AMPK Phosphorylation Is Controlled by Glucose Transport Rate in a PKA-Independent Manner
Abstract
1. Introduction
2. Results
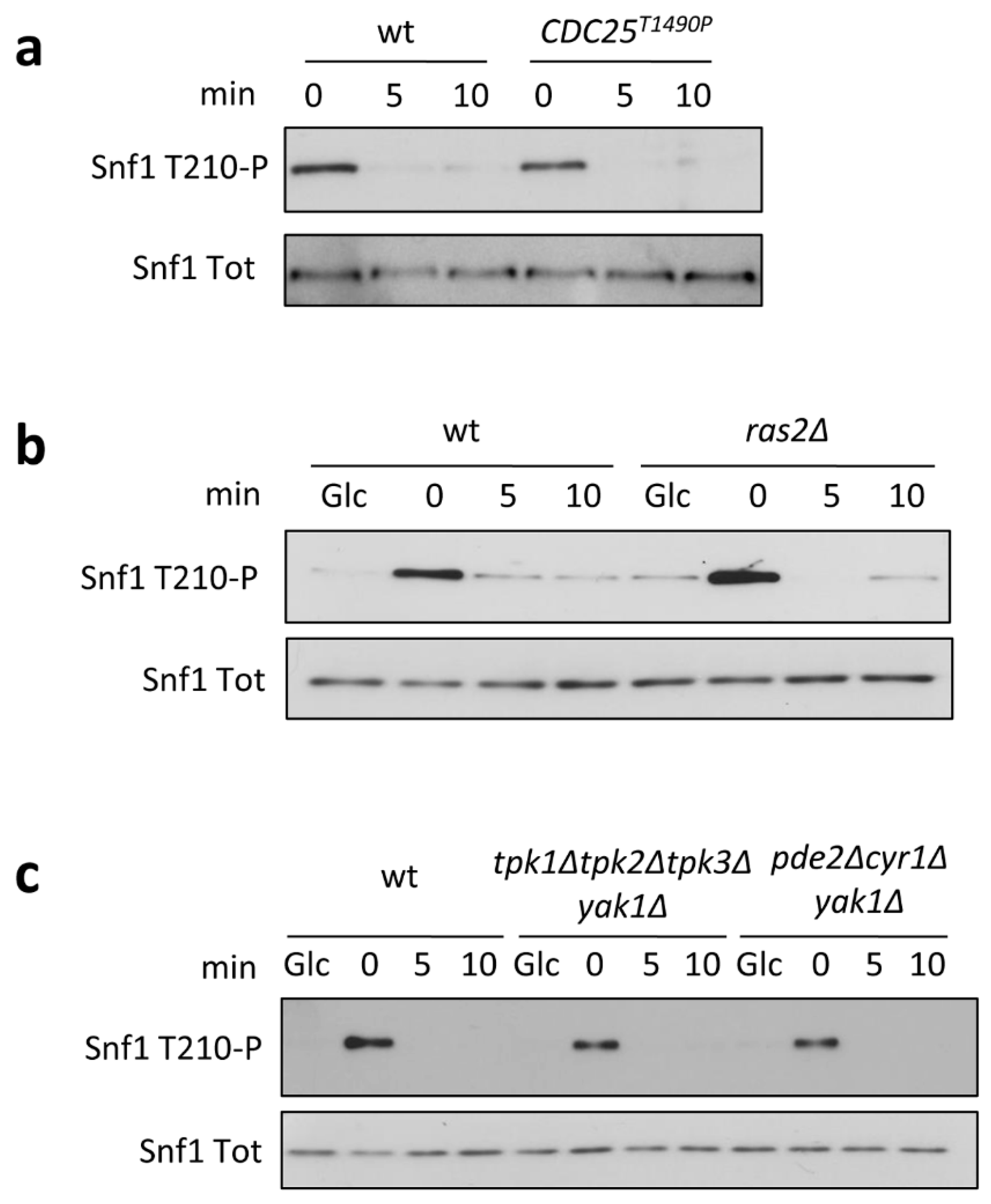
2.1. Glucose Inhibition of Snf1/AMPK Is Independent from the Ras/PKA Pathway
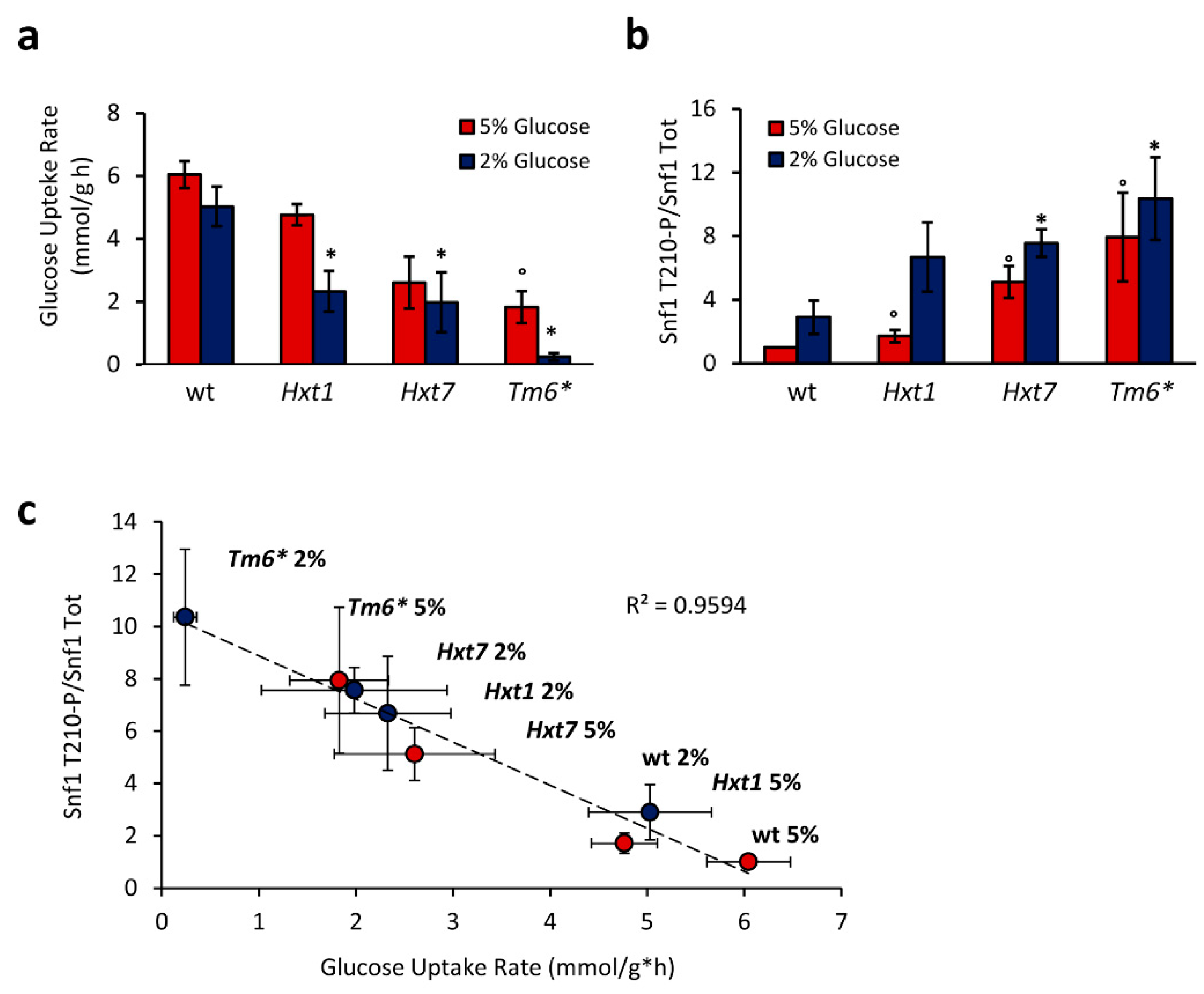
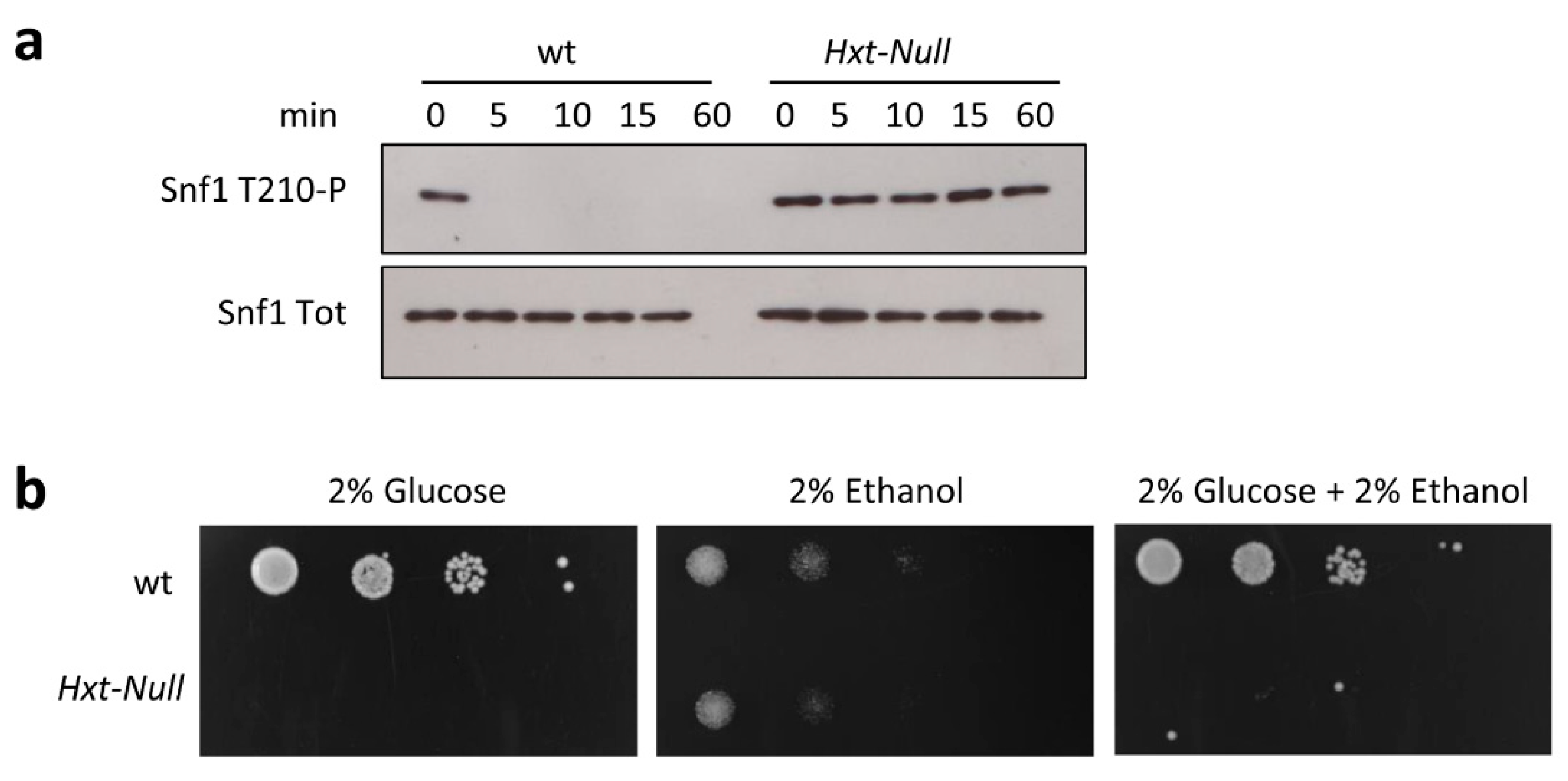
2.2. Snf1/AMPK Phosphorylation Is Correlated with Glucose Transport Rate
2.3. G6P, and Not F1,6BP, Influences Snf1/AMPK Phosphorylation
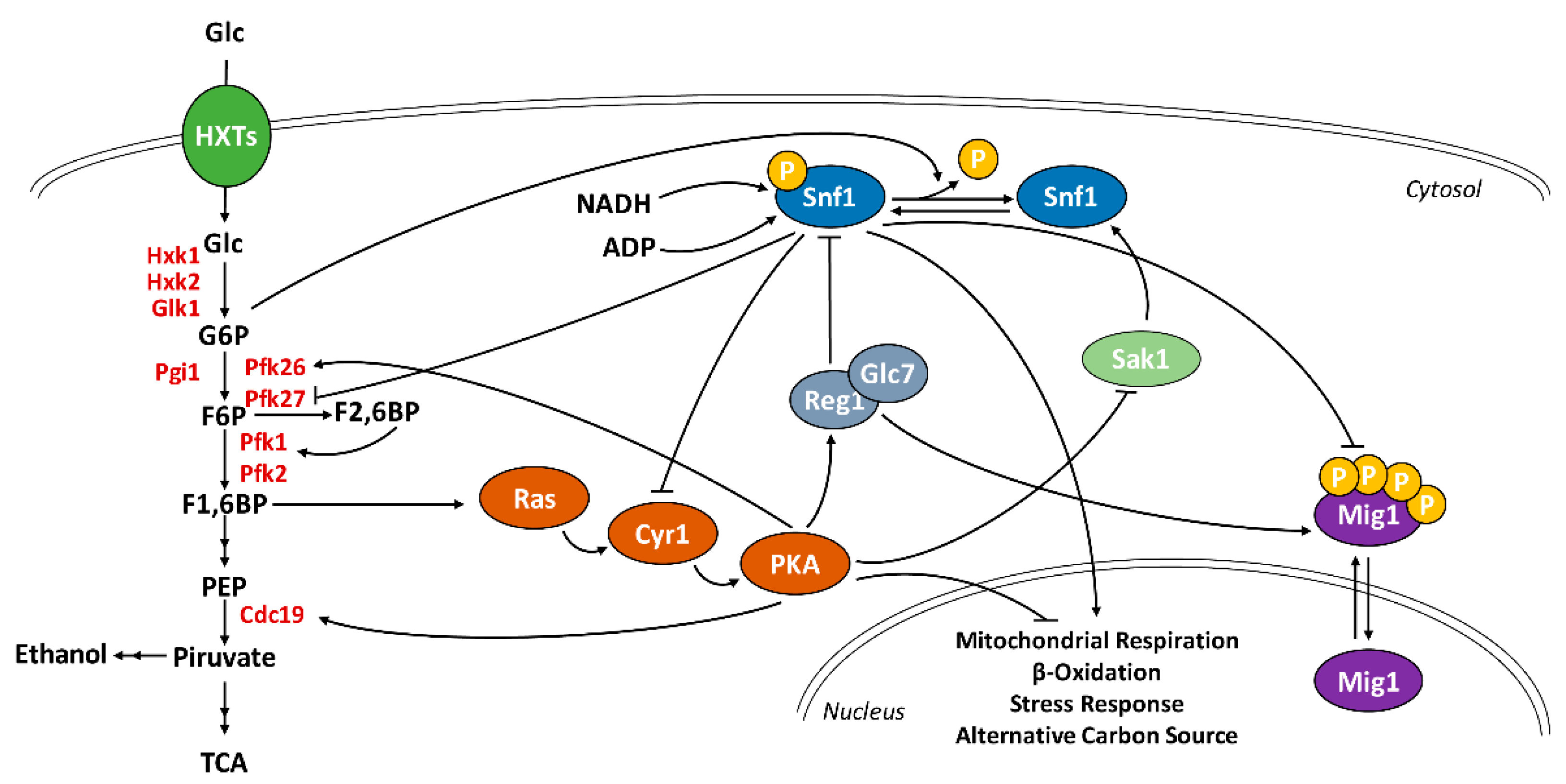
3. Discussion
4. Materials and Methods
4.1. The Yeast Strains and Growth Conditions
4.2. Protein Extraction, Immunoblotting, and Densitometric Analysis
4.3. Glucose Consumption Rate
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orth, J.D.; Thiele, I.; Palsson, B. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- Kitano, H. Foundations of Systems Biology; MIT Press: Cambridge, MA, USA, 2001; ISBN 9780262112666. [Google Scholar]
- Nielsen, J. Yeast Systems Biology: Model Organism and Cell Factory. Biotechnol. J. 2019, 14, e1800421. [Google Scholar] [CrossRef] [PubMed]
- Busti, S.; Coccetti, P.; Alberghina, L.; Vanoni, M. Glucose Signaling-Mediated Coordination of Cell Growth and Cell Cycle in Saccharomyces cerevisiae. Sensors 2010, 10, 6195–6240. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Schothorst, J.; Kankipati, H.N.; Van Zeebroeck, G.; Rubio-Texeira, M.; Thevelein, J.M. Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2014, 38, 254–299. [Google Scholar] [CrossRef]
