Enhanced Performance of Zn/Br Flow Battery Using N-Methyl-N-Propylmorpholinium Bromide as Complexing Agent
Abstract
:1. Introduction
2. Results and Discussion
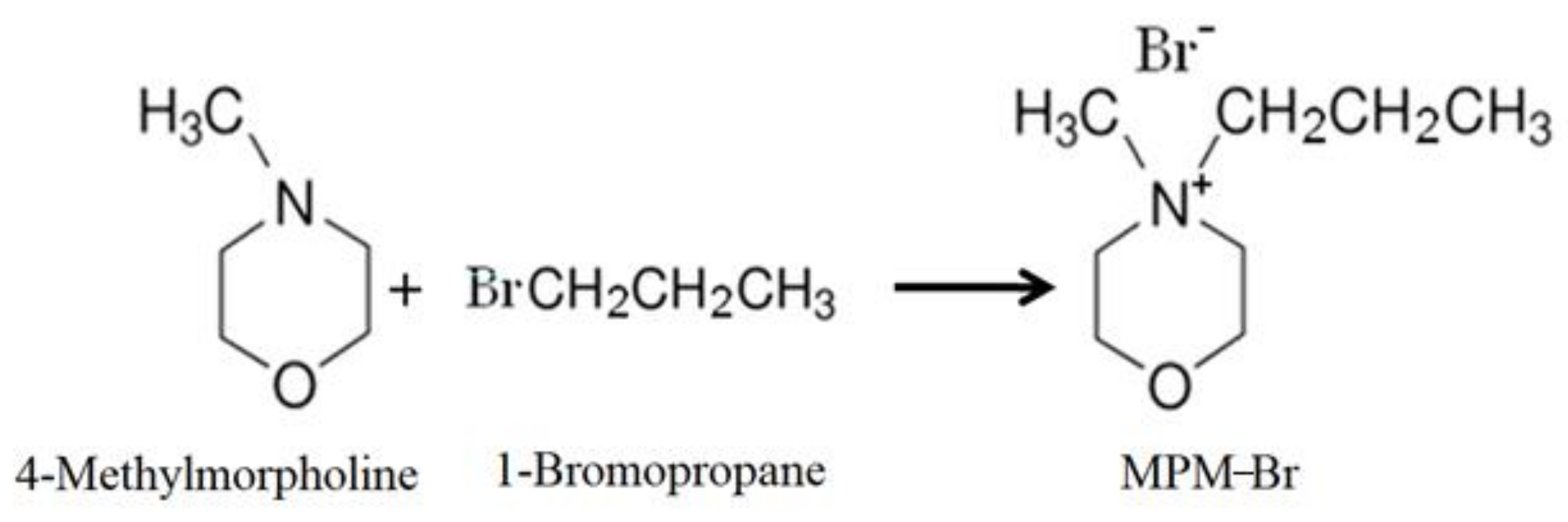
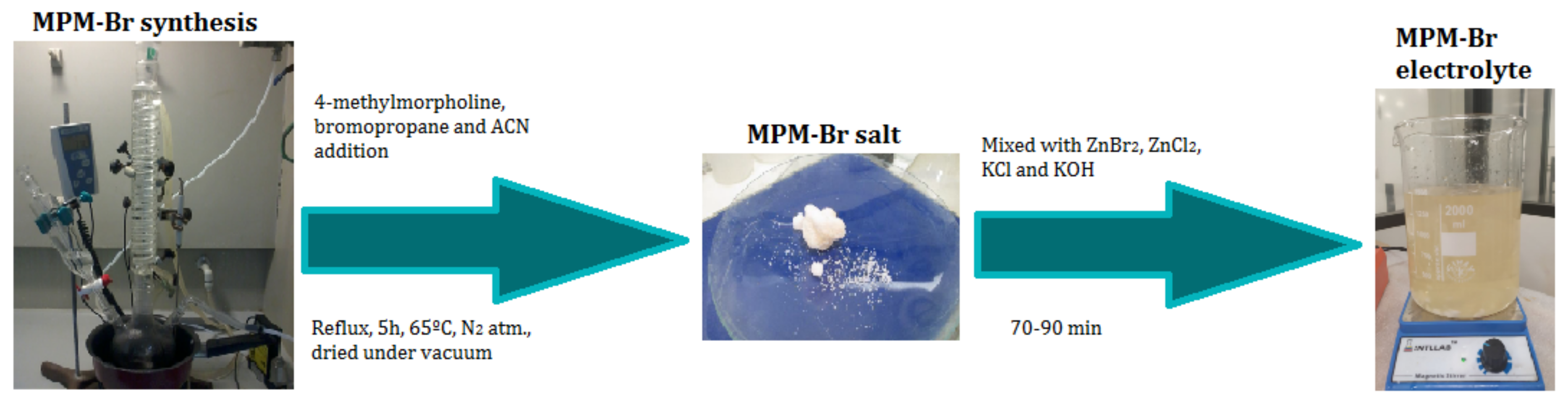
2.1. Synthesis of MPM-Br Salt
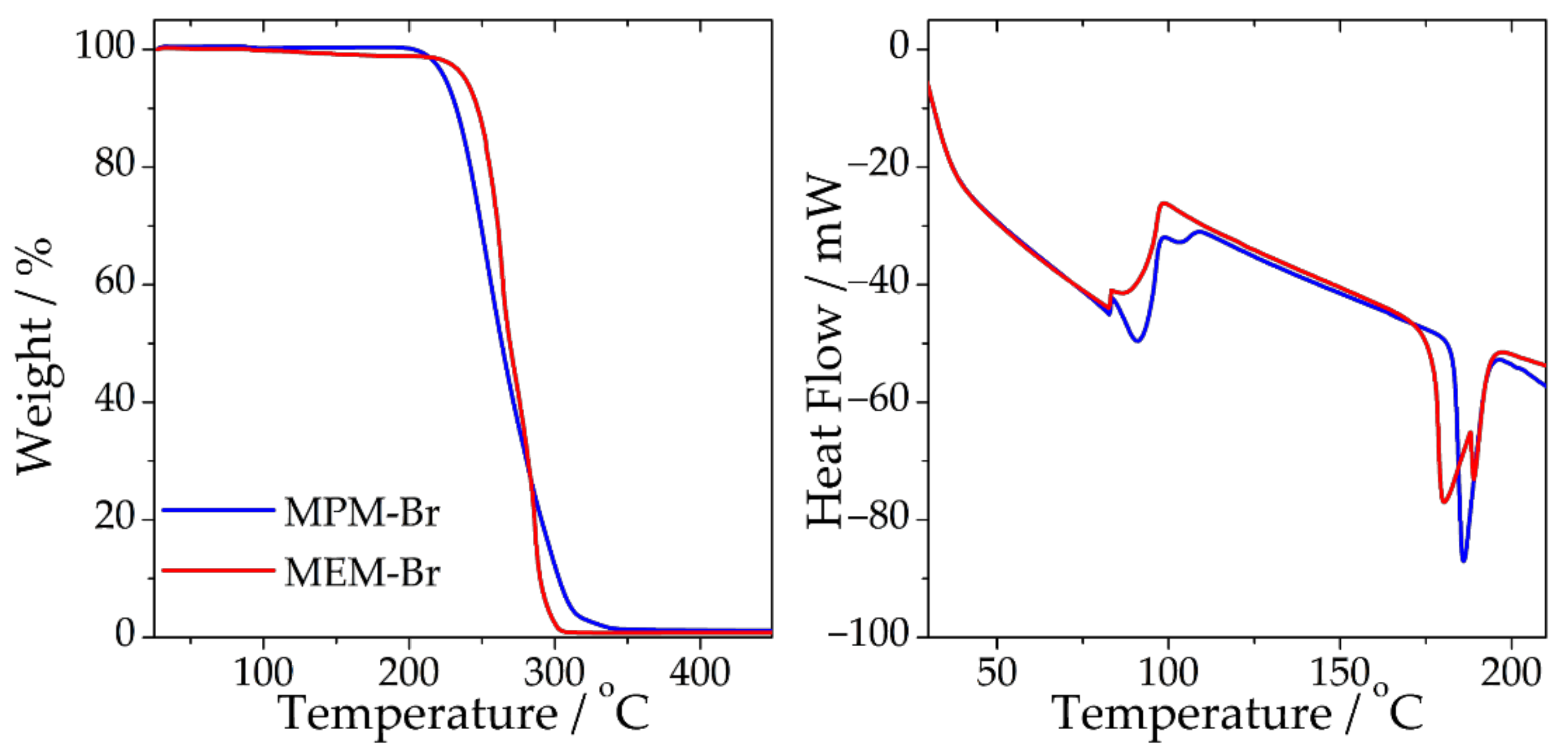
2.2. Physicochemical Properties of MPM-Br
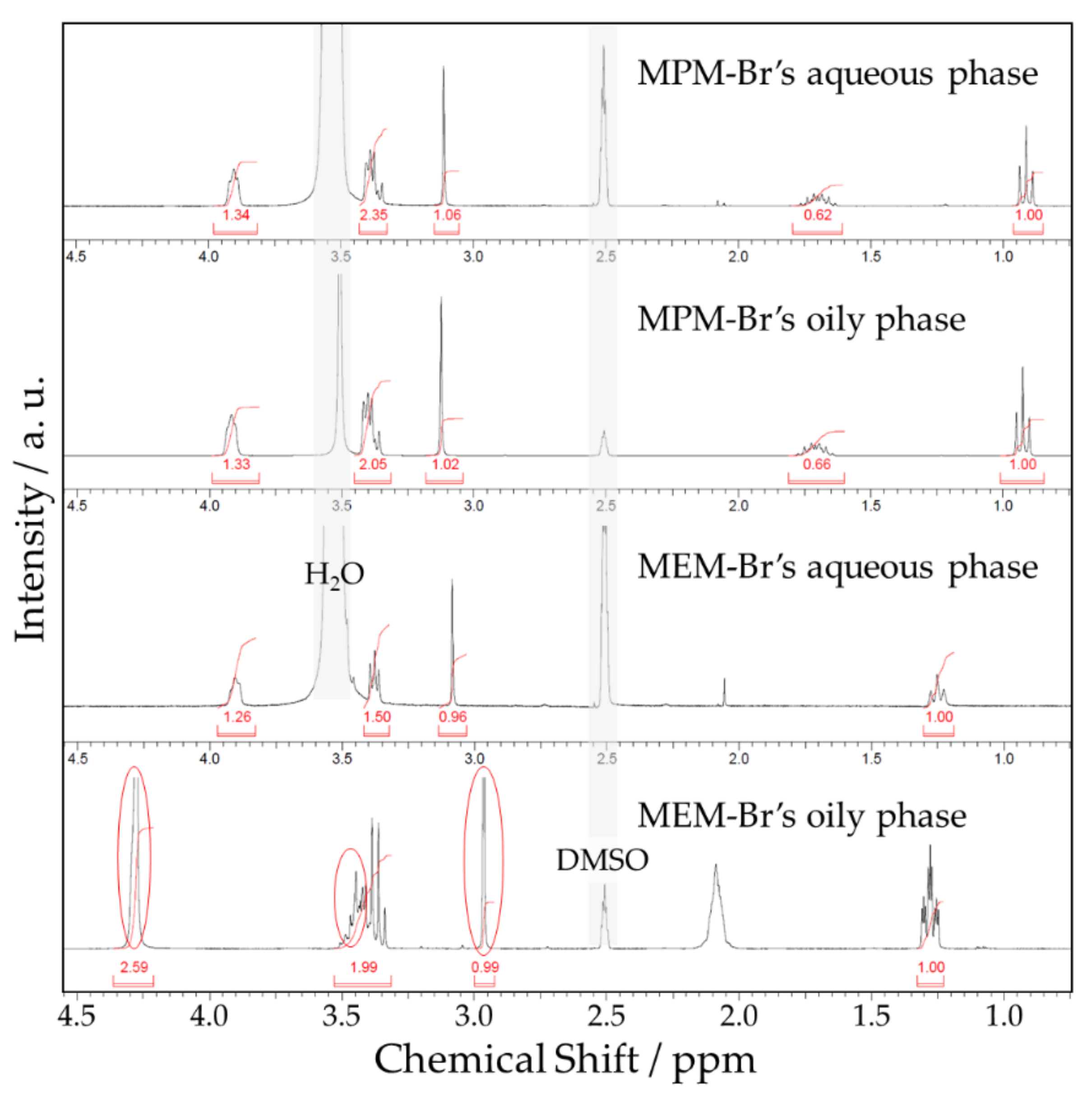
2.3. Physicochemical Properties of Electrolytes
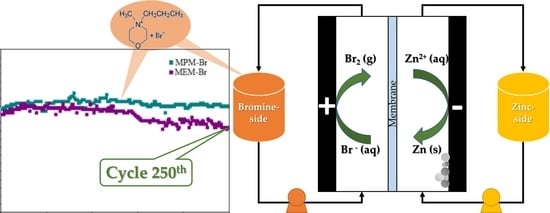
2.4. Electrochemical Characterisation
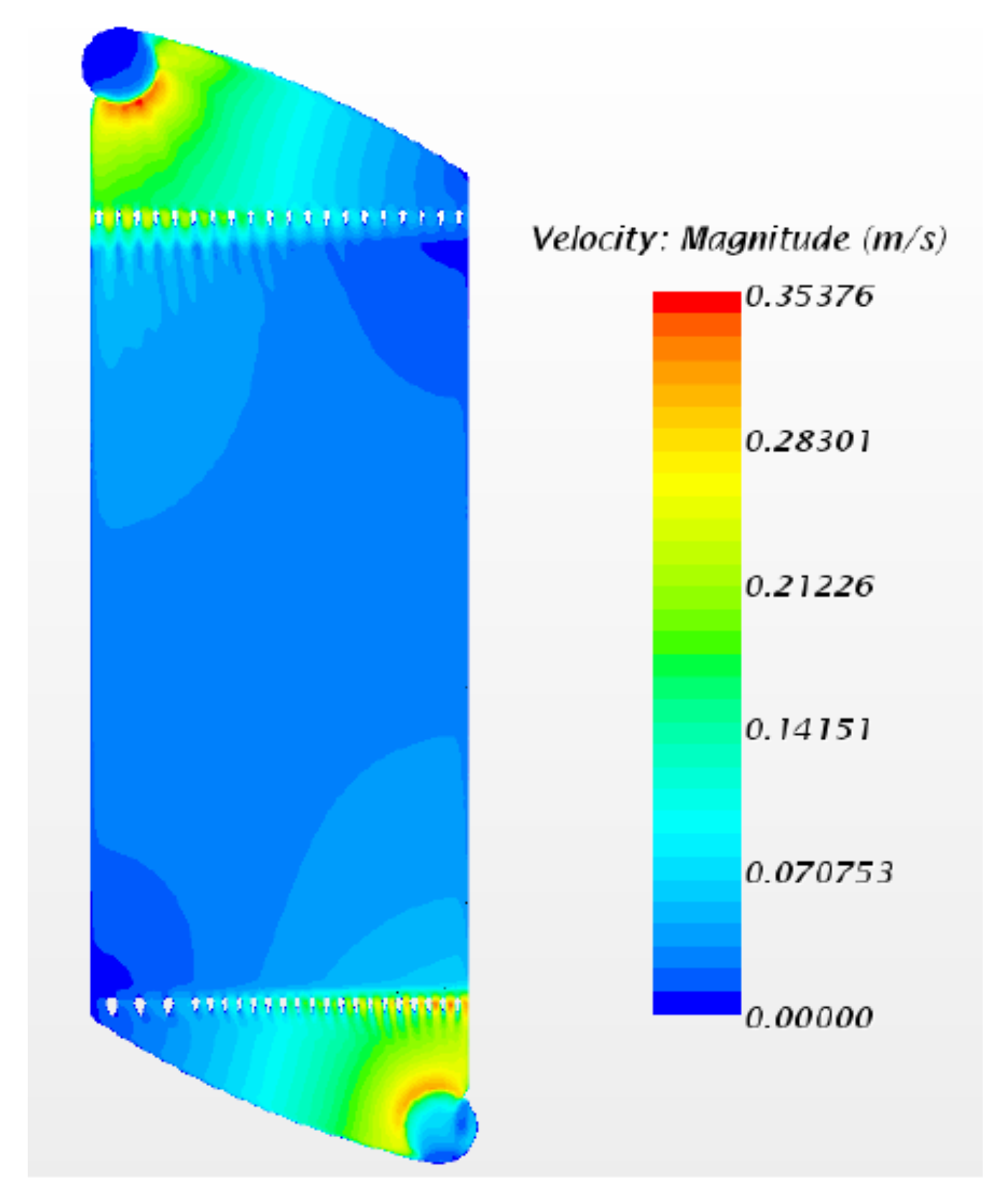
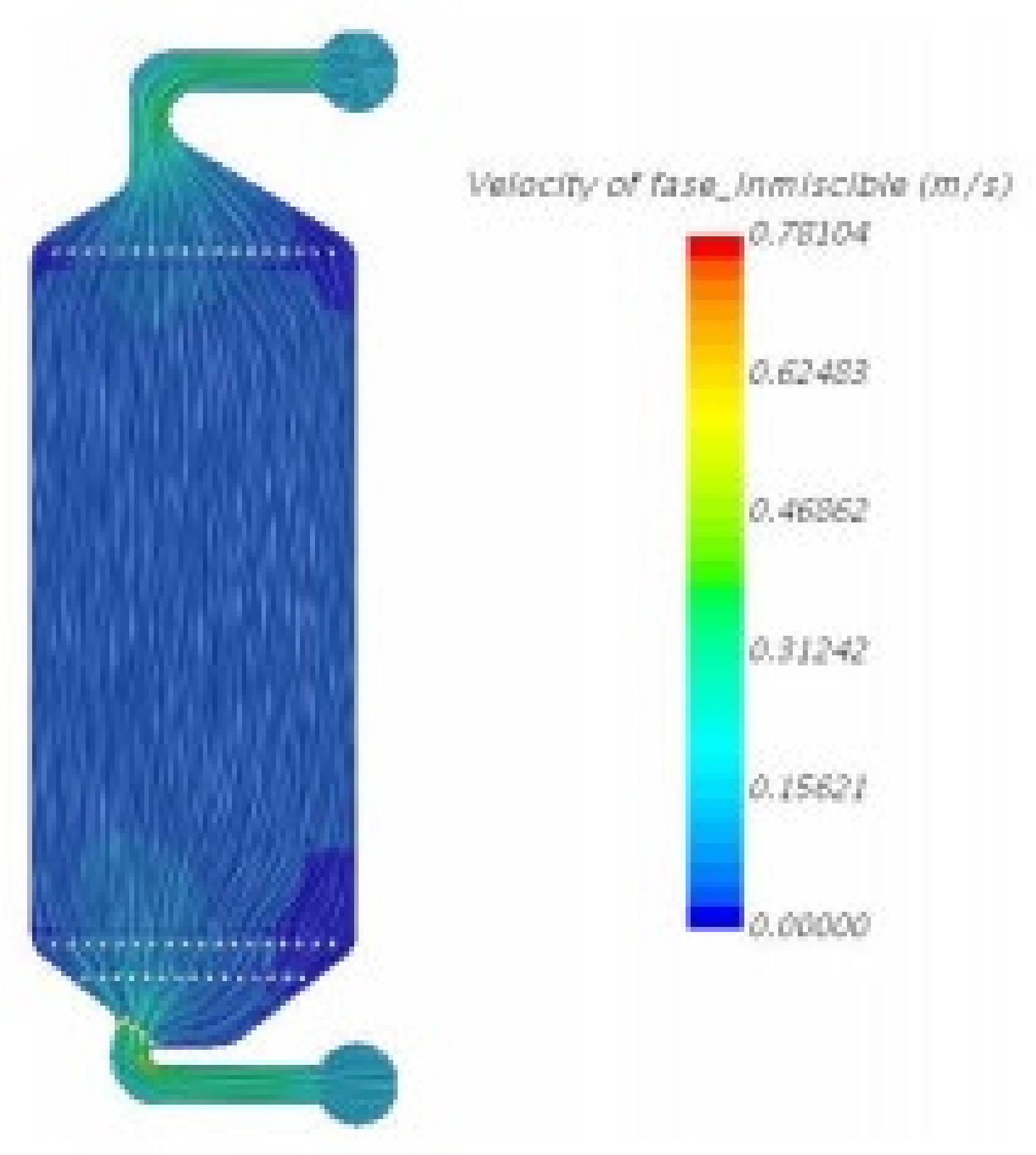
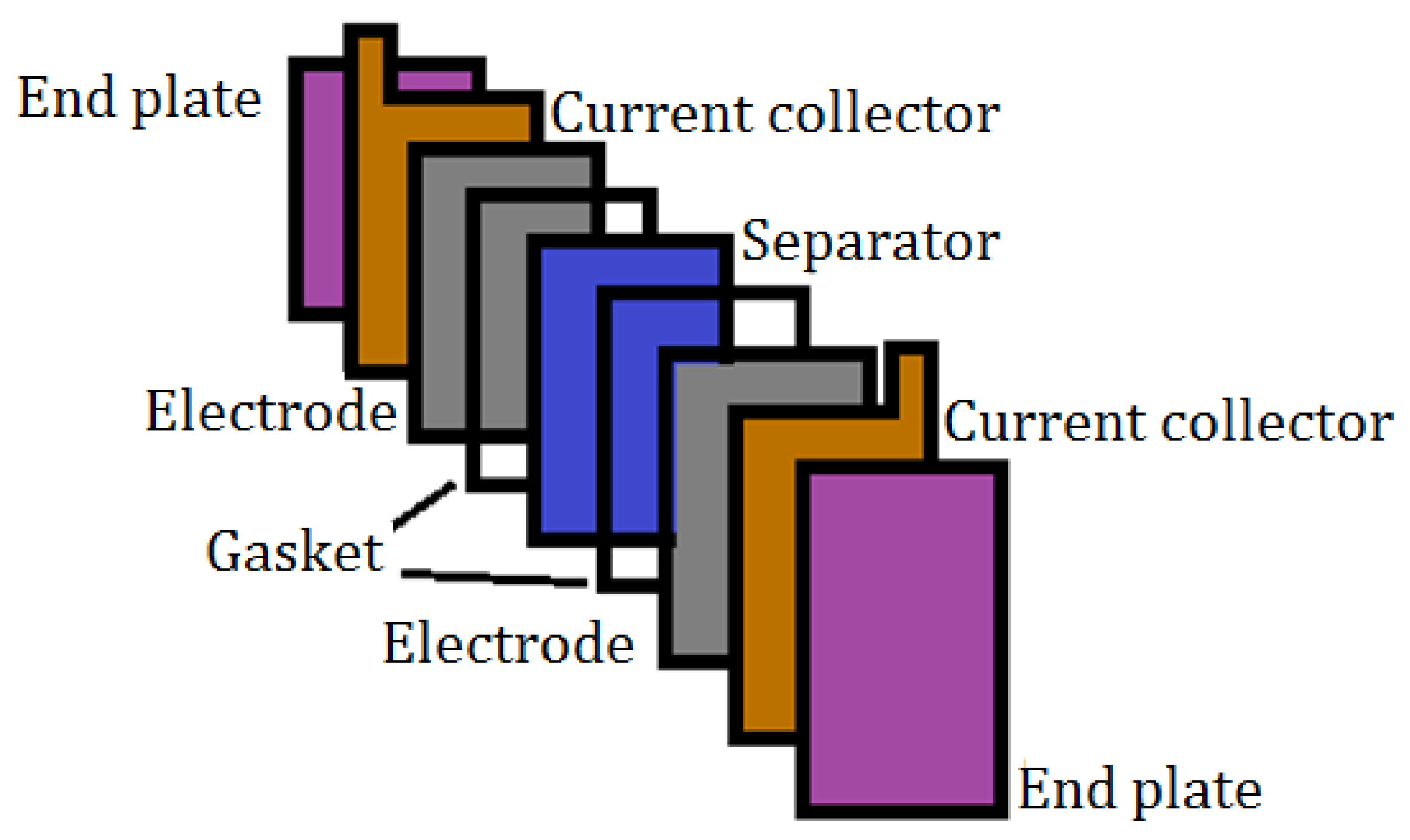
2.4.1. Design and Assembly of the Cell Prototype
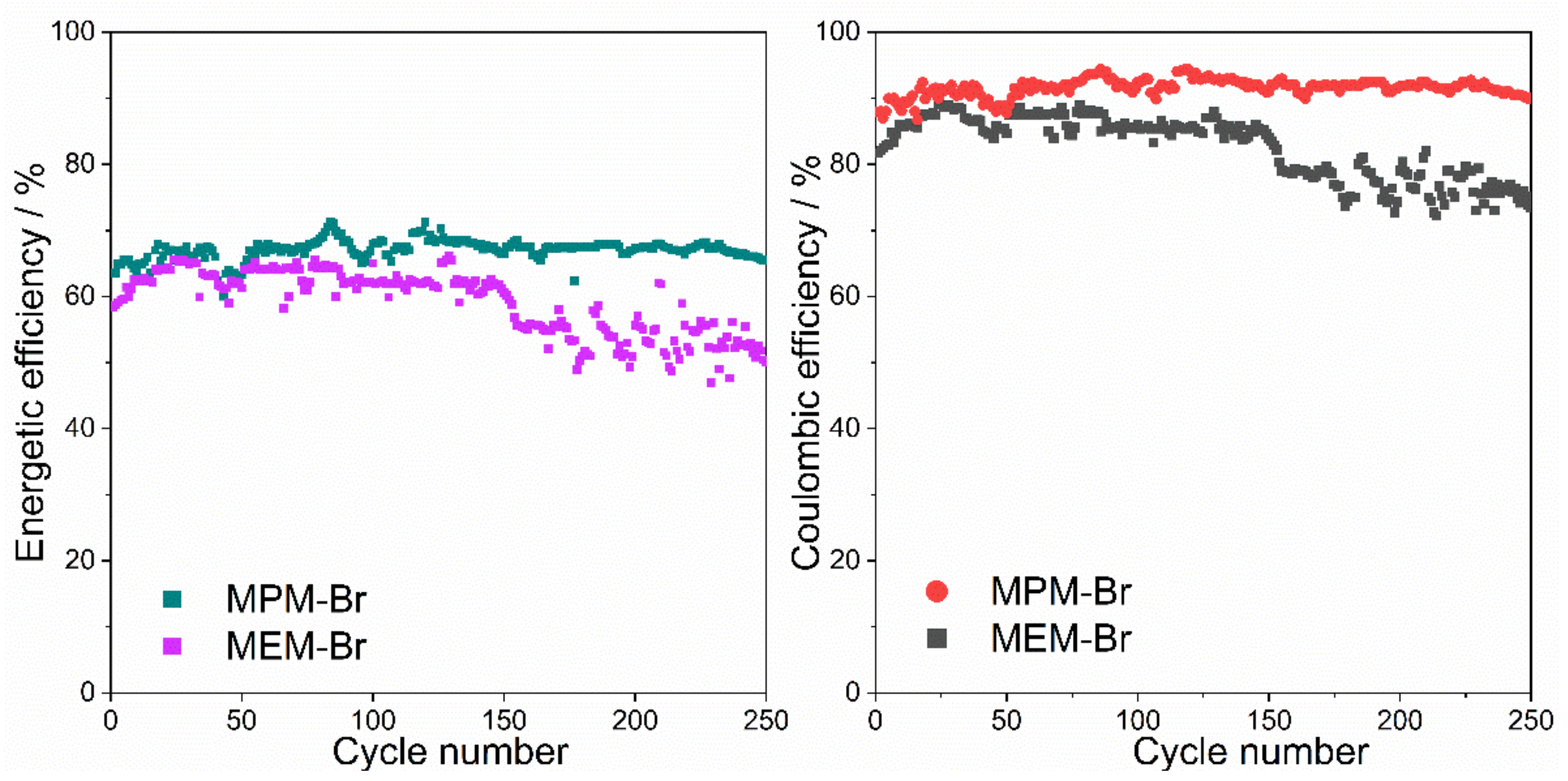
2.4.2. Electrochemical Performance
3. Materials and Methods
3.1. Electrolyte Preparation
3.2. Characterisation of the Electrolyte
3.3. Electrochemical Characterisation
3.3.1. Design and Assembly of the Cell Prototype
