Activation of the Constitutive Androstane Receptor Inhibits Leukocyte Adhesiveness to Dysfunctional Endothelium
Abstract
:1. Introduction
2. Results
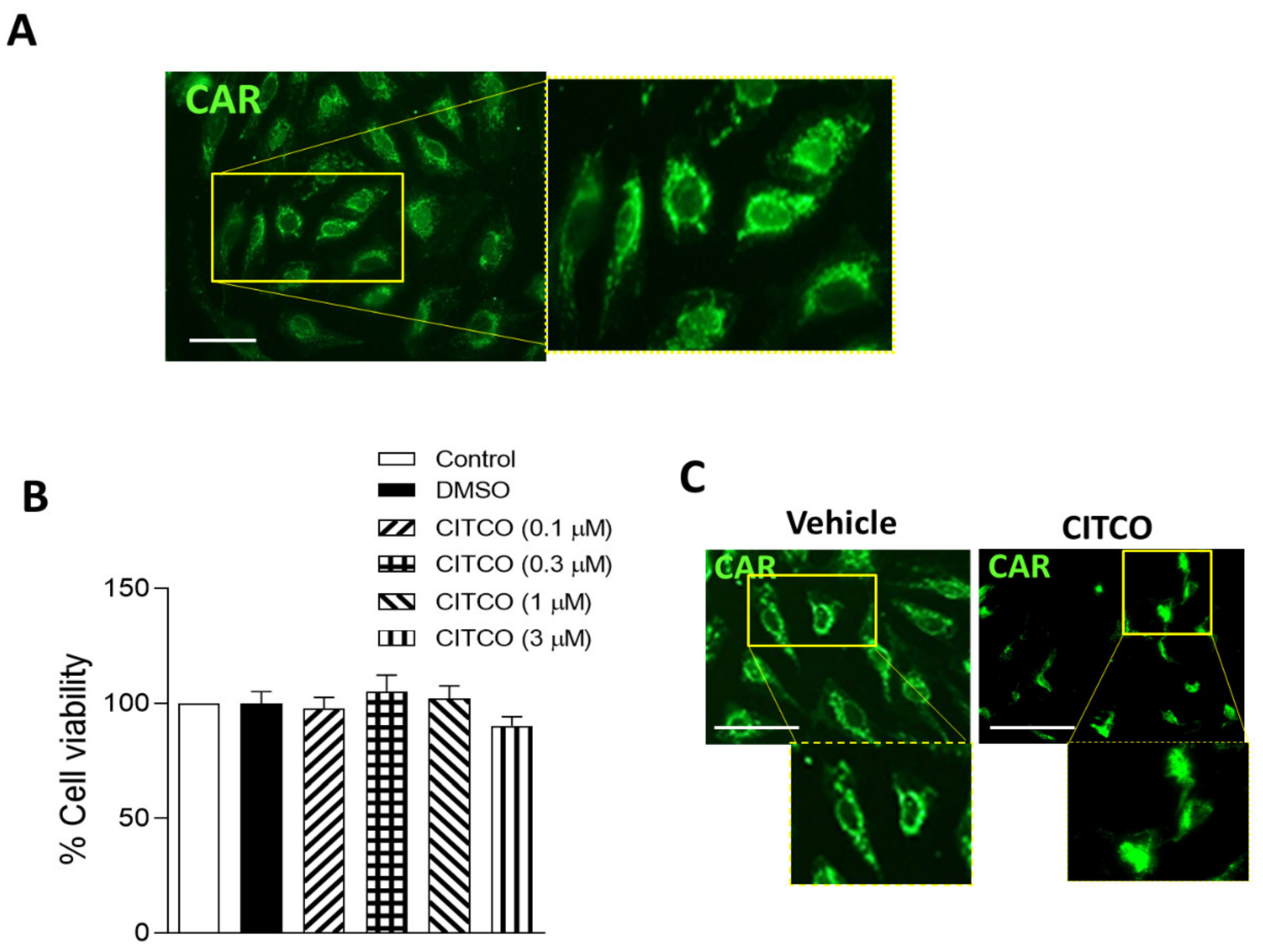
2.1. CAR Is Expressed in Human Endothelial Cells
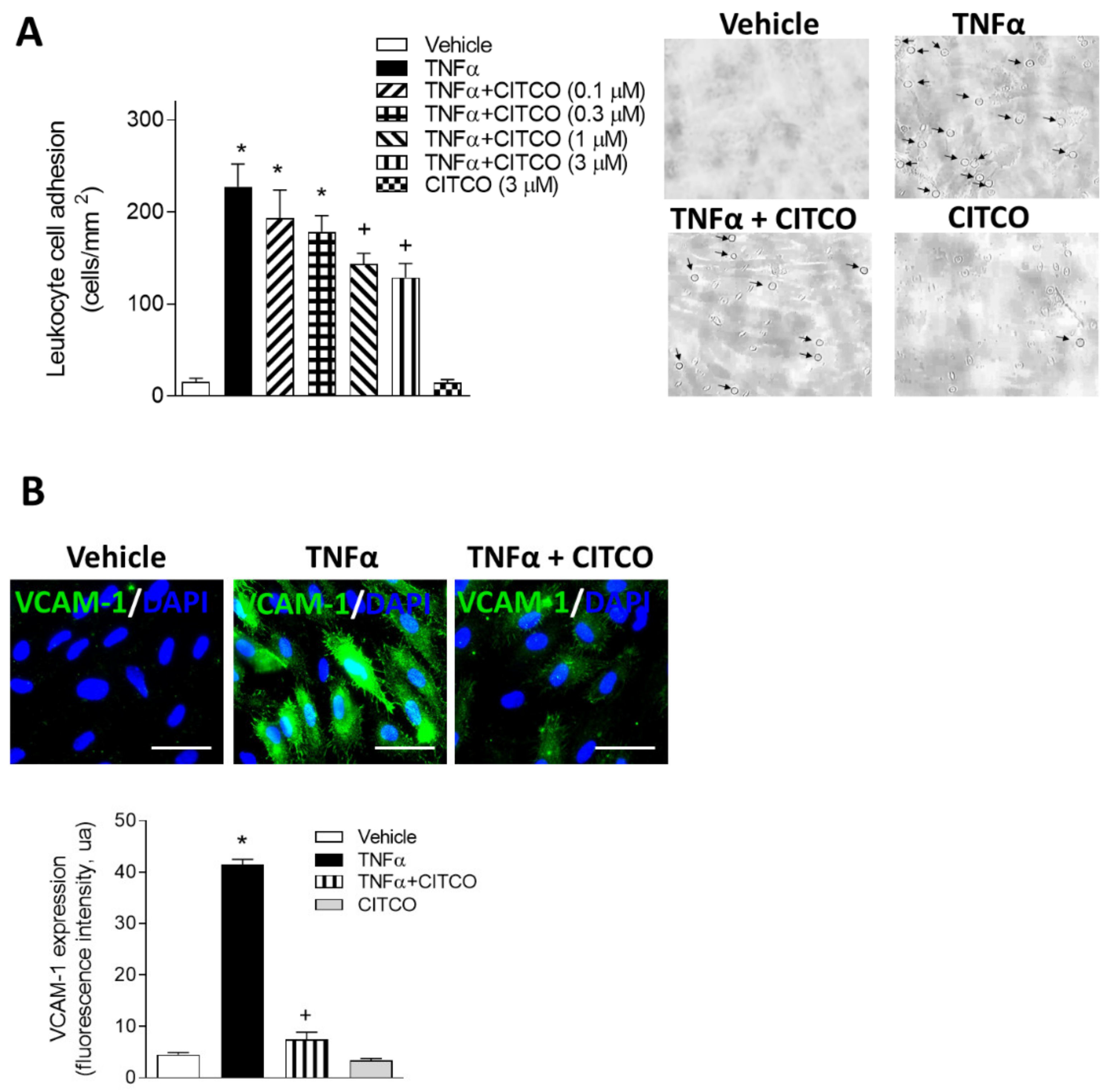
2.2. CITCO Inhibits TNFα-Induced Leukocyte–Endothelial Cell Interactions under Flow Conditions
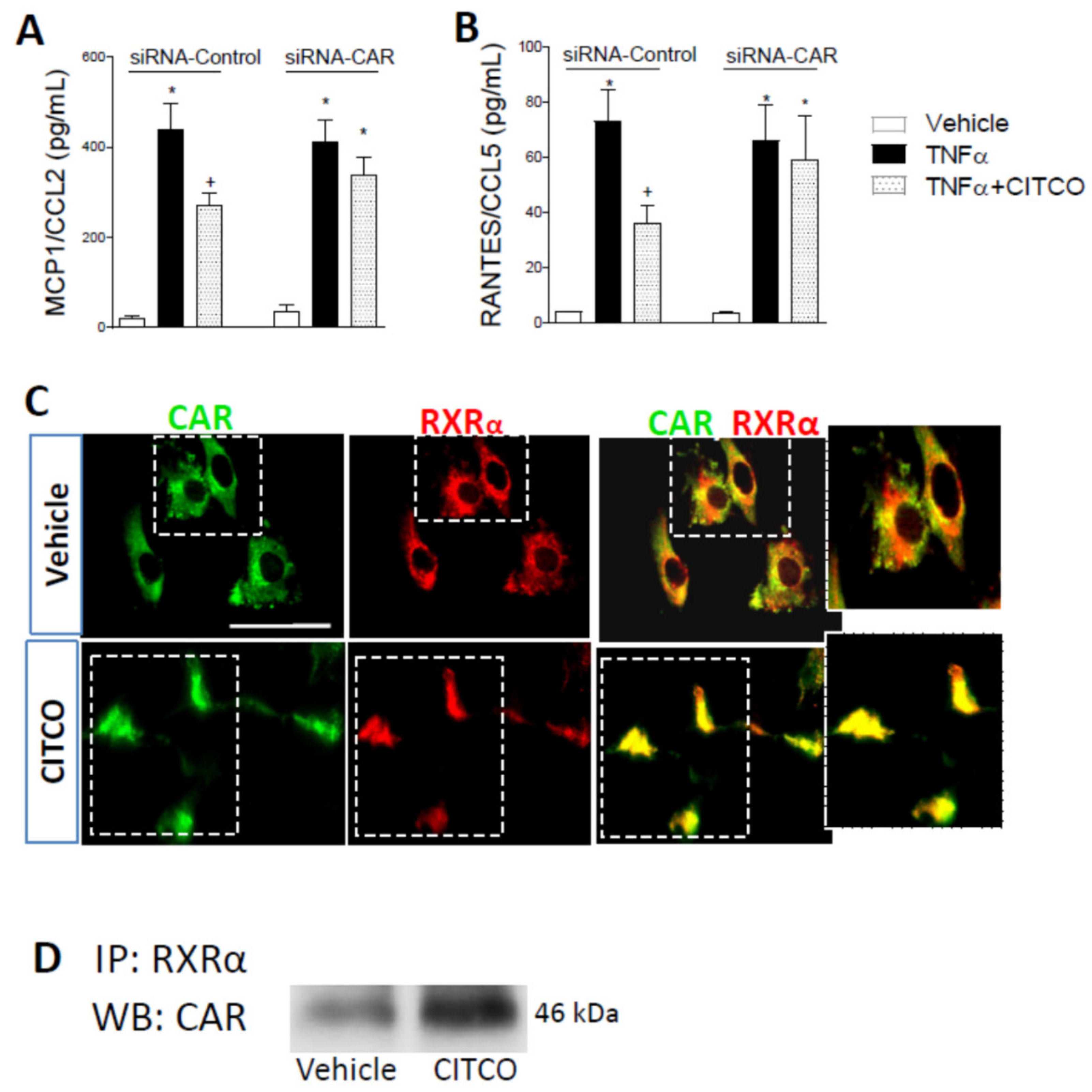
2.3. CAR Silencing by siRNA Blocks the Anti-Inflammatory Effect of CITCO in Endothelial Cells
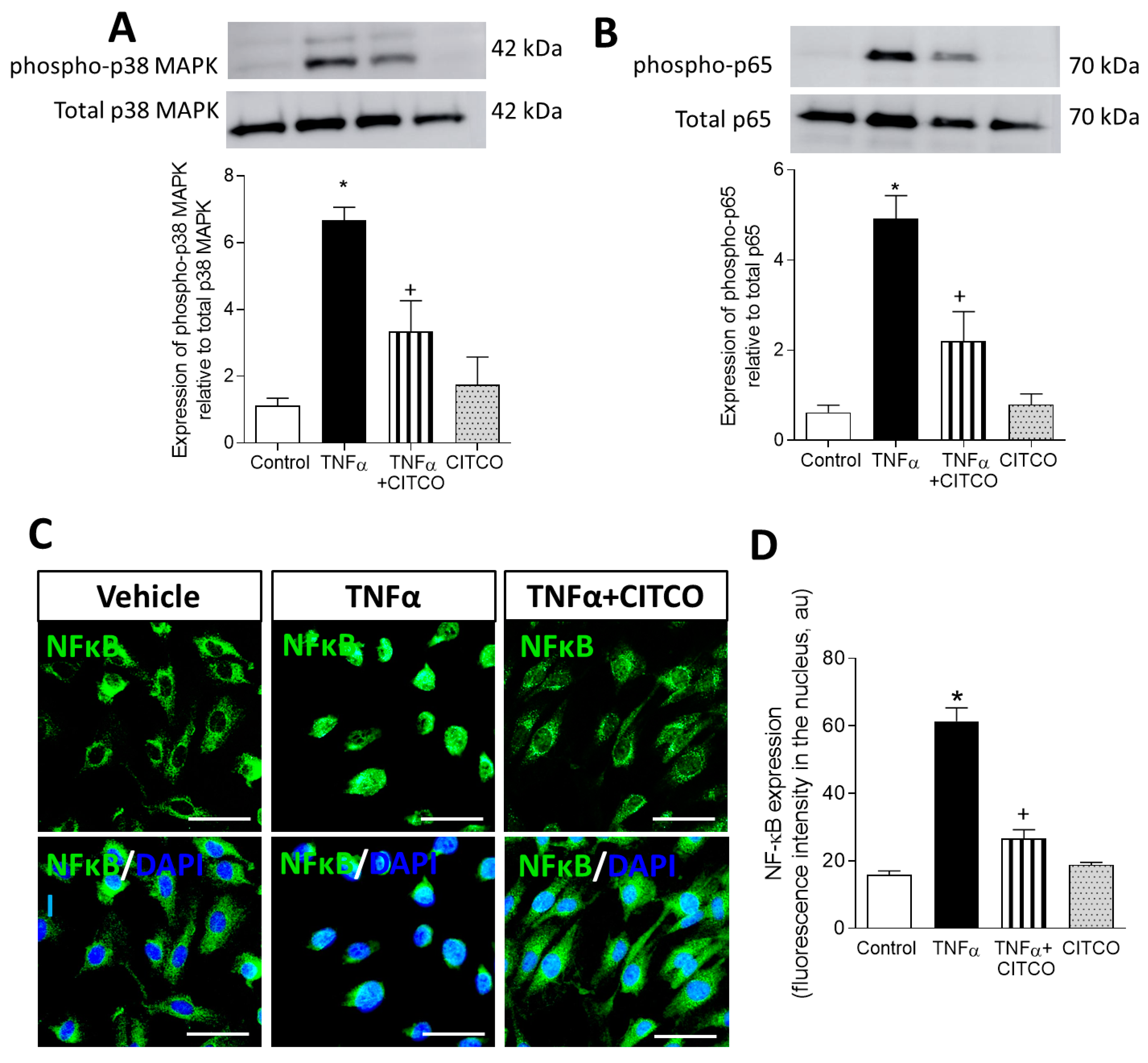
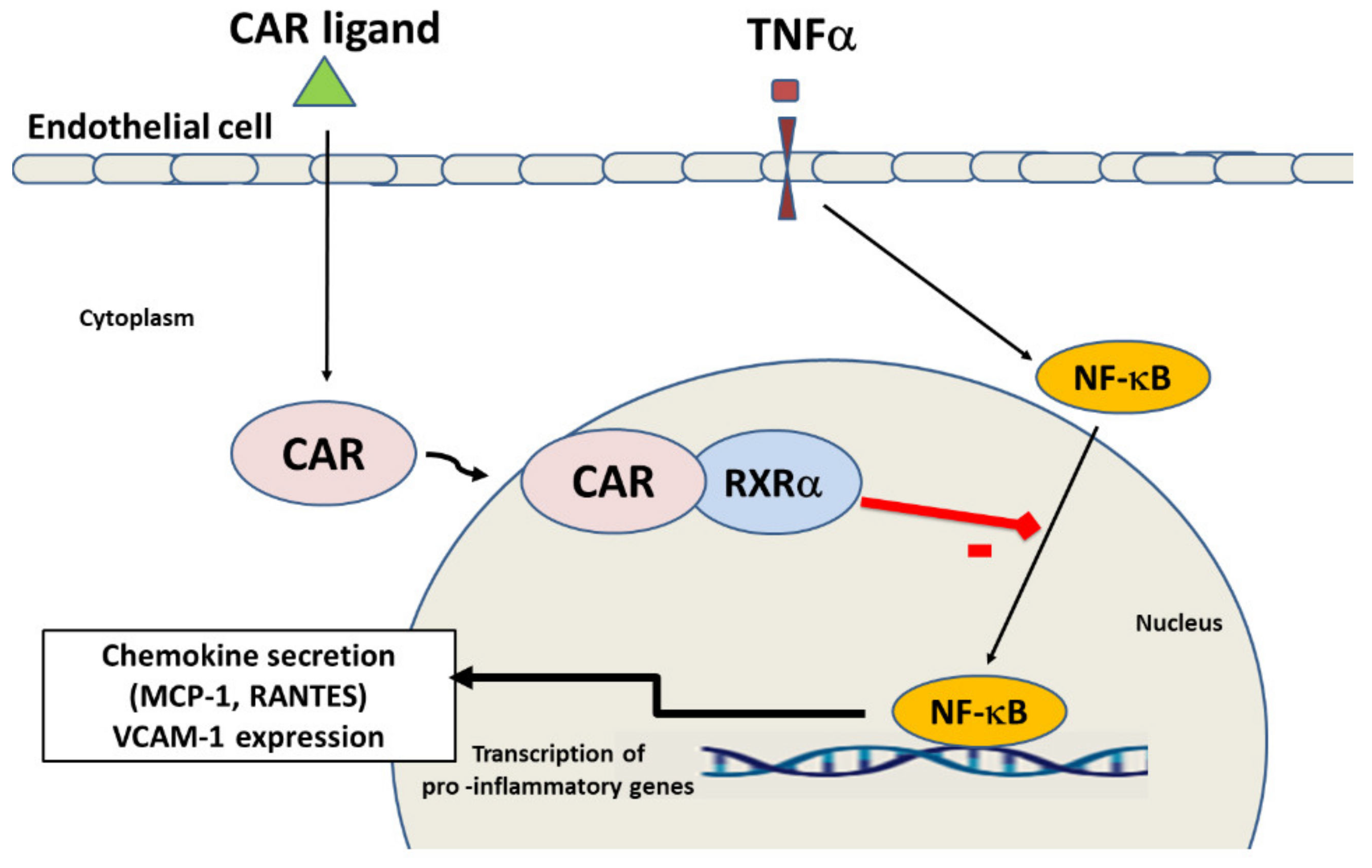
2.4. CAR Activation Inhibits p38 MAPK and NF-κB and Signalling Pathways
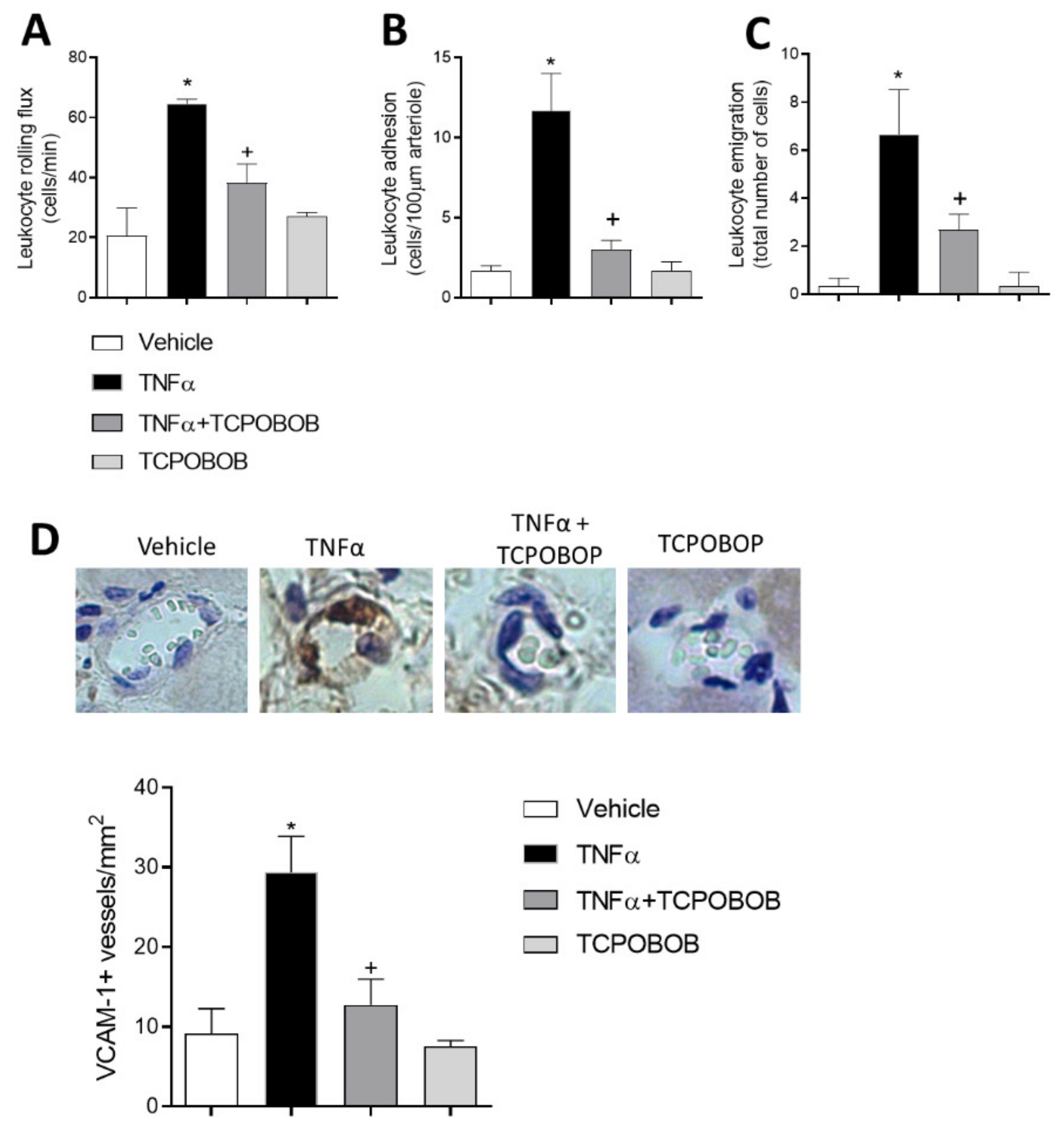
2.5. CAR Agonism Inhibits Leukocyte–Endothelial Cell Interactions In Vivo
3. Discussion
4. Material and Methods
4.1. Endothelial Cell Culture
4.2. Viability Assay
4.3. Leukocyte–HUVEC Interactions under Flow Conditions
4.4. Chemokine Detection
4.5. Immunofluorescence Analysis
4.6. Western Blotting
4.7. CAR Silencing via Small Interfering RNA
4.8. Immunoprecipitation
4.9. Intravital Microscopy Studies
4.10. Immunohistochemistry
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAR | Constitutive androstane receptor |
| TNF | Tumoral necrosis factor |
| MCP-1 | Monocyte chemoattractant protein-1 |
| RANTES | Regulated upon Activation, Normal T cell Expressed and presumably Secreted |
| HUVEC | Human Umbilical Vein Endothelial Cells |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| MAPK | Mitogen activated protein kinase |
