Piezoelectric Microvibration Mitigates Estrogen Loss-Induced Osteoporosis and Promotes Piezo1, MicroRNA-29a, and Wnt3a Signaling in Osteoblasts
Abstract
:1. Introduction
2. Results
2.1. PMVS Intervention Downregulated Serum Bone Resorption Marker CTX-1 Levels
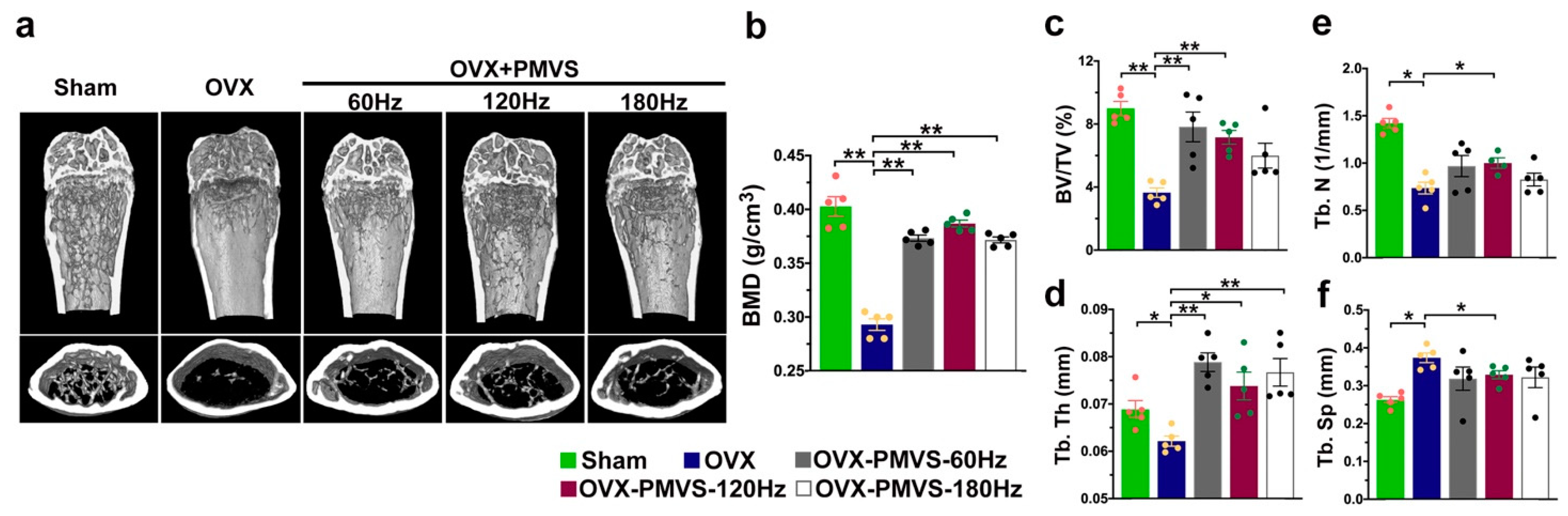
2.2. PMVS Intervention Preserved Bone Mass, Trabecular and Cortical Bone Microstructure
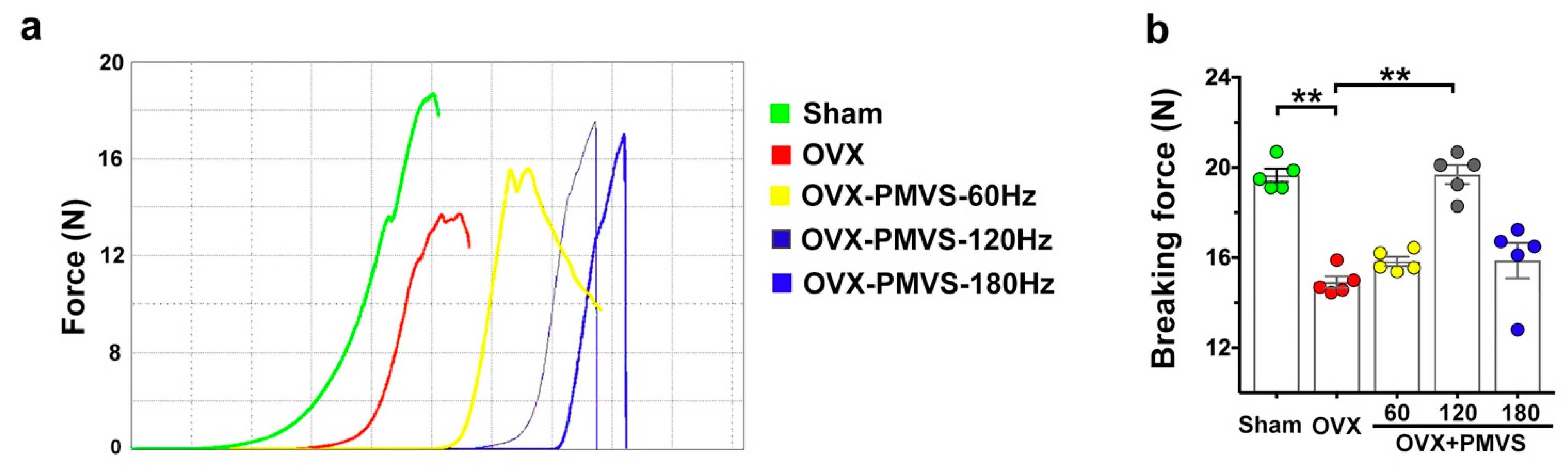
2.3. PMVS Intervention Attenuated OVX-Induced Biomechanics Inhibition
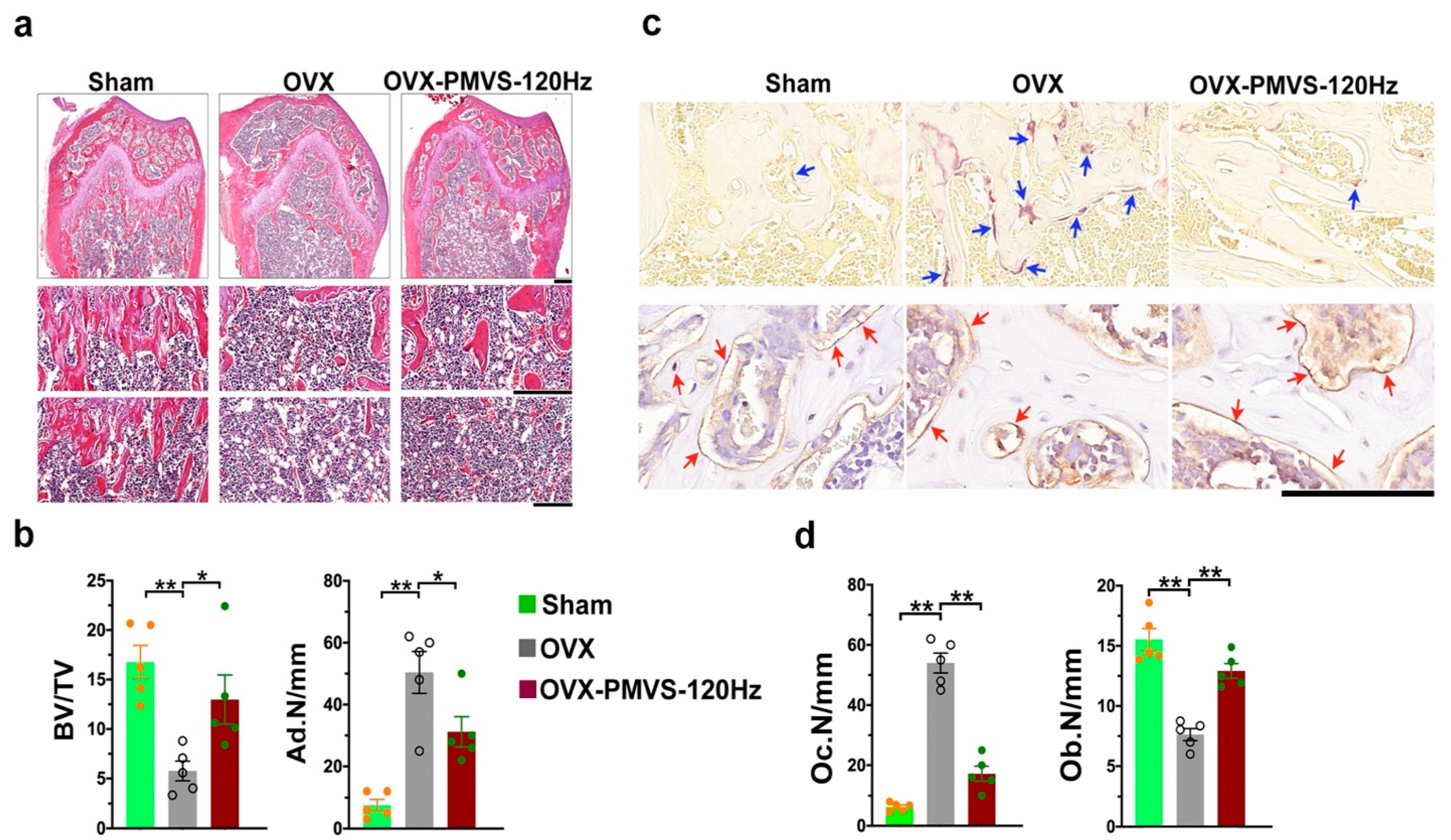
2.4. PMVS Intervention Improved Trabecular Morphology and Osteoclast Formation
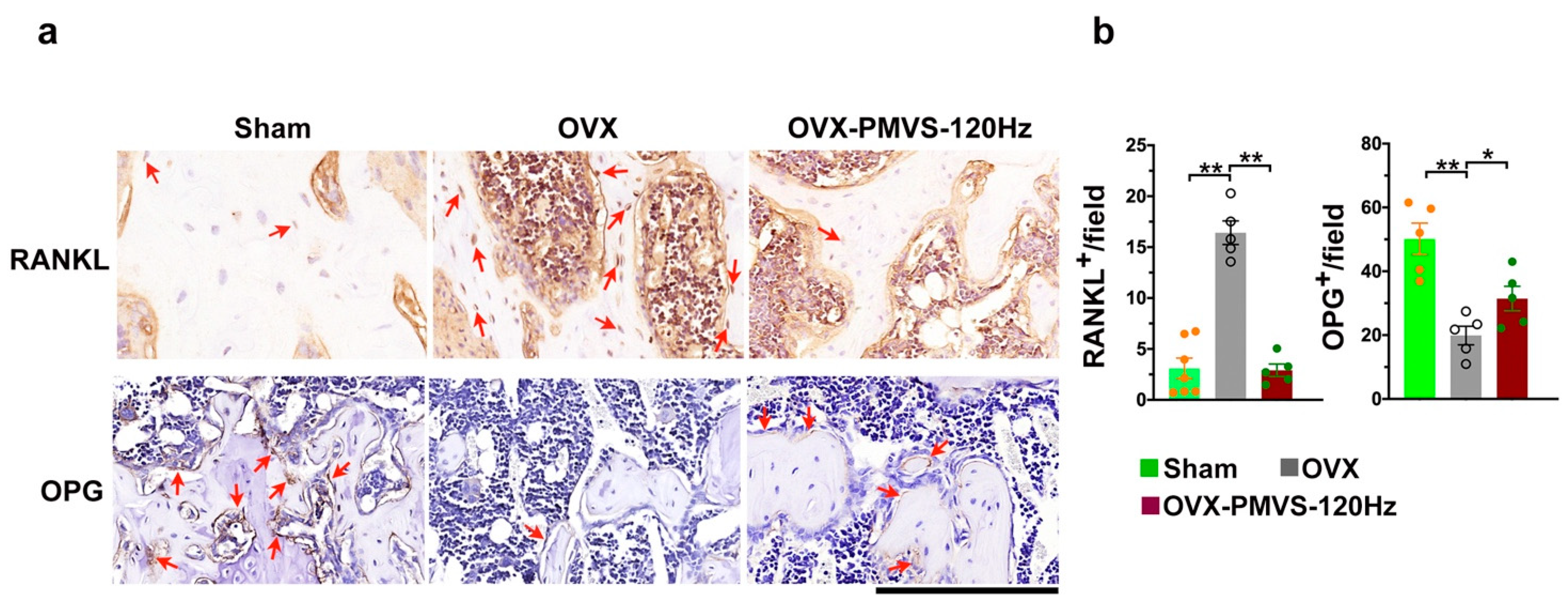
2.5. PMVS Intervention Affected RANKL and OPG
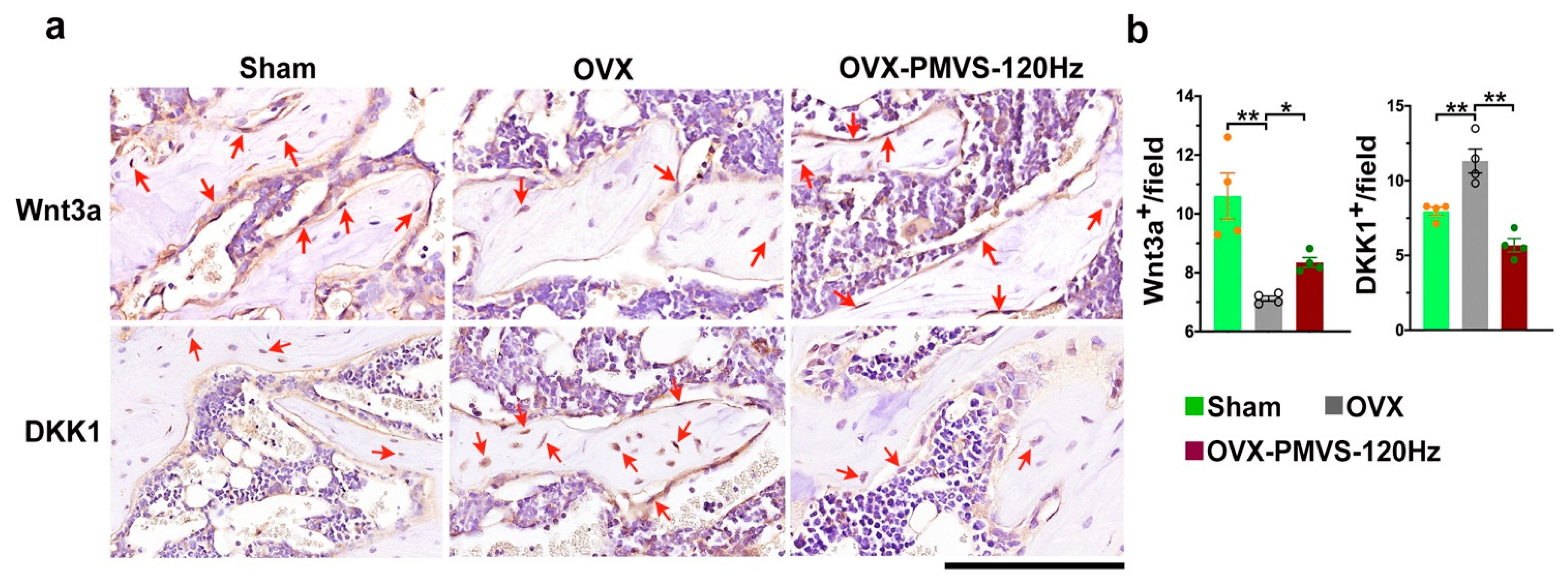
2.6. PMVS Intervention Affected Wnt3a and Dkk1 Expression
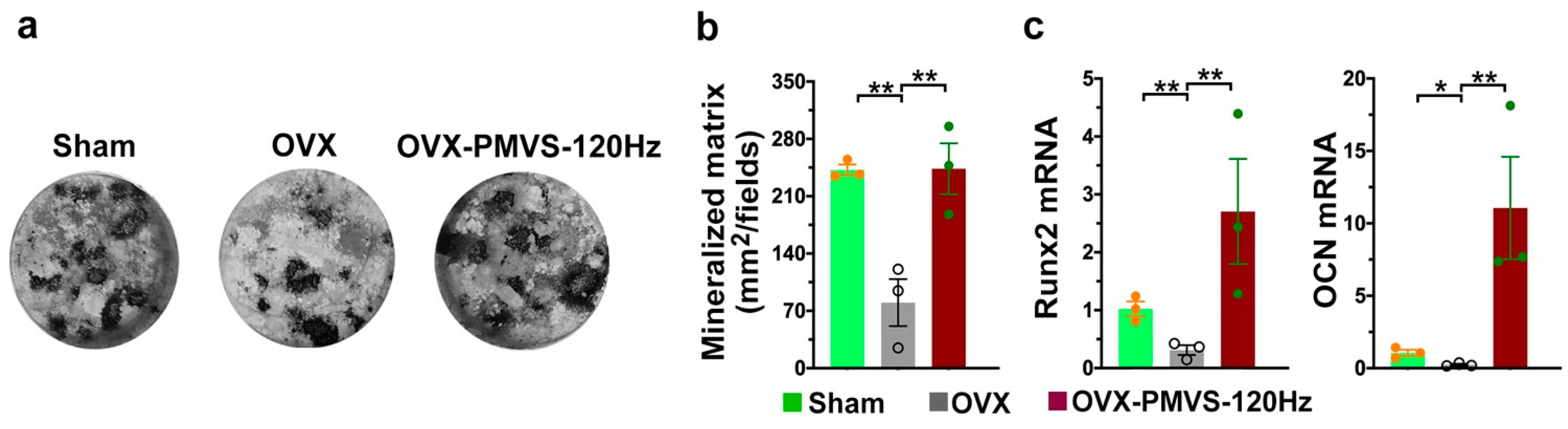
2.7. PMVS Intervention Preserved Osteogenesis of Bone-Marrow Mesenchymal Cells
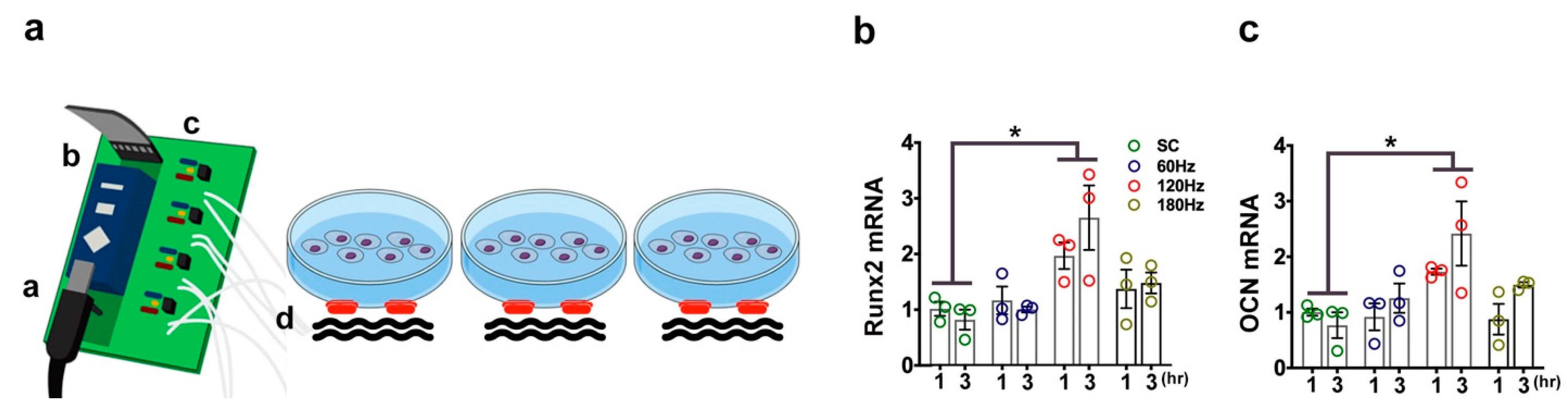
2.8. PMVS Intervention Promoted Osteogenic Gene Expression of MC3T3-E1 Osteoblasts
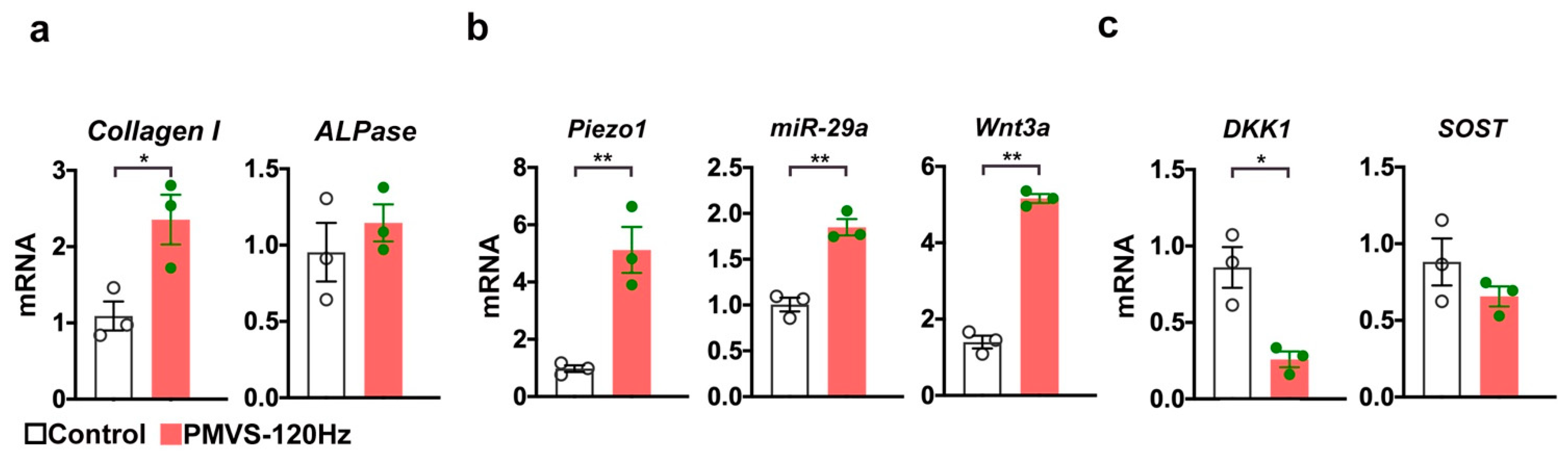
2.9. PMVS Intervention Promoted Mechanosensitive Signaling and Wnt Signaling in Osteoblasts
3. Discussion
4. Materials and Methods
4.1. Experimental Osteoporosis Models
4.2. PMVS Intervention
4.3. In vitro PMVS Treatment
4.4. Quantification of Serum CTX-1
4.5. Quantitative µCT Analysis
4.6. Biomechanical Analysis
4.7. Histology
