Myogenic Potential of Extracellular Matrix Derived from Decellularized Bovine Pericardium
Abstract
1. Introduction
2. Results
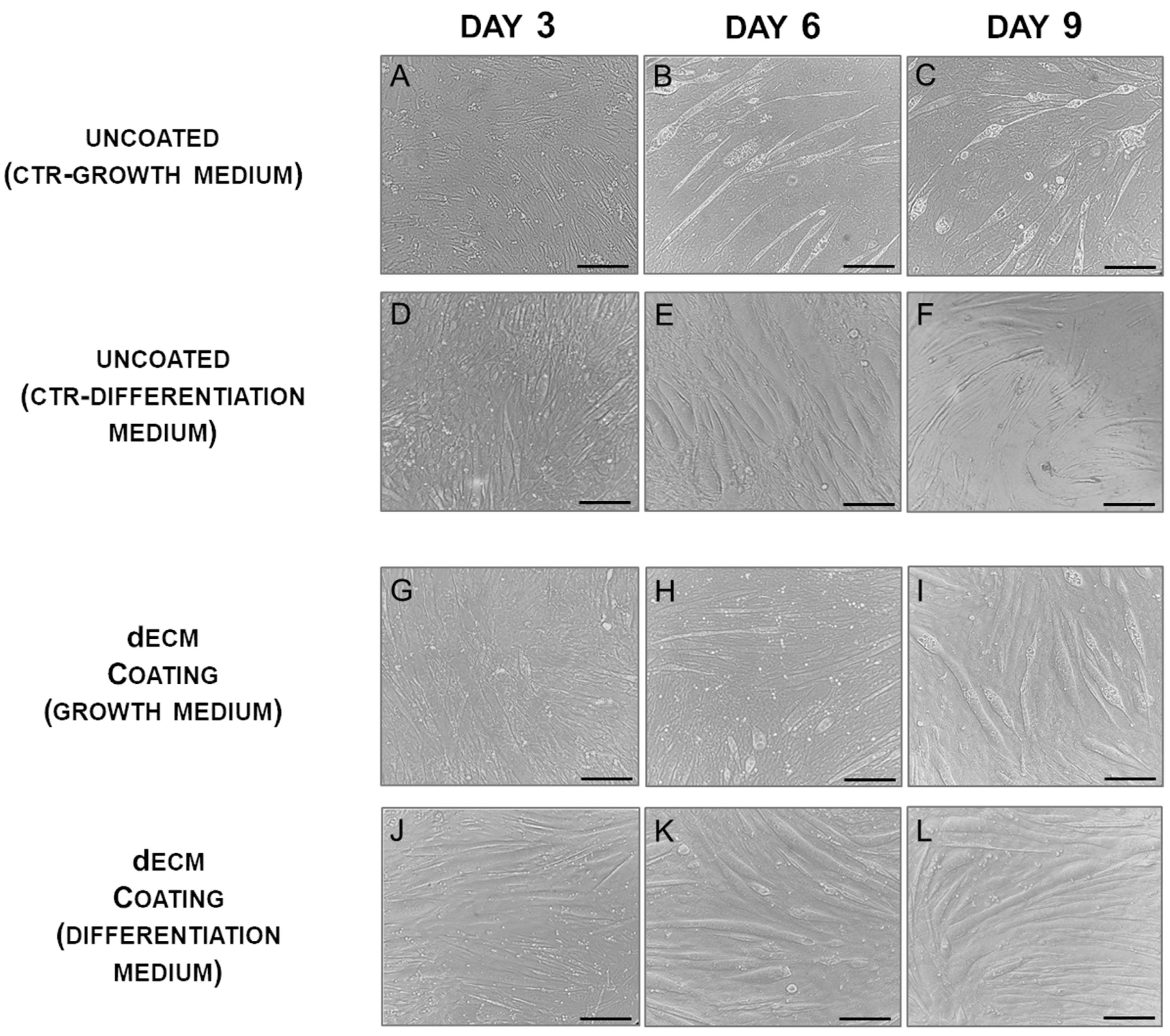
2.1. Myotubes Formation on dECM Coating
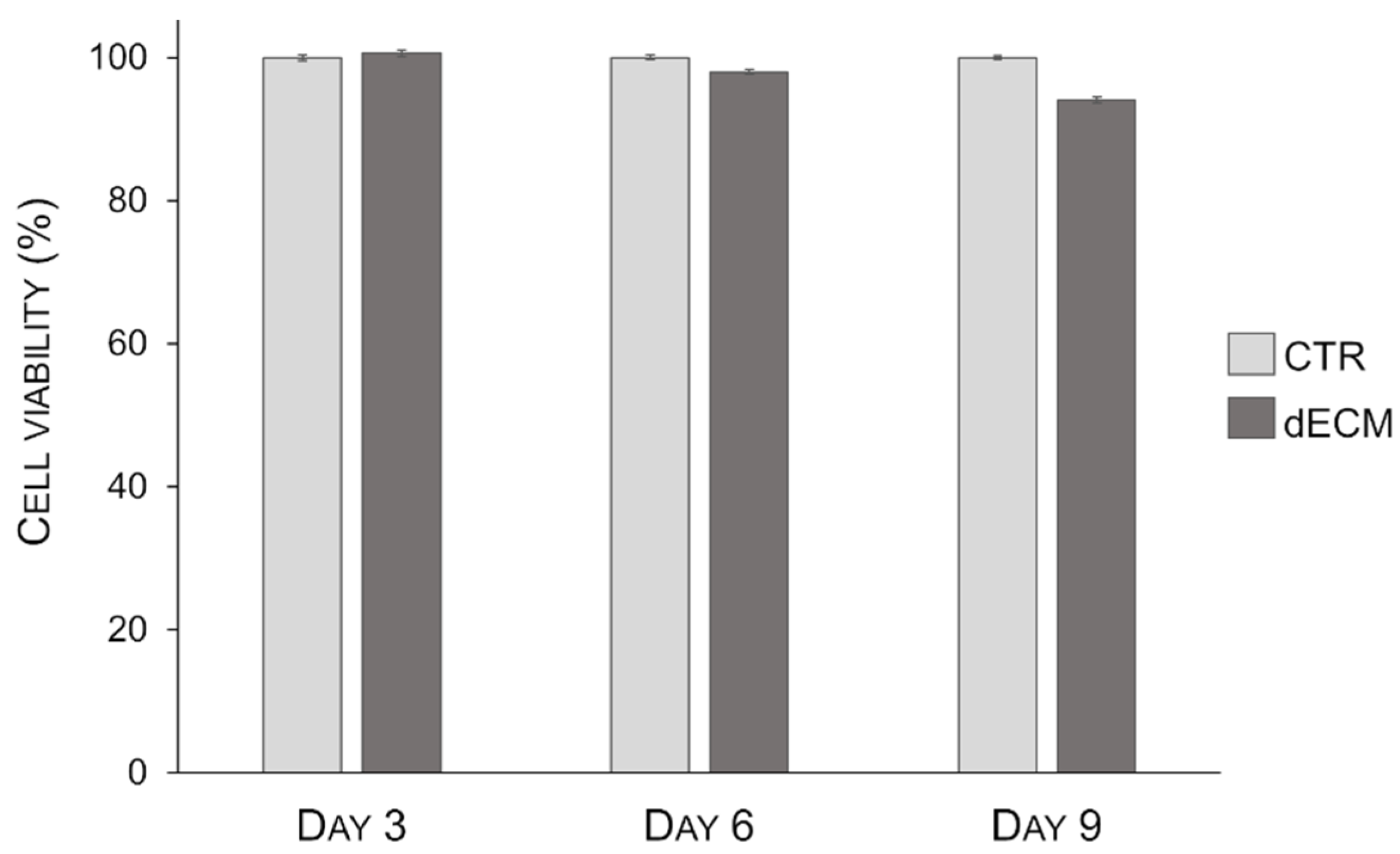
2.2. Myotubes Viability on dECM Coating
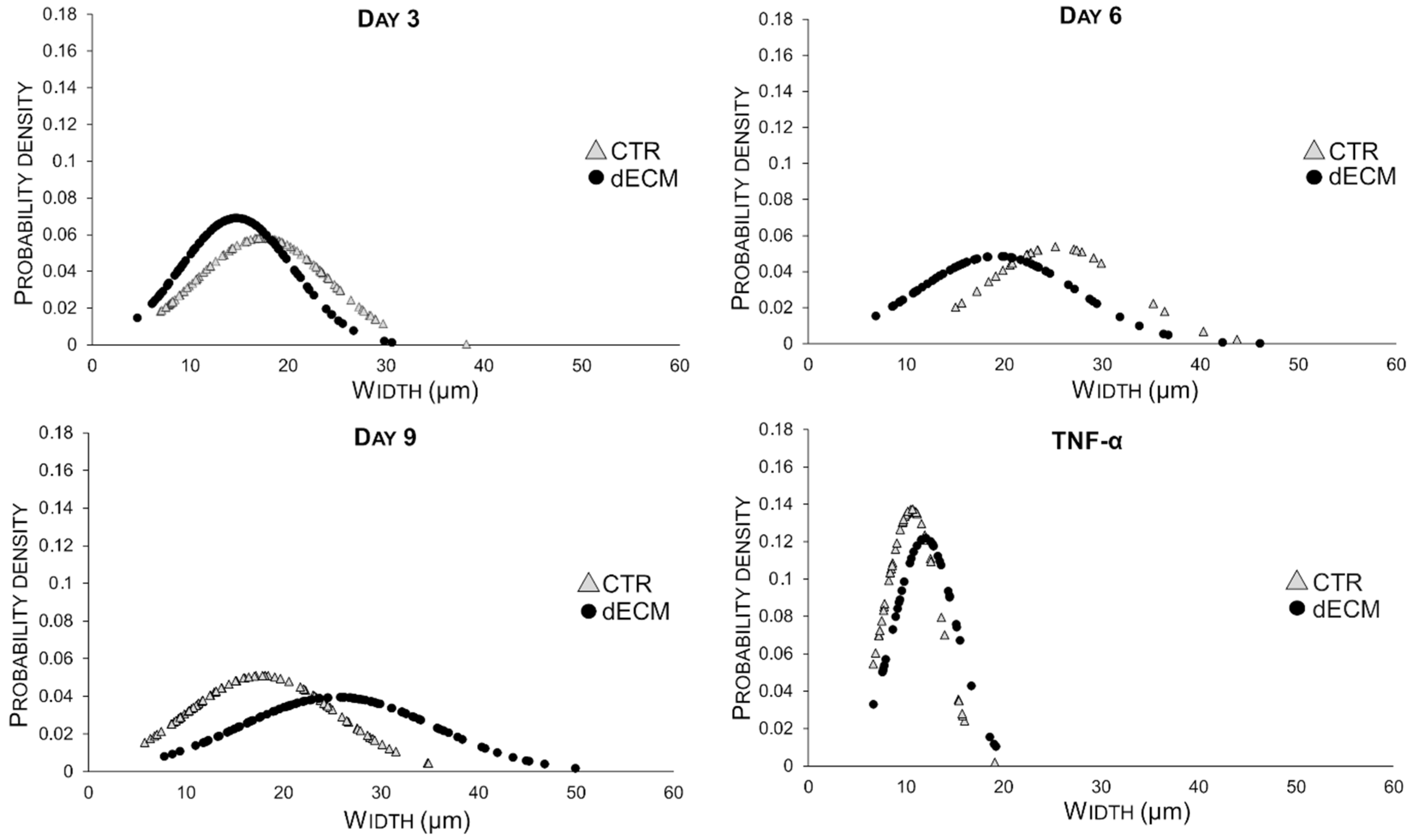
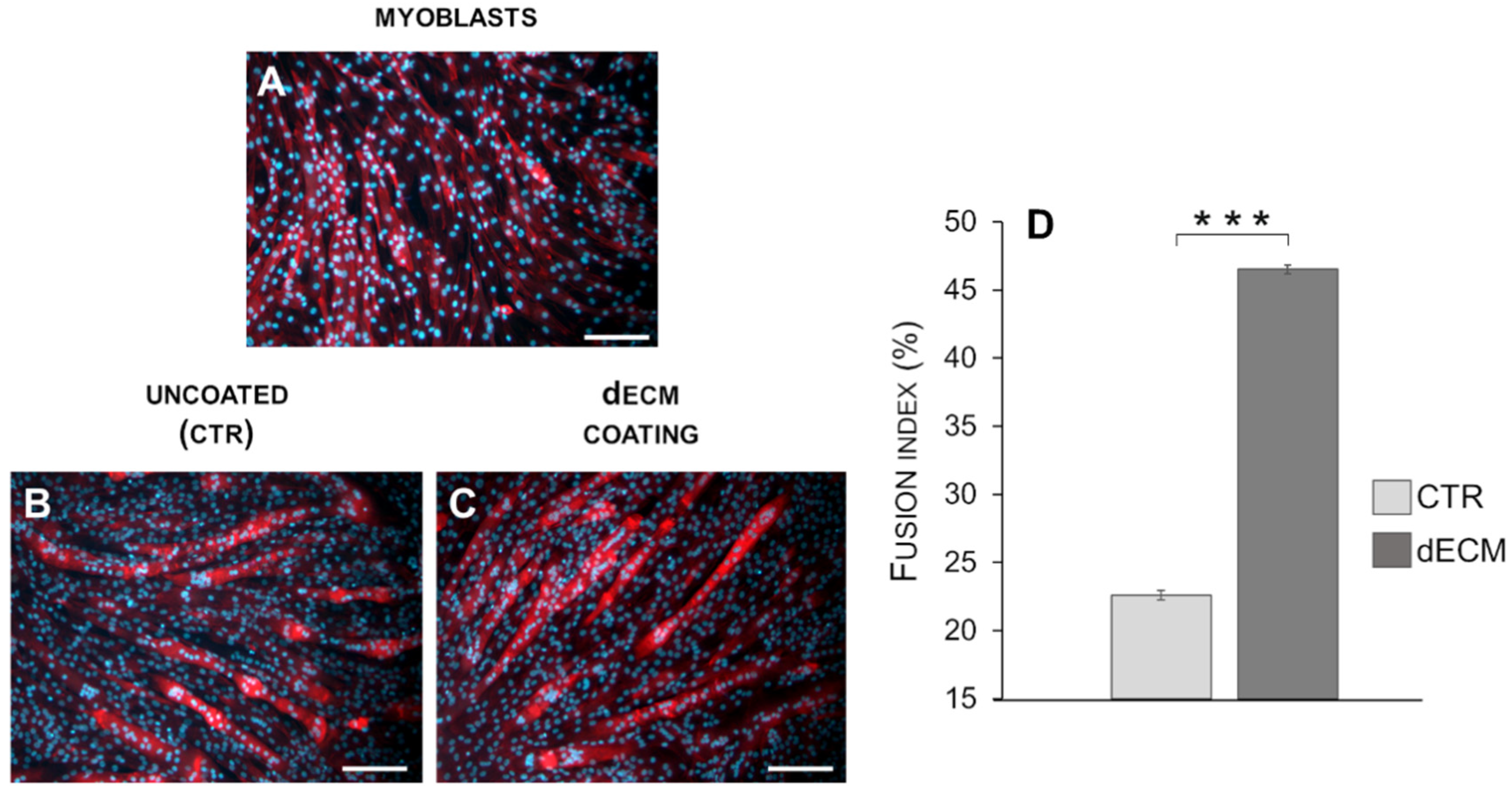
2.3. Myotubes Morphology and Fusion Index
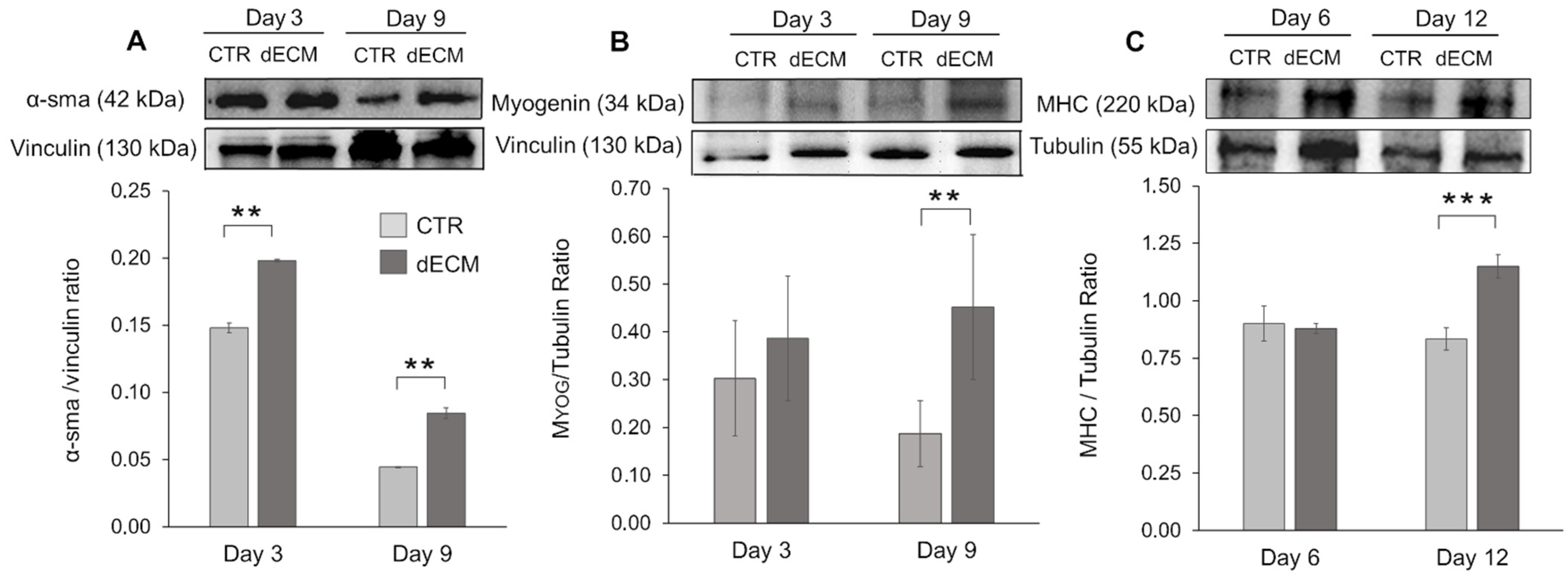
2.4. Myogenic Differentiation of dECM Coating
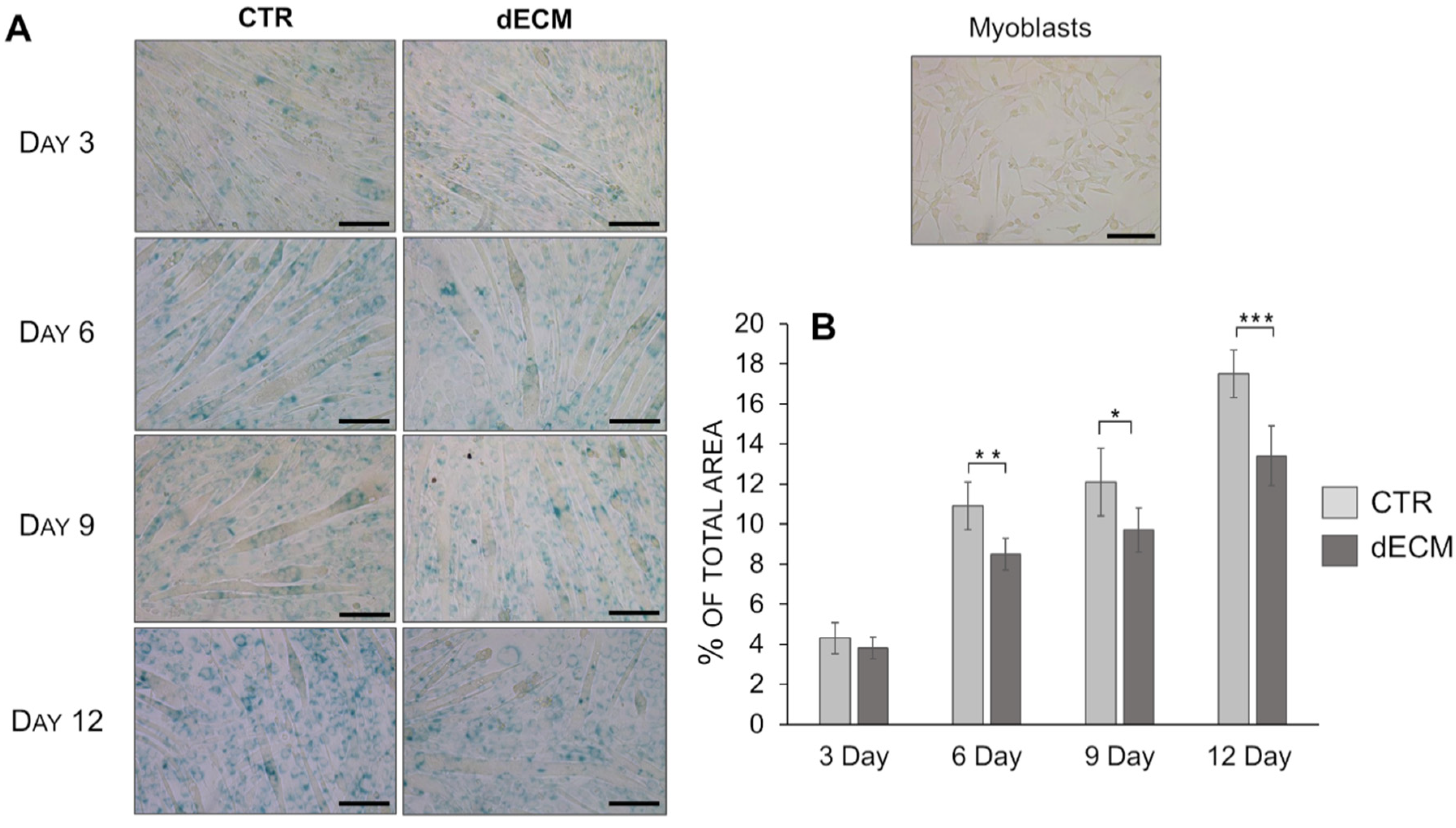
2.5. Senescence Evaluation
3. Discussion
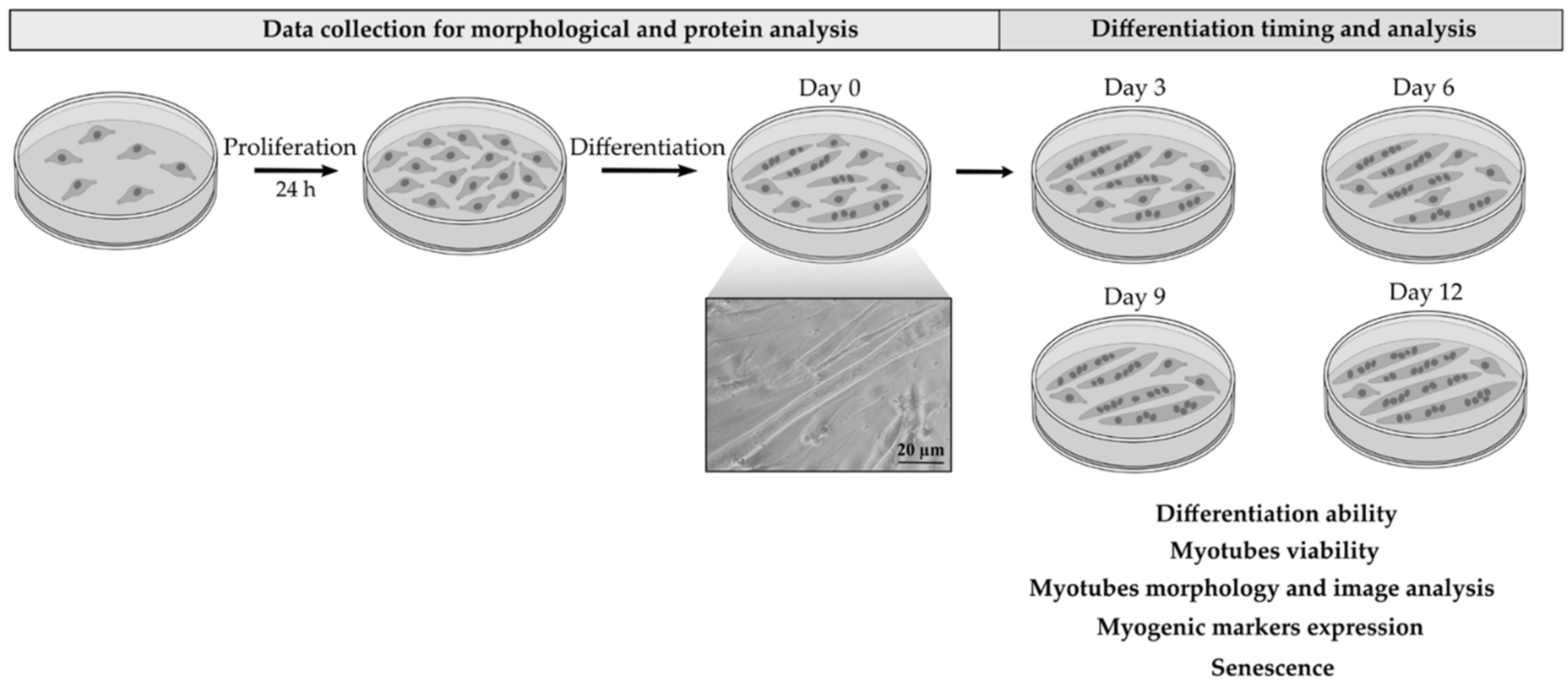
4. Materials and Methods
4.1. Decellularization of the Bovine Pericardial Extracellular Matrix
4.2. Cell Cultures
4.3. Cells Morphology
4.4. Cell Viability Assay
4.5. Morphometry and Image Analysis
4.6. Determination of Myogenic Differentiation by Western Blot
4.7. Senescence Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baghdadi, M.B.; Tajbakhsh, S. Regulation and phylogeny of skeletal muscle regeneration. Dev. Biol. 2018, 433, 200–209. [Google Scholar] [CrossRef]
- Tedesco, F.S.; Dellavalle, A.; Diaz-Manera, J.; Messina, G.; Cossu, G. Repairing skeletal muscle: Regenerative potential of skeletal muscle stem cells. J. Clin. Investig. 2010, 120, 11–19. [Google Scholar] [CrossRef]