3.3.2. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.V.; Julien, C.M.; Mauger, A.; Zaghib, K. Sulfide and Oxide Inorganic Solid Electrolytes for All-Solid-State Li Batteries: A Review. Nanomaterials 2020, 10, 1606. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Thakur, A.K.; Majumder, M.; Patole, S.P.; Zaghib, K.; Reddy, M.V. Metal-organic framework-based materials: Advances, exploits, and challenges in promoting post Li-ion battery technologies. Mater. Adv. 2021, 2, 2457–2482. [Google Scholar] [CrossRef]
- Rajarathnam, G.P.; Vassallo, A.M. The Zinc/Bromine Flow Battery; Springer: Singapore, 2016. [Google Scholar]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef] [Green Version]
- Linden, D.; Reddy, T.B. Handbook of Batteries, 3rd ed.; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Vanýsek, P.; Novák, V. Redox flow batteries as the means for energy storage. J. Energy Storage 2017, 13, 435–441. [Google Scholar] [CrossRef]
- Menictas, C.; Skyllas-Kazacos, M.; Lim, T.M. Advances in Batteries for Medium and Large-Scale Energy Storage; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- Wu, M.C.; Zhao, T.S.; Wei, L.; Jiang, H.R.; Zhang, R.H. Improved electrolyte for zinc-bromine flow batteries. J. Power Sources 2018, 384, 232–239. [Google Scholar] [CrossRef]