- Coccetti, P.; Nicastro, R.; Tripodi, F. Conventional and emerging roles of the energy sensor Snf1/AMPK in Saccharomyces cerevisiae. Microb. Cell 2018, 5, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, R.; Tripodi, F.; Gaggini, M.; Castoldi, A.; Reghellin, V.; Nonnis, S.; Tedeschi, G.; Coccetti, P. Snf1 Phosphorylates Adenylate Cyclase and Negatively Regulates Protein Kinase A-dependent Transcription in Saccharomyces cerevisiae. J. Biol. Chem. 2015, 290, 24715–24726. [Google Scholar] [CrossRef]
- Barrett, L.; Orlova, M.; Maziarz, M.; Kuchin, S. Protein Kinase a Contributes to the Negative Control of Snf1 Protein Kinase in Saccharomyces cerevisiae. Eukaryot. Cell 2011, 11, 119–128. [Google Scholar] [CrossRef][Green Version]
- Welkenhuysen, N.; Schnitzer, B.; Österberg, L.; Cvijovic, M. Robustness of Nutrient Signaling Is Maintained by Interconnectivity Between Signal Transduction Pathways. Front. Physiol. 2019, 9, 1964. [Google Scholar] [CrossRef] [PubMed]
- Huberts, D.H.E.W.; Niebel, B.; Heinemann, M. A flux-sensing mechanism could regulate the switch between respiration and fermentation. FEMS Yeast Res. 2011, 12, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Bozaquel-Morais, B.L.; Madeira, J.B.; Maya-Monteiro, C.M.; Masuda, C.A.; Montero-Lomelí, M. A New Fluorescence-Based Method Identifies Protein Phosphatases Regulating Lipid Droplet Metabolism. PLoS ONE 2010, 5, e13692. [Google Scholar] [CrossRef] [PubMed]
- Milanesi, R.; Coccetti, P.; Tripodi, F. The Regulatory Role of Key Metabolites in the Control of Cell Signaling. Biomolecules 2020, 10, 862. [Google Scholar] [CrossRef]
- Peeters, K.; Van Leemputte, F.; Fischer, B.; Bonini, B.M.; Quezada, H.; Tsytlonok, M.; Haesen, D.; Vanthienen, W.; Bernardes, N.; Gonzalez-Blas, C.B.; et al. Fructose-1,6-bisphosphate couples glycolytic flux to activation of Ras. Nat. Commun. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-S.; Hawley, S.A.; Zong, Y.; Li, M.; Wang, Z.; Gray, A.; Ma, T.; Cui, J.; Feng, J.-W.; Zhu, M.; et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 2017, 548, 112–116. [Google Scholar] [CrossRef]
