Square-Planar vs. Trigonal Bipyramidal Geometry in Pt(II) Complexes Containing Triazole-Based Glucose Ligands as Potential Anticancer Agents
Abstract
:1. Introduction
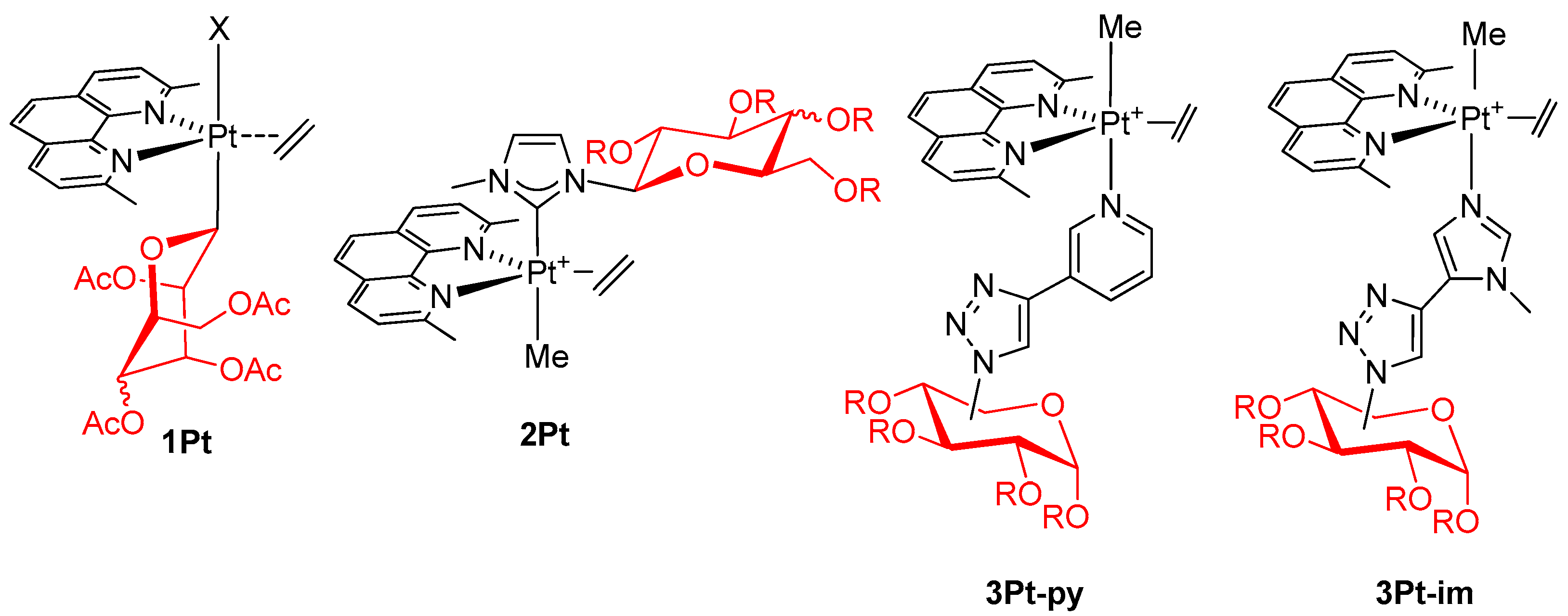
2. Results
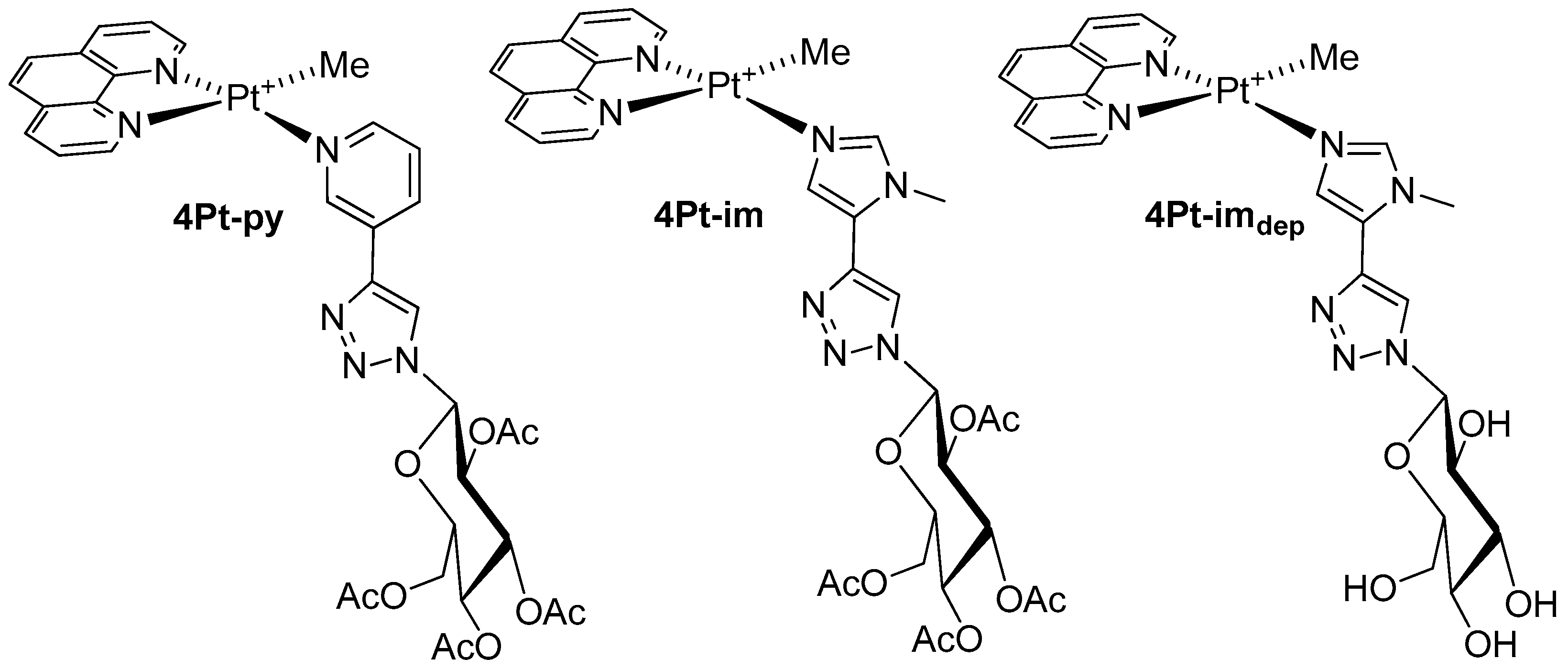
2.1. Synthesis and Characterization of Complexes of Type 4Pt
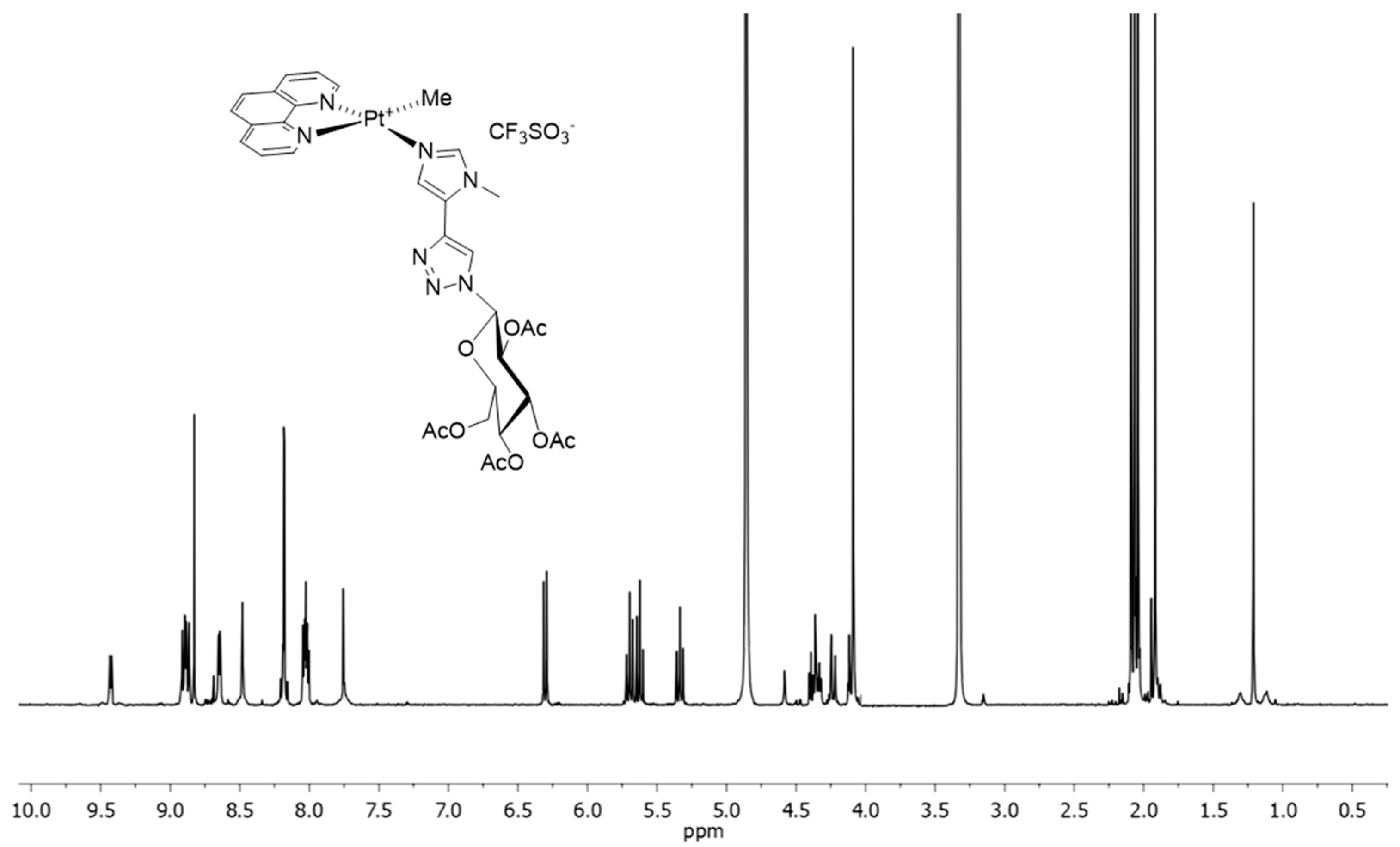
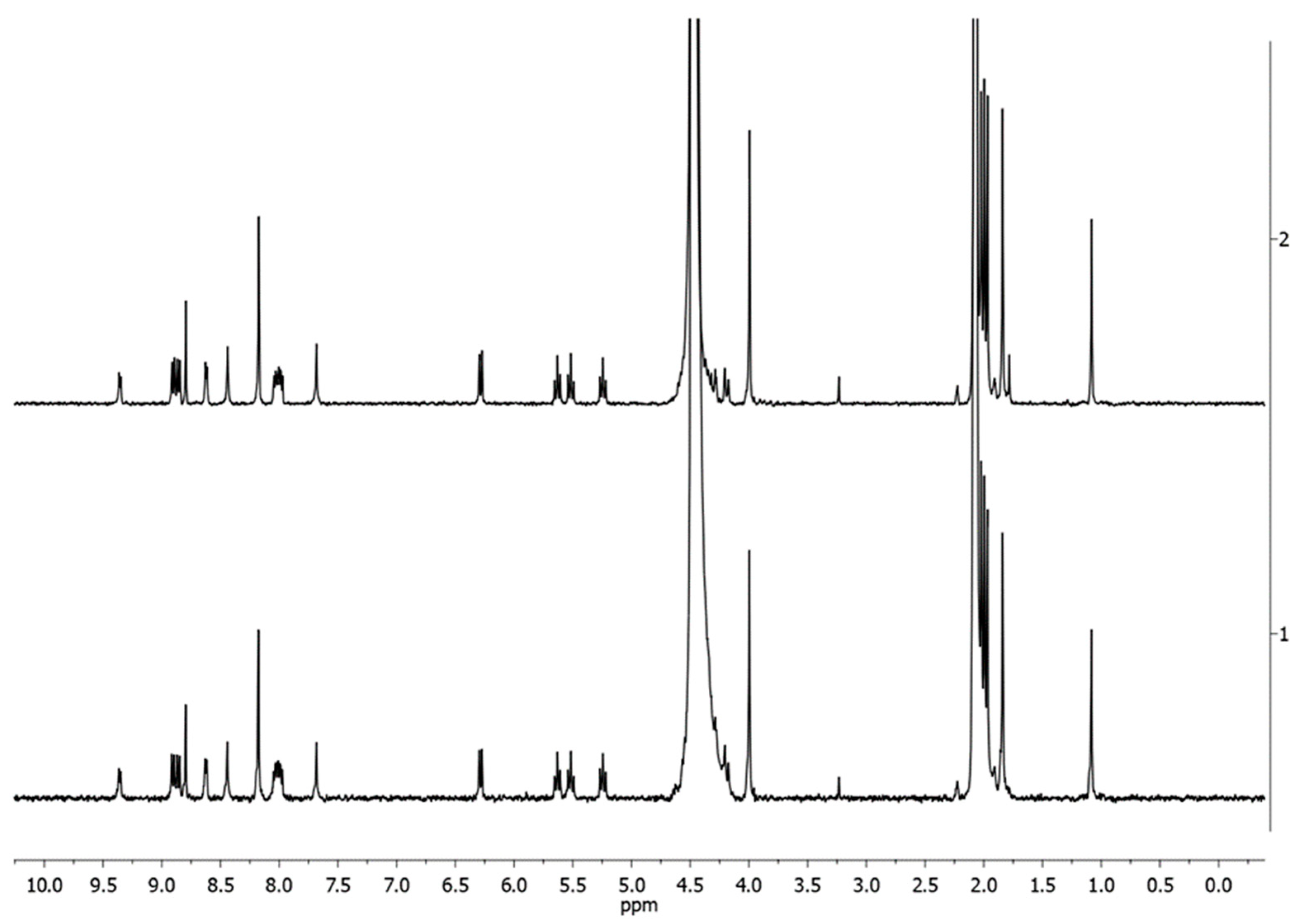
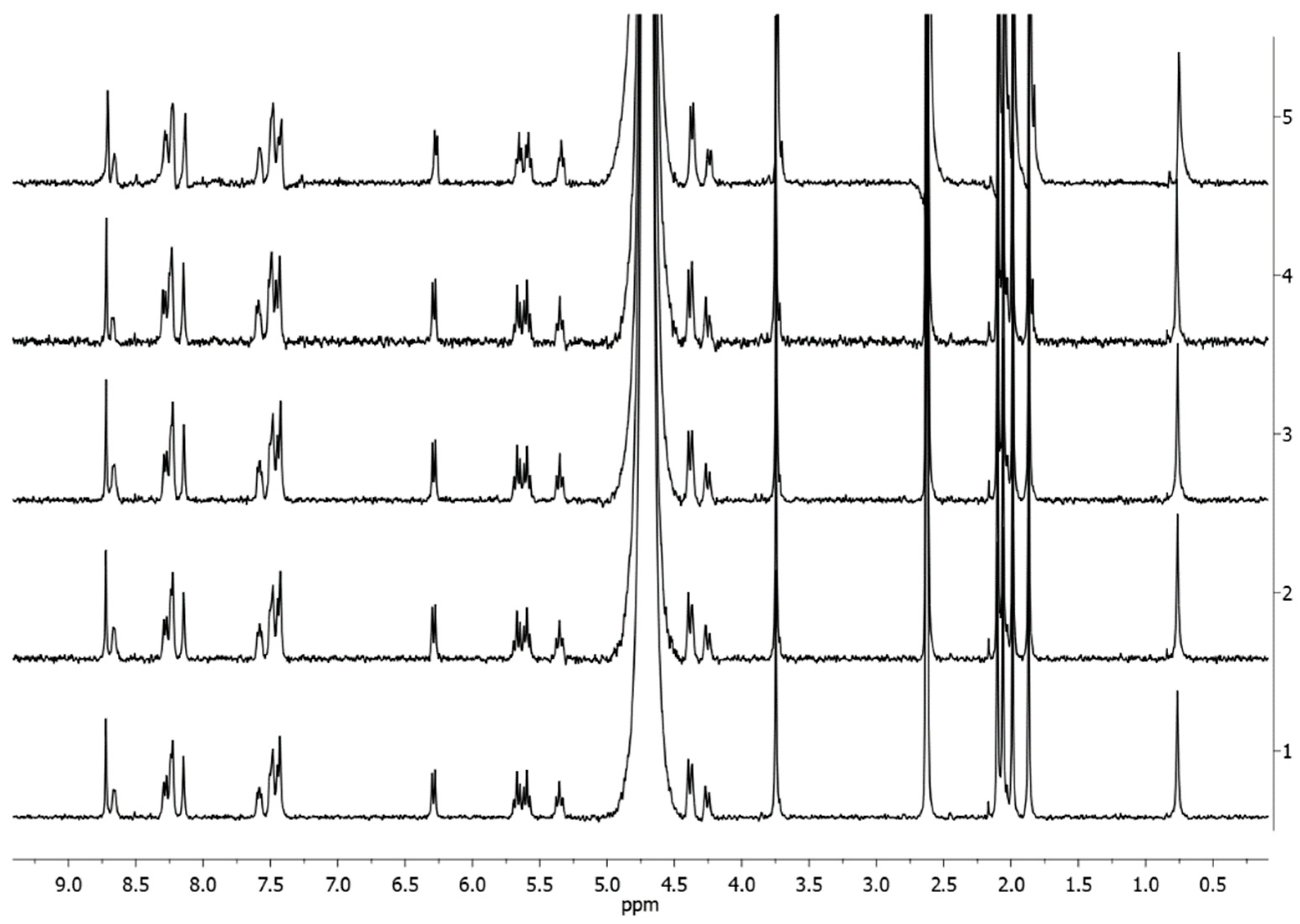
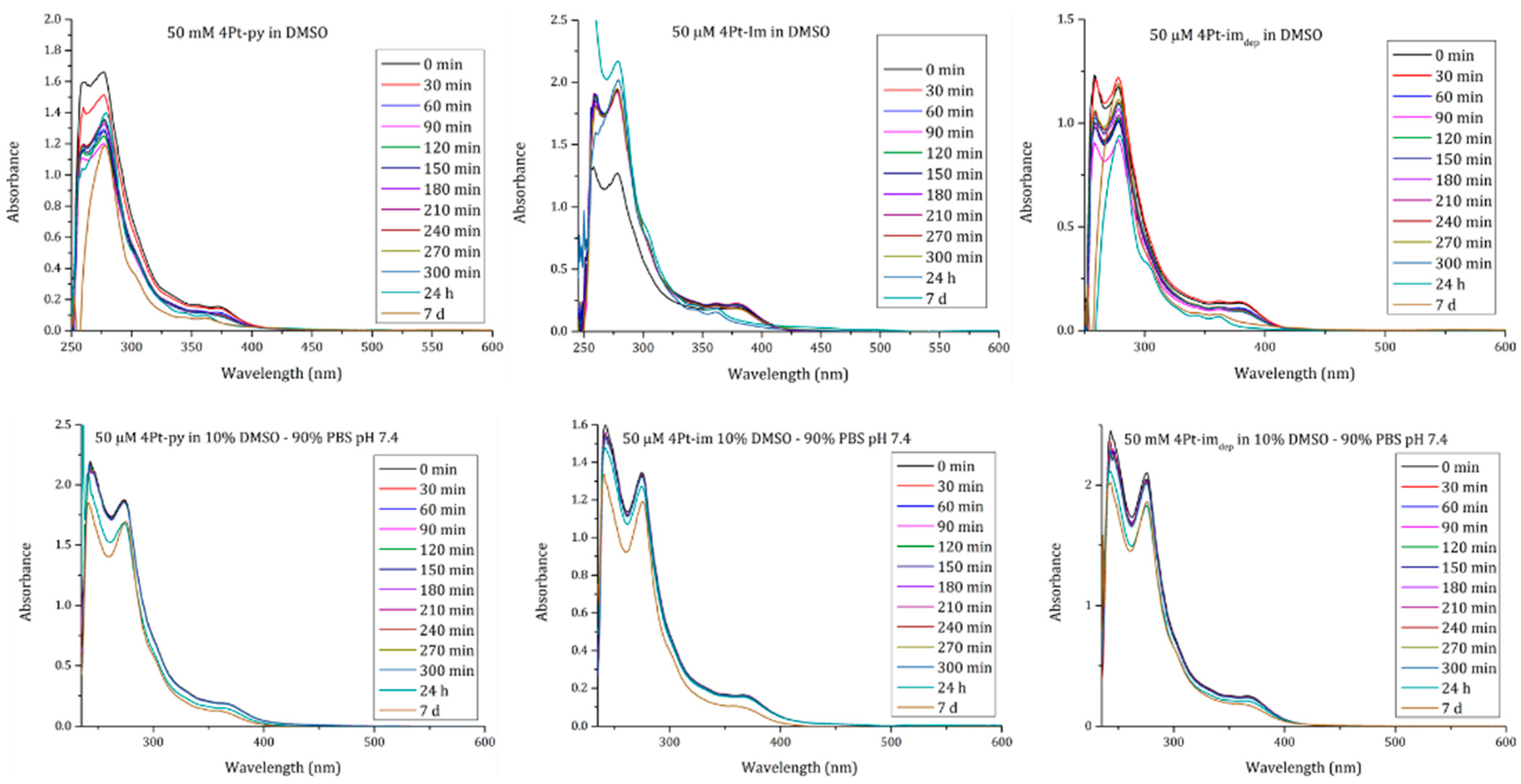
2.2. In-Solution Behaviour and Reactivity with Model Nucleophiles
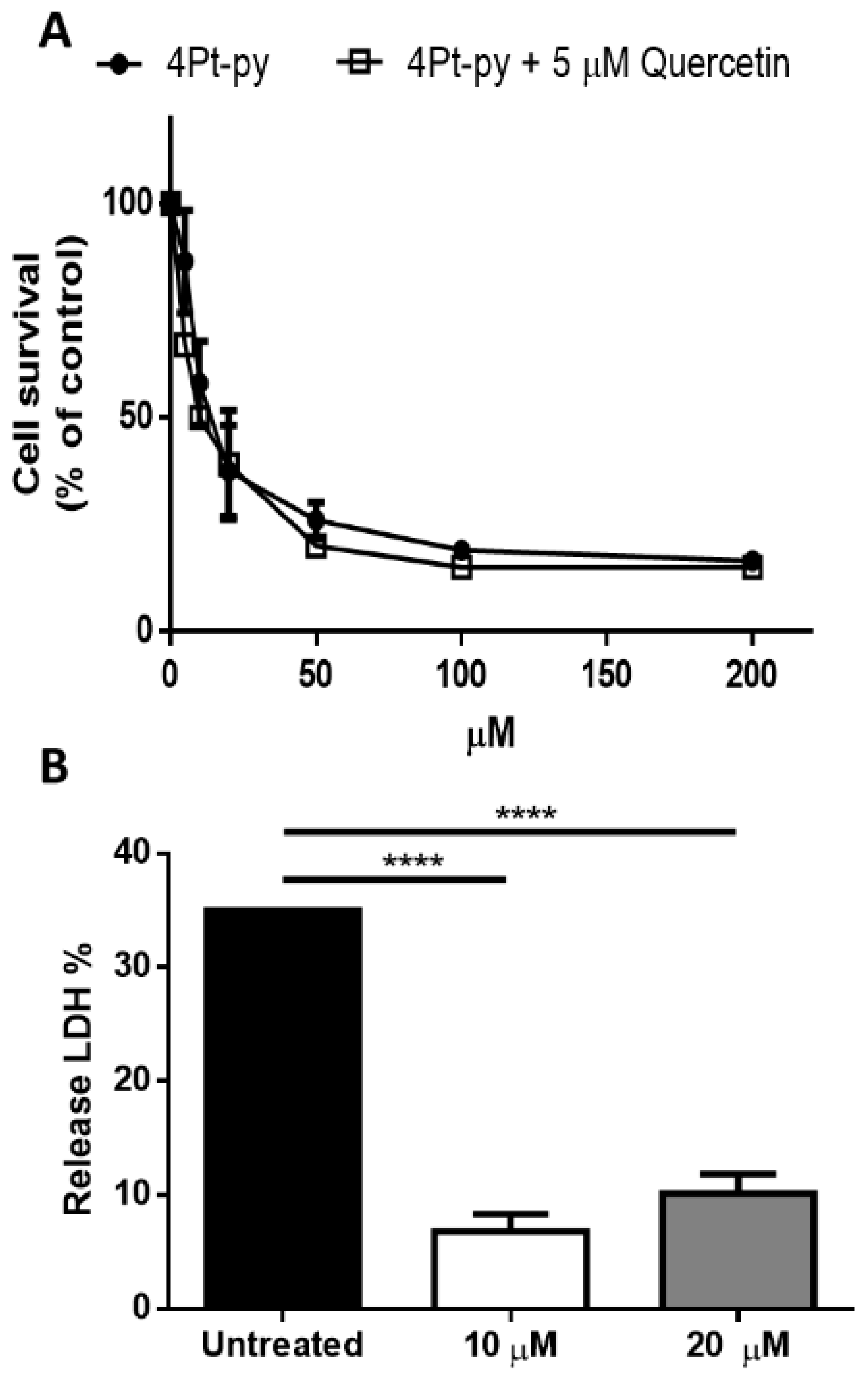
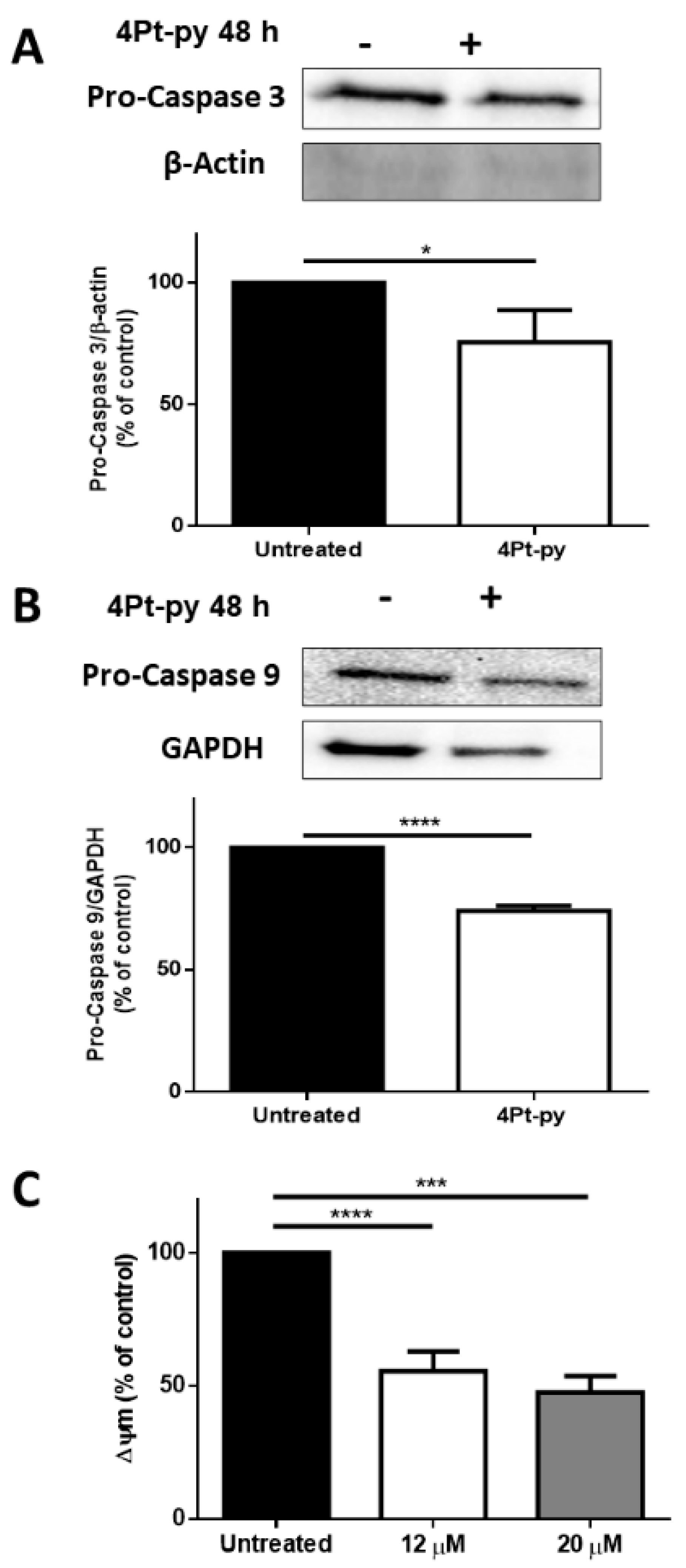
2.3. Cytotoxicity Studies
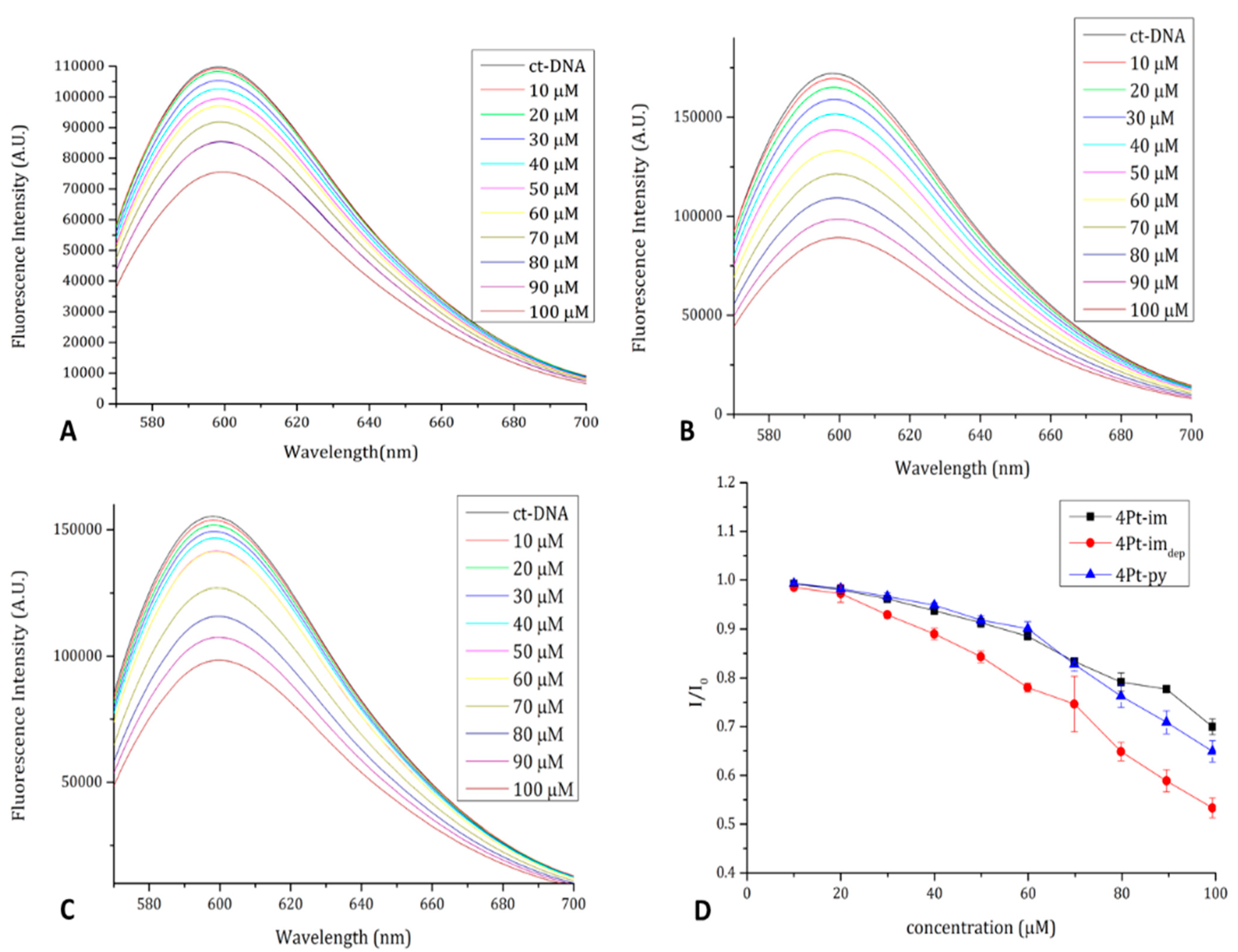
2.4. DNA Binding Properties
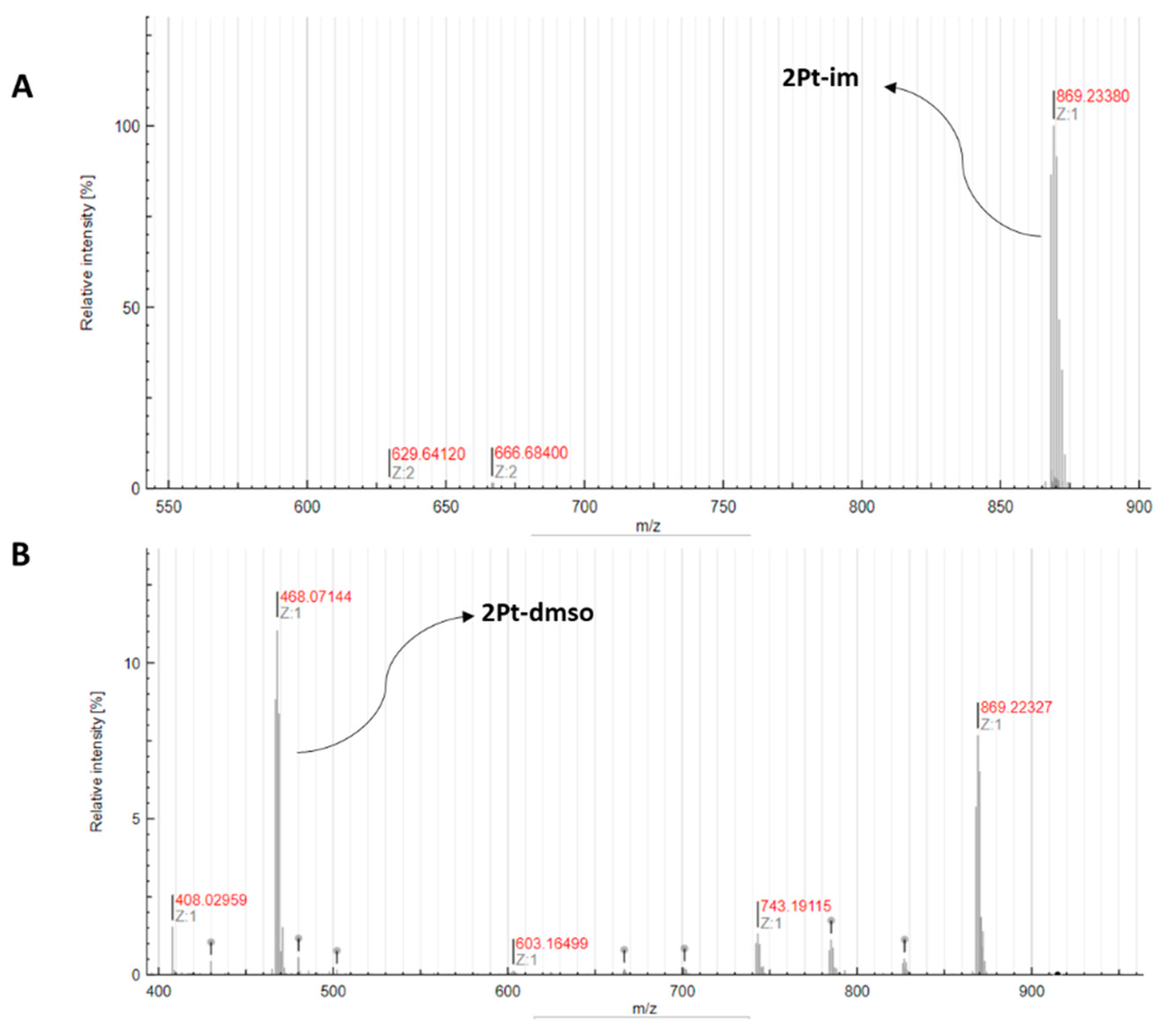
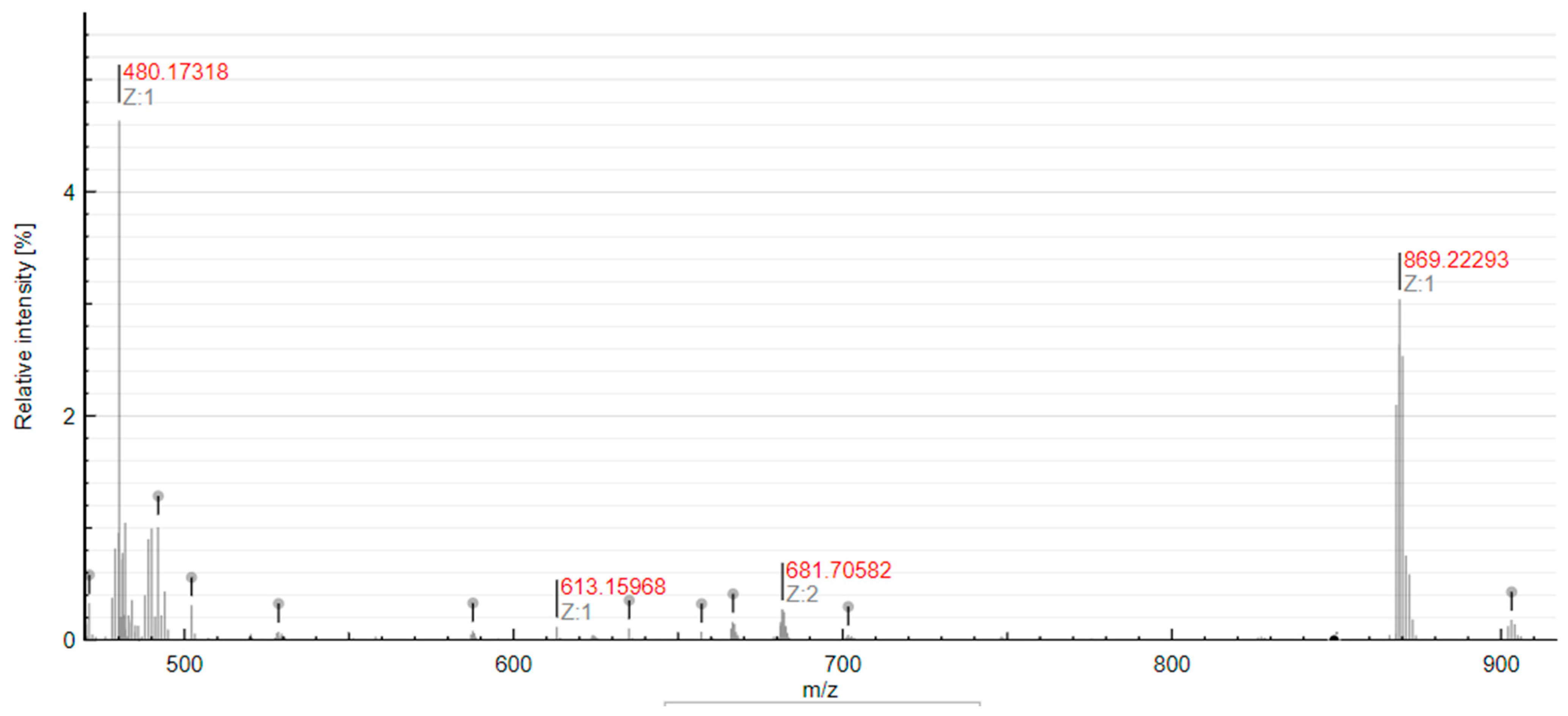
2.5. Reactivity with S-Donor Molecules
3. Materials and Methods
3.1. Synthesis of 4Pt-py and 4Pt-im
3.2. Synthesis of 4Pt-imdep
3.3. UV–Vis Absorption Spectroscopy
3.4. Cell Culture and the MTT Test
3.5. LDH Release
3.6. Western Blot Analyses
3.7. Analysis of Mitochondrial Membrane Potential
3.8. Ethidium Bromide Displacement Fluorescence Assay
3.9. 1H-NMR and ESI-MS In-Solution Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sadler, P.J. Advances in the Design of Organometallic Anticancer Complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Živković, M.D.; Kljun, J.; Ilic-Tomic, T.; Pavic, A.; Veselinović, A.; Manojlović, D.D.; Nikodinovic-Runic, J.; Turel, I. A New Class of Platinum(II) Complexes with the Phosphine Ligand Pta Which Show Potent Anticancer Activity. Inorg. Chem. Front. 2018, 5, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Qin, Q.-P.; Wang, Z.-F.; Huang, X.-L.; Tan, M.; Luo, Z.; Wang, S.; Zou, B.-Q.; Liang, H. Two Telomerase-Targeting Pt(II) Complexes of Jatrorrhizine and Berberine Derivatives Induce Apoptosis in Human Bladder Tumor Cells. Dalton Trans. 2019, 48, 15247–15254. [Google Scholar] [CrossRef]
