Cripto-1 as a Key Factor in Tumor Progression, Epithelial to Mesenchymal Transition and Cancer Stem Cells
Abstract
1. Cripto-1: Role in Cell Biology
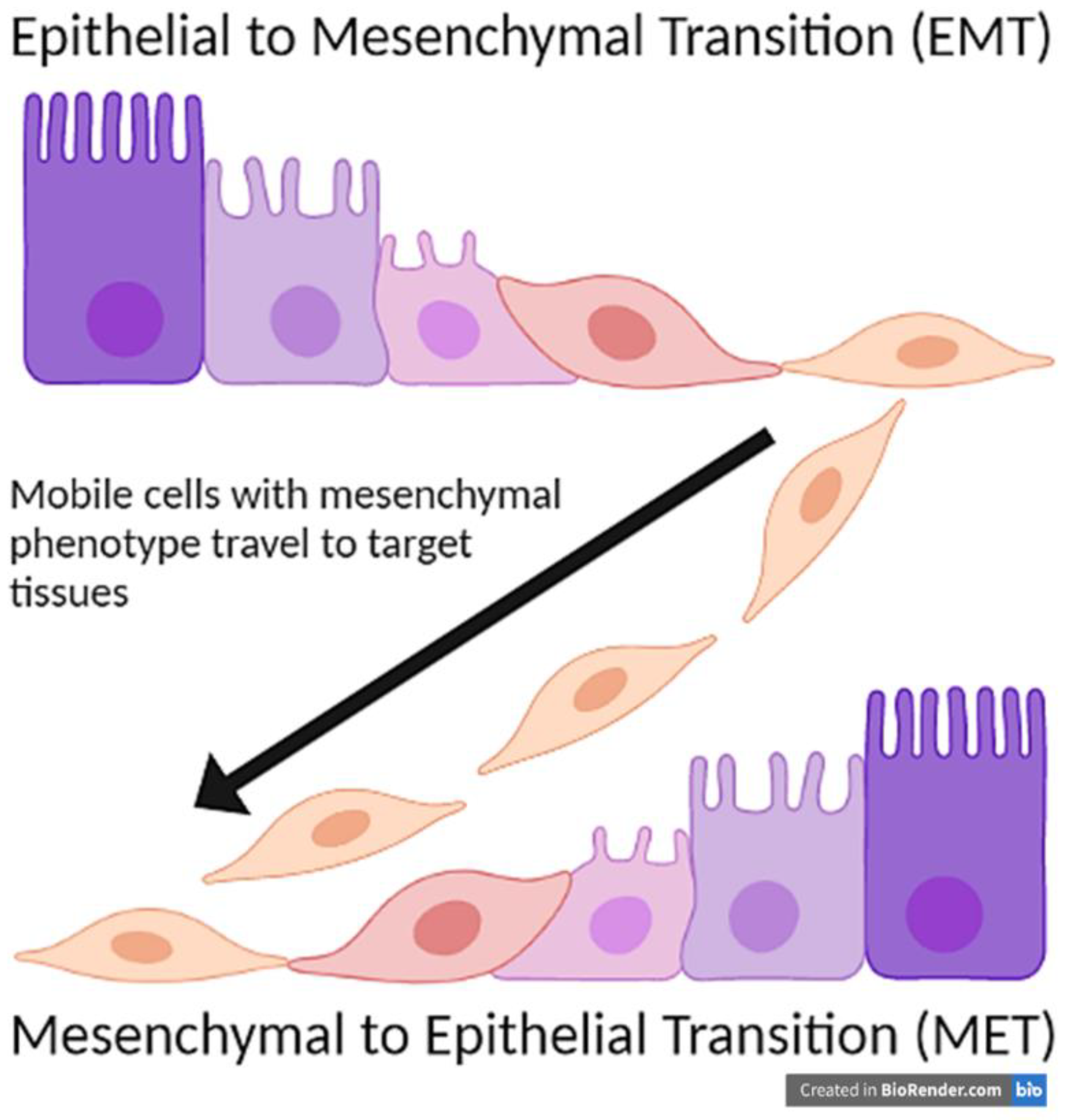
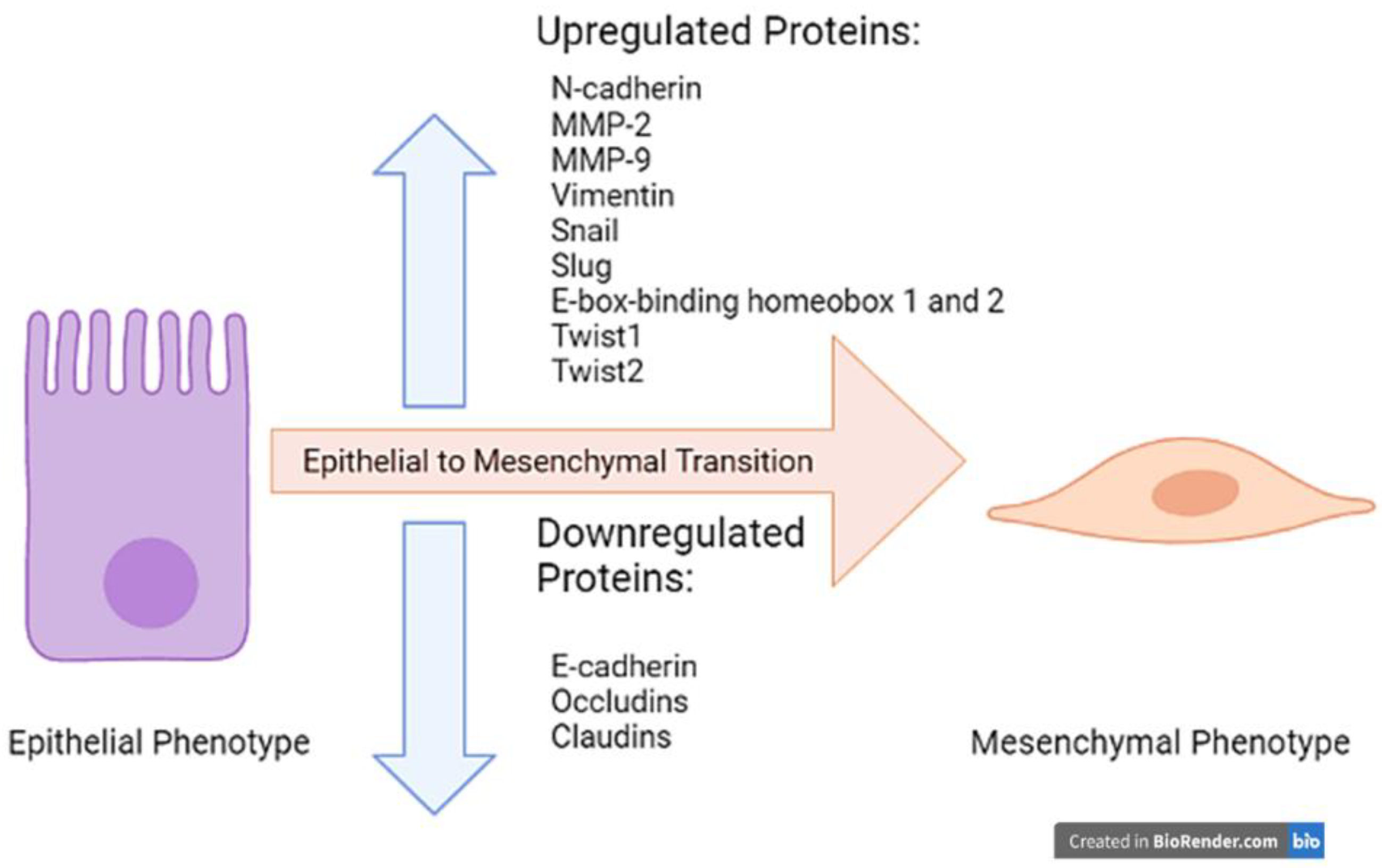
2. Epithelial to Mesenchymal Transition (EMT)
3. Cripto-1: Role in EMT and Cancer Stem Cells (CSC)
4. Cripto-1: Involvement in Different Types of Cancer
4.1. Breast Cancer
4.2. Lung Cancer
4.3. Gastrointestinal Tract Cancers
4.4. Liver Cancer
4.5. Renal Cancer
4.6. Reproductive System Cancers
4.7. Cutaneous Melanoma
5. Cripto-1 as a Therapeutic Target in Cancer
Funding
Conflicts of Interest
References
- Wang, B.; Yan, J.; Peng, Z.; Wang, J.; Liu, S.; Xie, X.; Ma, X. Teratocarcinoma derived growth factor 1 (TDGF1) sequence variants in patients with congenital heart defect. Int. J. Cardiol. 2011, 146, 225–227. [Google Scholar] [CrossRef]
- Morkel, M.; Huelsken, J.; Wakamiya, M.; Ding, J.; van de Wetering, M.; Clevers, H.; Taketo, M.M.; Behringer, R.R.; Shen, M.M.; Birchmeier, W. Beta catenin regulates Cripto- and Wnt3-dependent gene expression programs in mouse axis and mesoderm formation. Development 2003, 130, 6283–6294. [Google Scholar] [CrossRef]
- Parisi, S.; D’Andrea, D.; Lago, C.T.; Adamson, E.D.; Persico, M.G.; Minchiotti, G. Nodal-dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J. Cell Biol. 2003, 163, 303–314. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, C.; Lonardo, E.; Iaconis, S.; Guardiola, O.; Liguoro, A.M.; Liguori, G.L.; Autiero, M.; Carmeliet, P.; Minchiotti, G. G protein-coupled receptor APJ and its ligand apelin act downstream of Cripto to specify embryonic stem cells toward the cardiac lineage through extracellular signal-regulated kinase/p70S6 kinase signaling pathway. Circ. Res. 2009, 105, 231–238. [Google Scholar] [CrossRef]