- Hong, S.-P.; Leiper, F.; Woods, A.; Carling, D.; Carlson, M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc. Natl. Acad. Sci. USA 2003, 100, 8839–8843. [Google Scholar] [CrossRef]
- Castermans, D.; Somers, I.; Kriel, J.; Louwet, W.; Wera, S.; Versele, M.; Janssens, V.; Thevelein, J.M. Glucose-induced posttranslational activation of protein phosphatases PP2A and PP1 in yeast. Cell Res. 2012, 22, 1058–1077. [Google Scholar] [CrossRef]
- Rubenstein, E.M.; McCartney, R.R.; Zhang, C.; Shokat, K.M.; Shirra, M.K.; Arndt, K.M.; Schmidt, M.C. Access Denied: Snf1 Activation Loop Phosphorylation Is Controlled by Availability of the Phosphorylated Threonine 210 to the PP1 Phosphatase. J. Biol. Chem. 2008, 283, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Uno, I.; Ishikawa, T.; Powers, S.; Kataoka, T.; Broek, D.; Cameron, S.; Broach, J.; Matsumoto, K.; Wigler, M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell 1985, 40, 27–36. [Google Scholar] [CrossRef]
- Wilson, W.; Hawley, S.A.; Hardie, D. Glucose repression/derepression in budding yeast: SNF1 protein kinase is activated by phosphorylation under derepressing conditions, and this correlates with a high AMP:ATP ratio. Curr. Biol. 1996, 6, 1426–1434. [Google Scholar] [CrossRef]
- Otterstedt, K.; Larsson, C.; Bill, R.; Ståhlberg, A.; Boles, E.; Hohmann, S.; Gustafsson, L. Switching the mode of metabolism in the yeast Saccharomyces cerevisiae. EMBO Rep. 2004, 5, 532–537. [Google Scholar] [CrossRef]