- Dutta, P.K.; Sharma, R.; Kumari, S.; Dubey, R.D.; Sarkar, S.; Paulraj, J.; Vijaykumar, G.; Pandey, M.; Sravanti, L.; Samarla, M.; et al. A Safe and Efficacious Pt(II) Anticancer Prodrug: Design, Synthesis, in Vitro Efficacy, the Role of Carrier Ligands and in Vivo Tumour Growth Inhibition. Chem. Commun. 2019, 55, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, A.; Kundu, A.; Ghosh, S.; Suntharalingam, K. A Triangular Platinum(II) Multinuclear Complex with Cytotoxicity Towards Breast Cancer Stem Cells. Angew. Chem. Int. Ed. 2019, 58, 12059–12064. [Google Scholar] [CrossRef]
- Singh, K.; Gangrade, A.; Jana, A.; Mandal, B.B.; Das, N. Design, Synthesis, Characterization, and Antiproliferative Activity of Organoplatinum Compounds Bearing a 1,2,3-Triazole Ring. ACS Omega 2019, 4, 835–841. [Google Scholar] [CrossRef]
- Maji, M.; Karmakar, S.; Gupta, A.; Mukherjee, A. Oxamusplatin: A Cytotoxic Pt(II) Complex of a Nitrogen Mustard with Resistance to Thiol Based Sequestration Displays Enhanced Selectivity towards Cancer. Dalton Trans. 2020, 49, 2547–2558. [Google Scholar] [CrossRef]
- Tham, M.J.R.; Babak, M.V.; Ang, W.H. PlatinER: A Highly Potent Anticancer Platinum(II) Complex That Induces Endoplasmic Reticulum Stress Driven Immunogenic Cell Death. Angew. Chem. Int. Ed. 2020, 59, 19070–19078. [Google Scholar] [CrossRef]
- Imran, M.; Rehman, Z.; Hogarth, G.; Tocher, D.A.; Chaudhry, G.-S.; Butler, I.S.; Bélanger-Gariepy, F.; Kondratyuk, T. Two New Monofunctional platinum(ii) Dithiocarbamate Complexes: Phenanthriplatin-Type Axial Protection, Equatorial-Axial Conformational Isomerism, and Anticancer and DNA Binding Studies. Dalton Trans. 2020, 49, 15385–15396. [Google Scholar] [CrossRef]
- Adams, M.; Sullivan, M.P.; Tong, K.K.H.; Goldstone, D.C.; Hanif, M.; Jamieson, S.M.F.; Hartinger, C.G. Mustards-Derived Terpyridine–Platinum Complexes as Anticancer Agents: DNA Alkylation vs Coordination. Inorg. Chem. 2021, 60, 2414–2424. [Google Scholar] [CrossRef] [PubMed]