- Kim, M.; Yun, D.; Jeon, J. Effect of a bromine complex agent on electrochemical performances of zinc electrodeposition and electrodissolution in Zinc–Bromide flow battery. J. Power Sources 2019, 438, 227020. [Google Scholar] [CrossRef]
- Schneider, M.; Rajarathnam, G.P.; Easton, M.E.; Masters, A.F.; Maschmeyer, T.; Vassallo, A.M. The influence of novel bromine sequestration agents on zinc/bromine flow battery performance. RSC Adv. 2016, 6, 110548–110556. [Google Scholar] [CrossRef]
- Jeon, J.-D.; Yang, H.S.; Shim, J.; Kim, H.S.; Yang, J.H. Dual function of quaternary ammonium in Zn/Br redox flow battery: Capturing the bromine and lowering the charge transfer resistance. Electrochim. Acta 2014, 127, 397–402. [Google Scholar] [CrossRef]
- Yang, J.H.; Yang, H.S.; Ra, H.W.; Shim, J.; Jeon, J.-D. Effect of a surface active agent on performance of zinc/bromine redox flow batteries: Improvement in current efficiency and system stability. J. Power Sources 2015, 275, 294–297. [Google Scholar] [CrossRef]
- Cha, J.-H.; Kim, K.-S.; Choi, S.; Yeon, S.-H.; Lee, H.; Kim, H.S.; Kim, H. Thermal and Electrochemical Properties of Morpholinium Salts with Bromide Anion. Korean J. Chem. Eng. 2005, 22, 945–948. [Google Scholar] [CrossRef]
- Marcinkowski, L.; Olszewska, T.; Kloskowski, A.; Warmińska, D. Apparent Molar Volumes and Expansivities of Ionic Liquids Based on N-Alkyl-N-methylmorpholinium Cations in Acetonitrile. J. Chem. Eng. Data 2014, 59, 718–725. [Google Scholar] [CrossRef]
- Domańska, U.; Karpińska, M.; Zawadzki, M. Activity coefficients at infinite dilution for organic solutes and water in 1-ethyl-1-methylpyrrolidinium lactate. J. Chem. Thermodyn. 2015, 89, 127–133. [Google Scholar] [CrossRef]
- Bryans, D.; McMillan, B.G.; Spicer, M.; Wark, A.; Berlouis, L. Complexing Additives to Reduce the Immiscible Phase Formed in the Hybrid ZnBr2 Flow Battery. J. Electrochem. Soc. 2017, 164, A3342–A3348. [Google Scholar] [CrossRef] [Green Version]
- Poon, G.; Parasuraman, A.; Lim, T.M.; Skyllas-Kazacos, M. Evaluation of N-ethyl-N-methyl-morpholinium bromide and N-ethyl-N-methyl-pyrrolidinium bromide as bromine complexing agents in vanadium bromide redox flow batteries. Electrochim. Acta 2013, 107, 388–396. [Google Scholar] [CrossRef]