- Elbing, K.; Ståhlberg, A.; Hohmann, S.; Gustafsson, L. Transcriptional responses to glucose at different glycolytic rates in Saccharomyces cerevisiae. JBIC J. Biol. Inorg. Chem. 2004, 271, 4855–4864. [Google Scholar] [CrossRef]
- Reifenberger, E.; Freidel, K.; Ciriacy, M. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on qlycolytic flux. Mol. Microbiol. 1995, 16, 157–167. [Google Scholar] [CrossRef]
- Kayikci, Ö.; Nielsen, J. Glucose repression in Saccharomyces cerevisiae. FEMS Yeast Res. 2015, 15, fov068. [Google Scholar] [CrossRef]
- Heinisch, J. Construction and physiological characterization of mutants disrupted in the phosphofructokinase genes of Saccharomyces cerevisiae. Curr. Genet. 1986, 11, 227–234. [Google Scholar] [CrossRef]
- Kümmel, A.; Ewald, J.C.; Fendt, S.-M.; Jol, S.J.; Picotti, P.; Aebersold, R.; Sauer, U.; Zamboni, N.; Heinemann, M. Differential glucose repression in common yeast strains in response to HXK2 deletion. FEMS Yeast Res. 2010, 10, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Peláez, R.; Herrero, P.; Moreno, F. Functional domains of yeast hexokinase. Biochem. J. 2010, 432, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Minokoshi, Y.; Alquier, T.; Furukawa, N.; Kim, Y.-B.; Lee, A.; Xue, B.; Mu, J.; Foufelle, F.; Ferre, P.; Birnbaum, M.; et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004, 428, 569–574. [Google Scholar] [CrossRef]
- López, M. AMPK Wars: The VMH Strikes Back, Return of the PVH. Trends Endocrinol. Metab. 2018, 29, 135–137. [Google Scholar] [CrossRef]
- Stark, R.; Ashley, S.E.; Andrews, Z.B. AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol. Cell. Endocrinol. 2013, 366, 215–223. [Google Scholar] [CrossRef]
- Lamberigts, C.; Wang, Y.; Dierckx, T.; Buys, N.; Everaert, N.; Buyse, J. The influence of thyroid state on hypothalamic AMP-activated protein kinase pathways in broilers. Gen. Comp. Endocrinol. 2021, 311, 113838. [Google Scholar] [CrossRef]