- Schier, A.; Shen, M. Nodal signalling in vertebrate development. Nature 2000, 403, 385–389. [Google Scholar] [CrossRef]
- Bianco, C.; Rangel, M.C.; Castro, N.P.; Nagaoka, T.; Rollman, K.; Gonzales, M.; Salomon, D.S. Role of Cripto-1 in stem cell maintenance and malignant progression. Am. J. Pathol. 2010, 177, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Strizzi, L.; Bianco, C.; Normanno, N.; Seno, M.; Wechselberger, C.; Wallace-Jones, B.; Khan, N.I.; Hirota, M.; Sun, Y.; Sanicola, M.; et al. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J. Cell Physiol. 2004, 201, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Di Bari, M.G.; Ginsburg, E.; Plant, J.; Strizzi, L.; Salomon, D.S.; Vonderhaar, B.K. Msx2 induces epithelial-mesenchymal transition in mouse mammary epithelial cells through upregulation of Cripto-1. J. Cell Physiol. 2009, 219, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, Z.; Yang, K.; Liu, R.; Xu, Y. Cripto-1 promotes epithelial-mesenchymal transition in prostate cancer via Wnt/β-catenin signaling. Oncol. Rep. 2017, 37, 1521–1528. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Gao, L.; Zhang, L.; Zhu, K.; Cheng, R.; Wang, C. Clinical significance of cripto-1 expression in lung adenocarcinoma. Oncotarget 2017, 8, 79087–79098. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Maiello, M.R.; Carriero, M.V.; Rehman, A.; Wechselberger, C.; Arra, C.; Strizzi, L.; Sanicola, M.; et al. Cripto-1 overexpression leads to enhanced invasiveness and resistance to anoikis in human MCF-7 breast cancer cells. J. Cell Physiol. 2004, 198, 31–39. [Google Scholar] [CrossRef]