- Hyeraci, M.; Scalcon, V.; Folda, A.; Labella, L.; Marchetti, F.; Samaritani, S.; Rigobello, M.P.; Dalla Via, L. New Platinum(II) Complexes Affecting Different Biomolecular Targets in Resistant Ovarian Carcinoma Cells. Chem. Med. Chem. 2021, 16, 1956–1966. [Google Scholar] [CrossRef]
- Ding, S.; Hackett, C.L.; Liu, F.; Hackett, R.G.; Bierbach, U. Evaluation of a Platinum–Acridine Anticancer Agent and Its Liposomal Formulation in an in Vivo Model of Lung Adenocarcinoma. Chem. Med. Chem. 2021, 16, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenny, R.G.; Marmion, C.J. Toward Multi-Targeted Platinum and Ruthenium Drugs—A New Paradigm in Cancer Drug Treatment Regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Szablewski, L. Expression of Glucose Transporters in Cancers. Biochim. Biophys. Acta (BBA) Rev. Cancer 2013, 1835, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Pettenuzzo, A.; Pigot, R.; Ronconi, L. Metal-Based Glycoconjugates and Their Potential in Targeted Anticancer Chemotherapy. Metallodrugs 2016, 1, 36–61. [Google Scholar] [CrossRef] [Green Version]
- Hartinger, C.; Nazarov, A.; Ashraf, S.; Dyson, P.; Keppler, B. Carbohydrate-Metal Complexes and Their Potential as Anticancer Agents. Curr. Med. Chem. 2008, 15, 2574–2591. [Google Scholar] [CrossRef]
- Patra, M.; Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. A Potent Glucose-Platinum Conjugate Exploits Glucose Transporters and Preferentially Accumulates in Cancer Cells. Angew. Chem. Int. Ed. 2016, 55, 2550–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Gao, X.; Liu, R.; Yang, J.; Zhang, M.; Mi, Y.; Shi, Y.; Gao, Q. Design, Synthesis of Novel Platinum(II) Glycoconjugates, and Evaluation of Their Antitumor Effects. Chem. Biol. Drug Des. 2016, 87, 867–877. [Google Scholar] [CrossRef]
- Wu, M.; Li, H.; Liu, R.; Gao, X.; Zhang, M.; Liu, P.; Fu, Z.; Yang, J.; Zhang-Negrerie, D.; Gao, Q. Galactose Conjugated Platinum(II) Complex Targeting the Warburg Effect for Treatment of Non-Small Cell Lung Cancer and Colon Cancer. Eur. J. Med. Chem. 2016, 110, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, S.; Shi, Y.; Huang, Z.; Mi, Y.; Mi, Q.; Yang, J.; Gao, Q. Mechanistic and Biological Characteristics of Different Sugar Conjugated 2-Methyl Malonatoplatinum(II) Complexes as New Tumor Targeting Agents. Eur. J. Med. Chem. 2017, 125, 372–384. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Gao, X.; Mi, Q.; Zhao, H.; Gao, Q. Mannose-Conjugated Platinum Complexes Reveals Effective Tumor Targeting Mediated by Glucose Transporter 1. Biochem. Biophys. Res. Commun. 2017, 487, 34–40. [Google Scholar] [CrossRef]
- Hildebrandt, J.; Trautwein, R.; Kritsch, D.; Häfner, N.; Görls, H.; Dürst, M.; Runnebaum, I.B.; Weigand, W. Synthesis, Characterization and Biological Investigation of Platinum(II) Complexes with Asparagusic Acid Derivatives as Ligands. Dalton Trans. 2019, 48, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Bononi, G.; Iacopini, D.; Cicio, G.; Di Pietro, S.; Granchi, C.; Di Bussolo, V.; Minutolo, F. Glycoconjugated Metal Complexes as Cancer Diagnostic and Therapeutic Agents. Chem. Med. Chem. 2021, 16, 30–64. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; D’Amora, A.; De Feo, G.; Ferraro, G.; Giorgio, A.; Petruk, G.; Monti, D.M.; Merlino, A.; Ruffo, F. Five-Coordinate Platinum(II) Compounds Containing Sugar Ligands: Synthesis, Characterization, Cytotoxic Activity, and Interaction with Biological Macromolecules. Inorg. Chem. 2018, 57, 3133–3143. [Google Scholar] [CrossRef]
- Cucciolito, M.E.; De Luca Bossa, F.; Esposito, R.; Ferraro, G.; Iadonisi, A.; Petruk, G.; D’Elia, L.; Romanetti, C.; Traboni, S.; Tuzi, A.; et al. C -Glycosylation in Platinum-Based Agents: A Viable Strategy to Improve Cytotoxicity and Selectivity. Inorg. Chem. Front. 2018, 5, 2921–2933. [Google Scholar] [CrossRef]
- Annunziata, A.; Cucciolito, M.E.; Esposito, R.; Imbimbo, P.; Petruk, G.; Ferraro, G.; Pinto, V.; Tuzi, A.; Monti, D.M.; Merlino, A.; et al. A Highly Efficient and Selective Antitumor Agent Based on a Glucoconjugated Carbene Platinum(II) Complex. Dalton Trans. 2019, 48, 7794–7800. [Google Scholar] [CrossRef]
