
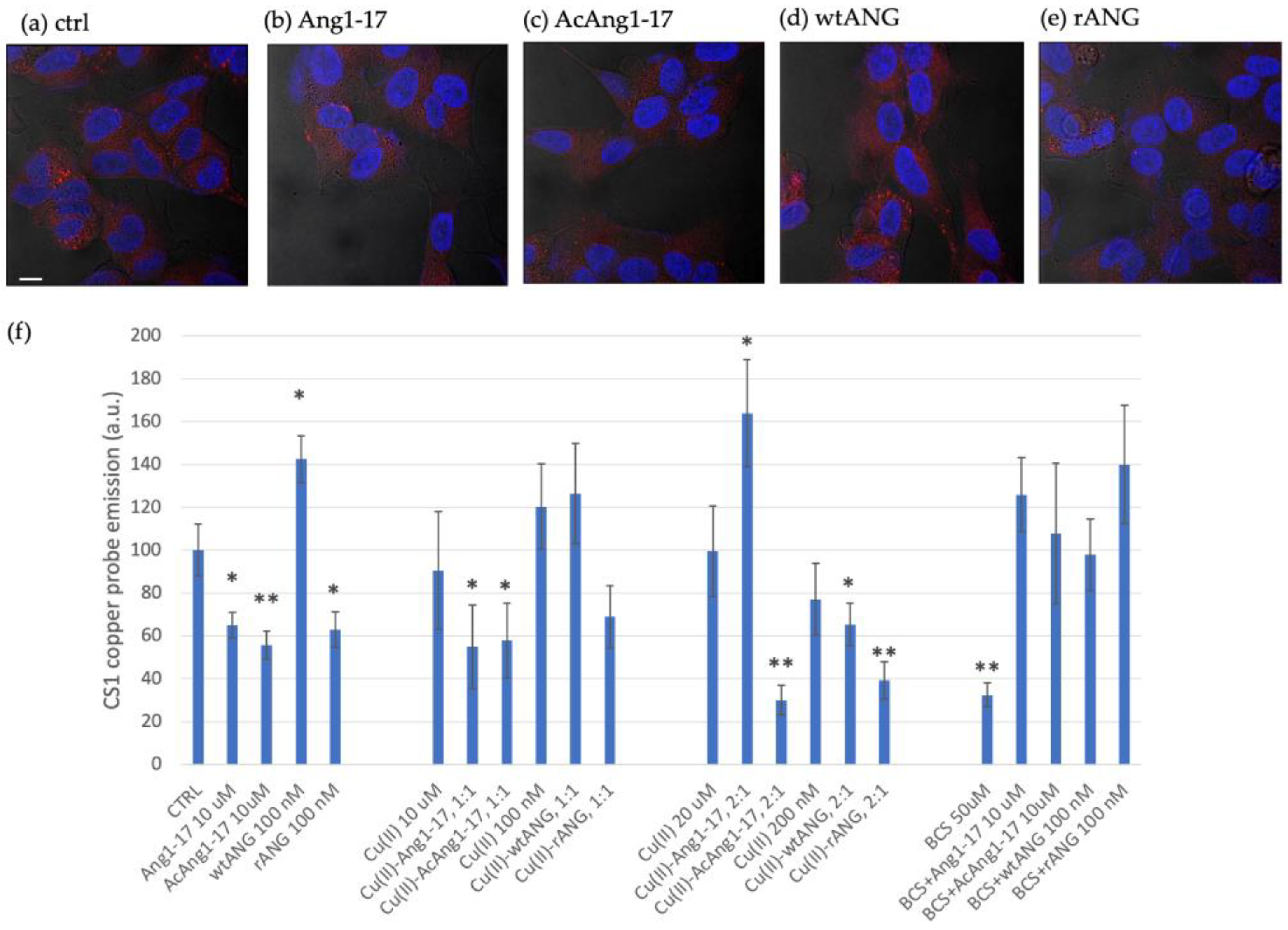
2.8. Peptides Effect on Intracellular Copper Contents in SH-SY5Y Cell Line
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Expression and Purification of Angiogenin
4.3. Electron Spin Resonance Spectroscopy (ESR)
4.4. Potentiometric Titrations
4.5. Ultraviolet-Visible (UV-vis) Measurements
4.6. Circular Dichroism (CD) Measurements
4.7. Voltammetric Study
4.8. Cell Cultures
4.9. Confocal Microscopy Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lyons, S.M.; Fay, M.M.; Akiyama, Y.; Anderson, P.J.; Ivanov, P. RNA biology of angiogenin: Current state and perspectives. RNA Biol. 2016, 14, 171–178. [Google Scholar] [CrossRef]
- Sheng, J.; Xu, Z. Three decades of research on angiogenin: A review and perspective. Acta Biochim. Biophys. Sin. 2016, 48, 399–410. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.N.; Kim, D.H. Decreased serum angiogenin level in Alzheimer’s disease. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 116–120. [Google Scholar] [CrossRef]
- Van Es, M.A.; Schelhaas, H.J.; van Vught, P.W.J.; Ticozzi, N.; Andersen, P.M.; Groen, E.J.N.; Schulte, C.; Blauw, H.M.; Koppers, M.; Diekstra, F.P.; et al. Angiogenin variants in Parkinson disease and amyotrophic lateral sclerosis. Ann. Neurol. 2011, 70, 964–973. [Google Scholar] [CrossRef]
- Bradshaw, W.J.; Rehman, S.; Pham, T.T.K.; Thiyagarajan, N.; Lee, R.L.; Subramanian, V.; Acharya, K.R. Structural insights into human angiogenin variants implicated in Parkinson’s disease and Amyotrophic Lateral Sclerosis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Prehn, J.H.M.; Jirström, E. Angiogenin and tRNA fragments in Parkinson’s disease and neurodegeneration. Acta Pharmacol. Sin. 2020, 41, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Tello-Montoliu, A.; Patel, J.V.; Lip, G.Y.H. Angiogenin: A review of the pathophysiology and potential clinical applications. J. Thromb. Haemost. 2006, 4, 1864–1874. [Google Scholar] [CrossRef]
- Campos-Melo, D.; Droppelmann, C.A.; Volkening, K.; Strong, M.J. RNA-binding proteins as molecular links between cancer and neurodegeneration. Biogerontology 2014, 15, 587–610. [Google Scholar] [CrossRef]
- Greenway, M.J.; Andersen, P.M.; Russ, C.; Ennis, S.; Cashman, S.; Donaghy, C.; Patterson, V.; Swingler, R.; Kieran, D.; Prehn, J.; et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat. Genet. 2006, 38, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Kishikawa, H.; Wu, D.; Hu, G.-F. Targeting angiogenin in therapy of amyotropic lateral sclerosis. Expert Opin. Ther. Targets 2008, 12, 1229–1242. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-Erriu, I.M.; Prehn, J.H.M. Molecular Mechanisms in Amyotrophic Lateral Sclerosis: The Role of Angiogenin, a Secreted RNase. Front. Neurosci. 