- Andreana, I.; Repellin, M.; Carton, F.; Kryza, D.; Briançon, S.; Chazaud, B.; Mounier, R.; Arpicco, S.; Malatesta, M.; Stella, B.; et al. Nanomedicine for Gene Delivery and Drug Repurposing in the Treatment of Muscular Dystrophies. Pharmaceutics 2021, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Saure, C.; Caminiti, C.; Weglinski, J.; de Castro Perez, F.; Monges, S. Energy expenditure, body composition, and prevalence of metabolic disorders in patients with Duchenne muscular dystrophy. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Pansarasa, O.; Rossi, D.M.; Berardinelli, A.; Cereda, C. Amyotrophic Lateral Sclerosis and Skeletal Muscle: An Update. Mol. Neurobiol. 2014, 49, 984–990. [Google Scholar] [CrossRef]
- Wu, R.; Lv, H.; Zhang, W.; Wang, Z.; Zuo, Y.; Liu, J.; Yuan, Y. Clinical and Pathological Variation of Charcot-Marie-Tooth 1A in a Large Chinese Cohort. BioMed Res. Int. 2017, 2017, 6481367. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Corona, B.T.; Chen, X.; Walters, T.J. A Standardized Rat Model of Volumetric Muscle Loss Injury for the Development of Tissue Engineering Therapies. BioResearch Open Access 2012, 1, 280–290. [Google Scholar] [CrossRef]