- Ebert, A.D.; Wechselberger, C.; Nees, M.; Clair, T.; Schaller, G.; Martinez-Lacaci, I.; Wallace-Jones, B.; Bianco, C.; Weitzel, H.K.; Salomon, D.S. Cripto-1-induced increase in vimentin expression is associated with enhanced migration of human Caski cervical carcinoma cells. Exp. Cell Res. 2000, 257, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wechselberger, C.; Ebert, A.D.; Bianco, C.; Khan, N.I.; Sun, Y.; Wallace-Jones, B.; Montesano, R.; Salomon, D.S. Cripto-1 enhances migration and branching morphogenesis of mouse mammary epithelial cells. Exp. Cell Res. 2001, 266, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Strizzi, L.; Rehman, A.; Normanno, N.; Wechselberger, C.; Sun, Y.; Khan, N.; Hirota, M.; Adkins, H.; Williams, K.; et al. A Nodal- and ALK4-independent signaling pathway activated by Cripto-1 through Glypican-1 and c-Src. Cancer Res. 2003, 63, 1192–1197. [Google Scholar]
- Ikenouchi, J.; Sasaki, H.; Tsukita, S.; Furuse, M. Loss of occludin affects tricellular localization of tricellulin. Mol. Biol. Cell. 2008, 19, 4687–4693. [Google Scholar] [CrossRef]
- Cavatorta, A.L.; Giri, A.A.; Banks, L.; Gardiol, D. Cloning and functional analysis of the promoter region of the human Disc large gene. Gene 2008, 424, 87–95. [Google Scholar] [CrossRef]
- Whiteman, E.L.; Liu, C.J.; Fearon, E.R.; Margolis, B. The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 2008, 27, 3875–3879. [Google Scholar] [CrossRef]
- Pećina-Šlaus, N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003, 3, 17. [Google Scholar] [CrossRef]