- Cathro, K.J.; Cedzynska, K.; Constable, D.C.; Hoobin, P.M. Selection of quaternary ammonium bromides for use in zinc/bromine cells. J. Power Sources 1986, 18, 349–370. [Google Scholar] [CrossRef]
- Küttinger, M.; Brunetaud, R.; Włodarczyk, J.K.; Fischer, P.; Tübke, J. Cycle behaviour of hydrogen bromine redox flow battery cells with bromine complexing agents. J. Power Sources 2021, 495, 229820. [Google Scholar] [CrossRef]
- Zawadzki, M.; Królikowska, M.; Antonowicz, J.; Lipiński, P.; Karpińska, M. Physicochemical and thermodynamic properties of the {1-alkyl-1-methylmorpholinium bromide, [C1Cn=3,4,5MOR]Br, or 1-methyl-1-pentylpiperidinium bromide, [C1C5PIP]Br + water} binary systems. J. Chem. Thermodyn. 2016, 98, 324–337. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, S.; Demberelnyamba, D.; Lee, H.; Oh, J.; Lee, B.-B.; Mun, S.-J. Ionic liquids based on N-alkyl-N-methylmorpholinium salts as potential electrolytes. Chem. Commun. 2004, 0, 828–829. [Google Scholar] [CrossRef]