- Annunziata, A.; Amoresano, A.; Cucciolito, M.E.; Esposito, R.; Ferraro, G.; Iacobucci, I.; Imbimbo, P.; Lucignano, R.; Melchiorre, M.; Monti, M.; et al. Pt(II) versus Pt(IV) in Carbene Glycoconjugate Antitumor Agents: Minimal Structural Variations and Great Performance Changes. Inorg. Chem. 2020, 59, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, A.; Cucciolito, M.E.; Imbimbo, P.; Silipo, A.; Ruffo, F. A Hydrophilic Olefin Pt(0) Complex Containing a Glucoconjugated 2-Iminopyridine Ligand: Synthesis, Characterization, Stereochemistry and Biological Activity. Inorg. Chim. Acta 2021, 516, 120092. [Google Scholar] [CrossRef]
- Annunziata, A.; Cucciolito, M.E.; Esposito, R.; Ferraro, G.; Monti, D.M.; Merlino, A.; Ruffo, F. Five-coordinate Pt(II) Compounds as Potential Anticancer Agents. Eur. J. Inorg. Chem. 2020, 11-12, 918–929. [Google Scholar] [CrossRef]
- Wang, F.-Y.; Liu, R.; Huang, K.-B.; Feng, H.-W.; Liu, Y.-N.; Liang, H. New Platinum(II)-Based DNA Intercalator: Synthesis, Characterization and Anticancer Activity. Inorg. Chem. Commun. 2019, 105, 182–187. [Google Scholar] [CrossRef]
- Pages, B.J.; Garbutcheon-Singh, K.B.; Aldrich-Wright, J.R. Platinum Intercalators of DNA as Anticancer Agents: Platinum Intercalators of DNA as Anticancer Agents. Eur. J. Inorg. Chem. 2017, 2017, 1613–1624. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Yao, X.; Watkins, N.H.; Rose, P.K.; Caruso, S.R.; Day, C.S.; Bierbach, U. Discovery of a Chiral DNA-Targeted Platinum–Acridine Agent with Potent Enantioselective Anticancer Activity. Angew. Chem. Int. Ed. 2020, 59, 21965–21970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yam, V.W.-W. Platinum(II) Non-Covalent Crosslinkers for Supramolecular DNA Hydrogels. Chem. Sci. 2020, 11, 3241–3249. [Google Scholar] [CrossRef] [Green Version]
- De Pascali, S.A.; Migoni, D.; Papadia, P.; Muscella, A.; Marsigliante, S.; Ciccarese, A.; Fanizzi, F.P. New Water-Soluble Platinum(II) Phenanthroline Complexes Tested as Cisplatin Analogues: First-Time Comparison of Cytotoxic Activity between Analogous Four- and Five-Coordinate Species. Dalton Trans. 2006, 42, 5077–5087. [Google Scholar] [CrossRef]