4.8. Immunohistochemisty
4.9. Ex Vivo Osteogenesis of Primary Bone-Marrow Stroma Cells
4.10. RT-PCR
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy metabolism of the osteoblast: Implications for osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef]
- Yang, T.L.; Shen, H.; Liu, A.; Dong, S.S.; Zhang, L.; Deng, F.Y.; Zhao, Q.; Deng, H.W. A road map for understanding molecular and genetic determinants of osteoporosis. Nat. Rev. Endocrinol. 2020, 16, 91–103. [Google Scholar] [CrossRef]
- Han, Y.; You, X.; Xing, W.; Zhang, Z.; Zou, W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Fowler, T.W.; Mitchell, T.L.; Janda, C.Y.; Xie, L.; Tu, S.; Chen, H.; Zhang, H.; Ye, J.; Ouyang, B.; Yuan, T.Z.; et al. Development of selective bispecific Wnt mimetics for bone loss and repair. Nat. Commun. 2021, 12, 3247. [Google Scholar] [CrossRef] [PubMed]
- Estell, E.G.; Rosen, C.J. Emerging insights into the comparative effectiveness of anabolic therapies for osteoporosis. Nat Rev Endocrinol. 2021, 17, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; Crittenden, D.B.; Adachi, J.D.; Binkley, N.; Czerwinski, E.; Ferrari, S.; Hofbauer, L.C.; Lau, E.; Lewiecki, E.M.; Miyauchi, A.; et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016, 375, 1532–1543. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, P.; Liu, Y.; Wu, Y.; Chen, Y.; Guo, Y.; Zhang, S.; Zheng, X.; Zhou, L.; Liu, W.; et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nat. Commun. 2020, 11, 5596. [Google Scholar] [CrossRef]