- Choi, S.; Kim, K.-S.; Lee, H.; Oh, J.S.; Lee, B.-B. Synthesis and ionic conductivities of lithium-doped morpholinium salts. Korean J. Chem. Eng. 2005, 22, 281–284. [Google Scholar] [CrossRef]
- Kautek, W.; Conradi, A.; Sahre, M.; Fabjan, C.; Drobits, J.; Bauer, G.; Schuster, P. In Situ Investigations of Bromine-Storing Complex Formation in a Zinc-Flow Battery at Gold Electrodes. J. Electrochem. Soc. 1999, 146, 3211. [Google Scholar] [CrossRef]
- Skoog, D.A.; Skoog, A.; Crouch, S.R.; Holler, F.J.; Crouch, S.H. Principles of Instrumental Analysis, 7th ed.; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Zawadzki, M.; Królikowska, M.; Lipiński, P. Physicochemical and thermodynamic characterization of N-alkyl-N-methylpyrrolidinium bromides and its aqueous solutions. Thermochim. Acta 2014, 589, 148–157. [Google Scholar] [CrossRef]
- Lancry, E.; Magnes, B.-Z.; Ben-David, I.; Freiberg, M. New Bromine Complexing Agents for Bromide Based Batteries. ECS Trans. 2013, 53, 107. [Google Scholar] [CrossRef]
- Engineering Simulation & 3D Design Software|Ansys. Available online: https://www.ansys.com/ (accessed on 23 July 2021).