| NF-κb | Transcription nuclear factor kappa B |
References
- Liang, Y.; Wang, M.; Wang, C.; Liu, Y.; Naruse, K.; Takahashi, K. The Mechanisms of the Development of Atherosclerosis in Prediabetes. Int. J. Mol. Sci. 2021, 22, 4018. [Google Scholar] [CrossRef]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, T.J.; van Buul, J.D.; Huveneers, S.; Quax, P.H.A.; de Vries, M.R. Endothelial Barrier Function and Leukocyte Transmigration in Atherosclerosis. Biomedicines 2021, 9, 328. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Küblbeck, J.; Niskanen, J.; Honkakoski, P. Metabolism-Disrupting Chemicals and the Constitutive Androstane Receptor CAR. Cells 2020, 9, 2036. [Google Scholar] [CrossRef] [PubMed]
- Daujat-Chavanieu, M.; Gerbal-Chaloin, S. Regulation of CAR and PXR Expression in Health and Disease. Cells 2020, 9, 2395. [Google Scholar] [CrossRef]
- Bae, S.D.W.; Nguyen, R.; Qiao, L.; George, J. Role of the constitutive androstane receptor (CAR) in human liver cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188516. [Google Scholar] [CrossRef]
- Chen, M.L.; Huang, X.; Wang, H.; Hegner, C.; Liu, Y.; Shang, J.; Eliason, A.; Diao, H.; Park, H.; Frey, B.; et al. CAR directs T cell adaptation to bile acids in the small intestine. Nature 2021, 593, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Feng, Y.; Xu, M.; Yu, C.; Xie, W. Gadd45b is required in part for the anti-obesity effect of constitutive androstane receptor (CAR). Acta Pharm. Sin. B 2021, 11, 434–441. [Google Scholar] [CrossRef]