- Li, J.Y.; Yu, M.; Pal, S.; Tyagi, A.M.; Dar, H.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J. Clin. Investig. 2020, 130, 1767–1781. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cauley, J.A.; Giangregorio, L. Physical activity and skeletal health in adults. Lancet Diabetes Endocrinol. 2020, 8, 150–162. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin mediates effects on bone and fat via alphaV integrin receptors. Cell 2019, 178, 507–508. [Google Scholar] [CrossRef] [PubMed]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin prevents disuse-induced osteocyte apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, D.; Li, J.; Yang, S.; Xu, J.; Yokota, H.; Zhang, P. Wnt3a involved in the mechanical loading on improvement of bone remodeling and angiogenesis in a postmenopausal osteoporosis mouse model. FASEB J. 2019, 33, 8913–8924. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, C.S.; Johncola, A.J.; Batzdorf, A.S.; Jones, B.C.; Al Mukaddam, M.; Sexton, K.; Shults, J.; Leonard, M.B.; Snyder, P.J.; Wehrli, F.W. Effect of low-intensity vibration on bone strength, microstructure, and adiposity in pre-osteoporotic postmenopausal women: A randomized placebo-controlled trial. J. Bone Miner. Res. 2021, 36, 673–684. [Google Scholar] [CrossRef]