- Suresh, S.; Kesavan, T.; Munaiah, Y.; Arulraj, I.; Dheenadayalan, S.; Ragupathy, P. Zinc–bromine hybrid flow battery: Effect of zinc utilization and performance characteristics. RSC Adv. 2014, 4, 37947–37953. [Google Scholar] [CrossRef]




| BCA | Structure | Precursors | Synthesis Conditions | Dried Under Vacuum | [C] | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Tª (°C) | Time | Reflux | Atmosphere | ||||||
| N-ethyl-N-methylmorpholinium bromide |  | 4-methylmorpholine, bromoethane, acetonitrile | 65 | 5 h | Yes | N2 | Yes | 1–3 M | [15,16] |
| 1-ethyl-1-methylpyrrolidinium bromide | 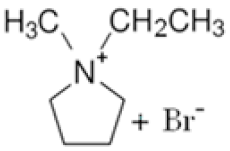 | N-methylpyrrolidine, bromoethane, acetonitrile | RT | 24 h | No | No | Yes | 1–3 M | [5,17] |
| N-propyl-N-methylmorpholinium bromide | 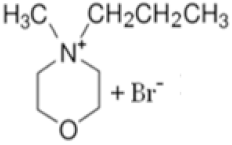 | 4-methylmorpholine, bromopropane, acetonitrile | 65 | 5 h | Yes | N2 | Yes | 1–3 M | [15] |
| 1-(carboxymethyl) pyridine-1-ium bromide | 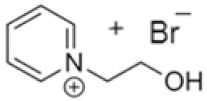 | Pyridine, 2-bromoacetic acid, ethyl acetate | RT | 4 h | Yes | No | No | 1–3 M | [5,18] |
| 1-(2-carboximethyl)-1-methylmorpholinium bromide | 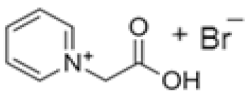 | 4-methylmorpholine, 3-chloropropanoic acid | RT | 4 h | No | No | Yes | 1–3 M | [5,18] |
| 1-(2-carboximethyl)-1-methylpyrrolidinium bromide | 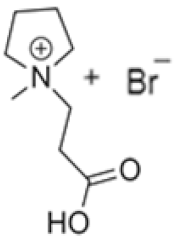 | N-methylpyrrolidine, 3-chloropropanoic acid | RT | 4 h | No | No | Yes | 1–3 M | [5,18] |
| Temp. (°C) | Solvent (mL/g Reactants) | Adduct Excess (%) | N2 | Reflux | Crystallisation (h) | Yield (%) |
|---|---|---|---|---|---|---|
| 70 | 1.41 | 14 | Yes | Yes | 12 | 68 |
| 70 | 1.11 | 60 | Yes | Yes | 12 | 85 |
| 70 | 0 | 60 | Yes | Yes | 12 | 19 |
| 50 | 1.11 * | 60 | Yes | Yes | 12 | 10 |
| 70 | 0.55 | 60 | Yes | Yes | 2 | 90 |
| 25 | 0.59 | 50 | No | No | 120 | 89 |
| Temp. (°C) | Solvent (mL/g Reactants) | Adduct Excess (%) | N2 | Time (h) | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| N.R. | N.R.* | 300 | N.R. | N.R. | 90 | [17] |
| 60 | 2.74 | 35 | Yes | 5 | 90 | [25] |
| N.R. | 3.22 | 0 | Yes | 5 | 90 | [26] |
| 70 | 3.22 | 0 | Yes | 5 | 90 | [27] |
| 70 | 1.61 | 0 | Yes | 5 | 78 | This work |
| Functional Group | Frequency Range (cm−1) | Frequency in MPM-Br Spectra * (cm−1) |