- Morrow, C.M.; Mruk, D.; Cheng, C.Y.; Hess, R.A. Claudin and occludin expression and function in the seminiferous epithelium. Philos. Transl. R. Soc. Lond. B Biol. Sci. 2010, 365, 1679–1696. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Platten, M.; Weller, M. Glioma cell invasion: Regulation of metalloproteinase activity by TGF-beta. J. Neurooncol. 2001, 53, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Tokura, Y. Epithelial-mesenchymal transition in the skin. J. Dermatol. Sci. 2011, 61, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Van Bokhoven, A.; Van Leenders, G.J.L.H.; Ruijter, E.T.G.; Jansen, C.F.J.; Bussemakers, M.J.G.; Schalken, J.A. Cadherin switching in human prostate cancer progression. Cancer Res. 2000, 60, 3650–3654. [Google Scholar] [PubMed]
- Li, G.; Herlyn, M. Dynamics of intercellular communication during melanoma development. Mol. Med. Today 2000, 6, 163–169. [Google Scholar] [CrossRef]
- Derycke, L.D.; Bracke, M.E. N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int. J. Dev. Biol. 2004, 48, 463–476. [Google Scholar] [CrossRef]
- Gerhardt, H.; Liebner, S.; Redies, C.; Wolburg, H. N-cadherin expression in endothelial cells during early angiogenesis in the eye and brain of the chicken: Relation to blood-retina and blood-brain barrier development. Eur. J. Neurosci. 1999, 11, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef]
- Hay, E.D. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 2005, 233, 706–720. [Google Scholar] [CrossRef]
- Gilbert, S.F. Developmental Biology, 6th ed.; Paraxial Mesoderm: The Somites and Their Derivatives; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10085 (accessed on 23 July 2021).