- Liphardt, A.M.; Schipilow, J.; Hanley, D.A.; Boyd, S.K. Bone quality in osteopenic postmenopausal women is not improved after 12 months of whole-body vibration training. Osteoporos. Int. 2015, 26, 911–920. [Google Scholar] [CrossRef]
- Wen, J.; Bao, M.; Tang, M.; He, X.; Yao, X.; Li, L. Low magnitude vibration alleviates age-related bone loss by inhibiting cell senescence of osteogenic cells in naturally senescent rats. Aging 2021, 13, 12031–12045. [Google Scholar] [CrossRef]
- Pamon, T.; Bhandal, V.; Adler, B.J.; Ete Chan, M.; Rubin, C.T. Low-intensity vibration increases cartilage thickness in obese mice. J. Orthop. Res. 2018, 36, 751–759. [Google Scholar] [CrossRef] [Green Version]
- García-López, S.; Villanueva, R.E.; Massó-Rojas, F.; Páez-Arenas, A.; Meikle, M.C. Micro-vibrations at 30 Hz on bone cells cultivated in vitro produce soluble factors for osteoclast inhibition and osteoblast activity. Arch. Oral Biol. 2020, 110, 104594. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Bellido, T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat. Rev. Endocrinol. 2016, 12, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.S.; Ko, J.Y.; Chen, Y.S.; Ke, H.J.; Hsieh, C.K.; Kuo, C.W.; Wang, S.Y.; Huang, B.W.; Tseng, J.G.; Wang, F.S. MicroRNA-29a represses osteoclast formation and protects against osteoporosis by regulating PCAF-mediated RANKL and CXCL12. Cell Death Dis. 