- Calvanese, L.; Cucciolito, M.E.; D’Amora, A.; D’Auria, G.; Esposito, A.; Esposito, R.; Falcigno, L.; Ruffo, F. Recognition of Prochiral Sulfides in Five-Coordinate PtII Complexes. Eur. J. Inorg. Chem. 2015, 2015, 4068–4075. [Google Scholar] [CrossRef]
- Esposito, R.; Calvanese, L.; Cucciolito, M.E.; D’Auria, G.; Falcigno, L.; Fiorini, V.; Pezzella, P.; Roviello, G.; Stagni, S.; Talarico, G.; et al. Oxidative Coupling of Imino, Amide Platinum(II) Complexes Yields Highly Conjugated Blue Dimers. Organometallics 2017, 36, 384–390. [Google Scholar] [CrossRef]
- Vera, J.C.; Reyes, A.M.; Velásquez, F.V.; Rivas, C.I.; Zhang, R.H.; Strobel, P.; Slebe, J.C.; Núñez-Alarcón, J.; Golde, D.W. Direct Inhibition of the Hexose Transporter GLUT1 by Tyrosine Kinase Inhibitors. Biochemistry 2001, 40, 777–790. [Google Scholar] [CrossRef]
- Guo, Y.; He, Y.; Wu, S.; Zhang, S.; Song, D.; Zhu, Z.; Guo, Z.; Wang, X. Enhancing Cytotoxicity of a Monofunctional Platinum Complex via a Dual-DNA-Damage Approach. Inorg. Chem. 2019, 58, 13150–13160. [Google Scholar] [CrossRef]
- Dabbish, E.; Russo, N.; Sicilia, E. Rationalization of the Superior Anticancer Activity of Phenanthriplatin: An In-Depth Computational Exploration. Chem. Eur. J. 2020, 26, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Suryadi, J.; Bierbach, U. Cellular Recognition and Repair of Monofunctional–Intercalative Platinum–DNA Adducts. Chem. Res. Toxicol. 2015, 28, 2170–2178. [Google Scholar] [CrossRef] [Green Version]
- Aseman, M.D.; Aryamanesh, S.; Shojaeifard, Z.; Hemmateenejad, B.; Nabavizadeh, S.M. Cycloplatinated(II) Derivatives of Mercaptopurine Capable of Binding Interactions with HSA/DNA. Inorg. Chem. 2019, 58, 16154–16170. [Google Scholar] [CrossRef]
- Li, C.; Xu, F.; Zhao, Y.; Zheng, W.; Zeng, W.; Luo, Q.; Wang, Z.; Wu, K.; Du, J.; Wang, F. Platinum(II) Terpyridine Anticancer Complexes Possessing Multiple Mode of DNA Interaction and EGFR Inhibiting Activity. Front. Chem. 2020, 8, 210. [Google Scholar] [CrossRef]