- Vazirian, M.; Nabavi, S.M.; Jafari, S.; Manayi, A. Natural activators of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) and their pharmacological activities. Food Chem. Toxicol. 2018, 122, 69–79. [Google Scholar] [CrossRef]
- Lyons, C.L.; Roche, H.M. Nutritional Modulation of AMPK-Impact upon Metabolic-Inflammation. Int. J. Mol. Sci. 2018, 19, 3092. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, K.U. Role of hypothalamic 5′-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J. Mol. Med. 2005, 83, 514–520. [Google Scholar] [CrossRef]
- Kim, M.-S.; Park, J.-Y.; Namkoong, C.; Jang, P.-G.; Ryu, J.-W.; Song, H.-S.; Yun, J.-Y.; Nam-Goong, I.S.; Ha, J.; Park, I.-S.; et al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 2004, 10, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Everaert, N.; Song, Z.; Decuypere, E.; Vermeulen, D.; Buyse, J. Alpha-lipoic acid impairs body weight gain of young broiler chicks via modulating peripheral AMPK. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 211, 34–40. [Google Scholar] [CrossRef]
- Targonsky, E.D.; Dai, F.; Koshkin, V.; Karaman, G.T.; Gyulkhandanyan, A.V.; Zhang, Y.; Chan, C.B.; Wheeler, M.B. α-Lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Diabetologia 2006, 49, 1587–1598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tripodi, F.; Nicastro, R.; Reghellin, V.; Coccetti, P. Post-translational modifications on yeast carbon metabolism: Regulatory mechanisms beyond transcriptional control. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 620–627. [Google Scholar] [CrossRef]