2019, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Stassen, O.M.J.A.; Ristori, T.; Sahlgren, C.M. Notch in mechanotransduction—From molecular mechanosensitivity to tissue mechanostasis. J. Cell Sci. 2020, 133, jcs250738. [Google Scholar] [CrossRef]
- Carina, V.; Della Bella, E.; Costa, V.; Bellavia, D.; Veronesi, F.; Cepollaro, S.; Fini, M.; Giavaresi, G. Bone’s response to mechanical loading in aging and osteoporosis: Molecular mechanisms. Calcif. Tissue Int. 2020, 107, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulou, A.; Karamesinis, K.; Basdra, E.K. Mechanotransduction pathways in bone pathobiology. Biochim. Biophys. Acta 2015, 1852, 1700–1708. [Google Scholar] [CrossRef] [Green Version]
- Pagnotti, G.M.; Thompson, W.R.; Guise, T.A.; Rubin, C.T. Suppression of cancer-associated bone loss through dynamic mechanical loading. Bone 2021, 150, 115998. [Google Scholar] [CrossRef]
- Jing, D.; Yan, Z.; Cai, J.; Tong, S.; Li, X.; Guo, Z.; Luo, E. Low-1 level mechanical vibration improves bone microstructure, tissue mechanical properties and porous titanium implant osseointegration by promoting anabolic response in type 1 diabetic rabbits. Bone 2018, 106, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, T.; Wang, Z.; Chen, X.; Qu, S.; Weng, J.; Zhi, W.; Wang, J. Joint construction of micro-vibration stimulation and BCP scaffolds for enhanced bioactivity and self-adaptability tissue engineered bone grafts. J. Mater. Chem. B. 2020, 8, 4278–4288. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Ofotokun, I. Physiological and pathophysiological bone turnover—Role of the immune system. Nat. Rev. Endocrinol. 2016, 12, 518–532. [Google Scholar] [CrossRef]
- Stavenschi, E.; Labour, M.N.; Hoey, D.A. Oscillatory fluid flow induces the osteogenic lineage commitment of mesenchymal stem cells: The effect of shear stress magnitude, frequency, and duration. J. Biomech. 2017, 55, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Fukunaga, T.; Sasaki, K.; Seiryu, M.; Yoshizawa, M.; Takeshita, N.; Takano-Yamamoto, T. Vibration enhances osteoclastogenesis by inducing RANKL expression via NF-kappaB signaling in osteocytes. Bone 2019, 23, 56–66. [Google Scholar] [CrossRef]
- Bramlett, H.M.; Dietrich, W.D.; Marcillo, A.; Mawhinney, L.J.; Furones-Alonso, O.; Bregy, A.; Peng, Y.; Wu, Y.; Pan, J.; Wang, J.; et al. Effects of low intensity vibration on bone and muscle in rats with spinal cord injury. Osteoporos. Int. 2014, 25, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. Elife 2019, 8, e47454. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 2019, 8, e49631. [Google Scholar] [CrossRef]
- Fan, J.; Lee, C.S.; Kim, S.; Chen, C.; Aghaloo, T.; Lee, M. Generation of small RNA-modulated exosome mimetics for bone regeneration. ACS Nano 2020, 14, 11973–11984. [Google Scholar] [CrossRef]