2012, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strong, M.J. The evidence for altered RNA metabolism in amyotrophic lateral sclerosis (ALS). J. Neurol. Sci. 2010, 288, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Goncalves, K.A.; Li, S.; Kishikawa, H.; Sun, G.; Yang, H.; Vanli, N.; Wu, Y.; Jiang, Y.; Hu, M.G.; et al. Plexin-B2 Mediates Physiologic and Pathologic Functions of Angiogenin. Cell 2017, 171, 849–864.e825. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.P.; Hoang, T.T.; Ressler, V.T.; Raines, R.T. Human angiogenin is a potent cytotoxin in the absence of ribonuclease inhibitor. RNA 2018, 24, 1018–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strydom, D.J. The angiogenins. Cell. Mol. Life Sci. (CMLS) 1998, 54, 811–824. [Google Scholar] [CrossRef]
- Fett, J.W.; Strydom, D.J.; Lobb, R.R.; Alderman, E.M.; Bethune, J.L.; Riordan, J.F.; Vallee, B.L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry 2002, 24, 5480–5486. [Google Scholar] [CrossRef]
- Lee, H.-H.; Wang, Y.-N.; Hung, M.-C. Functional roles of the human ribonuclease A superfamily in RNA metabolism and membrane receptor biology. Mol. Asp. Med. 2019, 70, 106–116. [Google Scholar] [CrossRef]
- Strydom, D.J.; Fett, J.W.; Lobb, R.R.; Alderman, E.M.; Bethune, J.L.; Riordan, J.F.; Vallee, B.L. Amino acid sequence of human tumor derived angiogenin. Biochemistry 2002, 24, 5486–5494. [Google Scholar] [CrossRef]
- Kishimoto, K.; Liu, S.; Tsuji, T.; Olson, K.A.; Hu, G.-f. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene 2004, 24, 445–456. [Google Scholar] [CrossRef] [Green Version]
- Finney, L.; Mandava, S.; Ursos, L.; Zhang, W.; Rodi, D.; Vogt, S.; Legnini, D.; Maser, J.; Ikpatt, F.; Olopade, O.I.; et al. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 2247–2252. [Google Scholar] [CrossRef] [Green Version]
- Finney, L.; Vogt, S.; Fukai, T.; Glesne, D. Copper and Angiogenesis: Unravelling a Relationship Key to Cancer Progression. Clin. Exp. Pharmacol. Physiol. 2009, 36, 88–94. [Google Scholar] [CrossRef] [Green Version]
- La Mendola, D.; Giacomelli, C.; Rizzarelli, E. Intracellular Bioinorganic Chemistry and Cross Talk Among Different -Omics. Curr. Top. Med. Chem. 2016, 16, 3103–3130. [Google Scholar] [CrossRef] [PubMed]
- Mathys, Z.K.; White, A.R. Copper and Alzheimer’s Disease. Neurotox. Met. 2017, 18, 199–216. [Google Scholar]
- Ackerman, C.M.; Chang, C.J. Copper signaling in the brain and beyond. J. Biol. Chem. 2018, 293, 4628–4635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urso, E.; Maffia, M. Behind the Link between Copper and Angiogenesis: Established Mechanisms and an Overview on the Role of Vascular Copper Transport Systems. J. Vasc. Res. 2015, 52, 172–196. [Google Scholar] [CrossRef] [PubMed]
