Abstract
Cartilage is frequently damaged with a limited capacity for repair. Current treatment strategies are insufficient as they form fibrocartilage as opposed to hyaline cartilage, and do not prevent the progression of degenerative changes. There is increasing interest in the use of autologous mesenchymal stem cells (MSC) for tissue regeneration. MSCs that are used to treat articular cartilage defects must not only present a robust cartilaginous production capacity, but they also must not cause morbidity at the harvest site. In addition, they should be easy to isolate from the tissue and expand in culture without terminal differentiation. The source of MSCs is one of the most important factors that may affect treatment. The infrapatellar fat pad (IPFP) acts as an important reservoir for MSC and is located in the anterior compartment of the knee joint in the extra-synovial area. The IPFP is a rich source of MSCs, and in this review, we discuss studies that demonstrate that these cells have shown many advantages over other tissues in terms of ease of isolation, expansion, and chondrogenic differentiation. Future studies in articular cartilage repair strategies and suitable extraction as well as cell culture methods will extend the therapeutical application of IPFP-derived MSCs into additional orthopedic fields, such as osteoarthritis. This review provides the latest research concerning the use of IPFP-derived MSCs in the treatment of articular cartilage damage, providing critical information for the field to grow.
1. Introduction
1.1. Articular Cartilage
Articular cartilage is a specialized connective tissue that lacks blood vessels, nerves, and lymphatic tissue. Consequently, cartilage tissue has limited capacity for repair and the progression of focal cartilage defects leads to more generalized degenerative changes or osteoarthritis [1]. Articular cartilage damage is a disabling disease characterized by fibrillation and subsequent destruction of the articular cartilage surface, frequently including subchondral bone damage inducing more generalized changes [2]. The adjoining synovium in articular cartilage disease includes biomarkers for significant inflammation, including Substance P, which further stimulates a local inflammatory response [3,4]. Consequently, oedema and inflammation of the infrapatellar fat pad (IPFP) and the synovial membrane cause the progression of osteoarthritis as well as articular cartilage loss that often necessitates full joint replacement. Furthermore, the synovial membrane and IPFP may interact with each other, affecting the development and progression of osteoarthritis [4,5].
1.2. Past and Current Articular Cartilage Treatments
Cartilage defects cause a significant disease burden and a previous study indicated that more than of 60% of knees undergoing arthroscopy have articular defects [6]. If these defects are left untreated, or managed suboptimally, they lead to the progression of more widespread degenerative changes. There are several methods for treating articular cartilage defects depending on the anatomical location, extent, shape, and depth of the cartilage defect, and age of the patient [7] (Table 1). The operative treatment techniques mentioned in Table 1 result in the formation of the less desirable fibrocartilage, or hyaline-like cartilage, as opposed to hyaline cartilage. Fibrocartilage has suboptimal biomechanical properties and does not prevent the progression of the degenerative changes of osteoarthritis [8]. Studies on cell-based techniques, including Autologous Chondrocyte Implantation (ACI) and Matrix-Assisted ACI (MACI), have highlighted the disadvantages of using fully differentiated chondrocytes, such as their difficulty in extraction, isolation, expansion, and growth in vivo after implantation. It is of the highest importance to find alternative cell sources.

Table 1.
Non-operative and operative treatment techniques for articular cartilage defects.
1.3. Cell-Based Therapies
Recent advances in developing therapeutic strategies for treating articular cartilage defects [17,18] have focused on stem cell therapies and tissue regeneration to prevent the progression to osteoarthritis [19]. Stem cells have shown superiority in treating articular cartilage defects due to their ease of isolation, expansion, and culture in preliminary studies, and are a promising method for promoting articular cartilage regeneration [20]. Mesenchymal stem cells (MSCs), in contrast to autologous chondrocytes, possess a greater capacity to expand in vitro [19]. The use of MSCs does not raise ethical concerns, as is the case with embryonic stem cells. Many questions need to be answered before stem cells are routinely used for cartilage defects, including the optimal stem cell source, an optimal extraction, isolation, and expansion protocol, the need for scaffolds, and cost. Although adipose tissue-derived MSCs have a lower chondrogenic capacity in comparison with bone marrow mesenchymal stem cells [21], they can be harvested in a less invasive and cost-effective manner with liposuction. Due to this easier access, the therapeutic application of adipose tissue-derived MSCs is increasing [22]. For all of the above reasons, the IPFP has become an area of high interest in regenerative medicine since it stores MSC.
Despite the promise of the IPFP as a source of MSC for regenerating articular cartilage, very few reviews exist on this subject. In this review, for the first time, the authors detail several IPFP cell-based therapies, their current progress towards healing articular cartilage damage, and the most recent advances in the use of IPFP-derived MSCs for cartilage repair.
2. Advantages of Adipose Tissue-Derived MSCs for Articular Cartilage Repair
The presence of MSCs in adipose tissue from liposuction was first reported in 2001 by Zuck et al. [23]. Adipose tissue-derived MSCs have also demonstrated a greater ability to differentiate into other lineages in pre-clinical studies compared to umbilical cord stem cells [2,24]. Adipose tissue is one of the most easily accessible tissues for the extraction of MSCs and is often discarded after liposuction. Since increased BMI and adipose tissue content are related to articular cartilage damage, removal of adipose tissue through liposuction and subsequent isolation of adipose tissue-derived MSCs can be well suited for treating articular cartilage damage [25,26].