- Kang, K.S.; Robling, A.G. New Insights into Wnt-Lrp5/6-beta-catenin signaling in mechanotransduction. Front. Endocrinol. 2015, 5, 246. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Yan, Z.; Wang, D.; Yang, Y.; Ding, Y.; Luo, E.; Jing, D.; Cai, J. Pulsed electromagnetic fields ameliorate skeletal deterioration in bone mass, microarchitecture, and strength by enhancing canonical Wnt signaling-mediated bone formation in rats with spinal cord injury. J. Neurotrauma 2021, 38, 765–776. [Google Scholar] [CrossRef]
- Gerbaix, M.; Ammann, P.; Ferrari, S. Mechanically driven counter-regulation of cortical bone formation in response to sclerostin-neutralizing antibodies. J. Bone Miner. Res. 2021, 36, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Shao, X.; Yan, Z.; Liu, X.; Yang, Y.; Luo, E.; Jing, D. Differential skeletal response in adult and aged rats to independent and combinatorial stimulation with pulsed electromagnetic fields and mechanical vibration. FASEB J. 2020, 34, 3037–3050. [Google Scholar] [CrossRef]
- Kapinas, K.; Kessler, C.; Ricks, T.; Gronowicz, G.; Delany, A.M. miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J. Biol. Chem. 2010, 285, 25221–25231. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.S.; Wu, R.W.; Lain, W.S.; Tsai, T.C.; Chen, Y.S.; Sun, Y.C.; Ke, H.J.; Li, J.C.; Hwang, J.; Ko, J.Y. Sclerostin vaccination mitigates estrogen deficiency induction of bone mass loss and microstructure deterioration. Bone 2018, 112, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, R.