- Petcu, E.-B.; Andrei, A.; Popa-Wagner, A.; Uzoni, A.; Gaman, A.M. The Role of Oxidative Stress in Etiopathogenesis of Chemotherapy Induced Cognitive Impairment (CICI)-“Chemobrain”. Aging Dis. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Men, L.; Wang, J.; Zhang, Y.; Chickenyen, S.; Wang, Y.; Zhou, F. Redox Reactions of Copper Complexes Formed with Different β-Amyloid Peptides and Their Neuropathalogical Relevance. Biochemistry 2007, 46, 9270–9282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnham, K.J.; Bush, A.I. Metals in Alzheimer’s and Parkinson’s Diseases. Curr. Opin. Chem. Biol. 2008, 12, 222–228. [Google Scholar] [CrossRef]
- Girvan, P.; Teng, X.; Brooks, N.J.; Baldwin, G.S.; Ying, L. Redox Kinetics of the Amyloid-β-Cu Complex and Its Biological Implications. Biochemistry 2018, 57, 6228–6233. [Google Scholar] [CrossRef]
- Bacchella, C.; Dell’Acqua, S.; Nicolis, S.; Monzani, E.; Casella, L. Oxidase Reactivity of CuII Bound to N-Truncated Aβ Peptides Promoted by Dopamine. Int. J. Mol. Sci. 2021, 22, 5190. [Google Scholar] [CrossRef]
- Cheignon, C.; Jones, M.; Atrián-Blasco, E.; Kieffer, I.; Faller, P.; Collin, F.; Hureau, C. Identification of key structural features of the elusive Cu–Aβ complex that generates ROS in Alzheimer’s disease. Chem. Sci. 2017, 8, 5107–5118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmieu, C.; Guettas, D.; Conte-Daban, A.; Sabater, L.; Faller, P.; Hureau, C. Copper-Targeting Approaches in Alzheimer’s Disease: How To Improve the Fallouts Obtained from in Vitro Studies. Inorg. Chem. 2019, 58, 13509–13527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manoharan, S.; Guillemin, G.J.; Abiramasundari, R.S.; Essa, M.M.; Akbar, M.; Akbar, M.D. The Role of Reactive Oxygen Species in the Pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A Mini Review. Oxidative Med. Cell. Longev. 2016, 2016, 8590578. [Google Scholar] [CrossRef] [PubMed]
- Hemerková, P.; Vališ, M. Role of Oxidative Stress in the Pathogenesis of Amyotrophic Lateral Sclerosis: Antioxidant Metalloenzymes and Therapeutic Strategies. Biomolecules 2021, 11, 437. [Google Scholar] [CrossRef]
- Zheng, J.; Goch, W.; Bal, W. Numerical Simulations Reveal Randomness of Cu(II) Induced Aβ Peptide Dimerization under Conditions Present in Glutamatergic Synapses. PLoS ONE 2017, 12, e0170749. [Google Scholar] [CrossRef] [Green Version]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal. 2018, 16. [Google Scholar] [CrossRef] [Green Version]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868. [Google Scholar] [CrossRef]