- Scott, J.B.; Ward, C.L.; Corona, B.T.; Deschenes, M.R.; Harrison, B.S.; Saul, J.M.; Christ, G.J. Achieving Acetylcholine Receptor Clustering in Tissue-Engineered Skeletal Muscle Constructs In vitro through a Materials-Directed Agrin Delivery Approach. Front. Pharmacol. 2017, 7, 508. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.B.; Passipieri, J.A.; Siriwardane, M.; Ellenburg, M.D.; Vadhavkar, M.; Bergman, C.R.; Saul, J.M.; Tomblyn, S.; Burnett, L.; Christ, G.J. Cell and Growth Factor-Loaded Keratin Hydrogels for Treatment of Volumetric Muscle Loss in a Mouse Model. Tissue Eng. Part A 2017, 23, 572–584. [Google Scholar] [CrossRef]
- Wang, X.H.; Du, J.; Klein, J.D.; Bailey, J.L.; Mitch, W.E. Exercise ameliorates chronic kidney disease–induced defects in muscle protein metabolism and progenitor cell function. Kidney Int. 2009, 76, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Gates, C.; Huard, J. Management of Skeletal Muscle Injuries in Military Personnel. Oper. Tech. Sports Med. 2005, 13, 247–256. [Google Scholar] [CrossRef]
- Pollot, B.E.; Rathbone, C.R.; Wenke, J.C.; Guda, T. Natural polymeric hydrogel evaluation for skeletal muscle tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 672–679. [Google Scholar] [CrossRef]