-W.; Lian, W.-S.; Chen, Y.-S.; Ko, J.-Y.; Wang, S.-Y.; Jahr, H.; Wang, F.-S. Piezoelectric Microvibration Mitigates Estrogen Loss-Induced Osteoporosis and Promotes Piezo1, MicroRNA-29a, and Wnt3a Signaling in Osteoblasts. Int. J. Mol. Sci. 2021, 22, 9476. https://doi.org/10.3390/ijms22179476
Wu R-W, Lian W-S, Chen Y-S, Ko J-Y, Wang S-Y, Jahr H, Wang F-S. Piezoelectric Microvibration Mitigates Estrogen Loss-Induced Osteoporosis and Promotes Piezo1, MicroRNA-29a, and Wnt3a Signaling in Osteoblasts. International Journal of Molecular Sciences. 2021; 22(17):9476. https://doi.org/10.3390/ijms22179476
Chicago/Turabian StyleWu, Re-Wen, Wei-Shiung Lian, Yu-Shan Chen, Jih-Yang Ko, Shao-Yu Wang, Holger Jahr, and Feng-Sheng Wang. 2021. "Piezoelectric Microvibration Mitigates Estrogen Loss-Induced Osteoporosis and Promotes Piezo1, MicroRNA-29a, and Wnt3a Signaling in Osteoblasts" International Journal of Molecular Sciences 22, no. 17: 9476. https://doi.org/10.3390/ijms22179476
APA StyleWu, R.-W., Lian, W.-S., Chen, Y.-S., Ko, J.-Y., Wang, S.-Y., Jahr, H., & Wang, F.-S. (2021). Piezoelectric Microvibration Mitigates Estrogen Loss-Induced Osteoporosis and Promotes Piezo1, MicroRNA-29a, and Wnt3a Signaling in Osteoblasts. International Journal of Molecular Sciences, 22(17), 9476. https://doi.org/10.3390/ijms22179476