- Hoang, T.T.; Johnson, D.A.; Raines, R.T.; Johnson, J.A. Angiogenin activates the astrocytic Nrf2/antioxidant-response element pathway and thereby protects murine neurons from oxidative stress. J. Biol. Chem. 2019, 294, 15095–15103. [Google Scholar] [CrossRef]
- Giacomelli, C.; Trincavelli, M.L.; Satriano, C.; Hansson, Ö.; La Mendola, D.; Rizzarelli, E.; Martini, C. Copper (II) ions modulate Angiogenin activity in human endothelial cells. Int. J. Biochem. Cell Biol. 2015, 60, 185–196. [Google Scholar] [CrossRef]
- Badet, J.; Soncin, F.; Guitton, J.D.; Lamare, O.; Cartwright, T.; Barritault, D. Specific binding of angiogenin to calf pulmonary artery endothelial cells. Proc. Natl. Acad. Sci. USA 1989, 86, 8427–8431. [Google Scholar] [CrossRef] [Green Version]
- Soncin, F.; Guitton, J.-D.; Cartwright, T.; Badet, J. Interaction of Human Angiogenin with Copper Modulates Angiogenin Binding to Endothelial Cells. Biochem. Biophys. Res. Commun. 1997, 236, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Naletova, I.; Cucci, L.M.; D’Angeli, F.; Anfuso, C.D.; Magrì, A.; La Mendola, D.; Lupo, G.; Satriano, C. A Tunable Nanoplatform of Nanogold Functionalised with Angiogenin Peptides for Anti-Angiogenic Therapy of Brain Tumours. Cancers 2019, 11, 1322. [Google Scholar] [CrossRef] [Green Version]
- Cucci, L.M.; Trapani, G.; Hansson, Ö.; La Mendola, D.; Satriano, C. Gold Nanoparticles Functionalized with Angiogenin for Wound Care Application. Nanomaterials 2021, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- La Mendola, D.; Arnesano, F.; Hansson, Ö.; Giacomelli, C.; Calò, V.; Mangini, V.; Magrì, A.; Bellia, F.; Trincavelli, M.L.; Martini, C.; et al. Copper binding to naturally occurring, lactam form of angiogenin differs from that to recombinant protein, affecting their activity. Metallomics 2016, 8, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Magrì, A.; Munzone, A.; Peana, M.; Medici, S.; Zoroddu, M.; Hansson, O.; Satriano, C.; Rizzarelli, E.; La Mendola, D. Coordination Environment of Cu(II) Ions Bound to N-Terminal Peptide Fragments of Angiogenin Protein. Int. J. Mol. Sci. 2016, 17, 1240. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Zeng, L.; Domaille, D.W.; Chang, C.J. Preparation and use of Coppersensor-1, a synthetic fluorophore for live-cell copper imaging. Nat. Protoc. 2006, 1, 824–827. [Google Scholar] [CrossRef]
- Kowalik-Jankowska, T.; Ruta-Dolejsz, M.; Wiśniewska, K.; Łankiewicz, L. Coordination of copper(II) ions by the 11–20 and 11–28 fragments of human and mouse β-amyloid peptide. J. Inorg. Biochem. 2002, 92, 1–10. [Google Scholar] [CrossRef]
- Magrì, A.; Pietropaolo, A.; Tabbì, G.; La Mendola, D.; Rizzarelli, E. From Peptide Fragments to Whole Protein: Copper(II) Load and Coordination Features of IAPP. Chem.-A Eur. J. 2017, 23, 17898–17902. [Google Scholar] [CrossRef]