- Goldman, S.M.; Henderson, B.E.P.; Walters, T.J.; Corona, B.T. Co-delivery of a laminin-111 supplemented hyaluronic acid based hydrogel with minced muscle graft in the treatment of volumetric muscle loss injury. PLoS ONE 2018, 13, e0191245. [Google Scholar] [CrossRef]
- Lev, R.; Seliktar, D. Hydrogel biomaterials and their therapeutic potential for muscle injuries and muscular dystrophies. J. R. Soc. Interface 2018, 15, 20170380. [Google Scholar] [CrossRef] [PubMed]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.M.; Scott, T.E.; Browe, D.P.; McGaughey, T.A.; Wood, C.; Wolyniak, M.J.; Freeman, J.W. Hydrogels for Skeletal Muscle Regeneration. Regen. Eng. Transl. Med. 2020, 1–9. [Google Scholar] [CrossRef]
- Pati, F.; Ha, D.-H.; Jang, J.; Han, H.H.; Rhie, J.-W.; Cho, D.-W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 2015, 62, 164–175. [Google Scholar] [CrossRef]
- Travascio, F. Composition and Function of the Extracellular Matrix in the Human Body; BoD—Books on Demand; InTech: Rijeka, Croatia, 2016; ISBN 978-953-51-2415-3. [Google Scholar]
- Boettiger, D.; Enomoto-Iwamoto, M.; Yoon, H.Y.; Hofer, U.; Menko, A.S.; Chiquet-Ehrismann, R. Regulation of Integrin alpha 5 beta 1 Affinity during Myogenic Differentiation. Dev. Biol. 1995, 169, 261–272. [Google Scholar] [CrossRef]