- Chen, J.; Han, Q.; Pei, D. EMT and MET as paradigms for cell fate switching. J. Mol. Cell Biol. 2011, 4, 66–69. [Google Scholar] [CrossRef]
- Bataille, F.; Rohrmeier, C.; Bates, R.; Weber, A.; Rieder, F.; Brenmoehl, J.; Strauch, U.; Farkas, S.; Fürst, A.; Hofstädter, F.; et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn's disease. Inflamm. Bowel Dis. 2008, 14, 1514–1527. [Google Scholar] [CrossRef]
- de Castro, N.P.; Rangel, M.C.; Nagaoka, T.; Salomon, D.S.; Bianco, C. Cripto-1: An embryonic gene that promotes tumorigenesis. Future Oncol. 2010, 6, 1127–1142. [Google Scholar] [CrossRef]
- Sefton, M.; Johnson, M.; Clayton, L. Synthesis and phosphorylation of uvomorulin during mouse early development. Development 1992, 115, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, J.A.; Liu, D.; Wheeler, J.M.D.; Kim, H.C.; Beck, N.E.; Ilyas, M.; Karayiannakis, A.J.; Mortensen, N.J.; Kmiot, W.; Playford, R.J.; et al. Mutated epithelial cadherin is associated with increased tumorigenicity and loss of adhesion and of responsiveness to the motogenic trefoil factor 2 in colon carcinoma cells. Proc. Natl. Acad. Sci. USA 1999, 96, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Somarelli, J.A.; Schaeffer, D.; Marengo, M.S.; Bepler, T.; Rouse, D.; Ware, K.E.; Hish, A.J.; Zhao, Y.; Buckley, A.F.; Epstein, J.I.; et al. Distinct routes to metastasis: Plasticity-dependent and plasticity-independent pathways. Oncogene 2016, 35, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T. To differentiate or not--routes towards metastasis. Nat. Rev. Cancer 2012, 12, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Zhang, G.; Findlay, S.D.; Hess, D.A.; Postovit, L.M. Nodal promotes invasive phenotypes via a mitogen-activated protein kinase-dependent pathway. Oncogene 2014, 33, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Strizzi, L.; Margaryan, N.V.; Gilgur, A.; Hardy, K.M.; Normanno, N.; Salomon, D.S.; Hendrix, M.J. The significance of a Cripto-1 positive subpopulation of human melanoma cells exhibiting stem cell-like characteristics. Cell Cycle 2013, 12, 1450–1456. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, X.; Yu, X.; Bian, B.S.; Qian, F.; Hu, X.G.; Ji, C.D.; Yang, L.; Ren, Y.; Cui, W.; et al. Cripto-1 acts as a functional marker of cancer stem-like cells and predicts prognosis of the patients in esophageal squamous cell carcinoma. Mol. Cancer 2017, 16, 81. [Google Scholar] [CrossRef]
- Guo, W.; Keckesova, Z.; Donaher, J.L.; Shibue, T.; Tischler, V.; Reinhardt, F.; Itzkovitz, S.; Noske, A.; Zürrer-Härdi, U.; Bell, G.; et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell 2012, 148, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Scheel, C.; Weinberg, R.A. Phenotypic plasticity and epithelial-mesenchymal transitions in cancer and normal stem cells? Int. J. Cancer 2011, 129, 2310–2314. [Google Scholar] [CrossRef]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Margaryan, N.V.; Loessner, D.; Collins, A.; Kerr, K.M.; Turner, M.; Seftor, E.A.; Stephens, C.R.; Lai, J.; Postovit, L.M.; et al. Reactivation of embryonic nodal signaling is associated with tumor progression and promotes the growth of prostate cancer cells. Prostate 2011, 71, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Salomon, D.S.; Bianco, C.; Ebert, A.D.; Khan, N.I.; De Santis, M.; Normanno, N.; Wechselberger, C.; Seno, M.; Williams, K.; Sanicola, M.; et al. The EGF-CFC family: Novel epidermal growth factor-related proteins in development and cancer. Endocr. Relat. Cancer 2000, 7, 199–226. [Google Scholar] [CrossRef] [PubMed]