- Lozada, I.B.; Huang, B.; Stilgenbauer, M.; Beach, T.; Qiu, Z.; Zheng, Y.; Herbert, D.E. Monofunctional Platinum(II) Anticancer Complexes Based on Multidentate Phenanthridine-Containing Ligand Frameworks. Dalton Trans. 2020, 49, 6557–6560. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.; Tachibana, C.; Rahr Winther, J.; Appenzeller-Herzog, C. Intracellular Glutathione Pools Are Heterogeneously Concentrated. Redox Biol. 2013, 1, 508–513. [Google Scholar] [CrossRef] [Green Version]
- Oehlsen, M.E.; Qu, Y.; Farrell, N. Reaction of Polynuclear Platinum Antitumor Compounds with Reduced Glutathione Studied by Multinuclear (1H, 1H−15N Gradient Heteronuclear Single-Quantum Coherence, and 195Pt) NMR Spectroscopy. Inorg. Chem. 2003, 42, 5498–5506. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Wheate, N.J.; Pisani, M.J.; Aldrich-Wright, J.R. Degradation of Bidentate-Coordinated Platinum(II)-Based DNA Intercalators by Reduced l-Glutathione. J. Med. Chem. 2008, 51, 2787–2794. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.; Gasilova, N.; Sepulveda, F.; Patiny, L.; Dyson, P.J.; Menin, L. Aom 2S: A New Web-based Application for DNA/RNA Tandem Mass Spectrometry Data Interpretation. Rapid Commun. Mass Spectrom. 2020, 34, e8927. [Google Scholar] [CrossRef] [PubMed]
- Sucha, L.; Hroch, M.; Rezacova, M.; Rudolf, E.; Havelek, R.; Sispera, L.; Cmielova, J.; Kohlerova, R.; Bezrouk, A.; Tomsik, P. The cytotoxic effect of α-tomatine in MCF-7 human adenocarcinoma breast cancer cells depends on its interaction with cholesterol in incubation media and does not involve apoptosis induction. Oncol. Rep. 2013, 30, 2593–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| A. MTT IC50 48 h (µM) | ||||
| Cell Line | HaCaT | A431 | H9c2 | MCF7 |
| 4Pt-py | 66 ± 11 | 12 ± 2 | 22 ± 10 | 35 ± 4 |
| 4Pt-im | 27 ± 7 | 16 ± 8 | 53 ± 11 | 43 ± 7 |
| 4Pt-imdep | >200 | >200 | >200 | >200 |
| 3Pt-py29 | 0.80 ± 0.14 | 1.10 ± 0.14 | 0.35 ± 0.07 | 0.88 ± 0.11 |