- Kitano, H. Biological robustness. Nat. Rev. Genet. 2004, 5, 826–837. [Google Scholar] [CrossRef]
- Welkenhuysen, N.; Borgqvist, J.; Backman, M.; Bendrioua, L.; Goksör, M.; Adiels, C.B.; Cvijovic, M.; Hohmann, S. Single-cell study links metabolism with nutrient signaling and reveals sources of variability. BMC Syst. Biol. 2017, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- DeVit, M.J.; Johnston, M. The nuclear exportin Msn5 is required for nuclear export of the Mig1 glucose repressor of Saccharomyces cerevisiae. Curr. Biol. 1999, 9, 1231–1241. [Google Scholar] [CrossRef]
- Zhang, Y.; Primavesi, L.; Jhurreea, D.; Andralojc, P.J.; Mitchell, R.; Powers, S.J.; Schluepmann, H.; Delatte, T.; Wingler, A.; Paul, M.J. Inhibition of SNF1-Related Protein Kinase1 Activity and Regulation of Metabolic Pathways by Trehalose-6-Phosphate. Plant. Physiol. 2009, 149, 1860–1871. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Primavesi, L.; Patel, M.K.; Martinez-Barajas, E.; Powers, S.J.; Sagar, R.; Fevereiro, P.; Davis, B.G.; Paul, M.J. Inhibition of SnRK1 by metabolites: Tissue-dependent effects and cooperative inhibition by glucose 1-phosphate in combination with trehalose 6-phosphate. Plant. Physiol. Biochem. 2013, 63, 89–98. [Google Scholar] [CrossRef]
- Momcilovic, M.; Iram, S.; Liu, Y.; Carlson, M. Roles of the Glycogen-binding Domain and Snf4 in Glucose Inhibition of SNF1 Protein Kinase. J. Biol. Chem. 2008, 283, 19521–19529. [Google Scholar] [CrossRef]
- Xiao, B.; Sanders, M.J.; Underwood, E.; Heath, R.; Mayer, F.V.; Carmena, D.; Jing, C.; Walker, P.A.; Eccleston, J.F.; Haire, L.F.; et al. Structure of mammalian AMPK and its regulation by ADP. Nature 2011, 472, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Zhou, X.E.; Ke, J.; de Waal, P.; Gu, X.; Tan, M.H.E.; Wang, D.; Wu, D.; Xu, H.E.; et al. Structural basis of AMPK regulation by adenine nucleotides and glycogen. Cell Res. 2014, 25, 50–66. [Google Scholar] [CrossRef]