- Wechselberger, C.; Strizzi, L.; Kenney, N.; Hirota, M.; Sun, Y.; Ebert, A.; Orozco, O.; Bianco, C.; Khan, N.I.; Wallace-Jones, B.; et al. Human Cripto-1 overexpression in the mouse mammary gland results in the development of hyperplasia and adenocarcinoma. Oncogene 2005, 24, 4094–4105. [Google Scholar] [CrossRef] [PubMed]
- Bianco, C.; Strizzi, L.; Ebert, A.; Chang, C.; Rehman, A.; Normanno, N.; Guedez, L.; Salloum, R.; Ginsburg, E.; Sun, Y.; et al. Role of human cripto-1 in tumor angiogenesis. J. Natl. Cancer Inst. 2005, 97, 132–141. [Google Scholar] [CrossRef]
- Bianco, C.; Castro, N.P.; Baraty, C.; Rollman, K.; Held, N.; Rangel, M.C.; Karasawa, H.; Gonzales, M.; Strizzi, L.; Salomon, D.S. Regulation of human Cripto-1 expression by nuclear receptors and DNA promoter methylation in human embryonal and breast cancer cells. J. Cell Physiol. 2013, 228, 1174–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, H.; Chi, X.; Fan, Y.; Shi, Y.; Niu, J. High level of serum Cripto-1 in hepatocellular carcinoma, especially with hepatitis B virus infection. Medicine 2018, 97, e11781. [Google Scholar] [CrossRef]
- Yokoyama, K.; Kamata, N.; Hayashi, E.; Hoteiya, T.; Ueda, N.; Fujimoto, R.; Nagayama, M. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001, 37, 65–71. [Google Scholar] [CrossRef]
- Blanco, M.J.; Moreno-Bueno, G.; Sarrio, D.; Locascio, A.; Cano, A.; Palacios, J.; Nieto, M.A. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 2002, 21, 3241–3246. [Google Scholar] [CrossRef]
- Bianco, C.; Kannan, S.; De Santis, M.; Seno, M.; Tang, C.K.; Martinez-Lacaci, I.; Kim, N.; Wallace-Jones, B.; Lippman, M.E.; Ebert, A.D.; et al. Cripto-1 indirectly stimulates the tyrosine phosphorylation of erb B-4 through a novel receptor. J. Biol. Chem. 1999, 274, 8624–8629. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, Q.; Hu, H.; Wang, W.; Zhang, Q.; Li, L.; Wang, J.; Yang, R. Expression of Cripto-1 predicts poor prognosis in stage I non-small cell lung cancer. J. Cell Mol. Med. 2020, 24, 9705–9711. [Google Scholar] [CrossRef]
- Gudbergsson, J.M.; Duroux, M. An evaluation of different Cripto-1 antibodies and their variable results. J. Cell Biochem. 2020, 121, 545–556. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume 1032. [Google Scholar]
- Daraghma, H.; Untiveros, G.; Raskind, A.; Iaccarino, E.; Sandomenico, A.; Ruvo, M.; Arnouk, H.; Ciancio, M.J.; Cuevas-Nunez, M.; Strizzi, L. The role of Nodal and Cripto-1 in human oral squamous cell carcinoma. Oral Dis. 2021, 27, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.Y.; Zhang, L.H.; Jia, S.Q.; Shi, T.; Niu, Z.J.; Du, H.; Zhang, G.G.; Hu, Y.; Lu, A.P.; Li, J.Y.; et al. Positive association of up-regulated Cripto-1 and down-regulated E-cadherin with tumour progression and poor prognosis in gastric cancer. Histopathology 2008, 52, 560–568. [Google Scholar] [CrossRef]