- Buck, C.A.; Horwitz, A.F. Cell surface receptors for extracellular matrix molecules. Annu. Rev. Cell Biol. 1987, 3, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Eklund, L.; Piuhola, J.; Komulainen, J.; Sormunen, R.; Ongvarrasopone, C.; Fässler, R.; Muona, A.; Ilves, M.; Ruskoaho, H.; Takala, T.E.S.; et al. Lack of type XV collagen causes a skeletal myopathy and cardiovascular defects in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 1194–1199. [Google Scholar] [CrossRef]
- Menko, A.S.; Boettiger, D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell 1987, 51, 51–57. [Google Scholar] [CrossRef]
- Nandan, D.; Clarke, E.P.; Ball, E.H.; Sanwal, B.D. Ethyl-3,4-dihydroxybenzoate inhibits myoblast differentiation: Evidence for an essential role of collagen. J. Cell Biol. 1990, 110, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Osses, N.; Brandan, E. ECM is required for skeletal muscle differentiation independently of muscle regulatory factor expression. Am. J. Physiol. Cell Physiol. 2002, 282, C383–C394. [Google Scholar] [CrossRef]
- The Importance of Extracellular Matrix in Skeletal Muscle Development and Function|IntechOpen. Available online: https://www.intechopen.com/books/composition-and-function-of-the-extracellular-matrix-in-the-human-body/the-importance-of-extracellular-matrix-in-skeletal-muscle-development-and-function (accessed on 20 June 2021).