Due to the potentially wide applications of MSCs in regenerative medicine, it is essential to have access to a reliable and reproducible MSC source. Adipose tissue-derived MSCs are feasible and promising candidates for cell-based therapies [27], and they can be differentiated into adipose tissue, bone, cartilage, and muscle [28,29]. Studies have confirmed chondrogenic differentiation of human adipose tissue-derived MSC pellet cultures by the expression of target tissue markers [30]. After harvesting of adipose tissue using liposuction aspiration or needle biopsy, adipose tissue-derived MSCs were isolated from adipose tissue. The tissue was washed with phosphate-buffered saline and a penicillin/streptomycin solution before being minced. To further dissolve any adipose tissue clumps or aggregates, the adipose tissue was pipetted up and down numerous times to facilitate mechanical disruption of the extracellular matrix. The tissue was then placed in a plate of sterile tissue culture dishes and 0.05% of a collagenase digestion buffer for tissue digestion after the debris was removed. The supernatant was aspirated after collagenase inactivation with K-NAC medium supplemented and 10% fetal calf serum (FCS), and the cell pellet was resuspended in a K-NAC medium supplemented with 10% FCS. Following centrifugation, the cell suspension was blended and filtered using a 100-μm cell strainer. Lastly, cell pellets were plated onto a tissue culture plate and cultured in an incubator at 37 °C with 5% CO2 [22]. Such processes are now commonly used for ADSC isolation and can be used for articular cartilage repair.
Over the last two decades, stem cell-based therapies using adipose tissue-derived MSCs have been expanding, as supported by their strong therapeutic potential. Studies have reported that the effect of stem cells derived from different tissues differ according to the site of extraction [31]. Adipose tissue is easier to access than other tissues and obtaining MSCs from this tissue is less invasive [32]. Compared to bone marrow, the process of harvesting tissue from adipose tissue is less invasive, and studies suggest a greater cell yield per unit of tissue as well [33]. Studies suggest that adipose tissue-derived MSCs have a smaller cell body than bone marrow-derived MSCs and have different gene expression and cell surface receptors. Commonly used markers include CD90, CD44, CD29, CD105, CD13, CD34, CD73, CD166, CD10, CD49e, and CD59, which are all positive, while CD31, CD45, CD14, CD11b, CD19, CD56, and CD146 are all negative in adipose tissue-derived MSCs. In addition, the positive expression of HLA-ABC and STRO-1 as well as the negative expression of HLA-DR are also features of adipose tissue-derived MSCs [34]. ADSCs can all be passaged in vitro up to passage 10 with no karyotype abnormalities detected [35]. Unlike bone marrow-derived MSCs, the number, viability, and proliferation capacity of ADSCs do not appear to be related to patient age.
Despite the important advantage of adipose tissue-derived MSCs in that they are easier to harvest and isolate, and their increased ability to proliferate and differentiate into chondrocytes, an incomplete understanding of the processes and mechanisms of their differentiation have limited their clinical applications [24]. Studies have reported important differences between various MSC sources, and this has implications on the choice of cells for articular cartilage regeneration [36,37]. MSCs from various tissues vary in their proliferation and differentiation properties (Table 2). An important challenge with adipose tissue-derived MSCs is the creation of fibrous and hypertrophic cartilage instead of articular hyaline cartilage [36].

Table 2.
A summary of MSC properties with respect to their isolation, proliferation, and differentiation [37,38].
3. IPFP-Derived MSCs Used in Cartilage Repair
IPFP-derived MSCs are a subset of MSCs and may have higher cartilage regeneration potential than other MSCs due to their proximity to the knee joint and similarity to subcutaneous adipose tissue cells; they are also more easily accessible than other MSC tissues [39]. The IPFP is intracapsular and extra-synovial, and is located between the patellar tendon, the femoral condyle, and the tibial plateau [40]. The IPFP is a less invasive source of MSCs that can be easily accessed with less morbidity arthroscopically [40]. Obtaining subcutaneous fat by liposuction can obtain a large amount of adipose tissue, however, this can be associated with complications such as skin necrosis, scarring, hematoma, allergic reactions to drugs, temporary bruising, numbness, and nerve injury [41,42].
The cellular environment and adjacent tissues alter cell gene expression [43]. The IPFP is adjacent to the synovial membrane and the synovial fluid as well as articular cartilage, and is potentially influenced by this anatomical location in terms of stem cell differentiation [44]. Additionally, the IPFP cells are likely to be positively affected by biomolecules in the synovial membrane and synovial fluid including TGF-β1, vitamin C, and FGF [43]. Studies show that cartilage-derived morphogenic protein-1 (CDMP-1) and osteogenic protein-1 (OP-1) are more highly expressed in the synovial tissue than in articular cartilage tissue [33], and this can certainly affect IPFP-derived MSC numbers, differentiation, and function [45,46].