|---|---|---|
| C–H | 3010–3100 (aromatic) 2850–2970 (alkane) 1340–1470 (alkane) 690–900 (aromatic) | 3016 2960, 2874 1462 974, 937, 914, 881, 851, 748 |
| C–N | 1180–1360 | 1140, 1113 |
| C–O | 1050–1300 | 1140, 1113 |
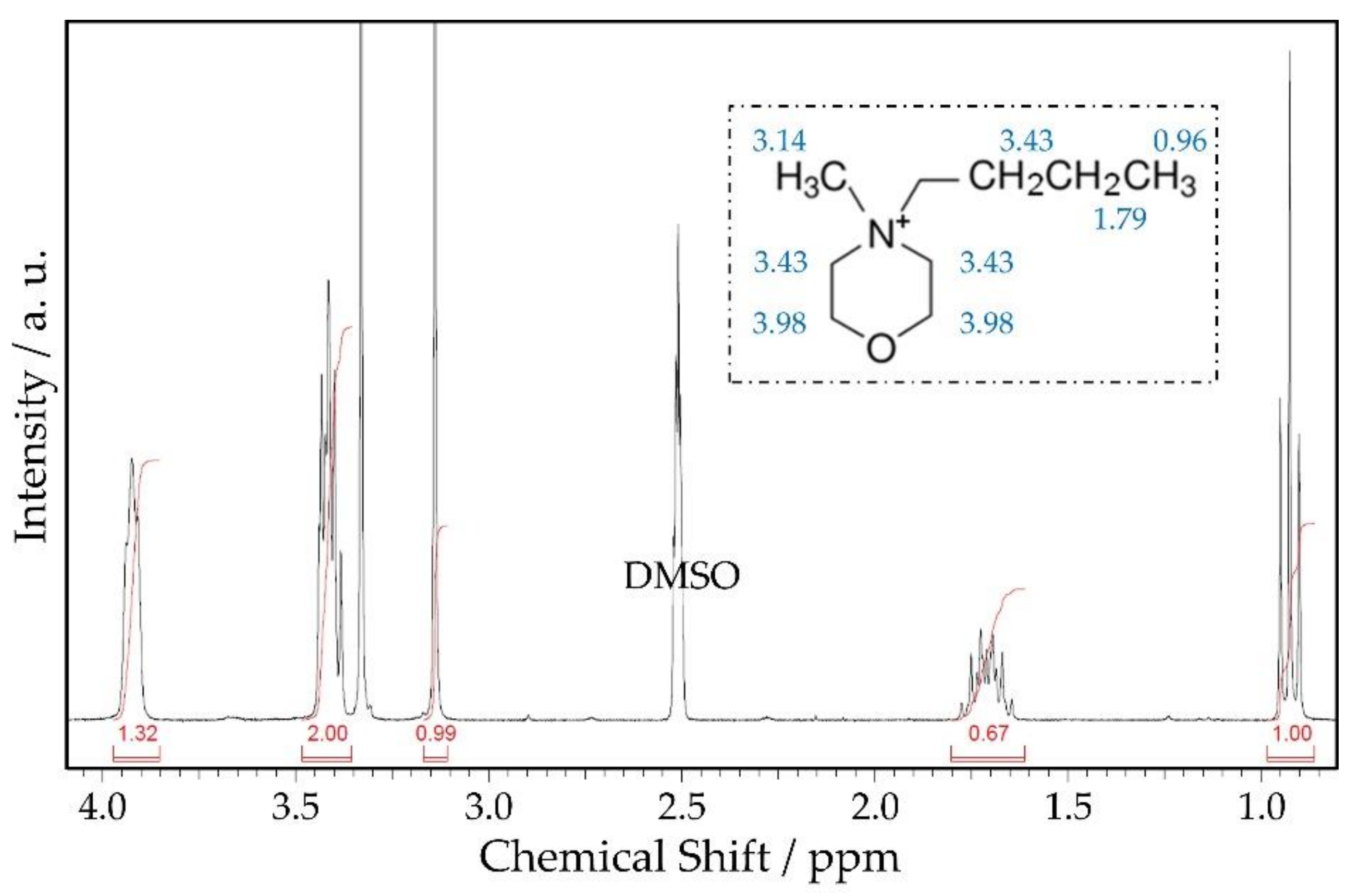
| Shift (ppm) | Functional Group | Shift (ppm) Ref. [20] | |
|---|---|---|---|
| 0.96 | -CH3 | Outer methyl group of the propyl branch | 1.03 |
| 1.79 | -CH2- | Inner methyl group of the propyl branch | 1.85 |
| 3.14 | -CH3 | Methyl branch bound to N | 3.22 |
| 3.43 | -CH2- | Three methyl groups bound to N: one from the propyl branch and two from inside the aromatic ring | 3.51 |
| 3.98 | -CH2- | Two methyl groups inside the aromatic ring bound to O | 4.08 |
| Complexing Salt | Conductivity (mS/cm) | pH | Density (g/mL) | Viscosity |
|---|---|---|---|---|
| MPM-Br | 94.6 | 4.4 | 1.67 | 3.58 |
| MEM-Br | 96.4 | 4.6 | 1.53 | 2.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Blasco, U.; Moreno, E.; Cólera, M.; Díaz-Carrasco, P.; Arrebola, J.C.; Caballero, A.; Morales, J.; Vargas, Ó.A. Enhanced Performance of Zn/Br Flow Battery Using N-Methyl-N-Propylmorpholinium Bromide as Complexing Agent. Int. J. Mol. Sci. 2021, 22, 9288. https://doi.org/10.3390/ijms22179288
Jiménez-Blasco U, Moreno E, Cólera M, Díaz-Carrasco P, Arrebola JC, Caballero A, Morales J, Vargas ÓA. Enhanced Performance of Zn/Br Flow Battery Using N-Methyl-N-Propylmorpholinium Bromide as Complexing Agent. International Journal of Molecular Sciences. 2021; 22(17):9288. https://doi.org/10.3390/ijms22179288
Chicago/Turabian StyleJiménez-Blasco, Uxua, Eduardo Moreno, Maura Cólera, Pilar Díaz-Carrasco, José C. Arrebola, Alvaro Caballero, Julián Morales, and Óscar A. Vargas. 2021. "Enhanced Performance of Zn/Br Flow Battery Using N-Methyl-N-Propylmorpholinium Bromide as Complexing Agent" International Journal of Molecular Sciences 22, no. 17: 9288. https://doi.org/10.3390/ijms22179288
APA StyleJiménez-Blasco, U., Moreno, E., Cólera, M., Díaz-Carrasco, P., Arrebola, J. C., Caballero, A., Morales, J., & Vargas, Ó. A. (2021). Enhanced Performance of Zn/Br Flow Battery Using N-Methyl-N-Propylmorpholinium Bromide as Complexing Agent. International Journal of Molecular Sciences, 22(17), 9288. https://doi.org/10.3390/ijms22179288