- Bianco, C.; Strizzi, L.; Mancino, M.; Rehman, A.; Hamada, S.; Watanabe, K.; De Luca, A.; Jones, B.; Balogh, G.; Russo, J.; et al. Identification of cripto-1 as a novel serologic marker for breast and colon cancer. Clin. Cancer Res. 2006, 12, 5158–5164. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Karasawa, H.; Suzuki, T.; Nakayama, S.; Katagiri, M.; Maeda, S.; Ohnuma, S.; Motoi, F.; Naitoh, T.; Unno, M. The Function and Prognostic Significance of Cripto-1 in Colorectal Cancer. Cancer Investig. 2020, 38, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Wei, W.; Xu, J.; Guo, Z.X.; Xiao, C.Z.; Zhang, Y.F.; Jian, P.E.; Wu, X.L.; Shi, M.; Guo, R.P. Elevated expression of Cripto-1 correlates with poor prognosis in hepatocellular carcinoma. Oncotarget 2015, 6, 35116–35128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xue, Y.J.; Chen, S.N.; Chen, W.G.; Wu, G.Q.; Liao, Y.F.; Xu, J.B.; Tang, H.; Yang, S.H.; He, S.Y.; Luo, Y.F.; et al. Cripto-1 expression in patients with clear cell renal cell carcinoma is associated with poor disease outcome. J. Exp. Clin. Cancer Res. 2019, 38, 378. [Google Scholar] [CrossRef]
- Pattillo, R.A.; Hussa, R.O.; Story, M.T.; Ruckert, A.C.; Shalaby, M.R.; Mattingly, R.F. Tumor antigen and human chorionic gonadotropin in CaSki cells: A new epidermoid cervical cancer cell line. Science 1977, 196, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Friedl, F.; Kimura, I.; Osato, T.; Ito, Y. Studies on a new human cell line (SiHa) derived from carcinoma of uterus. I. Its establishment and morphology. Proc. Soc. Exp. Biol. Med. 1970, 135, 543–545. [Google Scholar] [CrossRef]
- Yee, C.; Krishnan-Hewlett, I.; Baker, C.C.; Schlegel, R.; Howley, P.M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am. J. Pathol. 1985, 119, 361–366. [Google Scholar]
- Ebert, A.D.; Wechselberger, C.; Frank, S.; Wallace-Jones, B.; Seno, M.; Martinez-Lacaci, I.; Bianco, C.; De Santis, M.; Weitzel, H.K.; Salomon, D.S. Cripto-1 induces phosphatidylinositol 3'-kinase-dependent phosphorylation of AKT and glycogen synthase kinase 3beta in human cervical carcinoma cells. Cancer Res. 1999, 59, 4502–4505. [Google Scholar] [PubMed]
- D'Antonio, A.; Losito, S.; Pignata, S.; Grassi, M.; Perrone, F.; De Luca, A.; Tambaro, R.; Bianco, C.; Gullick, W.J.; Johnson, G.R.; et al. Transforming growth factor alpha, amphiregulin and cripto-1 are frequently expressed in advanced human ovarian carcinomas. Int. J. Oncol. 2002, 21, 941–948. [Google Scholar] [PubMed]
- Liu, Y.; Wang, J.; Yang, T.; Liu, R.; Xu, Y. Overexpression levels of cripto-1 predict poor prognosis in patients with prostate cancer following radical prostatectomy. Oncol. Lett. 2019, 18, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Lamura, L.; Strizzi, L.; Roma, C.; D’Antonio, A.; Margaryan, N.; Pirozzi, G.; Hsu, M.Y.; Botti, G.; Mari, E.; et al. Expression and functional role of CRIPTO-1 in cutaneous melanoma. Br. J. Cancer 2011, 105, 1030–1038. [Google Scholar] [CrossRef]
- Xing, P.X.; Hu, X.F.; Pietersz, G.A.; Hosick, H.L.; McKenzie, I.F. Cripto: A novel target for antibody-based cancer immunotherapy. Cancer Res. 2004, 64, 4018–4023. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.K.; Olson, D.L.; Sun, Y.; Wen, D.; Wortham, K.A.; Antognetti, G.; Cheung, A.E.; Orozco, O.E.; Yang, L.; Bailly, V.; et al. An antibody-cytotoxic conjugate, BIIB015, is a new targeted therapy for Cripto positive tumours. Eur. J. Cancer 2011, 47, 1736–1746. [Google Scholar] [CrossRef]