| 3Pt-im29 | 1.03 ± 0.25 | 1.10 ± 0.01 | 0.38 ± 0.04 | 0.63 ± 0.11 |
| 3Pt-imdep29 | 0.58 ± 0.04 | 1.00 ± 0.14 | 0.35 ± 0.07 | 0.60 ± 0.09 |
| Cisplatin | 6.6 ± 0.331 | 39 ± 1231 | 8 ± 2.1 | 18 ± 1.6 |
| B. | ||||
| Cell Line | HaCaT/A431 | HaCaT/MCF7 | H9c2/ A431 | H9c2/ MCF7 |
| 4Pt-py | 5.5 | 1.88 | 1.83 | 0.63 |
| 4Pt-im | 1.69 | 0.63 | 3.3 | 1.23 |
| 4Pt-imdep | N.A. | N.A. | N.A. | N.A. |
| 3Pt-py29 | 0.72 | 0.89 | 0.32 | 0.39 |
| 3Pt-im29 | 0.91 | 1.59 | 0.34 | 0.6 |
| 3Pt-imdep29 | 0.58 | 0.96 | 0.35 | 0.58 |
| Cisplatin | 0.17 | 0.82 | 0.2 | 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annunziata, A.; Liberti, D.; Bedini, E.; Cucciolito, M.E.; Loreto, D.; Monti, D.M.; Merlino, A.; Ruffo, F. Square-Planar vs. Trigonal Bipyramidal Geometry in Pt(II) Complexes Containing Triazole-Based Glucose Ligands as Potential Anticancer Agents. Int. J. Mol. Sci. 2021, 22, 8704. https://doi.org/10.3390/ijms22168704
Annunziata A, Liberti D, Bedini E, Cucciolito ME, Loreto D, Monti DM, Merlino A, Ruffo F. Square-Planar vs. Trigonal Bipyramidal Geometry in Pt(II) Complexes Containing Triazole-Based Glucose Ligands as Potential Anticancer Agents. International Journal of Molecular Sciences. 2021; 22(16):8704. https://doi.org/10.3390/ijms22168704
Chicago/Turabian StyleAnnunziata, Alfonso, Davide Liberti, Emiliano Bedini, Maria Elena Cucciolito, Domenico Loreto, Daria Maria Monti, Antonello Merlino, and Francesco Ruffo. 2021. "Square-Planar vs. Trigonal Bipyramidal Geometry in Pt(II) Complexes Containing Triazole-Based Glucose Ligands as Potential Anticancer Agents" International Journal of Molecular Sciences 22, no. 16: 8704. https://doi.org/10.3390/ijms22168704
APA StyleAnnunziata, A., Liberti, D., Bedini, E., Cucciolito, M. E., Loreto, D., Monti, D. M., Merlino, A., & Ruffo, F. (2021). Square-Planar vs. Trigonal Bipyramidal Geometry in Pt(II) Complexes Containing Triazole-Based Glucose Ligands as Potential Anticancer Agents. International Journal of Molecular Sciences, 22(16), 8704. https://doi.org/10.3390/ijms22168704