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef]
- Guldner, N.W.; Jasmund, I.; Zimmermann, H.; Heinlein, M.; Girndt, B.; Meier, V.; Flüß, F.; Rohde, D.; Gebert, A.; Sievers, H.-H. Detoxification and Endothelialization of Glutaraldehyde-Fixed Bovine Pericardium with Titanium Coating. Circulation 2009, 119, 1653–1660. [Google Scholar] [CrossRef]
- Chang, Y.; Tsai, C.-C.; Liang, H.-C.; Sung, H.-W. In vivo evaluation of cellular and acellular bovine pericardia fixed with a naturally occurring crosslinking agent (genipin). Biomaterials 2001, 23, 2447–2457. [Google Scholar] [CrossRef]
- Ricotti, L.; Polini, A.; Genchi, G.G.; Ciofani, G.; Iandolo, D.; Vazão, H.; Mattoli, V.; Ferreira, L.; Menciassi, A.; Pisignano, D. Proliferation and skeletal myotube formation capability of C2C12 and H9c2 cells on isotropic and anisotropic electrospun nanofibrous PHB scaffolds. Biomed. Mater. 2012, 7, 035010. [Google Scholar] [CrossRef]
- Yamamoto, D.L.; Csikasz, R.I.; Li, Y.; Sharma, G.; Hjort, K.; Karlsson, R.; Bengtsson, T. Myotube Formation on Micro-patterned Glass: Intracellular Organization and Protein Distribution in C2C12 Skeletal Muscle Cells. J. Histochem. Cytochem. 2008, 56, 881–892. [Google Scholar] [CrossRef]
- Springer, M.L.; Ozawa, C.R.; Blau, H.M. Transient production of α-smooth muscle actin by skeletal myoblasts during differentiation in culture and following intramuscular implantation. Cell Motil. Cytoskelet. 2002, 51, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Renna, L.V.; Cardani, R.; Botta, A.; Rossi, G.; Fossati, B.; Costa, E.; Meola, G. Premature senescence in primary muscle cultures of myotonic dystrophy type 2 is not associated with p16 induction. Eur. J. Histochem. 2014, 58, 2444. [Google Scholar] [CrossRef]
- Li, N.; Li, Y.; Gong, D.; Xia, C.; Liu, X.; Xu, Z. Efficient decellularization for bovine pericardium with extracellular matrix preservation and good biocompatibility. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, D.C.; Konieczny, S.F. Transcription factor families: Muscling in on the myogenic program. FASEB J. 1995, 9, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Lehka, L.; Rędowicz, M.J. Mechanisms regulating myoblast fusion: A multilevel interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef]
- Han, J.-W.; Lee, H.-J.; Bae, G.-U.; Kang, J.-S. Promyogenic function of Integrin/FAK signaling is mediated by Cdo, Cdc42 and MyoD. Cell. Signal. 2011, 23, 1162–1169. [Google Scholar] [CrossRef]
- McClure, M.J.; Clark, N.M.; Hyzy, S.L.; Chalfant, C.E.; Olivares-Navarrete, R.; Boyan, B.D.; Schwartz, Z. Role of integrin α7β1 signaling in myoblast differentiation on aligned polydioxanone scaffolds. Acta Biomater. 2016, 39, 44–54. [Google Scholar] [CrossRef]
- Olivero, D.K.; Furcht, L.T. Type IV Collagen, Laminin, and Fibronectin Promote the Adhesion and Migration of Rabbit Lens Epithelial Cells in Vitro. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2825–2834. [Google Scholar]