- Adkins, H.B.; Bianco, C.; Schiffer, S.G.; Rayhorn, P.; Zafari, M.; Cheung, A.E.; Orozco, O.; Olson, D.; De Luca, A.; Chen, L.L.; et al. Antibody blockade of the Cripto CFC domain suppresses tumor cell growth in vivo. J. Clin. Investig. 2003, 112, 575–587. [Google Scholar] [CrossRef]
- Ishii, H.; Zahra, M.H.; Takayanagi, A.; Seno, M. A novel artificially humanized Anti-Cripto-1 antibody suppressing cancer cell growth. Int. J. Mol. Sci. 2021, 22, 1709. [Google Scholar] [CrossRef]
- Ligtenberg, M.A.; Witt, K.; Galvez-Cancino, F.; Sette, A.; Lundqvist, A.; Lladser, A.; Kiessling, R. Cripto-1 vaccination elicits protective immunity against metastatic melanoma. Oncoimmunology 2016, 5, e1128613. [Google Scholar] [CrossRef][Green Version]
- Witt, K.; Ligtenberg, M.A.; Conti, L.; Lanzardo, S.; Ruiu, R.; Wallmann, T.; Tufvesson-Stiller, H.; Chambers, B.J.; Rolny, C.; Lladser, A.; et al. Cripto-1 Plasmid DNA vaccination targets metastasis and cancer stem cells in murine mammary carcinoma. Cancer Immunol. Res. 2018, 6, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, F.; Tortora, G.; Bianco, C.; Selvam, M.P.; Basolo, F.; Fontanini, G.; Pacifico, F.; Normanno, N.; Brandt, R.; Persico, M.G.; et al. Inhibition of CRIPTO expression and tumorigenicity in human colon cancer cells by antisense RNA and oligodeoxynucleotides. Oncogene 1994, 9, 291–298. [Google Scholar] [PubMed]
- De Luca, A.; Casamassimi, A.; Selvam, M.P.; Losito, S.; Ciardiello, F.; Agrawal, S.; Salomon, D.S.; Normanno, N. EGF-related peptides are involved in the proliferation and survival of MDA-MB-468 human breast carcinoma cells. Int. J. Cancer 1999, 80, 589–594. [Google Scholar] [CrossRef]
- De Luca, A.; Arra, C.; D’Antonio, A.; Casamassimi, A.; Losito, S.; Ferraro, P.; Ciardiello, F.; Salomon, D.S.; Normanno, N. Simultaneous blockage of different EGF-like growth factors results in efficient growth inhibition of human colon carcinoma xenografts. Oncogene 2000, 19, 5863–5871. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnouk, H.; Yum, G.; Shah, D. Cripto-1 as a Key Factor in Tumor Progression, Epithelial to Mesenchymal Transition and Cancer Stem Cells. Int. J. Mol. Sci. 2021, 22, 9280. https://doi.org/10.3390/ijms22179280
Arnouk H, Yum G, Shah D. Cripto-1 as a Key Factor in Tumor Progression, Epithelial to Mesenchymal Transition and Cancer Stem Cells. International Journal of Molecular Sciences. 2021; 22(17):9280. https://doi.org/10.3390/ijms22179280
Chicago/Turabian StyleArnouk, Hilal, Gloria Yum, and Dean Shah. 2021. "Cripto-1 as a Key Factor in Tumor Progression, Epithelial to Mesenchymal Transition and Cancer Stem Cells" International Journal of Molecular Sciences 22, no. 17: 9280. https://doi.org/10.3390/ijms22179280
APA StyleArnouk, H., Yum, G., & Shah, D. (2021). Cripto-1 as a Key Factor in Tumor Progression, Epithelial to Mesenchymal Transition and Cancer Stem Cells. International Journal of Molecular Sciences, 22(17), 9280. https://doi.org/10.3390/ijms22179280