- Gao, J.; He, J.; Zhai, Y.; Wada, T.; Xie, W. The constitutive androstane receptor is an anti-obesity nuclear receptor that improves insulin sensitivity. J. Biol. Chem. 2009, 284, 25984–25992. [Google Scholar] [CrossRef] [Green Version]
- Escudero, P.; Martinez de Maranon, A.; Collado, A.; Gonzalez-Navarro, H.; Hermenegildo, C.; Peiro, C.; Piqueras, L.; Sanz, M.J. Combined Sub-Optimal Doses of Rosuvastatin and Bexarotene Impair Angiotensin II-Induced Arterial Mononuclear Cell Adhesion Through Inhibition of Nox5 Signaling Pathways and Increased RXR/PPARalpha and RXR/PPARgamma Interactions. Antioxid. Redox Signal. 2015, 22, 901–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martorell, S.; Hueso, L.; Gonzalez-Navarro, H.; Collado, A.; Sanz, M.J.; Piqueras, L. Vitamin D Receptor Activation Reduces Angiotensin-II-Induced Dissecting Abdominal Aortic Aneurysm in Apolipoprotein E-Knockout Mice. Arter. Thromb. Vasc. Biol. 2016, 36, 1587–1597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [Green Version]
- Escudero, P.; Navarro, A.; Ferrando, C.; Furio, E.; Gonzalez-Navarro, H.; Juez, M.; Sanz, M.J.; Piqueras, L. Combined treatment with bexarotene and rosuvastatin reduces angiotensin-II-induced abdominal aortic aneurysm in apoE(-/-) mice and angiogenesis. Br. J. Pharmacol. 2015, 172, 2946–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, B.; Saha, P.K.; Huang, W.; Chen, W.; Abu-Elheiga, L.A.; Wakil, S.J.; Stevens, R.D.; Ilkayeva, O.; Newgard, C.B.; Chan, L.; et al. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc. Natl. Acad. Sci. USA 2009, 106, 18831–18836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peluso, I.; Palmery, M. The relationship between body weight and inflammation: Lesson from anti-TNF-alpha antibody therapy. Hum. Immunol. 2016, 77, 47–53. [Google Scholar] [CrossRef]
- Kwaifa, I.K.; Bahari, H.; Yong, Y.K.; Noor, S.M. Endothelial Dysfunction in Obesity-Induced Inflammation: Molecular Mechanisms and Clinical Implications. Biomolecules 2020, 10, 291. [Google Scholar] [CrossRef] [Green Version]
- Virdis, A.; Colucci, R.; Bernardini, N.; Blandizzi, C.; Taddei, S.; Masi, S. Microvascular Endothelial Dysfunction in Human Obesity: Role of TNF-alpha. J. Clin. Endocrinol. Metab. 2019, 104, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Shimobayashi, M.; Albert, V.; Woelnerhanssen, B.; Frei, I.C.; Weissenberger, D.; Meyer-Gerspach, A.C.; Clement, N.; Moes, S.; Colombi, M.; Meier, J.A.; et al. Insulin resistance causes inflammation in adipose tissue. J. Clin. Investig. 2018, 128, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Karstoft, K.; Pedersen, B.K. Exercise and type 2 diabetes: Focus on metabolism and inflammation. Immunol. Cell Biol. 2016, 94, 146–150. [Google Scholar] [CrossRef]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus-Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Xie, Q.; Ma, D.; Wang, W. Effects of ET-1 and TNF-alpha levels on the cardiac function and prognosis in rats with chronic heart failure. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 11004–11010. [Google Scholar]