The general characteristics of IPFP and adipose tissue-derived MSCs are similar, but the differentiation capacity of IPFP-derived MSCs compared to a chondrogenic and osteogenic lineage is higher than that of adipose tissue-derived cells. SOX-9, collagen type II, and aggrecan gene expression are higher in IPFP-derived MSCs than adipose tissue-derived MSCs, which may explain their more optimal use for articular cartilage regeneration applications [47].
IPFP, and specifically the macrophages from the tissue, can be candidates for the future treatment of cartilage defects and chondrogenesis [42]. Importantly, polarization of macrophages to pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes led to an upsurge in TGF-β (a pro-chondrogenic and anti-inflammatory cytokine) that may play a key role in the differentiation of MSCs and subsequent cartilage repair.
In summary, the use of IPFP-derived MSCs to regenerate articular cartilage has led to encouraging results. The collaboration of researchers from many disciplines and different fields will help address current challenges concerning the therapeutic role of IPFP-derived MSCs, including the best isolation process, and should pave the way for the improved treatment of articular cartilage. Future detailed studies regarding articular cartilage repair strategies focusing on suitable material and methods may eventually extend the application of IPFP-derived MSCs to a future therapeutic role in osteoarthritis.
Many MSCs when injected into the body migrate away from damaged tissue to healthy tissue and a suitable carrier may be needed to “anchor” the MSCs in place [48]. The identification of the optimal cell source as well as the optimal carrier material for these cells plays an important role in the success of stem cell-based regenerative strategies (Table 3).

Table 3.
Some important studies on IPFP-derived MSCs for articular cartilage repair that use a carrier.
The studies mentioned above and other important studies in the literature that used IPFP-derived MSCs for regenerative applications in chondrogenesis are listed in Table 4.

Table 4.
Studies on IPFP-derived MSC proliferation and chondrogenic differentiation.
4. Clinical Applications of IPFP-Derived MSCs for Articular Cartilage Repair
From a therapeutic perspective, clinical studies have already been conducted using adipose tissue and IPFP-derived MSCs (Table 5) [66]. In a clinical study, an average of 1.89 × 106 MSCs isolated from osteoarthritic knees were combined with 3 mL of platelet-rich plasma and placed in a novel biological scaffold. Twenty five patients (eight men and seventeen women) with knee osteoarthritis received these percutaneously administered injections combined with arthroscopic debridement [45,66]. The constructs, along with platelet-rich plasma, were transplanted into articular cartilage defects to facilitate articular cartilage repair. This study showed that at a one-year follow-up, patient knee pain grading scores, including the Tegner activity scale, the mean Lysholm, and visual analog scale (VAS), all significantly improved, with 92% of 25 patients showing an improvement in these scores.

Table 5.
Clinical studies on the use of MSCs for articular cartilage regeneration.
The use of IPFP-derived MSCs in such clinical studies led our group to use adipose-derived MSCs with polycaprolactone to form a 3D “cartilage-like” chondrogenic structure which was then transplanted into small cartilage defects in the knees of sheep. Tissue was isolated from the IPFP of five male sheep and after 6 months, amorphous proliferative tissue was regenerated in the defect area. Real time RT-PCR analysis proved collagen protein expression as well as greater cartilage regeneration compared to controls [69,70]. In this study, the ideal size of a cartilage defect was designated as 1 mm in depth and 4 mm in diameter. A 3D IPFP-derived MSC/polycaprolactone structure was directly transplanted into the defects. Within 6 months, the 3D graft improved cartilage formation and regenerated the cartilage without any inflammatory reaction and in the newly formed cartilage tissue, polygonal chondrocyte clusters were shown. As a result, histological analysis, including staining of safranin O, morphological assays, and the expression of type I and type II collagen, confirmed hyaline-like cartilage formation but without integration into the surrounding cartilage [68].
One of the most prominent clinical studies to date [68] described the safety and effects of an intra-articular injection of adipose tissue-derived MSCs prepared from abdominal subcutaneous fat obtained by liposuction [71] on 18 patients with knee osteoarthritis. At the final six-month follow-up, results demonstrated the formation of new cartilage in the knee joint at the medial femoral and tibial condyles, and a decrease in cartilage defect size using Magnetic Resonance Imaging (MRI).
Dufrane et al. used human adipose tissue-derived MSCs with a demineralized bone matrix to generate a 3D, bone-like construction and transplanted them into large bone defects in six patients. After three months, bone growth was observed and the anatomy and function of the defect was restored without any adverse effects [72]. In another study, human adipose tissue-derived MSCs in combination with platelet-rich plasma, hyaluronic acid, and CaCl2 were injected into the knees of osteoarthritis patients. Bone and cartilage regeneration were observed over three months and showed increased cartilage volume and bone regeneration [67]. Histological assessment confirmed the formation of hyaline-like cartilage integrated into subchondral bone. Poor integration of cartilage into bone is one of the most common problems in articular cartilage repair [67,73]. Furthermore, the efficiency and safety of an intra-articular injection of adipose tissue-derived MSCs in osteoarthritis patients was also investigated [67].