- Rajković, S.; Kállay, C.; Serényi, R.; Malandrinos, G.; Hadjiliadis, N.; Sanna, D.; Sóvágó, I. Complex formation processes of terminally protected peptides containing two or three histidyl residues. Characterization of the mixed metal complexes of peptides. Dalton Trans. 2008. [Google Scholar] [CrossRef]
- La Mendola, D.; Magrì, A.; Campagna, T.; Campitiello, M.A.; Raiola, L.; Isernia, C.; Hansson, Ö.; Bonomo, R.P.; Rizzarelli, E. A Doppel α-Helix Peptide Fragment Mimics the Copper(II) Interactions with the Whole Protein. Chem.-A Eur. J. 2010, 16, 6212–6223. [Google Scholar] [CrossRef]
- La Mendola, D.; Magrì, A.; Vagliasindi, L.I.; Hansson, Ö.; Bonomo, R.P.; Rizzarelli, E. Copper(ii) complex formation with a linear peptide encompassing the putative cell binding site of angiogenin. Dalton Trans. 2010, 39. [Google Scholar] [CrossRef]
- Sóvágó, I.; Ősz, K. Metal ion selectivity of oligopeptides. Dalton Trans. 2006, 3841–3854. [Google Scholar] [CrossRef]
- Łuczkowski, M.; Kozlowski, H.; Stawikowski, M.; Rolka, K.; Gaggelli, E.; Valensin, D.; Valensin, G. Is the monomeric prion octapeptide repeat PHGGGWGQ a specific ligand for Cu2+ ions? J. Chem. Soc. Dalton Trans. 2002, 2269–2274. [Google Scholar] [CrossRef]
- Jankowska, E.; Pietruszka, M.; Kowalik-Jankowska, T. Coordination of copper(ii) ions by the fragments of neuropeptide gamma containing D1, H9, H12residues and products of copper-catalyzed oxidation. Dalton Trans. 2012, 41, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Tabbì, G.; Giuffrida, A.; Bonomo, R.P. Determination of formal redox potentials in aqueous solution of copper(II) complexes with ligands having nitrogen and oxygen donor atoms and comparison with their EPR and UV–Vis spectral features. J. Inorg. Biochem. 2013, 128, 137–145. [Google Scholar] [CrossRef] [PubMed]
- White, A.R.; Barnham, K.J.; Huang, X.; Voltakis, I.; Beyreuther, K.; Masters, C.L.; Cherny, R.A.; Bush, A.I.; Cappai, R. Iron inhibits neurotoxicity induced by trace copper and biological reductants. JBIC J. Biol. Inorg. Chem. 2004, 9, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Gordge, M.P.; Meyer, D.J.; Hothersall, J.; Neild, G.H.; Payne, N.N.; Noronha-Dutra, A. Copper chelation-induced reduction of the biological activity of S-nitrosothiols. Br. J. Pharmacol. 1995, 114, 1083–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandini, G.; Satriano, C.; Pietropaolo, A.; Gianì, F.; Travaglia, A.; La Mendola, D.; Nicoletti, V.G.; Rizzarelli, E. The Inorganic Side of NGF: Copper(II) and Zinc(II) Affect the NGF Mimicking Signaling of the N-Terminus Peptides Encompassing the Recognition Domain of TrkA Receptor. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Lutsenko, S. Copper trafficking to the secretory pathway. Metallomics 2016, 8, 840–852. [Google Scholar] [CrossRef] [Green Version]