- Li, W.; Yang, X.; Zheng, T.; Xing, S.; Wu, Y.; Bian, F.; Wu, G.; Li, Y.; Li, J.; Bai, X.; et al. TNF-alpha stimulates endothelial palmitic acid transcytosis and promotes insulin resistance. Sci. Rep. 2017, 7, 44659. [Google Scholar] [CrossRef] [PubMed]
- Munjal, A.; Khandia, R. Atherosclerosis: Orchestrating cells and biomolecules involved in its activation and inhibition. Adv. Protein. Chem. Struct. Biol. 2020, 120, 85–122. [Google Scholar] [PubMed]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Udalova, I.; Monaco, C.; Nanchahal, J.; Feldmann, M. Anti-TNF Therapy. Microbiol. Spectr. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, D.W.; Patel, R.P. Endothelial heterogeneity and adhesion molecules N-glycosylation: Implications in leukocyte trafficking in inflammation. Glycobiology 2013, 23, 622–633. [Google Scholar] [CrossRef] [Green Version]
- Weber, K.S.; von Hundelshausen, P.; Clark-Lewis, I.; Weber, P.C.; Weber, C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur. J. Immunol. 1999, 29, 700–712. [Google Scholar] [CrossRef]
- Gencer, S.; Evans, B.R.; van der Vorst, E.P.C.; Döring, Y.; Weber, C. Inflammatory Chemokines in Atherosclerosis. Cells 2021, 10, 226. [Google Scholar] [CrossRef]
- Uehara, D.; Tojima, H.; Kakizaki, S.; Yamazaki, Y.; Horiguchi, N.; Takizawa, D.; Sato, K.; Yamada, M.; Uraoka, T. Constitutive androstane receptor and pregnane X receptor cooperatively ameliorate DSS-induced colitis. Dig. Liver Dis. 2019, 51, 226–235. [Google Scholar] [CrossRef]