In summary, these studies in the literature not only support the use of IPFP-derived MSC for osteoarthritis therapy and articular cartilage regeneration, but they also provide encouraging clinical results. There are, however, some limitations to the use of IPFP-derived MSC in clinical applications; in human models, researchers typically obtain IPFP tissues from patients that have been diagnosed with osteoarthritis, which affects the function and differentiation activity of IPFP-derived MSC [63,64].
MSCs have shown promise in regenerative medicine and tissue engineering. In the field of regenerative medicine, cell therapy methods involving direct injection, cell seeding on scaffolds, and transplantation of stem cells to the site of the defect have been applied [67]. Much of the evidence in this review supports the use of MSCs together with scaffolds comprising a proper biomimetic structure [74].
Before using MSCs, it is necessary to choose the specific tissue and MSCs for more efficient cartilage regeneration [75]. Identifying the anatomical location of the tissue to harvest MSCs from and the characterization methods to use for the MSCs are promising future directions for this field [76]. Adipose-derived MSCs that are used in cell therapy are obtained mostly from subcutaneous adipose tissue by liposuction. Previous studies have demonstrated that the mechanical disruption associated with liposuction may adversely affect MSCs and may reduce their proliferative capacity [51,77,78]. The IPFP is a rich source of MSCs for articular cartilage regeneration and contains a high number of chondroprogenitor cells [45,72]. Evidence suggests that the IPFP location [79] adjacent to the synovial membrane and fluid potentiates its use for articular cartilage regeneration [80,81,82]. The literature suggests that the proliferation and differentiation potential of IPFP-derived MSCs is independent of age, while MSCs from other sources generally undergo an age-related decline in potential [66]. Recently, IPFP-derived MSCs have been used in cell-based therapies for healing cartilage defects, as reported by Ashton et al. [83].
The self-repair capacity of hyaline cartilage is very limited. Although small subchondral defects may spontaneously repair with the production of hyaline cartilage, larger chondral defects generally heal with fibrocartilage [39]. Fibrocartilage is histochemically and biomechanically inferior to normal hyaline cartilage. Cartilage defects frequently progress to more generalized osteoarthritic changes [84]. Interestingly, a study has shown that hyaline cartilage may not be the definitive repair tissue in the healing of articular cartilage defects [85], and it may be produced at an intermediary stage; in the process of ossification, hyaline cartilage is formed first. So, detailed studies are necessary for articular cartilage repair. In addition, the use of suitable harvesting methods would improve the efficacy of IPFP-derived MSCs in their future therapeutic role in regenerative medicine [86]. Wei et al. reported that the immunohistochemical analysis of IPFP and adipose tissue indicated that IPFP contains more macrophages [65] (Figure 1). Previous studies have shown that pro-inflammatory macrophages prevent chondrogenesis and induce MSCs to produce a fibrocartilage matrix [87,88]. Therefore, due to the various challenges and opinions regarding the therapeutic role of IPFP-derived MSCs, further studies are needed [21].
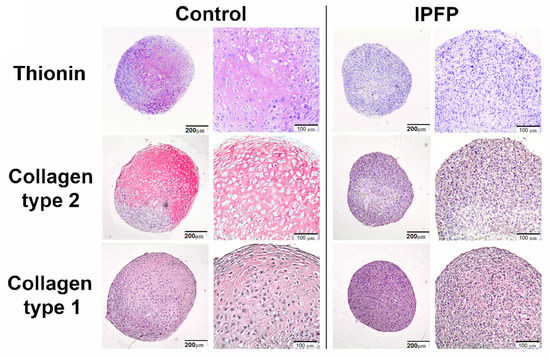
Figure 1.
IPFP inhibits chondrogenic differentiation of MSCs. Immunohistochemically stained images of pellets from MSC donors for glycosaminoglycans with thionin or collagen type II and collagen type I showing IPFP decreased thionin and collagen type II, whereas collagen type I staining was not affected. Reproduced with permission [65]. Copyright 2015, Elsevier.
5. Conclusions
In summary, the use of IPFP-derived MSCs to regenerate articular cartilage has achieved better results than MSCs from other sources. The collaboration of researchers from many disciplines and different fields will help solve current challenges facing the therapeutic applications of IPFP-derived MSCs and may create new therapies for the treatment of articular cartilage defects.