- Öhrvik, H.; Thiele, D.J. How copper traverses cellular membranes through the mammalian copper transporter 1, Ctr1. Ann. N. Y. Acad. Sci. 2014, 1314, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalik-Jankowska, T.; Ruta, M.; Wiśniewska, K.; Łankiewicz, L. Coordination abilities of the 1–16 and 1–28 fragments of β-amyloid peptide towards copper(II) ions: A combined potentiometric and spectroscopic study. J. Inorg. Biochem. 2003, 95, 270–282. [Google Scholar] [CrossRef]
- La Mendola, D.; Farkas, D.; Bellia, F.; Magrì, A.; Travaglia, A.; Hansson, Ö.; Rizzarelli, E. Probing the Copper(II) Binding Features of Angiogenin. Similarities and Differences between a N-Terminus Peptide Fragment and the Recombinant Human Protein. Inorg. Chem. 2012, 51, 128–141. [Google Scholar] [CrossRef]
- Sanna, D.; Micera, G.; Kállay, C.; Rigó, V.; Sóvágó, I. Copper(ii) complexes of N-terminal protected tri- and tetra-peptides containing histidine residues. Dalton Trans. 2004, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Jankowska, T.; Rajewska, A.; Wiśniewska, K.; Grzonka, Z.; Jezierska, J. Coordination abilities of N-terminal fragments of α-synuclein towards copper(II) ions: A combined potentiometric and spectroscopic study. J. Inorg. Biochem. 2005, 99, 2282–2291. [Google Scholar] [CrossRef]
- Kállay, C.; Nagy, Z.; Várnagy, K.; Malandrinos, G.; Hadjiliadis, N.; Sóvágó, I. Thermodynamic and Structural Characterization of the Copper(II) Complexes of Peptides Containing Both Histidyl and Aspartyl Residues. Bioinorg. Chem. Appl. 2007, 2007, 30394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magrì, A.; Grasso, G.; Corti, F.; Finetti, F.; Greco, V.; Santoro, A.M.; Sciuto, S.; La Mendola, D.; Morbidelli, L.; Rizzarelli, E. Peptides derived from the histidine–proline rich glycoprotein bind copper ions and exhibit anti-angiogenic properties. Dalton Trans. 2018, 47, 9492–9503. [Google Scholar] [CrossRef]
- Holloway, D.E.; Hares, M.C.; Shapiro, R.; Subramanian, V.; Acharya, K.R. High-Level Expression of Three Members of the Murine Angiogenin Family in Escherichia coli and Purification of the Recombinant Proteins. Protein Expr. Purif. 2001, 22, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-H.; Kang, D.-K.; Chang, S.-I.; Scheraga, H.A.; Shin, H.-C. High level production of bovine angiogenin in E. coli by an efficient refolding procedure. Biotechnol. Lett. 2004, 26, 1501–1504. [Google Scholar] [CrossRef]