- Collado, A.; Marques, P.; Escudero, P.; Rius, C.; Domingo, E.; Martinez-Hervas, S.; Real, J.T.; Ascaso, J.F.; Piqueras, L.; Sanz, M.J. Functional role of endothelial CXCL16/CXCR6-platelet-leucocyte axis in angiotensin II-associated metabolic disorders. Cardiovasc. Res. 2018, 114, 1764–1775. [Google Scholar] [CrossRef]
- Hueso, L.; Ortega, R.; Selles, F.; Wu-Xiong, N.Y.; Ortega, J.; Civera, M.; Ascaso, J.F.; Sanz, M.J.; Real, J.T.; Piqueras, L. Upregulation of angiostatic chemokines IP-10/CXCL10 and I-TAC/CXCL11 in human obesity and their implication for adipose tissue angiogenesis. Int. J. Obes. 2018, 42, 1406–1417. [Google Scholar] [CrossRef]
- Piqueras, L.; Sanz, M.J. Angiotensin II and leukocyte trafficking: New insights for an old vascular mediator. Role of redox-signaling pathways. Free Radic. Biol. Med. 2020, 157, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Suino, K.; Peng, L.; Reynolds, R.; Li, Y.; Cha, J.Y.; Repa, J.J.; Kliewer, S.A.; Xu, H.E. The nuclear xenobiotic receptor CAR: Structural determinants of constitutive activation and heterodimerization. Mol. Cell 2004, 16, 893–905. [Google Scholar] [PubMed]
- Tzameli, I.; Pissios, P.; Schuetz, E.G.; Moore, D.D. The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol. Cell Biol. 2000, 20, 2951–2958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sberna, A.L.; Assem, M.; Xiao, R.; Ayers, S.; Gautier, T.; Guiu, B.; Deckert, V.; Chevriaux, A.; Grober, J.; Le Guern, N.; et al. Constitutive androstane receptor activation decreases plasma apolipoprotein B-containing lipoproteins and atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2232–2239. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.Y.; Klaassen, C.D. RNA-Seq reveals common and unique PXR- and CAR-target gene signatures in the mouse liver transcriptome. Biochim. Biophys. Acta 2016, 1859, 1198–1217. [Google Scholar] [CrossRef] [Green Version]
- Gabbia, D.; Pozzo, L.; Zigiotto, G.; Roverso, M.; Sacchi, D.; Dalla Pozza, A.; Carrara, M.; Bogialli, S.; Floreani, A.; Guido, M.; et al. Dexamethasone counteracts hepatic inflammation and oxidative stress in cholestatic rats via CAR activation. PLoS ONE 2018, 13, e0204336. [Google Scholar] [CrossRef]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Minick, C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef]
- Piqueras, L.; Sanz, M.J.; Perretti, M.; Morcillo, E.; Norling, L.; Mitchell, J.A.; Li, Y.; Bishop-Bailey, D. Activation of PPARbeta/delta inhibits leukocyte recruitment, cell adhesion molecule expression, and chemokine release. J. Leukoc. Bio. 2009, 86, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.U.; Lister, K.J.; Yang, Y.H.; Issekutz, A.; Hickey, M.J. TNF regulates leukocyte-endothelial cell interactions and microvascular dysfunction during immune complex-mediated inflammation. Br. J. Pharmacol. 2005, 144, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sberna, A.L.; Assem, M.; Gautier, T.; Grober, J.; Guiu, B.; Jeannin, A.; Pais de Barros, J.P.; Athias, A.; Lagrost, L.; Masson, D. Constitutive androstane receptor activation stimulates faecal bile acid excretion and reverse cholesterol transport in mice. J. Hepatol. 