Author Contributions
Conceptualization, P.V., and A.E.; validation, T.J.W., A.C.C.K. and E.A., A.J.M.; writing—original draft preparation, R.M., E.A.; writing—review and editing, T.J.W., W.S.K., A.J.M.; visualization, R.M., and W.S.K.; supervision, P.V., T.J.W., W.S.K. and A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are thankful to the Maragheh University of Medical Sciences, Maragheh, Iran.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- Jain, K.B.; Ravikumar, P. Recent advances in treatments of cartilage regeneration for knee osteoarthritis. J. Drug Deliv. Sci. Technol. 2020, 60, 102014. [Google Scholar] [CrossRef]
- Di Martino, A.; Kon, E.; Perdisa, F.; Sessa, A.; Filardo, G.; Neri, M.P.; Bragonzoni, L.; Marcacci, M. Surgical treatment of early knee osteoarthritis with a cell-free osteochondral scaffold: Results at 24 months of follow-up. Injury 2015, 46, S33–S38. [Google Scholar] [CrossRef]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. Inflammation and fibrosis in adipose tissue of osteoarthritic joints. Nat. Rev. Rheumatol. 2017, 13, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Widuchowski, W.; Widuchowski, J.; Trzaska, T. Articular cartilage defects: Study of 25,124 knee arthroscopies. Knee 2007, 14, 177–182. [Google Scholar] [CrossRef]
- Nam, Y.; Rim, Y.A.; Lee, J.; Ju, J.H. Current therapeutic strategies for stem cell-based cartilage regeneration. Stem Cells Int. 2018, 1–20. [Google Scholar] [CrossRef]
- Falah, M.; Nierenberg, G.; Soudry, M.; Hayden, M.; Volpin, G. Treatment of articular cartilage lesions of the knee. Int. Orthop. 2010, 34, 621–630. [Google Scholar] [CrossRef]
- Jung, S.; Petelska, A.; Beldowski, P.; Augé, W.K.; Casey, T.; Walczak, D.; Lemke, K.; Gadomski, A. Hyaluronic acid and phospholipid interactions useful for repaired articular cartilage surfaces—A mini review toward tribological surgical adjuvants. Colloid Polym. Sci. 2017, 295, 403–412. [Google Scholar] [CrossRef]
- Johnson, L.L. Arthroscopic abrasion arthroplasty: A review. Clin. Orthop. Relat. Res. 2001, 391, S306–S317. [Google Scholar] [CrossRef]
- Laupattarakasem, W.; Laopaiboon, M.; Laupattarakasem, P.; Sumananont, C. Arthroscopic debridement for knee osteoarthritis. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Beris, A.E.; Lykissas, M.G.; Papageorgiou, C.D.; Georgoulis, A.D. Advances in articular cartilage repair. Injury 2005, 36, S14–S23. [Google Scholar] [CrossRef]
- Caffey, S.; McPherson, E.; Moore, B.; Hedman, T.; Vangsness, C.T. Effects of radiofrequency energy on human articular cartilage: An analysis of 5 systems. Am. J. Sports Med. 2005, 33, 1035–1039. [Google Scholar] [CrossRef]
- Kon, E.; Verdonk, P.; Condello, V.; Delcogliano, M.; Dhollander, A.; Filardo, G.; Pignotti, E.; Marcacci, M. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: Systematic clinical data review and study quality analysis. Am. J. Sports Med. 2009, 37 (Suppl. 1), 156–166. [Google Scholar] [CrossRef]
- Steadman, J.R.; Briggs, K.K.; Rodrigo, J.J.; Kocher, M.S.; Gill, T.J.; Rodkey, W.G. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, 477–484. [Google Scholar] [CrossRef]
- Haene, R.; Qamirani, E.; Story, R.A.; Pinsker, E.; Daniels, T.R. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. JBJS 2012, 94, 1105–1110. [Google Scholar] [CrossRef]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of damaged articular cartilage: Current approaches and future directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Maleki Dizaj, S.; Sharifi, S.; Salatin, S.; Rahbar Saadat, Y.; Zununi Vahed, S.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The use of nanomaterials in tissue engineering for cartilage regeneration; current approaches and future perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Guo, W.; Wang, M.; Hao, C.; Gao, S.; Zhang, X.; Li, X.; Chen, M.; Jing, X. Human umbilical cord Wharton’s jelly mesenchymal stem cells combined with an acellular cartilage extracellular matrix scaffold improve cartilage repair compared with microfracture in a caprine model. Osteoarthr. Cartil. 2018, 26, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.-T.; Feng, Y.; Jia, H.-H.; Zhao, M.; Yu, H. Application of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee: A concise review. World J. Stem Cells 2019, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.L.; Wang, J. Cell-based articular cartilage repair: The link between development and regeneration. Osteoarthr. Cartil. 2015, 23, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Helmick, C.G.; Felson, D.T.; Lawrence, R.C.; Gabriel, S.; Hirsch, R.; Kwoh, C.K.; Liang, M.H.; Kremers, H.M.; Mayes, M.D.; Merkel, P.A. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum. 2008, 58, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; L’Heureux, N.; Elisseeff, J.H. Stem Cell and Tissue Engineering; World Scientific: Singapore, 2011. [Google Scholar]