- Amodeo, G.A.; Rudolph, M.J.; Tong, L. Crystal structure of the heterotrimer core of Saccharomyces cerevisiae AMPK homologue SNF. Nature 2007, 449, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.V.; Heath, R.; Underwood, E.; Sanders, M.J.; Carmena, D.; McCartney, R.R.; Leiper, F.; Xiao, B.; Jing, C.; Walker, P.A.; et al. ADP Regulates SNF1, the Saccharomyces cerevisiae Homolog of AMP-Activated Protein Kinase. Cell Metab. 2011, 14, 707–714. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanesi, R.; Tripodi, F.; Vertemara, J.; Tisi, R.; Coccetti, P. AMPK Phosphorylation Is Controlled by Glucose Transport Rate in a PKA-Independent Manner. Int. J. Mol. Sci. 2021, 22, 9483. https://doi.org/10.3390/ijms22179483
Milanesi R, Tripodi F, Vertemara J, Tisi R, Coccetti P. AMPK Phosphorylation Is Controlled by Glucose Transport Rate in a PKA-Independent Manner. International Journal of Molecular Sciences. 2021; 22(17):9483. https://doi.org/10.3390/ijms22179483
Chicago/Turabian StyleMilanesi, Riccardo, Farida Tripodi, Jacopo Vertemara, Renata Tisi, and Paola Coccetti. 2021. "AMPK Phosphorylation Is Controlled by Glucose Transport Rate in a PKA-Independent Manner" International Journal of Molecular Sciences 22, no. 17: 9483. https://doi.org/10.3390/ijms22179483
APA StyleMilanesi, R., Tripodi, F., Vertemara, J., Tisi, R., & Coccetti, P. (2021). AMPK Phosphorylation Is Controlled by Glucose Transport Rate in a PKA-Independent Manner. International Journal of Molecular Sciences, 22(17), 9483. https://doi.org/10.3390/ijms22179483