- La Mendola, D.; Magrì, A.; Hansson, Ö.; Bonomo, R.P.; Rizzarelli, E. Copper(II) complexes with peptide fragments encompassing the sequence 122–130 of human doppel protein. J. Inorg. Biochem. 2009, 103, 758–765. [Google Scholar] [CrossRef]
- Lund, A.; Callens, F.; Sagstuen, E. Determination of the g-, hyperfine coupling- and zero-field splitting tensors in EPR and ENDOR using extended Matlab codes. J. Magn. Reson. 2021, 325. [Google Scholar] [CrossRef]
- Pilbrow, J.R.; Winfield, M.E. Computer simulation of low symmetry E.S.R. spectra due to vitamin B12rand model systems. Mol. Phys. 1973, 25, 1073–1092. [Google Scholar] [CrossRef]
- Bellia, F.; La Mendola, D.; Maccarrone, G.; Mineo, P.; Vitalini, D.; Scamporrino, E.; Sortino, S.; Vecchio, G.; Rizzarelli, E. Copper(II) complexes with β-cyclodextrin–homocarnosine conjugates and their antioxidant activity. Inorg. Chim. Acta 2007, 360, 945–954. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Kaifer, A.E.; Bard, A.J. Micellar effects on the reductive electrochemistry of methylviologen. J. Phys. Chem. 2002, 89, 4876–4880. [Google Scholar] [CrossRef]
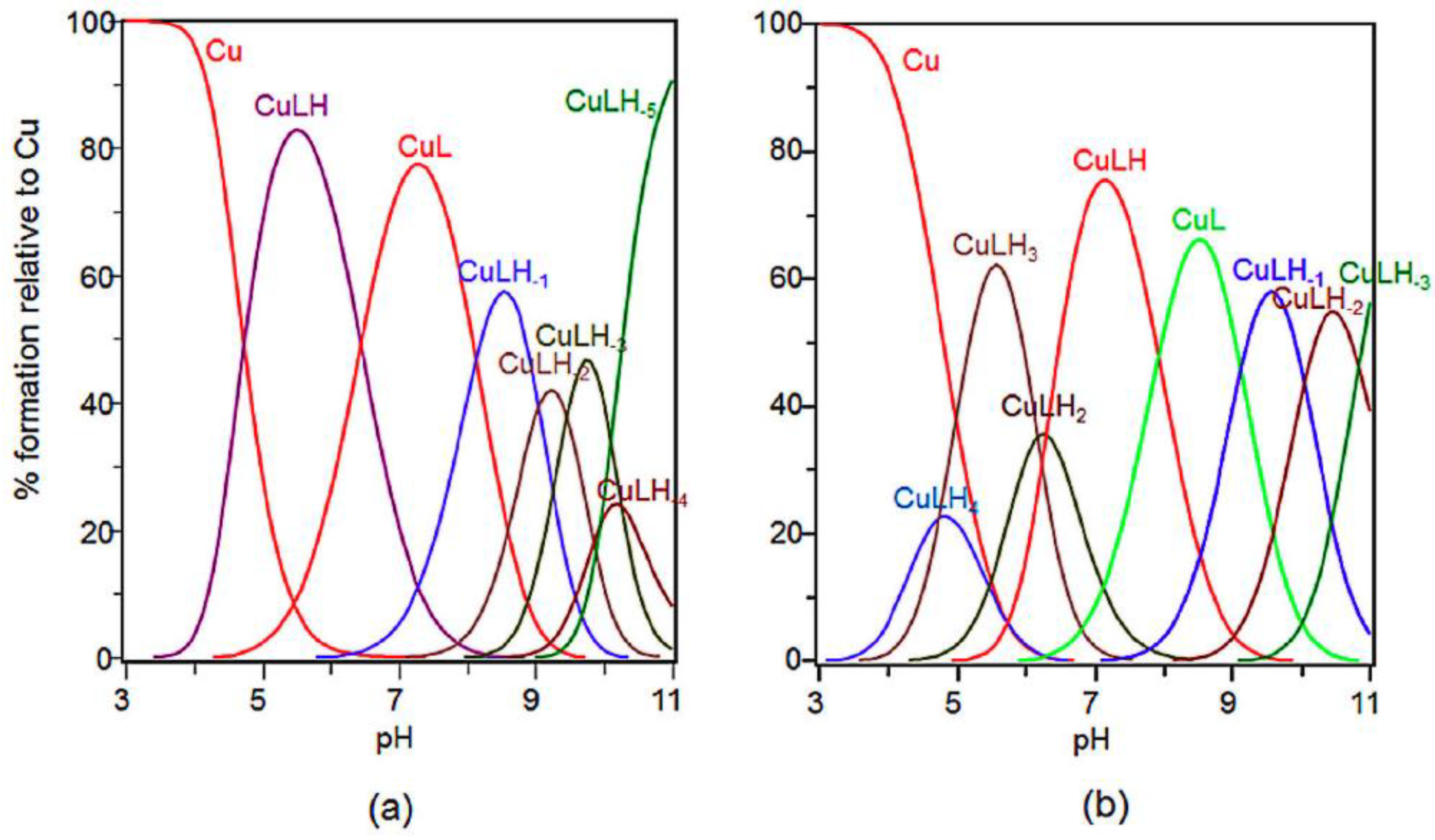
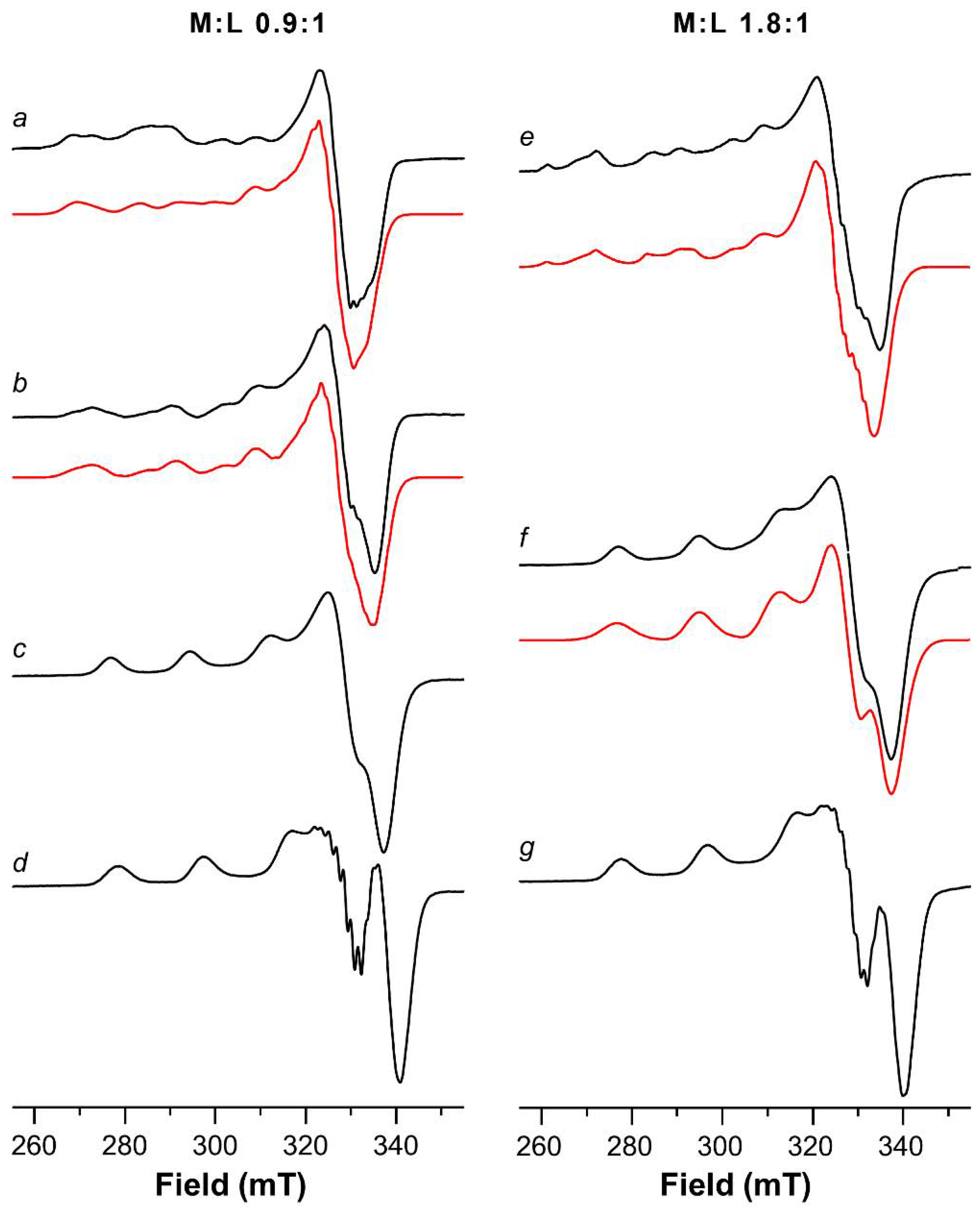
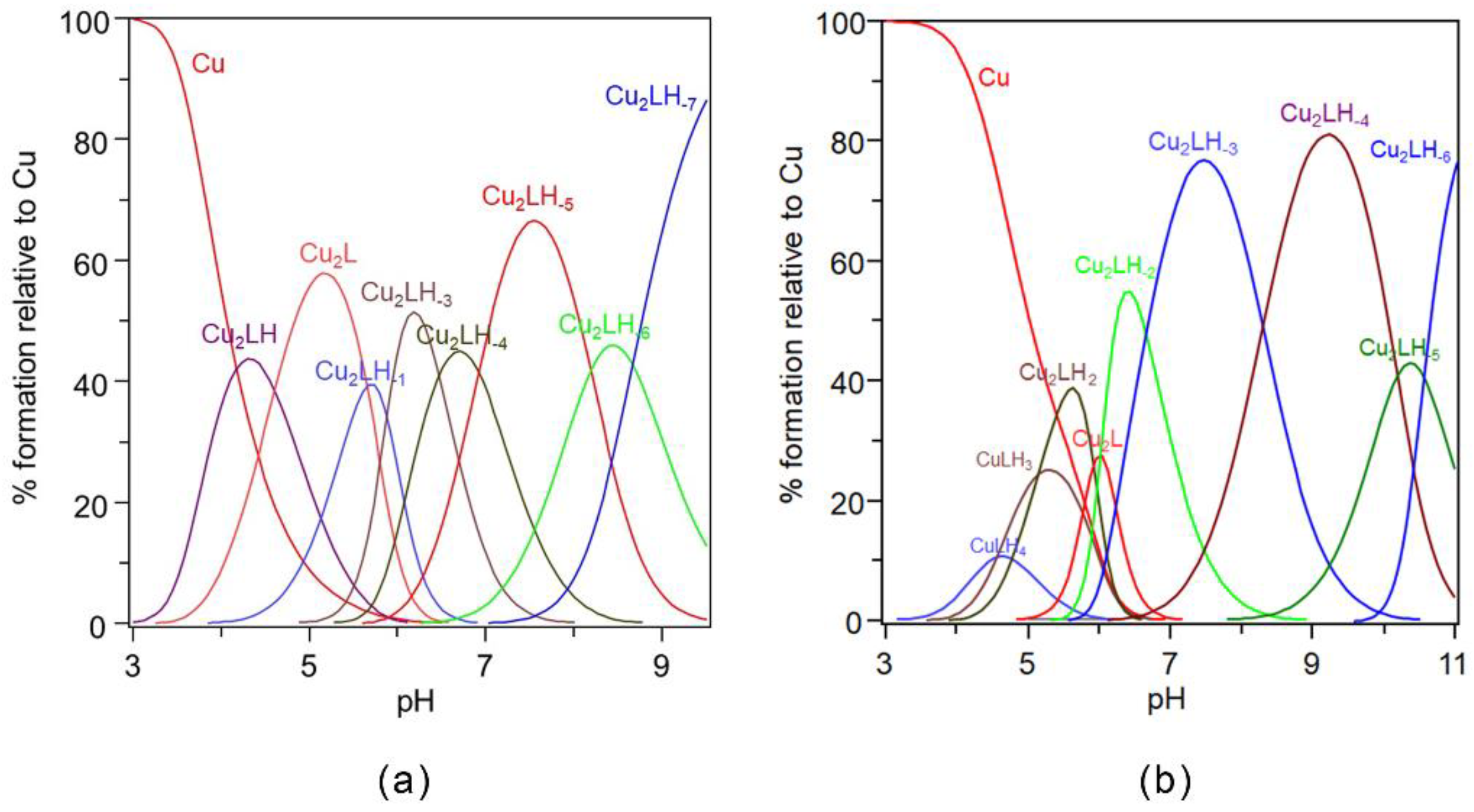

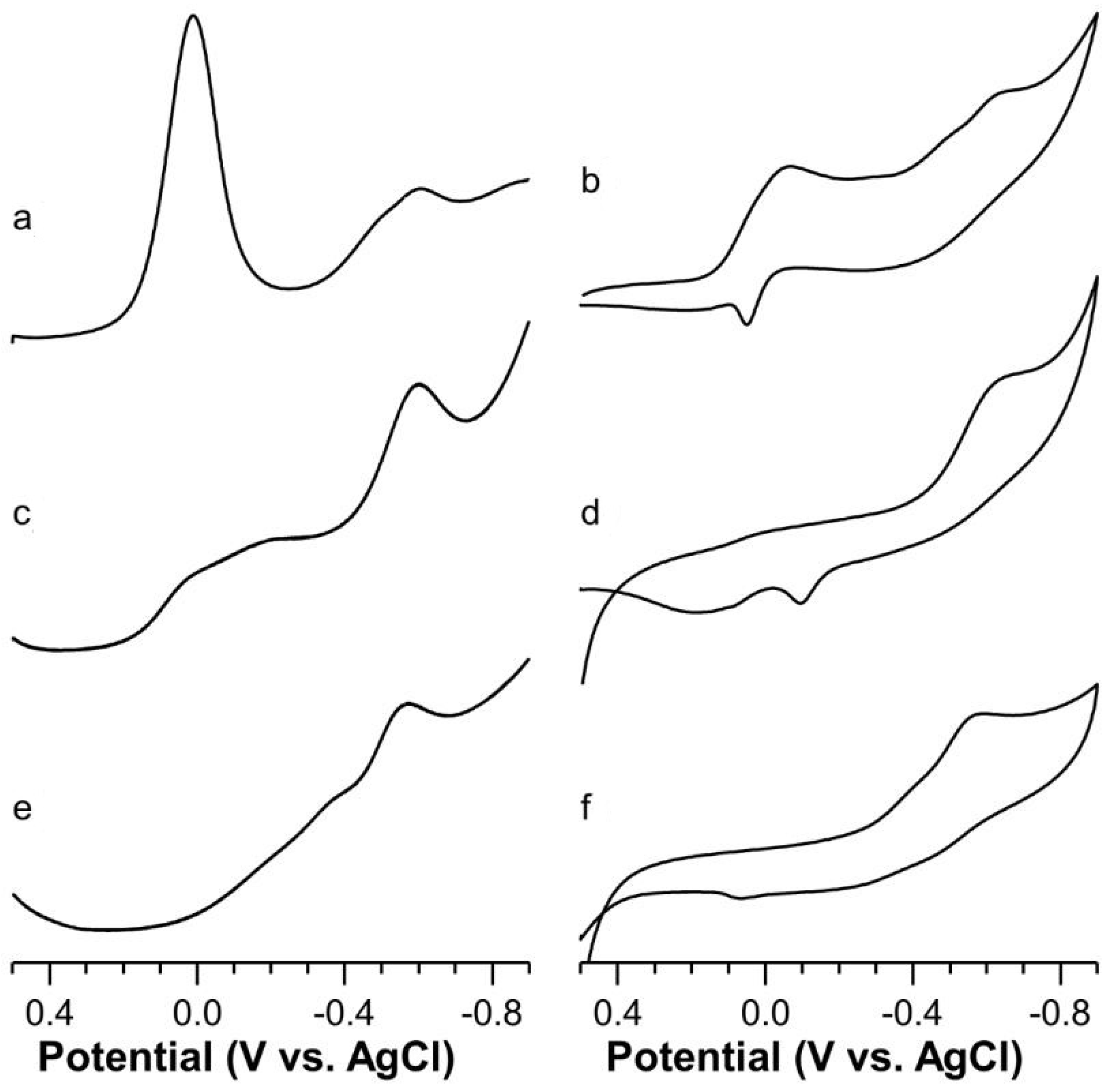
| Species [CupLqHr] | log β * | log K | UV (λmax, εmax) (nm, M−1 cm−1) | CD (λ, ∆ε) (nm, M−1 cm−1) |
|---|---|---|---|---|
| 211 | 17.28 (1) | - | - | - |
| 210 | 12.67 (2) | 4.60 | 650 (110) | 266 (+0.25), 299 (−1.15), 602 (+0.25) |
| 21-1 | 7.02 (2) | 5.65 | - | - |
| 21-3 | −4.74 (2) | 5.88 × 2 | 602 (227) | 260 (+7.43), 303 (−1.45), 342 (+0.45), 532 (+0.33), 625 (−0.33) |
| 21-4 | −11.21 (3) | 6.47 | - | - |
| 21-5 | −18.13 (3) | 6.92 | See text | 261 (+8.90), 301 (−0.73), 321 (+0.35), 350 (−0.35), 491 (−1.00), 669 (+0.78) |
| 21-6 | −26.33 (3) | 8.19 | - | - |
| 21-7 | −34.99 (3) | 8.66 | 532 (380) | 270 (+3.31), 302 (+0.97), 343 (−0.98), 514 (−0.93), 661 (+1.06) |
| Species [CupLqHr] | log β * | log K | UV (λmax, εmax) (nm, M−1 cm−1) | CD (λ, ∆ε) (nm, M−1 cm−1) |
|---|---|---|---|---|
| 114 | 40.38 (1) | - | - ** | - ** |
| 113 | 35.82 (1) | 4.56 | 650 (60) ** | 258 (2.52); 331 (0.30); 598 (−0.24) ** |
| 212 | 33.50 (2) | - | See text | 334 (+0.04); 528 (+0.03), |
| 21-0 | 21.95 (2) | (5.77 × 2) | - | - |
| 21-2 | 9.56 (3) | (6.19 × 2) | See text | 260 (+6.69); 326 (+0.47); 486 (−0.33); 546 (+0.28); 609 (−0.19); 681 (+0.30) |
| 21-3 | 2.94 (3) | 6.62 | 532 (250) | 262 (+7.30); 320 (+0.70), 360 (−0.27), 495 (−1.08), 560 (+0.32), 665 (+0.90) |
| 21-4 | −5.36 (6) | 8.30 | 528 (242) | 264 (+7.95); 347 (−1.08); 492 (−1.12); 650 (+1.35) |
| 21-5 | −15.55 (8) | 10.19 | - | - |
| 21-6 | −26.10 (5) | 10.65 | 528 (230) | 265 (+8.05); 347 (−0.66); 501 (−2.06); 646 (+1.30) |