2011, 55, 154–161. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Riera, M.; Ortega, R.; Hueso, L.; Montesinos, M.C.; Gomez-Cabrera, M.C.; Sanz, M.J.; Real, J.T.; Piqueras, L. Activation of the Constitutive Androstane Receptor Inhibits Leukocyte Adhesiveness to Dysfunctional Endothelium. Int. J. Mol. Sci. 2021, 22, 9267. https://doi.org/10.3390/ijms22179267
López-Riera M, Ortega R, Hueso L, Montesinos MC, Gomez-Cabrera MC, Sanz MJ, Real JT, Piqueras L. Activation of the Constitutive Androstane Receptor Inhibits Leukocyte Adhesiveness to Dysfunctional Endothelium. International Journal of Molecular Sciences. 2021; 22(17):9267. https://doi.org/10.3390/ijms22179267
Chicago/Turabian StyleLópez-Riera, Mireia, Rebeca Ortega, Luisa Hueso, María Carmen Montesinos, Mari Carmen Gomez-Cabrera, María Jesús Sanz, José T. Real, and Laura Piqueras. 2021. "Activation of the Constitutive Androstane Receptor Inhibits Leukocyte Adhesiveness to Dysfunctional Endothelium" International Journal of Molecular Sciences 22, no. 17: 9267. https://doi.org/10.3390/ijms22179267
APA StyleLópez-Riera, M., Ortega, R., Hueso, L., Montesinos, M. C., Gomez-Cabrera, M. C., Sanz, M. J., Real, J. T., & Piqueras, L. (2021). Activation of the Constitutive Androstane Receptor Inhibits Leukocyte Adhesiveness to Dysfunctional Endothelium. International Journal of Molecular Sciences, 22(17), 9267. https://doi.org/10.3390/ijms22179267