- Foster, R.F.; Thompson, J.M.; Kaufman, S.J. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: Analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev. Biol. 1987, 122, 11–20. [Google Scholar] [CrossRef]
- Bettadapur, A.; Suh, G.C.; Geisse, N.A.; Wang, E.R.; Hua, C.; Huber, H.A.; Viscio, A.A.; Kim, J.Y.; Strickland, J.B.; McCain, M.L. Prolonged Culture of Aligned Skeletal Myotubes on Micromolded Gelatin Hydrogels. Sci. Rep. 2016, 6, 28855. [Google Scholar] [CrossRef]
- Xing, Q.; Parvizi, M.; Higuita, M.L.; Griffiths, L.G. Basement membrane proteins modulate cell migration on bovine pericardium extracellular matrix scaffold. Sci. Rep. 2021, 11, 4607. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Horie, M.; Nagasaka, S.; Ohsumi, S.; Shimizu, K.; Honda, H.; Nagamori, E.; Fujita, H.; Kawamoto, T. Effect of cell-extracellular matrix interaction on myogenic characteristics and artificial skeletal muscle tissue. J. Biosci. Bioeng. 2020, 130, 98–105. [Google Scholar] [CrossRef]
- Bois, P.R.; Grosveld, G.C. FKHR (FOXO1a) is required for myotube fusion of primary mouse myoblasts. EMBO J. 2003, 22, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, R.; Schmidt, A.; Linton, J.M.; Wang, D.; Backus, C.; Denda, S.; Müller, U.; Reichardt, L.F. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin α8β1 in the embryonic kidney. J. Cell Biol. 2001, 154, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, O.; Periasamy, M.; Kan, M.; Matsuda, R. Cis-4-hydroxy-l-proline and ethyl-3,4-dihydroxybenzoate prevent myogenesis of C2C12 muscle cells and block myoD1 and myogenin expression. Exp. Cell Res. 1992, 200, 70–76. [Google Scholar] [CrossRef]
- Grounds, M.D. Complexity of Extracellular Matrix and Skeletal Muscle Regeneration. In Skeletal Muscle Repair and Regeneration; Advances in Muscle Research; Springer: Dordrecht, The Netherlands, 2008; Volume 3, pp. 269–302. ISBN 978-1-4020-6767-9. [Google Scholar]
- Urciuolo, A.; Quarta, M.; Morbidoni, V.; Gattazzo, F.; Molon, S.; Grumati, P.; Montemurro, F.; Tedesco, F.S.; Blaauw, B.; Cossu, G.; et al. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013, 4, 1964. [Google Scholar] [CrossRef]
- Fuentealba, L.; Carey, D.J.; Brandan, E. Antisense Inhibition of Syndecan-3 Expression during Skeletal Muscle Differentiation Accelerates Myogenesis through a Basic Fibroblast Growth Factor-dependent Mechanism. J. Biol. Chem. 1999, 274, 37876–37884. [Google Scholar] [CrossRef][Green Version]
- Larraín, J.; Carey, D.J.; Brandan, E. Syndecan-1 Expression Inhibits Myoblast Differentiation through a Basic Fibroblast Growth Factor-dependent Mechanism. J. Biol. Chem. 1998, 273, 32288–32296. [Google Scholar] [CrossRef]
- Riquelme, C.; Larraín, J.; Schönherr, E.; Henriquez, J.P.; Kresse, H.; Brandan, E. Antisense Inhibition of Decorin Expression in Myoblasts Decreases Cell Responsiveness to Transforming Growth Factor β and Accelerates Skeletal Muscle Differentiation. J. Biol. Chem. 2001, 276, 3589–3596. [Google Scholar] [CrossRef]