| Cu(II)-Ang1-17 1:1 | ||||
| pH | g|| | A|| | Donor Set | E°f (SWV) |
| 3.9 | 2.424 | 124 | 6H2O | |
| 2.250 | 185 | NH2, N−, OCOO− | ||
| 2.295 | 176 | 2Nim, OCOO− | ||
| 2.335 | 146 | NH2, Nim, OCOO− | ||
| 4.5–5.3 | 2.295 | 176 | 2Nim, OCOO− | −0.515 |
| 2.250 | 185 | NH2, NIm, N−, OCOO− | −0.592 | |
| 5.3–8.2 | 2.224 | 186 | NH2, NIm, 2N− | −0.585 |
| 9.2 | 2.195 | 203 | Nim, 3N− | −0.550 |
| 10.5 | 2.195 | 201 | Nim, 3N− | −0.550 |
| Cu(II)-Ang1-17 2:1 | ||||
| 5.2–6.2 | 2.245 | 186 | NH2, N−, OCOO− | −0.590 |
| 2.295 | 176 | 2Nim, OCOO− | −0.515 | |
| 6.2–7.9 | 2.228 | 184 | NIm, 3N− | −0.534 |
| 2.208 | 187 | NH2, Nim, 2N− | −0.618 | |
| 8.5 | 2.209 | 194 | ||
| 9.2–11 | 2.196 | 205 | Nim, 3N−NH2, 3N− | −0.571 |
| Cu(II)-AcAng1-17 1:1 | ||||
| 3.8 | 2.424 | 124 | 6H2O | |
| 2.332 | 158 | Nim, OCOO− | −0.209 | |
| 2.295 | 176 | 2Nim, OCOO− | −0.515 | |
| 5.0 | 2.295 | 176 | 2Nim, OCOO− | |
| 6.0 | 2.266 | 180 | 2Nim, N− | −0.594 |
| 2.212 | 196 | 2Nim, 2N− | −0.662 | |
| 6.9–8.1 | 2.212 | 196 | 2Nim, 2N− | −0.656 |
| 9.3 | 2.206 | 200 | Nim, 3N− | −0.580 |
| 10.8 | 2.199 | 205 | Nim, 3N− | −0.615 |
| Cu(II)-AcAng1-17 2:1 | ||||
| 4.3–5.6 | 2.332 | 158 | Nim, OCOO− | |
| 2.290 | 170 | Nim, N−, OCOO− | ||
| 6.4 | 2.220 | 184 | Nim, 2N− | −0.565 |
| 2.200 | 190 | Nim, 3N− | −0.631 | |
| 7.6 | 2.205 | 190 | Nim, 3N− | −0.631 |
| 2.195 | 198 | Nim, 3N− | −0.582 | |
| 8.5–9.5 | 2.190 | 198 | Nim, 3N− | |
| 10.8 | 2.195 | 205 | Nim, 3N− | |
| 2.185 | 195 | Nim, 3N− | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabbì, G.; Cucci, L.M.; Pinzino, C.; Munzone, A.; Marzo, T.; Pizzanelli, S.; Satriano, C.; Magrì, A.; La Mendola, D. Peptides Derived from Angiogenin Regulate Cellular Copper Uptake. Int. J. Mol. Sci. 2021, 22, 9530. https://doi.org/10.3390/ijms22179530
Tabbì G, Cucci LM, Pinzino C, Munzone A, Marzo T, Pizzanelli S, Satriano C, Magrì A, La Mendola D. Peptides Derived from Angiogenin Regulate Cellular Copper Uptake. International Journal of Molecular Sciences. 2021; 22(17):9530. https://doi.org/10.3390/ijms22179530
Chicago/Turabian StyleTabbì, Giovanni, Lorena Maria Cucci, Calogero Pinzino, Alessia Munzone, Tiziano Marzo, Silvia Pizzanelli, Cristina Satriano, Antonio Magrì, and Diego La Mendola. 2021. "Peptides Derived from Angiogenin Regulate Cellular Copper Uptake" International Journal of Molecular Sciences 22, no. 17: 9530. https://doi.org/10.3390/ijms22179530
APA StyleTabbì, G., Cucci, L. M., Pinzino, C., Munzone, A., Marzo, T., Pizzanelli, S., Satriano, C., Magrì, A., & La Mendola, D. (2021). Peptides Derived from Angiogenin Regulate Cellular Copper Uptake. International Journal of Molecular Sciences, 22(17), 9530. https://doi.org/10.3390/ijms22179530