- Siciliano, C.; Bordin, A.; Ibrahim, M.; Chimenti, I.; Cassiano, F.; Gatto, I.; Mangino, G.; Coccia, A.; Miglietta, S.; Bastianelli, D. The adipose tissue of origin influences the biological potential of human adipose stromal cells isolated from mediastinal and subcutaneous fat depots. Stem Cell Res. 2016, 17, 342–351. [Google Scholar] [CrossRef][Green Version]
- Rai, M.F.; Sandell, L.J.; Barrack, T.N.; Cai, L.; Tycksen, E.D.; Tang, S.Y.; Silva, M.J.; Barrack, R.L. A microarray study of articular cartilage in relation to obesity and severity of knee osteoarthritis. Cartilage 2020, 11, 458–472. [Google Scholar] [CrossRef]
- Thijssen, E.; Van Caam, A.; Van Der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Askari, F.; Solouk, A.; Shafieian, M.; Seifalian, A.M. Stem cells for tissue engineered vascular bypass grafts. Artif. Cells Nanomed. Biotechnol. 2017, 45, 999–1010. [Google Scholar] [CrossRef]
- Sharma, R.R.; Pollock, K.; Hubel, A.; McKenna, D. Mesenchymal stem or stromal cells: A review of clinical applications and manufacturing practices. Transfusion 2014, 54, 1418–1437. [Google Scholar] [CrossRef]
- Zuttion, M.S.S.R.; Wenceslau, C.V.; Lemos, P.A.; Takimura, C.; Kerkis, I. Adipose tissue-derived stem cells and the importance of animal model standardization for pre-clinical trials. Rev. Bras. Cardiol. Invasiva 2013, 21, 281–287. [Google Scholar] [CrossRef]
- Technau, A.; Froelich, K.; Hagen, R.; Kleinsasser, N. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy 2011, 13, 310–317. [Google Scholar] [CrossRef]
- Hirano, A.; Sano, M.; Urushihata, N.; Tanemura, H.; Oki, K.; Suzaki, E. Assessment of safety and feasibility of human allogeneic adipose-derived mesenchymal stem cells in a pediatric patient. Pediatric Res. 2018, 84, 575–577. [Google Scholar] [CrossRef]
- Gimble, J.M.; Guilak, F. Adipose-derived adult stem cells: Isolation, characterization, and differentiation potential. Cytotherapy 2003, 5, 362–369. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kim, J.; Cho, J.H.; Chung, H.M.; Chae, J.I. Comparative analysis of human mesenchymal stem cells derived from bone marrow, placenta, and adipose tissue as sources of cell therapy. J. Cell. Biochem. 2016, 117, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Mildmay-White, A.; Khan, W. Cell surface markers on adipose-derived stem cells: A systematic review. Curr. Stem Cell Res. Ther. 2017, 12, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Kabiri, M.; Langroudi, L.; Soleimani, M.; Ai, J. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J. Biomed. Mater. Res. Part A 2016, 104, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Orth, P.; Rey-Rico, A.; Venkatesan, J.K.; Madry, H.; Cucchiarini, M. Current perspectives in stem cell research for knee cartilage repair. Stem Cells Cloning Adv. Appl. 2014, 7, 1. [Google Scholar]
- Han, I.; Kwon, B.-S.; Park, H.-K.; Kim, K.S. Differentiation potential of mesenchymal stem cells is related to their intrinsic mechanical properties. Int. Neurourol. J. 2017, 21 (Suppl. 1), S24. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Johnson, C.; McConnell, J. Evaluation and treatment of disorders of the infrapatellar fat pad. Sports Med. 2012, 42, 51–67. [Google Scholar] [CrossRef]
- Pei, M. Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential. Biomaterials 2017, 117, 10–23. [Google Scholar] [CrossRef]
- Hindle, P.; Khan, N.; Biant, L.; Péault, B. The infrapatellar fat pad as a source of perivascular stem cells with increased chondrogenic potential for regenerative medicine. Stem Cells Transl. Med. 2017, 6, 77–87. [Google Scholar] [CrossRef]
- Belluzzi, E.; El Hadi, H.; Granzotto, M.; Rossato, M.; Ramonda, R.; Macchi, V.; De Caro, R.; Vettor, R.; Favero, M. Systemic and local adipose tissue in knee osteoarthritis. J. Cell. Physiol. 2017, 232, 1971–1978. [Google Scholar] [CrossRef]
- Vahedi, P.; Madadi, M.; Ferdoosi Khosroshahi, A. Comparative study of the proliferation potency of adipose tissue derived stem cells from infrapatellar area, subcutaneous adipose tissue and tail fat pad in a sheep animal model. Daneshvar Med. Basic Clin. Res. J. 2020, 28, 1–11. [Google Scholar]
- Pang, H.-L.; Zhao, Q.-Q.; Ma, Y.; Song, Y.-L.; Min, J.; Lu, J.-R.; Li, H.; Zhao, D.-Q. Long noncoding RNA H19 participates in the regulation of adipose-derived stem cells cartilage differentiation. Stem Cells Int. 2019, 1–33. [Google Scholar] [CrossRef]
- Koh, Y.-G.; Choi, Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef]
- Kouidhi, M.; Villageois, P.; Mounier, C.M.; Ménigot, C.; Rival, Y.; Piwnica, D.; Aubert, J.; Chignon-Sicard, B.; Dani, C. Characterization of human knee and chin adipose-derived stromal cells. Stem Cells Int. 2015, 1–12. [Google Scholar] [CrossRef]