- Stern, M.M.; Myers, R.L.; Hammam, N.; Stern, K.A.; Eberli, D.; Kritchevsky, S.B.; Soker, S.; Van Dyke, M. The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials 2009, 30, 2393–2399. [Google Scholar] [CrossRef]
- Denes, L.T.; Riley, L.A.; Mijares, J.R.; Arboleda, J.D.; McKee, K.; Esser, K.A.; Wang, E.T. Culturing C2C12 myotubes on micromolded gelatin hydrogels accelerates myotube maturation. Skelet. Muscle 2019, 9, 17. [Google Scholar] [CrossRef]
- Palade, J.; Pal, A.; Rawls, A.; Stabenfeldt, S.; Wilson-Rawls, J. Molecular analysis of muscle progenitor cells on extracellular matrix coatings and hydrogels. Acta Biomater. 2019, 97, 296–309. [Google Scholar] [CrossRef]
- Bigot, A.; Klein, A.F.; Gasnier, E.; Jacquemin, V.; Ravassard, P.; Butler-Browne, G.; Mouly, V.; Furling, D. Large CTG Repeats Trigger p16-Dependent Premature Senescence in Myotonic Dystrophy Type 1 Muscle Precursor Cells. Am. J. Pathol. 2009, 174, 1435–1442. [Google Scholar] [CrossRef]
- Deane, C.S.; Hughes, D.C.; Sculthorpe, N.; Lewis, M.P.; Stewart, C.E.; Sharples, A.P. Impaired hypertrophy in myoblasts is improved with testosterone administration. J. Steroid Biochem. Mol. Biol. 2013, 138, 152–161. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
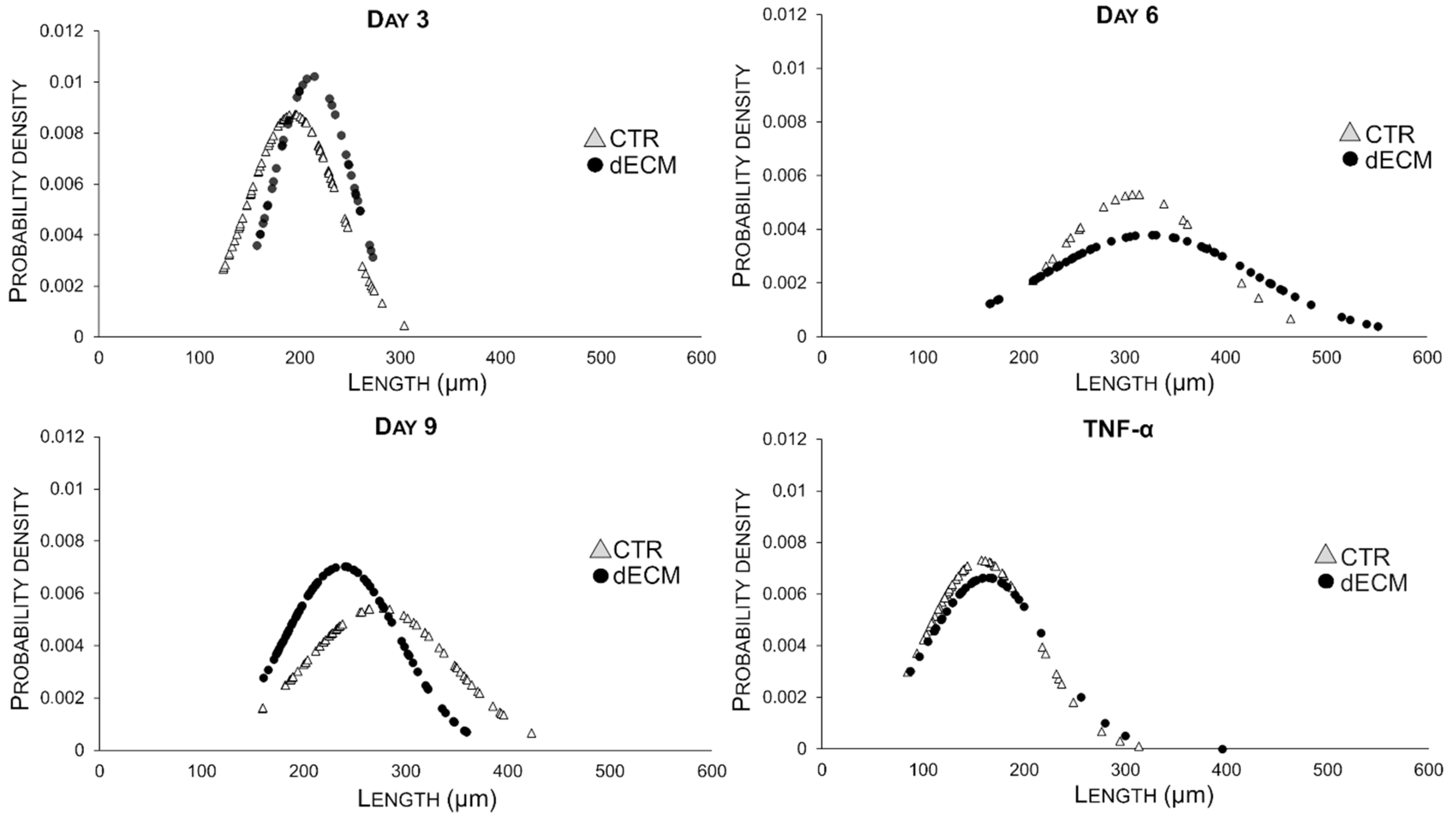
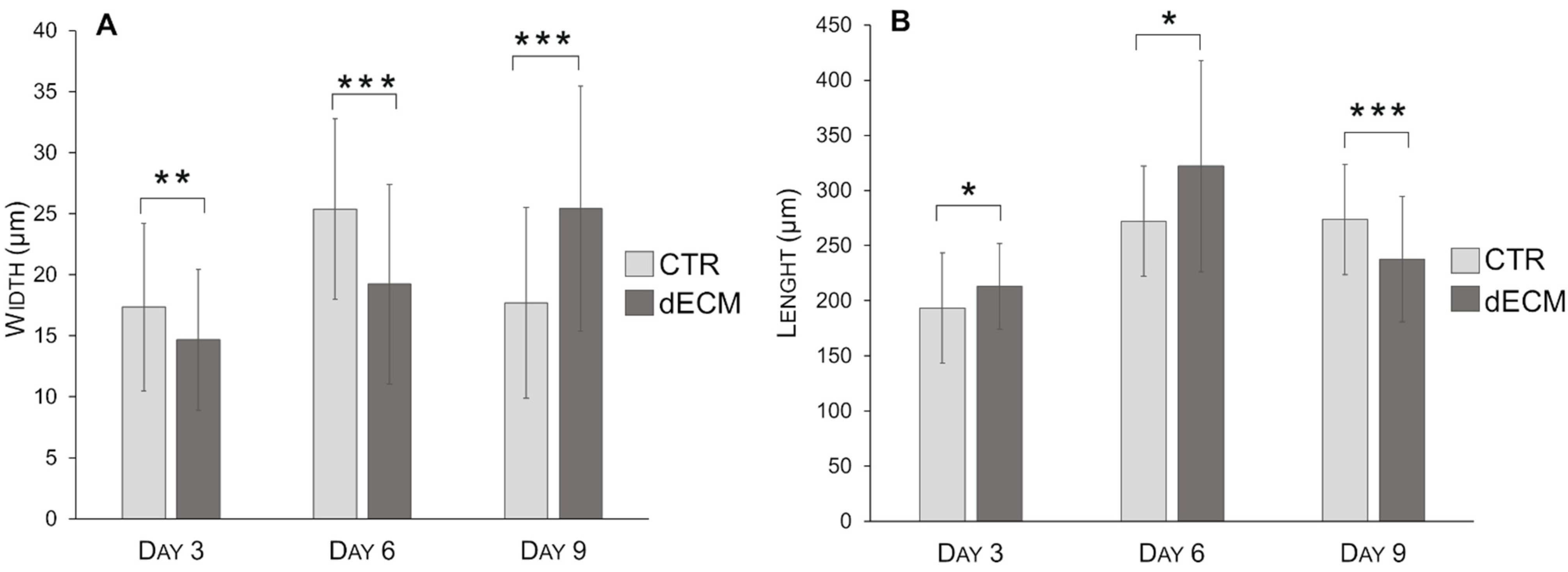
| Myotubes Width (µm) | CTR | dECM | p Values | Myotubes Length (µm) | CTR | dECM | p Values |
|---|---|---|---|---|---|---|---|
| Day 3 | 17.30 ± 6.86 | 14.67 ± 5.77 | ** | Day 3 | 193.45 ± 45.54 | 213.13 ± 38.90 | * |
| Day 6 | 25.39 ± 7.39 | 19.23 ± 8.19 | *** | Day 6 | 310.84 ± 75.27 | 324.34 ±105.31 | *** |
| Day 9 | 17.70 ± 7.81 | 25.43 ± 10.04 | *** | Day 9 | 273.57 ± 73.16 | 237.83 ± 56.70 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carton, F.; Di Francesco, D.; Fusaro, L.; Zanella, E.; Apostolo, C.; Oltolina, F.; Cotella, D.; Prat, M.; Boccafoschi, F. Myogenic Potential of Extracellular Matrix Derived from Decellularized Bovine Pericardium. Int. J. Mol. Sci. 2021, 22, 9406. https://doi.org/10.3390/ijms22179406
Carton F, Di Francesco D, Fusaro L, Zanella E, Apostolo C, Oltolina F, Cotella D, Prat M, Boccafoschi F. Myogenic Potential of Extracellular Matrix Derived from Decellularized Bovine Pericardium. International Journal of Molecular Sciences. 2021; 22(17):9406. https://doi.org/10.3390/ijms22179406
Chicago/Turabian StyleCarton, Flavia, Dalila Di Francesco, Luca Fusaro, Emma Zanella, Claudio Apostolo, Francesca Oltolina, Diego Cotella, Maria Prat, and Francesca Boccafoschi. 2021. "Myogenic Potential of Extracellular Matrix Derived from Decellularized Bovine Pericardium" International Journal of Molecular Sciences 22, no. 17: 9406. https://doi.org/10.3390/ijms22179406
APA StyleCarton, F., Di Francesco, D., Fusaro, L., Zanella, E., Apostolo, C., Oltolina, F., Cotella, D., Prat, M., & Boccafoschi, F. (2021). Myogenic Potential of Extracellular Matrix Derived from Decellularized Bovine Pericardium. International Journal of Molecular Sciences, 22(17), 9406. https://doi.org/10.3390/ijms22179406