- Garcia, J.; Mennan, C.; McCarthy, H.S.; Roberts, S.; Richardson, J.B.; Wright, K.T. Chondrogenic potency analyses of donor-matched chondrocytes and mesenchymal stem cells derived from bone marrow, infrapatellar fat pad, and subcutaneous fat. Stem Cells Int. 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, Y.; Li, H.; Wang, R.; Yang, D.; Li, B.; Cao, X.; Fu, J. Transplanted dental pulp stem cells migrate to injured area and express neural markers in a rat model of cerebral ischemia. Cell. Physiol. Biochem. 2018, 45, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.T.; Vinardell, T.; Thorpe, S.D.; Haugh, M.G.; Jones, E.; McGonagle, D.; Kelly, D.J. Functional properties of cartilaginous tissues engineered from infrapatellar fat pad-derived mesenchymal stem cells. J. Biomech. 2010, 43, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M.; Buckley, C.T.; Kelly, D.J. A growth factor delivery system for chondrogenic induction of infrapatellar fat pad-derived stem cells in fibrin hydrogels. Biotechnol. Appl. Biochem. 2011, 58, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Felimban, R.; Traianedes, K.; Moulton, S.E.; Wallace, G.G.; Chung, J.; Quigley, A.; Choong, P.F.; Myers, D.E. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PLoS ONE 2014, 9, e99410. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Traianedes, K.; Choong, P.F.; Myers, D.E. Chondrogenesis of human infrapatellar fat pad stem cells on acellular dermal matrix. Front. Surg. 2016, 3, 3. [Google Scholar] [CrossRef]
- Vahedi, P.; Jarolmasjed, S.; Shafaei, H.; Roshangar, L.; Rad, J.S.; Ahmadian, E. In vivo articular cartilage regeneration through infrapatellar adipose tissue derived stem cell in nanofiber polycaprolactone scaffold. Tissue Cell 2019, 57, 49–56. [Google Scholar] [CrossRef]
- Hemstapat, R.; Sriwatananukulkit, O.; Tawonsawatruk, T.; Rattanapinyopituk, K.; Luangwattanawilai, T.; Srikaew, N. Regenerative Effect of Adipose-Derived Mesenchymal Stem Cells on Pain in a Rat Model of Osteochondral Defect. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Sriwatananukulkit, O.; Tawonsawatruk, T.; Rattanapinyopituk, K.; Luangwattanawilai, T.; Srikaew, N.; Hemstapat, R. Scaffold-Free Cartilage Construct from Infrapatellar Fat Pad Stem Cells for Cartilage Restoration. Tissue Eng. Part A 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Chen, I.-H.; Yang, Y.-T.; Chen, Y.-R.; Yang, K.-C. Infrapatellar Fat Pads–Derived Stem Cell Is a Favorable Cell Source for Articular Cartilage Tissue Engineering: An In Vitro and Ex Vivo Study Based on 3D Organized Self-Assembled Biomimetic Scaffold. Cartilage 2021, 1947603520988153. [Google Scholar] [CrossRef]
- Buckley, C.T.; Kelly, D.J. Expansion in the presence of FGF-2 enhances the functional development of cartilaginous tissues engineered using infrapatellar fat pad derived MSCs. J. Mech. Behav. Biomed. Mater. 2012, 11, 102–111. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nakagawa, T.; Reddi, A.H. Mesenchymal progenitor cells derived from synovium and infrapatellar fat pad as a source for superficial zone cartilage tissue engineering: Analysis of superficial zone protein/lubricin expression. Tissue Eng. Part A 2010, 16, 317–325. [Google Scholar] [CrossRef]
- Vinardell, T.; Buckley, C.; Thorpe, S.; Kelly, D. Composition–function relations of cartilaginous tissues engineered from chondrocytes and mesenchymal stem cells isolated from bone marrow and infrapatellar fat pad. J. Tissue Eng. Regen. Med. 2011, 5, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Muneta, T.; Sakaguchi, Y.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Higher chondrogenic potential of fibrous synovium–and adipose synovium–derived cells compared with subcutaneous fat–derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis Rheum. 2006, 54, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Lopa, S.; Colombini, A.; Stanco, D.; De Girolamo, L.; Sansone, V.; Moretti, M. Donor-matched mesenchymal stem cells from knee infrapatellar and subcutaneous adipose tissue of osteoarthritic donors display differential chondrogenic and osteogenic commitment. Eur. Cell Mater. 2014, 27, 298–311. [Google Scholar] [PubMed]
- Vahedi, P.; Soleimanirad, J.; Roshangar, L.; Shafaei, H.; Jarolmasjed, S.; Charoudeh, H.N. Advantages of sheep infrapatellar fat pad adipose tissue derived stem cells in tissue engineering. Adv. Pharm. Bull. 2016, 6, 105. [Google Scholar] [CrossRef]
- Stocco, E.; Barbon, S.; Piccione, M.; Belluzzi, E.; Petrelli, L.; Pozzuoli, A.; Ramonda, R.; Rossato, M.; Favero, M.; Ruggieri, P. Infrapatellar fat pad stem cells responsiveness to microenvironment in osteoarthritis: From morphology to function. Front. Cell Dev. Biol. 2019, 7, 323. [Google Scholar] [CrossRef] [PubMed]
- Tangchitphisut, P.; Srikaew, N.; Numhom, S.; Tangprasittipap, A.; Woratanarat, P.; Wongsak, S.; Kijkunasathian, C.; Hongeng, S.; Murray, I.; Tawonsawatruk, T. Infrapatellar fat pad: An alternative source of adipose-derived mesenchymal stem cells. Arthritis 2016, 2016, 4019873. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Rudjito, R.; Fahy, N.; Bos, K.; Verhaar, J.; Clockaerts, S.; Bastiaansen-Jenniskens, Y.; Van Osch, G. The infrapatellar fat pad from diseased joints inhibits chondrogenesis of mesenchymal stem cells. Osteoarthr. Cartil. 2015, 23, A145–A146. [Google Scholar] [CrossRef]
- Im, G. Clinical use of stem cells in orthopaedics. Eur. Cell Mater. 2017, 33, 183–196. [Google Scholar] [CrossRef]
- Pak, J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: A case series. J. Med. Case Rep. 2011, 5, 1–8. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Vahedi, P.; Roshangar, L.; Jarolmasjed, S.; Shafaei, H.; Samadi, N.; Soleimanirad, J. Effect of low-intensity pulsed ultrasound on regenerative potential of transplanted ASCs-PCL construct in articular cartilage defects in sheep. Indian J. Anim. Sci. 2016, 86, 1111–1114. [Google Scholar]
- Vahedi, P.; Jarolmasjed, S.; Soleimani, A. Transplantation of ASCs-Poly (ε-Caprolactone) Nanofiber Scaffold and Evaluate the Effect of Mechanical Loading of Walking on Articular Cartilage Repair in Sheep Model. Cell Tissue Biol. 2021, 15, 199–207. [Google Scholar] [CrossRef]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- Dufrane, D.; Docquier, P.-L.; Delloye, C.; Poirel, H.A.; André, W.; Aouassar, N. Scaffold-free three-dimensional graft from autologous adipose-derived stem cells for large bone defect reconstruction: Clinical proof of concept. Medicine 2015, 94, e2220. [Google Scholar] [CrossRef]
- Doner, G.P.; Noyes, F.R. Arthroscopic resection of fat pad lesions and infrapatellar contractures. Arthrosc. Tech. 2014, 3, e413–e416. [Google Scholar] [CrossRef]
- da Silva, M.A.; Crawford, A.; Mundy, J.; Correlo, V.; Sol, P.; Bhattacharya, M.; Hatton, P.V.; Reis, R.; Neves, N. Chitosan/polyester-based scaffolds for cartilage tissue engineering: Assessment of extracellular matrix formation. Acta Biomater. 2010, 6, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Almeida, H.V.; Mulhall, K.J.; O’Brien, F.J.; Kelly, D.J. Stem cells display a donor dependent response to escalating levels of growth factor release from extracellular matrix-derived scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 2979–2987. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, T.; Chen, H.Y.; Panebianco, C.; Kaya, K.D.; Brooks, M.J.; Gieser, L.; Morgan, N.Y.; Pohida, T.; Swaroop, A. Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Rep. 2018, 10, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, A.; Lynch, A.P.; Ahearne, M. Self-Assembled Infrapatellar Fat-Pad Progenitor Cells on a Poly-ε-Caprolactone Film for Cartilage Regeneration. Artif. Organs 2016, 40, 376–384. [Google Scholar] [CrossRef]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef]
- Pizzute, T.; Lynch, K.; Pei, M. Impact of tissue-specific stem cells on lineage-specific differentiation: A focus on the musculoskeletal system. Stem Cell Rev. Rep. 2015, 11, 119–132. [Google Scholar] [CrossRef]
- Ding, D.-C.; Wu, K.-C.; Chou, H.-L.; Hung, W.-T.; Liu, H.-W.; Chu, T.-Y. Human infrapatellar fat pad-derived stromal cells have more potent differentiation capacity than other mesenchymal cells and can be enhanced by hyaluronan. Cell Transplant. 2015, 24, 1221–1232. [Google Scholar] [CrossRef]
- McConnell, J. Running injuries: The infrapatellar fat pad and plica injuries. Phys. Med. Rehabil. Clin. 2016, 27, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Eymard, F.; Chevalier, X. Inflammation of the infrapatellar fat pad. Jt. Bone Spine 2016, 83, 389–393. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Zupan, J.; Riemen, A.H.; Kania, K.; Ansboro, S.; White, N.; Clark, S.M.; De Bari, C. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Mak, J.; Jablonski, C.; Leonard, C.; Dunn, J.F.; Raharjo, E.; Matyas, J.; Biernaskie, J.; Krawetz, R. Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci. Rep. 2016, 6, 1–12. [Google Scholar]
- Ashton, B.A.; Allen, T.D.; Howlett, C.; Eaglesom, C.; Hattori, A.; Owen, M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin. Orthop. Relat. Res. 1980, 294–307. [Google Scholar] [CrossRef]
- Shelbourne, K.D.; Jari, S.; Gray, T. Outcome of untreated traumatic articular cartilage defects of the knee: A natural history study. JBJS 2003, 85 (Suppl. 2), 8–16. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage-Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).